Abstract
Hepatocellular carcinoma (HCC) is an intrinsically chemotherapy refractory malignancy. Development of effective therapeutic regimens would be facilitated by improved preclinical HCC models. Currently, most models consist of subcutaneous human tumor transplants in immunodeficient mice; however, these do not reproduce the extensive liver disease associated with HCC or metastasize. To address this deficiency, we developed an orthotopic model. Human HCC cells were transfected with the gene encoding secretable β-subunit human choriogonadotropin (β-hCG), which was used as a surrogate marker of tumor burden. The HCC cells were implanted into the left liver lobe of severe combined immunodeficient (SCID) mice, after which the efficacy of different therapies was evaluated on established, but liver-confined human Hep3B cell line HCC. Treatments included sorafenib or metronomic chemotherapy using cyclophosphamide (CTX), UFT, an oral 5-fluorouracil prodrug, or doxorubicin either alone or in various combinations, with or without an antiangiogenic agent, DC101, an anti-vascular endothelial growth factor receptor-2 antibody. Sorafenib inhibited tumor growth in a dose-dependent manner but caused severe weight loss in SCID mice, thus necessitating use of DC101 in subsequent experiments. Although less toxicity was observed using either single or doublet metronomic chemotherapy without any added antiangiogenic agent, none, provided survival benefit. In contrast, significantly improved overall survival was observed using various combinations of metronomic chemotherapy regimens such as UFT + CTX with DC101. In conclusion, using this model of liver-confined but advanced HCC suggests that the efficacy of a targeted antiangiogenic drug or metronomic chemotherapy can be mutually enhanced by concurrent combination treatment.
Introduction
A recurring concern regarding experimental therapeutic studies in oncology using various types of tumor models in mice is the frequent discrepancy of the antitumor efficacy results obtained using such models, which are often impressive, with the much more modest or negative results obtained in subsequent clinical trials [1–4]. There are a number of possible factors that can help explain this fundamental gap in translational research, one of which is the presence, or not, of advanced metastatic disease [1]. Typically, most patients in phase 1, 2, and 3 clinical trials have advanced disease involving high-volume visceral metastases in one or multiple organ sites; often, such patients have received previous therapies and thus have “refractory” disease. Successful treatment of such metastatic patients with new therapeutic regimens/drugs is much more difficult compared with patients with low-volume, asymptomatic micrometastatic disease or small, surgically resectable solitary tumors, whether primary or metastatic in nature. Yet, virtually all experimental therapy preclinical investigations in mice have historically involved models of localized, encapsulated primary tumors, usually implanted subcutaneously, as well as spontaneously arising tumors in genetically engineered mouse models or low-volume microscopic metastases at the time treatment was initiated [1].
To address this fundamental problem, we have been developing new models of advanced disease, including visceral metastases, using human tumor xenografts in immune deficient mice for testing various new drugs and treatment strategies, especially long-term low-dose metronomic chemotherapy regimens [5–8]. The therapeutic outcomes are compared with those testing the same therapy using the identical tumor cell line grown as a primary tumor transplant, either subcutaneously or orthotopically. The first such model we developed involved breast cancer using the human MDA-MB-231 cell line [5]; and the second, metastatic melanoma using the WM-239 cell line [6]. In both cases, the basic strategy involved serial selection of highly metastatic variant sublines using a multistep procedure of orthotopic primary tumor implantation, surgical resection of the established primary tumor, and the recovery of emergent lung metastases months later [5,6,9]. More recently, we have also generated Her-2/erbB-2-positive highly metastatic variants of MDA-MB-231, which are “tagged” with markers to allow serial assessment of disease burden and response to therapy, for example, the secreted β-subunit of human choriogonadotropin hormone (β-hCG) measured in the urine [8–10] or luciferase, allowing whole-body bioluminescent imaging of tumor [11].
