Abstract
Background
In this study, the effect of novel taxane SB-T-1216 and paclitaxel were compared on drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES human breast cancer cells.
Materials and Methods
Cell growth and survival were evaluated after 96-hour incubation with tested concentrations of taxanes. The effect on the formation of microtubule bundles was assessed employing fluorescence microscopy and on the cell cycle employing flow cytometric analysis. The activity of caspases was assessed employing commercial colorimetric kits.
Results
The IC50 (concentration resulting in 50% of living cells in comparison with the control) of SB-T-1216 in drug-sensitive cells was 0.6 nM versus 1 nM for paclitaxel. However, the IC50 of SB-T-1216 in drug-resistant cells was 1.8 nM versus 300 nM for paclitaxel. Both SB-T-1216 and paclitaxel at death-inducing concentrations induced the formation of microtubule bundles in drug-sensitive as well as drug-resistant cells. Cell death induced in drug-sensitive and drug-resistant cells by paclitaxel was associated with the accumulation of cells in the G2/M phase. On the contrary, cell death induced by SB-T-1216 took place without the accumulation of cells in the G2/M phase but with a decreased number of G1 cells and the accumulation of hypodiploid cells. Both SB-T-1216 and paclitaxel activated caspase-3, caspase-9, caspase-2 and caspase-8 in drug-sensitive as well as drug-resistant cells.
Conclusion
Cell death induced by both paclitaxel and novel taxane SB-T-1216 in breast cancer cells is associated with caspase activation and with the formation of interphase microtubule bundles. Novel taxane SB-T-1216, but not paclitaxel, seems to be capable of inducing cell death without the accumulation of cells in the G2/M phase.
Keywords: Cell death, taxane SB-T-1216, paclitaxel, breast cancer cells, microtubule bundles, cell cycle, caspases
Classical taxanes, such as paclitaxel (Taxol®) and docetaxel (Taxotere®), have been successfully used in the therapy not only of breast and ovarian cancer, but also of other types of cancer. Paclitaxel was originally isolated from the bark of the Pacific Yew (Taxus brevifolia) white docetaxel is a semisynthetic taxane (1–5).
Microtubules are polymers of tubulin heterodimers containing α and β subunits (6). Microtubules comprise one of the major components of the cytoskeleton of cells and are involved in many cellular processes including mitotic division (7). At the onset of mitosis, cytoplasmic microtubules undergo dramatic rearrangement to form mitotic spindles. Regular bipolar spindles play a pivotal role in the precise segregation of chromosomes. On the other hand, aberrant multipolar spindles are not compatible with cell viability. Most cells derived from defective mitosis are supposed to undergo apoptosis (8). Taxanes are known as mitotic poisons due to their binding to the β subunit of the tubulin heterodimer. This binding accelerates the polymerization of tubulin, stabilizes microtubules and inhibits their depolymerization (6, 9–11). Thus the interaction of taxanes with microtubules results in the formation of microtubule bundles in interphase cells and the formation of asters instead of mitotic spindles during mitosis. In this way, taxanes are thought to block progression through the M-phase of the cell cycle (12, 13). However, the relationship of the mitotic arrest to the induction of cell death by taxanes is unclear (14–16).
Novel synthetic paclitaxel analogs represent new generation taxanes. Some of them have been found to be more effective or more suitable than paclitaxel and docetaxel in the treatment of various types of cancer cells. They are particularly more effective in drug-resistant cancer cells (4, 17–21). Thus novel taxanes provide us with a better chance in the therapy of such types of cancer as breast and ovarian. Some novel taxanes have been found to exhibit a significant activity towards microtubules (13, 19).
We have shown previously that novel taxane SB-T-1216 is found to be more effective, particularly against drug-resistant breast cancer cells, than paclitaxel (18). In the present study, the effect of novel taxane SB-T-1216 and paclitaxel on the formation of microtubule bundles and cell cycle progression, as well as on the activity of caspase-3, caspase-9, caspase-2 and caspase-8, were compared in drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES human breast cancer cells.
Materials and Methods
Materials
Paclitaxel was obtained from Bristol-Myers Squibb (Princeton, NJ, USA). SB-T-1216 was synthesized in the laboratory of Professor I. Ojima (Stony Brook, NY, USA) (20). Indocarbocyanate (Cy3)-conjugated monoclonal anti-tubulin antibody (recognizing an epitope in the C-terminal of β-tubulin) was obtained from Sigma (St. Louis, MO, USA).
Cells and culture conditions
The human breast carcinoma cell lines MDA-MB-435 and NCI/ADR-RES, were obtained from the National Cancer Institute (Frederick, MD, USA). Cells were maintained in a culture medium at 37 °C in a humidified atmosphere of 5% CO2 in air. The culture medium was RPMI-1640 medium containing extra L-glutamine (300 μg/ml), sodium pyruvate (110 μg/ml), HEPES (15 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) as described elsewhere (22) supplemented with 10% fetal bovine serum (M. Kysilka, Brno, Czech Republic). For the experiments, cells were harvested employing trypsin (0.2%) + EDTA (0.02%) in PBS.