Using these aforementioned models, we have reported a number of actual or potentially clinically relevant observations. For example, monotherapy with targeted biologic drugs such as trastuzumab failed to induce a survival effect when used as a monotherapy to treat mice with advanced established human breast cancer metastases in severe combined immunodeficient (SCID) mice, whereas the expected growth delay was caused when used to treat localized primary tumors [9]. We have observed similar results using antiangiogenic drugs such as DC101, the antibody to vascular endothelial growth factor receptor-2 (VEGFR-2) [1]. Surprisingly, however, the opposite pattern has been observed when testing long-term oral “doublet” chemotherapy regimens, for example, low-dose cyclophosphamide (CTX) and low-dose UFT, a 5-fluorouracil (5-FU) prodrug, in an advanced human metastatic breast cancer model [5]. The remarkable antimetastatic effect of the CTX/UFT doublet combination was pivotal in the decision to initiate a phase 2 trial of daily low-dose metronomic capecitabine (another oral 5-FU prodrug) and CTX, combined with bevacizumab, for the treatment of metastatic breast cancer, the results of which were highly encouraging [12]. In another model, involving a highly metastatic melanoma variant, we observed the emergence of spontaneous brain metastases in long-term surviving mice treated with long-term metronomic vinblastine and CTX [6], a finding that reflects an increasingly common clinical phenomenon observed when certain types of cancer patients with visceral metastatic disease attain significant therapy-induced prolongation of survival [6,13–15].
With these precedents in mind, we decided to develop a new model of advanced human hepatocellular carcinoma (HCC) in immune deficient mice with which to study the impact of various investigational metronomic chemotherapy regimens and/or targeted antiangiogenic drugs such as sorafenib or DC101, the antimouse VEGFR-2 antibody. The decision to study HCC was based on several considerations. First, in addition to being one of the most lethal of all malignancies, until recently, there was no successful systemic therapy available to treat advanced HCC. However, sorafenib, a multitargeted antiangiogenic receptor tyrosine kinase inhibitor (RTKI) was recently approved for advanced HCC based on modest but statistically significant survival advantages observed in pivotal randomized phase 3 clinical trials [16,17]. In contrast, chemotherapy has marginal, if any, benefit when used at conventional doses and schedules [18,19]. The use of toxic conventional maximum tolerated dose chemotherapy is also made difficult by the comorbidity of liver dysfunction that often presents in HCC patients [20]. However, less toxic low-dose metronomic chemotherapy regimens might, in some circumstances, constitute an alternative and feasible form of chemotherapy for both advanced and early stage HCC, especially when combined with a targeted, biologic antiangiogenic drug, as shown in other systems [21,22]. To this end, we report here the details of an advanced orthotopic HCC therapy model we have developed and its use in long-term therapy investigations involving various empiric metronomic chemotherapy regimens when these are combined with a biologic VEGF pathway-targeting antiangiogenic drug. The model involves orthotopic transplantation of a human HCC cell line; however, in this case, the tumor(s) that arose in the liver were not resected, to allow locally advanced progression of the disease within this organ site. This approach was taken because most advanced HCC patients die of heavy disease burden confined to the liver without necessarily having evidence of macroscopic distant metastases. Here, we report promising results showing significant prolonged overall survival with minimal associated toxicity using doublet metronomic chemotherapy combinations such as UFT and CTX with DC101.
Materials and Methods
Cell Lines
The human HCC cell lines PLC and Hep3B were purchased from ATCC (Manassas, VA). They were maintained in Dulbecco's modified Eagle medium (DMEM) with high glucose (Hyclone, UT) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone) at 37°C in a humidified atmosphere containing 5% CO2. Hep3B and PLC cells were transfected with the hCG. pIRES vector and hCG-expressing variants (Hep3B-hCG and PLC-hCG) were obtained by puromycin selection. The hCG.pIRES vector carries the gene for β-hCG, and the secreted protein can be detected in the urine as a surrogate marker for tumor burden [10]. Clones (Hep3B-hCG and PLC-hCG) were used for the experiment based on the highest levels of protein expression. Cells are dissociated by trypsin, washed once in serum-containing DMEM followed by two washes in serum-free DMEM. Cells are resuspended in serum-free DMEM for implantation.