Cell growth and survival experiments
Cells maintained in the culture medium were harvested by low-speed centrifugation, washed with the culture medium and then seeded at 1×104 cells/100 μl of medium into the wells of a 96-well plastic plate. Cell growth and survival were evaluated after 96 h of incubation in the culture medium without taxane (control) and at a range of concentrations (0.01–100 nM) of SB-T-1216. The number of living cells was determined by hemacytometer counting after staining with trypan blue (23).
Propidium iodide staining analysis
Cells grown in the culture medium were harvested by low-speed centrifugation, washed with the culture medium and seeded at 3×106 cells/15 ml of culture medium into plastic culture dishes for 24-h preincubation, allowing cells to attach. The medium was changed using culture medium cells without taxane (control) and at selected concentrations of paclitaxel (30 nM for MDA-MB-435 cells, 3000 nM for NCI/ADR-RES cells) or SB-T-1216 (10 nM for MDA-MB-435 cells, 100 nM for NCI/ADR-RES). After the required incubation period (24, 48, 72 h), the cells were harvested by low-speed centrifugation, stained and analyzed as described elsewhere (23).
Immunofluorescence microscopy
For immunofluorescence microscopy, cells were seeded in culture medium onto glass slides for 24-h preincubation, allowing cells to attach. The medium was replaced after this time with culture medium without taxane (control) or at selected concentrations of paclitaxel or SB-T-1216. After incubation (24 h) attached cells were rinsed twice with microtubule-stabilizing buffer (MSB, 20 mM 2-(N-morpholino)ethanesulfonic acid adjusted to pH 6.9 with KOH, 2 mM EGTA, 2 mM MgCl2, and supplemented with 4% PEG 6000). Cells were fixed for 20 min at 37°C with 3% formaldehyde in MSB and then extracted for 4 min with 0.5% Triton®X-100 in MSB. After rinsing the glass slides with MSB, cells were incubated with the Cy3-conjugated anti-tubulin antibody (1:500) for 45 min at room temperature. The preparations were mounted in Mowiol 4–88 supplemented with 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI) used to label cell nuclei. Visualization was carried out with an Fluorescence A70 Provis microscope (Olympus, Hamburg, Germany) and images were captured and processed using a cooled CCD camera SensiCam (Olympus).
Measurement of caspase-3, caspase-9, caspase-2 and caspase-8 activities
Harvested cells were seeded at 5×106 cells/25 ml of culture medium into plastic culture flasks for 24-h preincubation allowing cells to attach after which the medium was changed to a relevant test medium. After 24 h of incubation in culture medium without taxane (control) or with paclitaxel or SB-T-1216, the cells were harvested by low-speed centrifugation and analyzed as described elsewhere (16, 24). Commercial colorimetric kits, Caspase-3/CPP32 Colorimetric Protease Assay, Caspase-9/Mch6/Apaf-3 Colorimetric Protease Assay and Caspase-8/FLICE Colorimetric Protease Assay from Biosource (Camarillo, CA, USA), as well as solutions from a Caspase-3 Colorimetric Protease Assay kit (Biosource) combined with the chromogenic caspase-2 substrate from Alexis Biochemicals (Lausen, Switzerland) were used. The total protein content in samples was determined by the bicinchoninic acid assay (25).
Statistical analysis
The statistical significance of differences was determined using Student’s t-test. P<0.05 was considered statistically significant.
Results
Effect of taxanes on growth and survival
The effect of paclitaxel on drug-sensitive MDA-MB-435 cells and drug-resistant NCI/ADR-RES cells was tested previously (16). Paclitaxel at a concentration of 3 nM and higher induced death of MDA-MB-435 cells within 96 h of incubation, i.e. the number of living cells after the incubation period was lower than the number of cells of the inoculus. The IC50 (concentration of taxane resulting in 50% of living cells in comparison with the control after 96 h of incubation) of paclitaxel was 1 nM. On the contrary, paclitaxel induced the death of NCI/ADR-RES cells at a concentration of 1000 nM and higher. The IC50 of paclitaxel was 300 nM. Approximately 300-fold higher concentration of paclitaxel was required to induce the death of drug-resistant NCI/ADR-RES cells than of drug-sensitive MDA-MB-435 cells. On the basis of these data, 30 nM paclitaxel for drug-sensitive MDA-MB-435 cells and 3,000 nM paclitaxel for drug-resistant NCI/ADR-RES cells were employed as death-inducing concentrations, i.e. the lowest concentrations with full death-inducing effect (16).