Animals
Female CB17 SCID (Charles River Canada, Quebec, Canada) or athymic nude (nu/nu) mice (Harlan, Indiana) between 6 and 8 weeks of age were used. All animals were housed in microisolator cages, and procedures were carried out in accordance with institutional guidelines for proper animal care and maintenance.
β-hCG Measurements
Urine β-hCG was measured with the commercially available Pathzyme Free β-hCG ELISA Kit (Omega Diagnostics Ltd, Scotland, UK), which allows for quantitative determination of β-hCG. Urine β-hCG levels were normalized by concomitant measurement of urine creatinine levels (using QuantiChrom Creatinine Assay Kit; BioAssay Systems, Hayward, CA) as detailed by Shih et al. [10]. Urine was collected by placing mice individually into small sterile aerated boxes for 2 hours.
Subcutaneous (Ectopic) Transplantation of Human HCC Cells
A bolus of 5 million Hep3B-hCG cells was injected subcutaneously into CB-17 SCID mice. Tumor size was assessed weekly by means of Vernier calipers and the following formula: (w1 x w2 x w2) / 2, where w1 and w2 are the length and width (mm), respectively.
Orthotopic Liver Implantation
Aseptic technique was used throughout the surgical procedure. The anesthetized mouse was laid on its back, and a 1-cm transverse incision was made through the skin and peritoneum of the left upper abdomen. A portion of the liver was exposed by applying gentle pressure on the abdomen. A bolus of 106 cells in a 10-µl volume was injected into the subsera of the liver using a Hamilton syringe and 30-gauge needle. After swabbing the area with sterile gauze, the pressure was removed from the abdomen allowing the liver to slip back into place. The peritoneum was closed with 4.0 absorbable sutures, and the skin was closed with wound clips.
β-hCG Immunostaining of Tumors
Formalin-fixed tumor and tissue sections were deparaffinized and rehydrated by standard histology protocols. The slides were then placed in preheated 10 mM citrate buffer (pH 6.0) in a pressure cooker and incubated for 20 to 40 minutes for antigen retrieval. After washing the slides in Tris-buffered saline twice for 2 minutes at room temperature, the slides were then immersed in 3% hydrogen peroxide for 15 minutes at 22°C to quench endogenous peroxidase activity. The specimens were preblocked for 30 minutes at room temperature in normal goat serum. Subsequently, the slides were incubated for 30 minutes with rabbit anti-β-hCG polyclonal antibody (dilution at 1:300; Dako, Glostrup, Denmark) at room temperature. After three washes in Tris-buffered saline, the signal was visualized by using the Zymed LAB-SA detection system (Zymed Laboratories, Inc, San Francisco, CA). Positive staining of β-hCG was indicated as reddish brown.
Drugs and Treatment Schedules Used for Therapy
The antiangiogenic drug sorafenib to sylate is an oral multitargeting RTKI and this was generously provided by Bayer (Leverkusen, Germany). Targets include VEGFR-2 and VEGFR-3 and platelet-derived growth factor receptors, C-RAF and B-RAF. Sorafenib was prepared fresh daily just before gavaging and was dissolved in cremophor EL/95% ethanol/water (12.5:12.5:75) as described previously [23,24]. The drug was administered by gavage, once daily for 35 days at dose levels of 15, 30, and 60mg/kg bodyweight starting at around day 12 to 14 when all animals in the study had evidence of established tumors averaging from 100 to 150 mg, which was estimated from the β-hCG analysis to be 12.27 ± 9.24 mIU/ml (Figure 2B), with five mice per group.
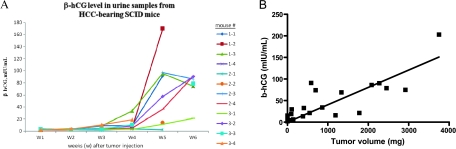
Figure 2.
Human β-hCG levels in urine samples from HCC-bearing SCID mice. (A) The relative HCC tumor burden is reflected by the level of β-hCG detected in urine samples (n = 12). Note that the latency period for growth of the HCC tumors is approximately 2 to 3 weeks. (B) Correlation of tumor mass and urine β-hCG levels from the pooled data obtained from the subcutaneous (n = 4) and orthotopic transplant models (n = 10).
For chemotherapy treatments, we used UFT, a 5-FU oral prodrug, which consists of a 4:1 molar combination of uracil and tegafur in 0.1% hydroxypropylmethyl cellulose [25,26].
Tegafur, uracil, and the hydroxypropylmethyl cellulose were generously supplied by Taiho Pharmaceutical Co, Ltd (Tokyo, Japan). UFT was prepared fresh daily just before gavaging. CTX (Baxter Oncology GmbH, Mississauga, Ontario, Canada) and doxorubicin (DOX) (Novopharm, Toronto, Ontario, Canada) were purchased from the institutional pharmacy. CTX was reconstituted as per manufacturer's instructions to a stock concentration of 20 mg/ml and administered through drinking water to provide an estimated dose of 20 mg/kg per day of CTX based on the estimated daily consumption of 3 ml for a 20-g mouse, as previously described [27]. DOX was freshly diluted with saline and administered intraperitoneally (0.6 or 0.5 mg/kg) three times a week. Another antiangiogenic drug, DC101, the antimouse VEGFR-2 monoclonal antibody, was used as previously described, that is, at a dose of 800 µg per mouse, injected intraperitoneally every 3 days [21].
Statistical Analysis
Results are reported as the mean (SD). Statistical significance of differences in the sorafenib therapy experiments was assessed by two-way analysis of variance. Survival curves were generated by the Kaplan-Meier method, and the statistical significance of differences in survival was assessed by χ2 test. All statistics was generated by using GraphPad Prism 4.00 software for windows, GraphPad Software, San Diego, CA (www.graphpad.com). The level of significance was set at P < .05.
Results
Noninvasive Serial Monitoring of Orthotopic Primary HCC Growth Using the β-hCG Marker
We first developed a preclinical orthotopic model of human HCC in SCID mice in which human HCC cells were implanted directly into the liver (Figure 1A). Monitoring changes in tumor size in this model using traditional methods such as caliper measurements is obviously not possible. Clearly, the ability to measure changes in tumor burden is important for such decisions such as when to initiate therapy if treatment of established liver disease is an objective. We therefore developed a noninvasive approach to measure and monitor HCC growth, which involves transplantation of tumor cells that have been transfected with the gene for β-hCG. The protein is secreted from the cells, detected in the urine, and can be used, similar in principle to prostate-specific antigen in prostate cancer, as a surrogate molecular marker of relative tumor burden and response to therapy [9]. To this end, we successfully tagged two HCC cell lines, PLC and Hep3B, with the gene for β-hCG.
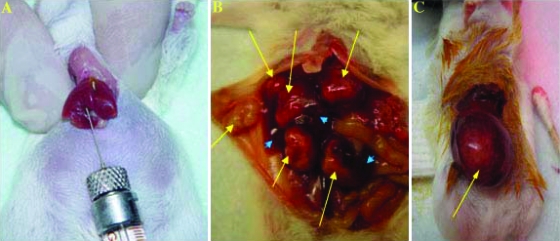
Figure 1.
Summary of the development of an orthotopic HCC model. (A) One million human HCC cells expressing β-hCG were implanted into the left lobe of the liver of SCID mouse, as described in Materials and Methods. (B) At week 6 after PLC-hCG cell implantation, multiple tumor nodules developed in liver, indicated by the yellow arrows. (C) Hep3B-hCG cells developed into a single tumor mass in the liver 6 weeks after cell implantation. Normal liver is indicated by blue arrowheads.