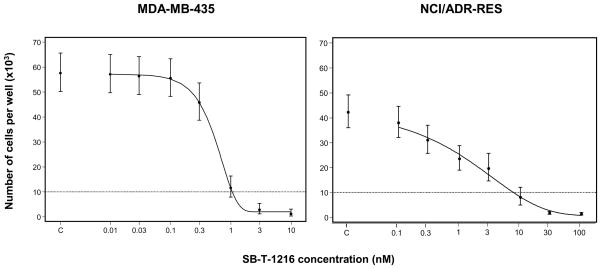
The effect of SB-T-1216 on the growth and survival of drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES cells was tested at a wide range of concentrations (0.01–100 nM). SB-T-1216 induced the death of MDA-MB-435 cells within 96 h of incubation at a concentration of 3 nM and higher. The IC50 of SB-T-1216 for MDA-MB-435 cells was 0.6 nM. In the case of NCI/ADR-RES cells, SB-T-1216 induced cell death at a concentration of 10 nM and higher. The IC50 of SB-T-1216 for NCI/ADR-RES cells was 1.8 nM (Figure 1). The data showed that only about 3-fold higher concentrations of SB-T-1216 were required to induce death in drug-resistant NCI/ADR-RES cells than in drug-sensitive MDA-MB-435 cells. The result indicates that SB-T-1216 was much more effective in drug-resistant NCI/ADR-RES cells than paclitaxel. On the basis of these data, the death-inducing concentrations of SB-T-1216, i.e. 10 nM for drug-sensitive MDA-MB-435 cells and 100 nM for drug-resistant NCI/ADR-RES cells were used. The death-inducing concentrations of paclitaxel and SB-T-1216 were used in further studies with MDA-MB-435 and NCI/ADR-RES cells.
Figure 1.
Effect of SB-T-1216 (0.01–100 nM) on the growth and survival of MDA-MB-435 and NCI/ADR-RES cells. Control cells (C) were incubated without SB-T-1216. The cells were seeded at 10×103 cells/100 μl of medium in the well. The number of cells of the inoculum is shown as a dotted line. The number of living cells was determined after 96 h of incubation (see Materials and Methods). Each point represents the mean of 8 separate cultures ± SEM.
Effect of taxanes on the formation of microtubule bundles
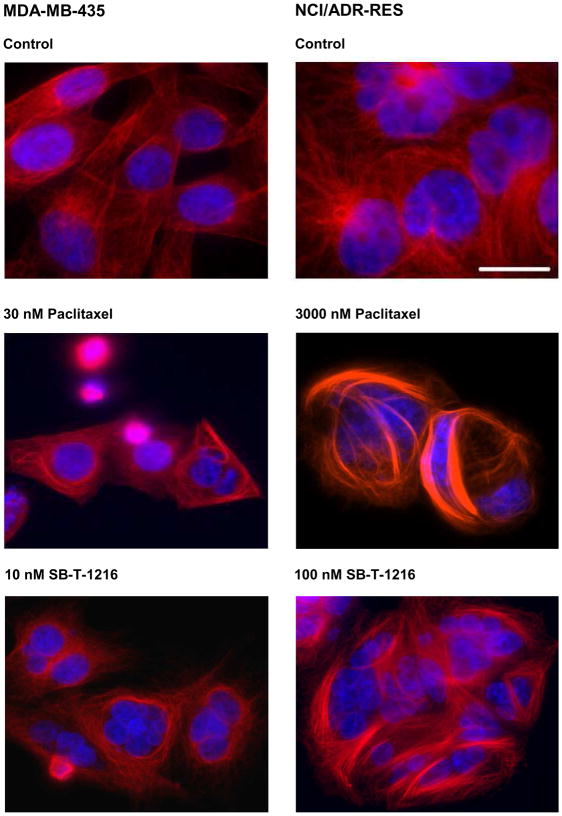
In drug-sensitive MDA-MB-435 cells, paclitaxel at 30 nM as well as SB-T-1216 at 10 nM induced the formation of microtubule bundles within 24 h of incubation (Figure 2). Similarly in drug-resistant NCI/ADR-RES cells, both paclitaxel and SB-T-1216 at 3,000 nM and 100 nM also induced the formation of microtubule bundles within 24 h of incubation (Figure 2).
Figure 2.
Effect of paclitaxel and SB-T-1216 at death-inducing concentrations on the formation of interphase microtubule bundles in drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES cells. Control cells were incubated without taxane. After 24 h of incubation, microtubules were detected by fluorescence microscopy following staining with Cy3-conjugated anti-tubulin antibody (red). Cell nuclei were stained with DAPI (blue) (see Materials and Methods). The scale bar represents 10 μm. The data shown were obtained in one representative experiment of two independent experiments.
Effect of taxanes on the cell cycle
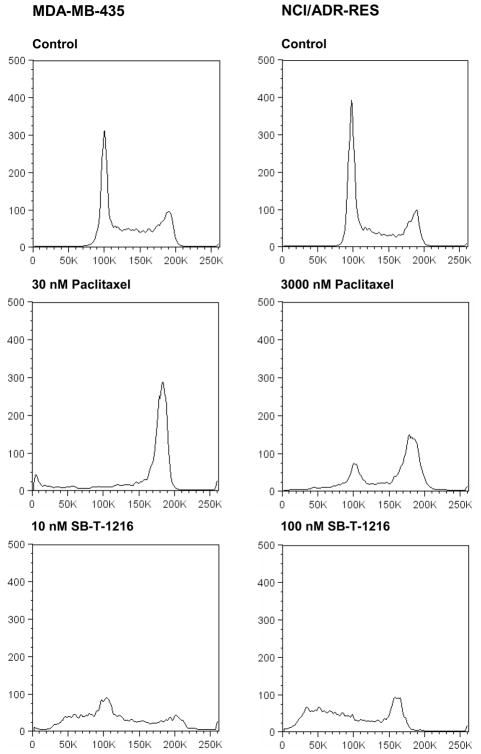
Flow cytometric analysis, after propidium iodide staining, showed that the application of paclitaxel at 30 nM resulted in nearly total accumulation of drug-sensitive MDA-MB-435 cells in the G2/M phase of the cell cycle after 24 h of incubation. On the contrary, the application of SB-T-1216 at 10 nM was without any accumulation of the cells in the G2/M phase. The G1 peak was significantly decreased and the accumulation of near-G1 hypodiploid cells/particles was apparent (Figure 3).
Figure 3.
Effect of paclitaxel and SB-T-1216 at death-inducing concentrations on the DNA histogram of drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES cells. Control cells were incubated without taxane. After 24 h of incubation, the cells were stained with propidium iodide (see Materials and Methods) and analyzed by flow cytometry.
Similarly, the application of paclitaxel at 3,000 nM resulted in the accumulation of drug-resistant NCI/ADR-RES cells in the G2/M phase after 24 h of incubation. The application of SB-T-1216 at 100 nM was again without accumulation of the cells in the G2/M phase. The G1 peak disappeared and the accumulation of near-G1 hypodiploid cells/particles was apparent (Figure 3). This characteristic effect of 100 nM SB-T-1216 without the accumulation in the G2/M phase was found after 24, 48 and 72 hour of incubation. The G2 peak even disappeared within 72-hour incubation (Figure 4).
Figure 4.
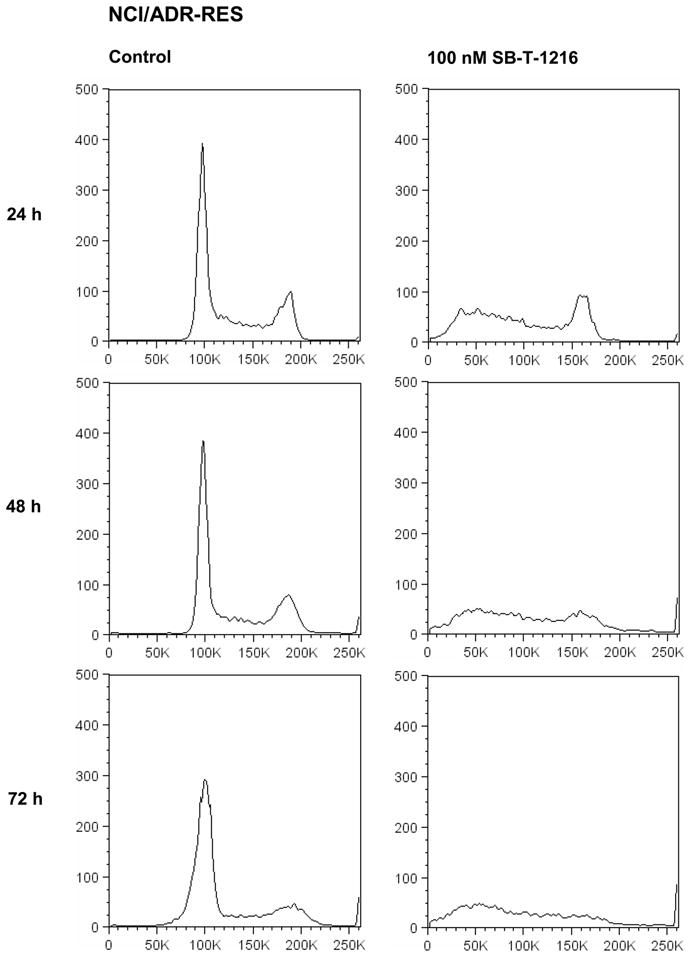
Effect of SB-T-1216 at the death-inducing concentration on the DNA histogram of drug-resistant NCI/ADR-RES cells. Control cells were incubated without taxane. After the incubation period (24, 48, 72 h), the cells were stained with propidium iodide (see Materials and Methods) and analyzed by flow cytometry.