Three mice were implanted with 106 PLC-hCG or Hep3B-hCG cells. All tumors grew locally in the liver, either as clusters of nodules (PLC; Figure 1B) or as a solitary nodule (Hep3B; Figure 1C). We chose Hep3B-hCG for the remainder of the experiments because solitary liver tumor nodules are much easier to surgically resect. As such, this model would be useful for future experiments involving surgical resection of the primary liver tumor followed either immediately by adjuvant therapy, or initiating therapy at later times for established metastatic disease, the results of which could then be compared with those obtained in this study. In addition, similar to HCC in humans, the Hep3B-hCG tumors growing in the liver have an abundant vasculature, more so than the PLC-hCG cell line (Tang et al., unpublished observations), thus making it ideal to investigate antiangiogenic drug treatments. Successful orthotopic implantation was achieved in 11 of 12 mice without signs of macroscopic incidence of tumor dissemination because of leakage of tumor cells from the site of implantation. Two mice died before the end point of the experiment (7 weeks after HCC implantation) because of large tumor burdens.
Levels of β-hCG seemed to reflect relative tumor burden/size in SCID mice during a 6-week period of observation (Figure 2A). One mouse (#2-1), in which no tumor developed, did not have detectable β-hCG levels (Figure 2A). As seen in Figure 2A, there was a 2- to 3-week latency period, after which tumor burden increased rapidly. On the basis of this result, we estimated that an appropriate time for initiating chemotherapy for established disease in this model would be between 2 and 3 weeks. In contrast, initiating treatment during the first week could be viewed as an experimental model of early stage (low-volume) disease therapy, which would be useful for future adjuvant-like therapy model experiments.
We also performed subcutaneous implantations of Hep3B-hCG in four mice to study tumor growth in this conventional treatment model. We collected urine samples and measured tumor size at the same time on a weekly basis. The rationale for this experiment was to obtain the timeline and pattern of tumor growth, which could be compared with the results in the orthotopic transplant model. One mouse did not develop a tumor, and again, this was paralleled by undetectable urine β-hCG levels. For the orthotopic model, the weight and urine samples to assess β-hCG levels were collected before termination of the experiment. We combined the data from ectopic (subcutaneous) and orthotopic models to evaluate the correlation of β-hCG with tumor size. The results (shown in Figure 2B) indicate a trend of a linear relationship between tumor size and β-hCG levels, although the correlation did not reach statistical significance (Figure 2B; regression coefficient, 0.3306; P < .0646). Thus, in addition to using β-hCG measurements, we included survival times in our subsequent experiments to evaluate the therapeutic impact of the various drugs we tested.
Suppressed β-hCG Levels Detected in Response to Sorafenib Treatment
Systemic therapy with sorafenib demonstrated efficacy in randomized phase 3 clinical trials of HCC patients [16,17], and it is now approved for this indication. We therefore decided to test this drug to determine whether it had efficacy in our preclinical HCC models. To do so, we implanted the Hep3B-hCG cell line subcutaneously or orthotopically, allowed the tumors to grow for 2 weeks, then treated the mice with 15, 30, or 60 mg/kg of sorafenib for 30 days. Subcutaneously grown HCC tumors responded to sorafenib therapy in a dose-dependent manner, with 60 mg/kg providing the best response (Figure 3A). This was accurately reflected by the detected β-hCG in the urine (Figure 3B), validating the use of this protein as a surrogate marker of tumor burden and response to therapy. On the basis of this observation, orthotopically grown HCC tumors also responded well to sorafenib therapy; whereas overall β-hCG levels were higher in mice with orthotopic tumors compared with subcutaneous tumors, only the controls showed a large increase in detectable β-hCG (Figure 3C). Unfortunately, sorafenib therapy showed significant toxicity—as evidenced by weight loss—which was more severe in mice with subcutaneous tumors compared with orthotopic tumor-bearing mice (Figure 3, D and E). This toxicity seems to be related to the use of many different RTKIs in SCID mice and is not restricted to sorafenib [28] (Tang et al., unpublished observations). Interestingly, tumor weights of the control group, taken on sacrifice at day 44, were significantly greater than those in the orthotopic model (Figure 3F) and may be a reflection of the tumor growing more robustly in its appropriate microenvironment.