Effect of taxanes on caspase-3, caspase-9, caspase-2 and caspase-8 activities
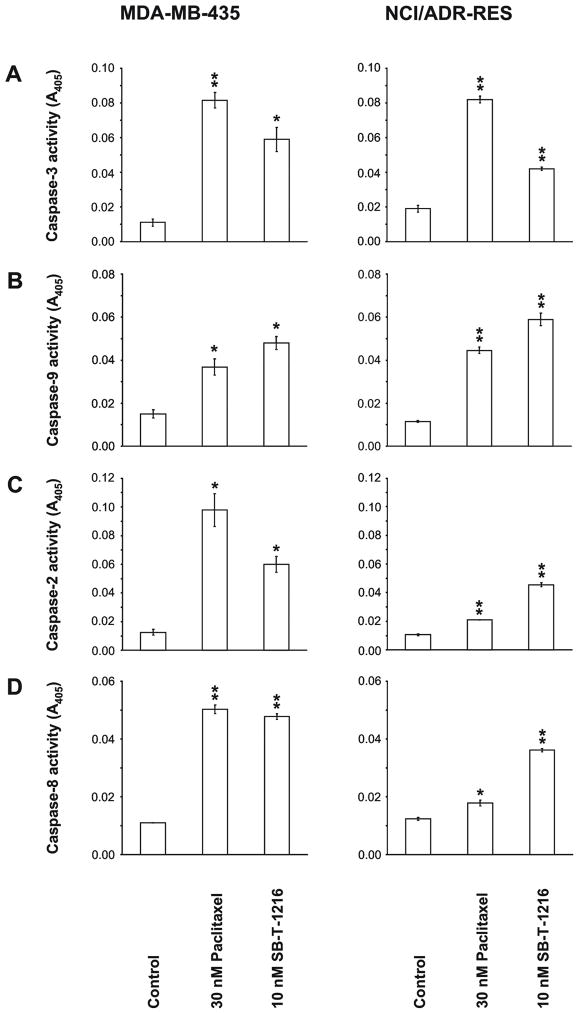
The employed colorimetric assays showed that after 24 h of incubation with paclitaxel or SB-T-1216 at the death-inducing concentrations (30 nM or 10 nM, respectively), the activity of caspase-3 increased significantly in drug-sensitive MDA-MB-435 cells. Caspase-3 activity increased approximately 7.5-fold after the application of paclitaxel (P<0.01) and approximately 5.5-fold after the application of SB-T-1216 (P<0.05). Similarly, caspase-3 activity increased significantly (P<0.01) in drug-resistant NCI/ADR-RES cells after incubation with paclitaxel or SB-T-1216 at the death-inducing concentrations (3,000 nM or 100 nM). The activity of caspase-3 increased approximately 4.5-fold in the case of paclitaxel and approximately 2-fold in the case of SB-T-1216 (Figure 5A).
Figure 5.
Effect of paclitaxel and SB-T-1216 at death-inducing concentrations on caspase activities in drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES cells. Control cells were incubated without taxane. After 24 h of incubation, the activity of caspase-3 (A), -9 (B), -2 (C) and -8 (D) were measured as the absorbance of the cleaved product of a respective chromogenic substrate at 405 nm, employing a commercial colorimetric kit (see Materials and Methods). Each column represents the mean of 2 experimental values ± SEM. *P<0.05, **P<0.01 when comparing with control values.
The activity of caspase-9 also increased significantly in both drug-sensitive MDA-MB-435 (P<0.05) and drug-resistant NCI/ADR-RES (P<0.01) cells after incubation with the death-inducing concentration of paclitaxel or SB-T-1216. The increase was approximately 2.5- to 4-fold for paclitaxel and approximately 3- to 5-fold for SB-T-1216 (Figure 5B).
Concerning caspase-2 activity in drug-sensitive MDA-MB-435 cells, a 7.5-fold increase was detected after incubation with the death-inducing concentration of paclitaxel (P<0.05) and a 4.5-fold increase after incubation with SB-T-1216 (P<0.05). In drug-resistant NCI/ADR-RES cells, we detected a smaller but still statistically significant 2-fold increase of caspase-2 activity after incubation with paclitaxel (P<0.01) and approximately 4.5-fold increase after incubation with SB-T-1216 (P<0.01) (Figure 5C).
A significant (P<0.01) approximately 4.5-fold increase of caspase-8 activity was detected in drug-sensitive MDA-MB-435 cells after incubation with death-inducing concentrations of both paclitaxel and SB-T-1216. In drug-resistant NCI/ADR-RES cells, again a smaller but still statistically significant 1.5-fold increase of caspase-8 activity was detected for paclitaxel (P<0.05) and an approximately 3-fold increase for SB-T-1216 (P<0.01) (Figure 5D).
Discussion
Taxanes are known to modify microtubule dynamics leading to the formation of interphase microtubule bundles and aberrant mitotic spindles. These events are believed to result in blocking of progression through the M-phase of the cell cycle (6, 9, 10, 13). It is also supposed that mitotic arrest could represent a mechanism by which taxanes induce cell death. However, this question has not been clearly answered yet (14–16).
We reported previously that novel taxane SB-T-1216 was more effective than classical taxanes against breast cancer cells (18). Our previous data (16) together with data of the present study (see Figure 1) demonstrate that SB-T-1216 is much more effective than paclitaxel against drug-resistant breast cancer cells and that SB-T-1216 could represent a potentially powerful tool for the treatment of drug-resistant breast cancer. Therefore, we were curious about the mechanism of the higher efficiency of SB-T-1216 and thus we compared the effects of SB-T-1216 and paclitaxel on the formation of interphase microtubule bundles, cell cycle progression and also on the activity of caspase-3, caspase-9, caspase-2 and caspase-8 in both drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES human breast cancer cells.
Both paclitaxel and SB-T-1216 at death-inducing concentrations induced the formation of interphase microtubule bundles in drug-sensitive MDA-MB-435 cells during 24-h incubation. Similarly paclitaxel and SB-T-1216 induced the formation of interphase microtubule bundles in drug-resistant NCI/ADR-RES cells (see Figure 2). It was shown previously that some novel taxanes can exhibit a signitificant activity towards microtubules (11, 13, 19, 21) and that they could interact with microtubules differently than paclitaxel (13). However, it does not seem that SB-T-1216 at death-inducing concentrations exerts different effect on the formation of microtubule bundles than paclitaxel in tested drug-sensitive and drug-resistant breast cancer cells. Thus our findings suggest that the induction of cell death by both taxanes in breast cancer cells is associated with analogous formation of interphase microtubule bundles.
Cell death induced in both MDA-MB-435 cells and NCI/ADR-RES cells at the death-inducing concentration of SB-T-1216 resulted in a markedly different cell cycle distribution from that of paclitaxel. The effect of paclitaxel was characterized by the accumulation of cells in the G2/M phase. On the contrary, the effect of SB-T-1216 was not associated with the accumulation in the G2/M phase and was characterized by the accumulation of hypodiploid cells (see Figure 3 and 4). These findings suggest that novel taxane SB-T-1216 at death-inducing concentrations, but not paclitaxel, induces cell death via a pathway different from that involving M-phase block. Some previous studies have suggested that taxanes can induce apoptosis independent of mitotic arrest (14, 26–28). This is usually related to lower taxane concentrations, while higher taxane concentrations cause cell death connected with mitotic arrest (27–29). Thus, it seems that mitotic arrest does not represent an indispensable prerequisite for taxane-induced cell death. Such indication is also supported by the finding that baccatin III, which contains the core taxane ring, can induce apoptosis independent of G2/M arrest (14). Our results suggest that the ability of SB-T-1216, but not of paclitaxel, to switch on the pathway independent of mitotic arrest in drug-resistant cells can explain, at least partly, the significantly higher potency of SB-T-1216 against drug-resistant cells.
The key executioner caspase-3 and upstream caspase-9 were activated in both drug-sensitive MDA-MB-435 and drug-resistant NCI/ADR-RES cells when cell death was induced by paclitaxel (16) as well as by SB-T-1216 (see Figure 5A and 5B). This fact indicates that the cell death induced by both taxanes could be related to the mitochondrial pathway of caspase activation as it was also suggested by others (30–34). However, significant activation of caspase-2 and caspase-8 was also found in both cell lines after the application of both taxanes at the death-inducing concentration (see Figure 5C and 5D). The role of caspase-2 in apoptosis induction is not clear yet but several lines of evidence point at caspase-2 as being a major player in apoptosis induction (35, 36). Several studies demonstrated caspase-2 activation in various types of cancer cells after apoptosis induction by taxanes (37–39). The involvement of caspase-2 activation in apoptosis of breast cancer cells induced by various stimuli was also demontrated (40–42). Concerning caspase-8 activation, the activation was shown during taxane-induced apoptosis in lymphoma and melanoma cells (38, 39) and during apoptosis induction by HOXA5 in breast cancer cells (40). The activation of caspase-8 suggests that the death receptor pathway of caspase activation could also be somehow involved in cell death induced by taxanes. Considering the activation of all discussed caspases, functional sequence of the activation of individual caspases remains to be elucidated in order to understand better mechanisms involved in apoptosis induction by taxanes in breast cancer cells.
Taken together, we conclude that cell death induced by both of the tested taxanes, namely paclitaxel and novel taxane SB-T-1216, in breast cancer cells is associated with the activation of several caspases and with the formation of interphase microtubule bundles. However, we suggest that novel taxane SB-T-1216, but not paclitaxel, can induce cell death that is not directly related to the accumulation of cells in the G2/M phase, via a pathway independent of M-phase block. The ability of SB-T-1216 to switch on such a pathway in drug-resistant cells could help to explain its significantly higher potency against those cells.
Acknowledgments
This work was supported by grant NR9426-3/2007 from the IGA, Ministry of Health of the Czech Republic, by grant 301/09/0362 from the Grant Agency of the Czech Republic, by grant 204/05/H023 from the Grant Agency of the Czech Republic, a grant from the National Cancer Institute, USA (CA103314 to I.O.) and a Faculty Development Award from the New York State Office of Science, Technology & Academic Research (to I.O.).
References
- 1.Rowinsky EK. The development and clinical utility of taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 2.Vaishampayan U, Parchment RE, Jasti BR, Hussain M. Taxanes: an overview of the pharmacokinetics and pharmacodynamics. Urology. 1999;54:22–29. doi: 10.1016/s0090-4295(99)00451-3. [DOI] [PubMed] [Google Scholar]
- 3.Choy H. Taxanes in combined modality therapy for solid tumors. Crit Rev Oncol Hematol. 2001;37:237–247. doi: 10.1016/s1040-8428(00)00112-8. [DOI] [PubMed] [Google Scholar]
- 4.Miller ML, Ojima I. Chemistry and chemical biology of taxane anticancer agents. Chem Record. 2001;1:195–211. doi: 10.1002/tcr.1008. [DOI] [PubMed] [Google Scholar]
- 5.Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxane-induced apoptosis. Curr Med Chem Anticancer Agents. 2003;3:291–306. doi: 10.2174/1568011033482422. [DOI] [PubMed] [Google Scholar]
- 6.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuszynski JA, Trpišová B, Sept D, Brown JA. Selected physical issues in the structure and function of microtubules. J Struct Biol. 1997;118:94–106. doi: 10.1006/jsbi.1997.3843. [DOI] [PubMed] [Google Scholar]
- 8.Brinkley BR. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 2001;11:18–21. doi: 10.1016/s0962-8924(00)01872-9. [DOI] [PubMed] [Google Scholar]
- 9.Spencer MC, Faulds D. Paclitaxel: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs. 1994;48:795–845. doi: 10.2165/00003495-199448050-00009. [DOI] [PubMed] [Google Scholar]
- 10.Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA. Insights into the mechanism of microtubule stabilization by Taxol. Proc Natl Acad Sci USA. 2006;103:10166–10173. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larroque AL, Dubois J, Thoret S, Aubert G, Chiaroni A, Guéritte F, Guénard D. Novel C2-3′ N-peptide-linked macrocyclic taxoids. Part 2: synthesis and biological activities of docetaxel analogues with a peptide side chain at C2 and their macrocyclic derivatives. Bioorg Med Chem. 2007;15:563–574. doi: 10.1016/j.bmc.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Sackett D, Fojo T. Taxanes. Cancer Chemother Biol Response Modif. 1997;17:59–79. [PubMed] [Google Scholar]
- 13.Jordan MA, Ojima I, Rosas F, Distefano M, Wilson L, Scambia G, Ferlini C. Effects of novel taxanes SB-T-1213 and IDN5109 on tubulin polymerization and mitosis. Chem Biol. 2002;9:93–101. doi: 10.1016/s1074-5521(01)00097-7. [DOI] [PubMed] [Google Scholar]
- 14.Fan W. Possible mechanisms of paclitaxel-induced apoptosis. Biochem Pharmacol. 1999;57:1215–1221. doi: 10.1016/s0006-2952(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 15.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlichová M, Koc M, Truksa J, Nad’ová Z, Václavíková R, Kovář J. Cell death induced by taxanes in breast cancer cells: cytochrome c is released in resistant but not in sensitive cells. Anticancer Res. 2005;25:4215–4224. [PubMed] [Google Scholar]
- 17.Nicoletti MI, Colombo T, Rossi C, Monardo C, Stura S, Zucchetti M, Riva A, Morazzoni P, Donati MB, Bombardelli E, D’Incalci M, Giavazzi R. IDN5109, a taxane with oral bioavailability and potent antitumor activity. Cancer Res. 2000;60:842–846. [PubMed] [Google Scholar]
- 18.Ehrlichová M, Václavíková R, Ojima I, Pepe A, Kuznetsova LV, Chen J, Truksa J, Kovář J, Gut I. Transport and cytotoxicity of paclitaxel, docetaxel, and novel taxanes in human breast cancer cells. N-S Arch Pharmacol. 2005;372:95–105. doi: 10.1007/s00210-005-1080-4. [DOI] [PubMed] [Google Scholar]
- 19.Ferlini C, Raspaglio G, Mozzetti S, Cicchillitti L, Filippetti F, Gallo D, Fattorusso C, Campiani G, Scambia G. The seco-taxane IDN5390 is able to target class III beta-tubulin and to overcome paclitaxel resistance. Cancer Res. 2005;65:2397–2405. doi: 10.1158/0008-5472.CAN-04-3065. [DOI] [PubMed] [Google Scholar]
- 20.(a) Geney R, Chen J, Ojima I. Recent advances in the new generation taxane anticancer agents. Med Chem. 2005;1:125–139. doi: 10.2174/1573406053175292. [DOI] [PubMed] [Google Scholar]; (b) Ojima I, Chen J, Sun L, Borella CP, Wang T, Miller ML, Lin S, Geng X, Kuznetsova L, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz SB, Mallen-St Clair J, Guerriero JL, Bar-Sagi D, Veith JM, Pera P, Bernacki RJ. Design, Synthesis and biological evaluation of new generation taxoids. J Med Chem. 2008;51:3203–3221. doi: 10.1021/jm800086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowinsky EK, Calvo E. Novel agents that target tubulin and related elements. Semin Oncol. 2006;33:421–435. doi: 10.1053/j.seminoncol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Musílková J, Kovář J. Additive stimulatory effect of extracellular calcium and potassium on non-transferrin ferric iron uptake by HeLa and K562 cells. Biochim Biophys Acta. 2001;1514:117–126. doi: 10.1016/s0005-2736(01)00367-4. [DOI] [PubMed] [Google Scholar]
- 23.Kovář J, Valenta T, Štýbrová H. Differing sensitivity of tumor cells to apoptosis induced by iron deprivation in vitro. In Vitro Cell Dev Biol Anim. 2001;37:450–458. doi: 10.1290/1071-2690(2001)037<0450:DSOTCT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Koc M, Nad’ová Z, Truksa J, Ehrlichová M, Kovář J. Iron deprivation induces apoptosis via mitochondrial changes related to Bax translocation. Apoptosis. 2005;10:381–393. doi: 10.1007/s10495-005-0812-8. [DOI] [PubMed] [Google Scholar]
- 25.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen JG, Yang CP, Cammer M, Horwitz SB. Gene expression and mitotic exit induced by microtubule-stabilizing drugs. Cancer Res. 2003;63:7891–7899. [PubMed] [Google Scholar]
- 27.Ikui AE, Yang CH, Matsumoto T, Horwitz SB. Low concentrations of taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle. 2005;4:1385–1388. doi: 10.4161/cc.4.10.2061. [DOI] [PubMed] [Google Scholar]
- 28.Estève MA, Carré M, Braguer D. Microtubules in apoptosis induction: are they necessary? Curr Cancer Drug Targets. 2007;7:325–334. doi: 10.2174/156800907783220480. [DOI] [PubMed] [Google Scholar]
- 29.Torres K, Horwitz SB. Mechanisms of taxol induced cell death are concentration dependent. Cancer Res. 1998;58:3620–3626. [PubMed] [Google Scholar]
- 30.Kottke TJ, Blajeski AL, Martins LM, Mesner PW, Jr, Davidson NE, Earnshaw WC, Armstrong DK, Kaufmann SH. Comparison of paclitaxel-, 5-fluoro-2′-deoxyuridine-, and epidermal growth factor (EGF)- induced apoptosis. Evidence for EGF-induced anokis. J Biol Chem. 1999;274:15927–15936. doi: 10.1074/jbc.274.22.15927. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich K, Wieder T, Von Haefen C, Radetzki S, Janicke R, Schulze-Osthoff K, Dorken B, Daniel PT. Overexpression of caspase-3 restores sensitivity for drug-induced apoptosis in breast cancer cell lines with acquired drug resistance. Oncogene. 2001;20:2749–2760. doi: 10.1038/sj.onc.1204342. [DOI] [PubMed] [Google Scholar]
- 32.André N, Carré M, Brasseur G, Pourroy B, Kovacic H, Briand C, Braguer D. Paclitaxel targets mitochondria upstream of caspase activation in intact human neuroblastoma cells. FEBS Lett. 2002;532:256–260. doi: 10.1016/s0014-5793(02)03691-8. [DOI] [PubMed] [Google Scholar]
- 33.Kolfschoten G, Hulscher TM, Duyndam MCA, Pinedo HM, Boven E. Variation in the kinetics of caspase-3 activation, Bcl-2 phosphorylation and apoptotic morphology in unselected human ovarian cancer cell lines as a response to docetaxel. Biochem Pharmacol. 2002;63:733–743. doi: 10.1016/s0006-2952(01)00895-4. [DOI] [PubMed] [Google Scholar]
- 34.Jiang S, Zu Y, Fu Y, Zhang Y, Efferth T. Activation of the mitochondria-driven pathway of apoptosis in human PC-3 prostate cancer cells by a novel hydrophilic paclitaxel derivative, 7-xylosyl-10-deacetylpaclitaxel. Int J Oncol. 2008;33:103–111. [PubMed] [Google Scholar]
- 35.Zhivotovsky B, Orrenius S. Caspase-2 function in response to DNA damage. Biochem Biophys Res Commun. 2005;331:859–867. doi: 10.1016/j.bbrc.2005.03.191. [DOI] [PubMed] [Google Scholar]
- 36.Ho LH, Read SH, Dorstyn L, Lambrusco L, Kumar S. Caspase-2 is required for cell death induced by cytoskeletal disruption. Oncogene. 2008;27:3393–3404. doi: 10.1038/sj.onc.1211005. [DOI] [PubMed] [Google Scholar]
- 37.Yuan SY, Hsu SL, Tsai KJ, Yang CR. Involvement of mitochondrial pathway in taxol-induced apoptosis of human T24 bladder cancer cells. Urol Res. 2002;30:282–288. doi: 10.1007/s00240-002-0263-4. [DOI] [PubMed] [Google Scholar]
- 38.Wang YF, Chen CY, Chung SF, Chiou YH, Lo HR. Involvement of oxidative stress and caspase activation in paclitaxel-induced apoptosis of primary effusion lymphoma cells. Cancer Chemother Pharmacol. 2004;54:322–330. doi: 10.1007/s00280-004-0831-0. [DOI] [PubMed] [Google Scholar]
- 39.Mhaidat NM, Wang Y, Kiejda KA, Zhang XD, Hersey P. Docetaxel-induced apoptosis in melanoma cells is dependent on activation of caspase-2. Mol Cancer Ther. 2007;6:752–761. doi: 10.1158/1535-7163.MCT-06-0564. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Chung S, Sukumar S. HOXA5-induced apoptosis in breast cancer cells is mediated by caspase 2 and 8. Mol Cell Biol. 2004;24:924–935. doi: 10.1128/MCB.24.2.924-935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansilla S, Priebe W, Portugal J. Mitotic catastrophe results in cell death by caspase-dependent and caspase-independent mechanisms. Cell Cycle. 2006;5:53–60. doi: 10.4161/cc.5.1.2267. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Nwachukwu JC, Li D, Vulin AI, Martinez-Caballero S, Kinnally KW, Samuels HH. The nuclear receptor interacting factor-3 transcriptional coregulator mediates rapid apoptosis in breast cancer cells through direct and bystander-mediated events. Cancer Res. 2007;67:1775–1782. doi: 10.1158/0008-5472.CAN-06-4034. [DOI] [PubMed] [Google Scholar]