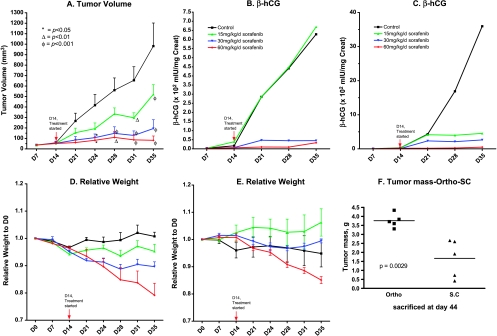
Figure 3.
Dose-response of sorafenib treatment in the HCC orthotopic and ectopic (subcutaneous) tumor therapy models (n = 5). (A) Tumor volumes in subcutaneous transplant model; sorafenib induced a dose-dependent inhibition of tumor volumes. (B) Relationship of β-hCG levels and relative tumor volume in the subcutaneous transplant model shown in Fig. 3A (n = 5). β-HCG levels correlated with tumor volumes. (C) Relationship of β-hCG levels and relative tumor volumes in HCC transplant models; β-hCG levels parallel relative tumor volumes in a dose-dependent manner. (D) Relative mouse weights in the subcutaneous HCC transplant model. Toxicity was detected after 7 days treatment with both the 30- and 60-mg/kg per day doses of sorafenib. (E) Relative weights detected in one orthotopic transplant model; toxicity became apparent after 7 days treatment at the 60 mg/kg/day dose. (F) Comparisons of tumor mass in the orthotopic and subcutaneous transplant models. The tumor mass in the orthotopic model control group is significantly greater compared with the subcutaneous transplant model group (P = .0029). The vertical arrows in panels A to E represent start of sorafenib treatment.
Histopathologic Detection of Hepatic Metastatic Foci after Hepatectomy Shows Stability of β-hCG as a Biomarker
To confirm the stability of β-hCG expression in the transfected HCC cells in vivo, we resected primary HCC tumors from the left liver lobes in two mice 7 weeks after implantation of Hep3B cells; mice were killed 8 weeks after hepatectomy. Immunohistochemical staining of anti-β-hCG (Figure 4) in paraffin sections showed the typical well-differentiated HCC cells in liver tissue. β-HCG in recurrent and metastatic HCC tumors was expressed predominantly in the cytoplasm of tumor cells (Figure 4, reddish brown).
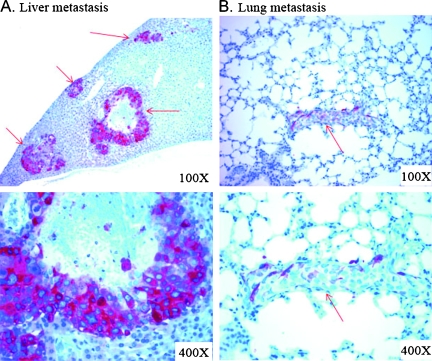
Figure 4.
Evidence of b-hCG expression in vivo and of metastatic and recurrent HCC after surgical resection of primary tumor mass by immunohistochemical staining. Hepatectomy was performed in two mice 7 weeks after implantation of Hep3B cells in the liver. Immunohistochemical staining was done with anti-β-hCG antibody on formalin-fixed paraffin sections. The liver (A) and lung (B) specimens were collected 8 weeks after hepatectomy. Reddish brown staining indicates positive β-hCG staining of HCC cells. Red arrows indicate HCC metastasis.
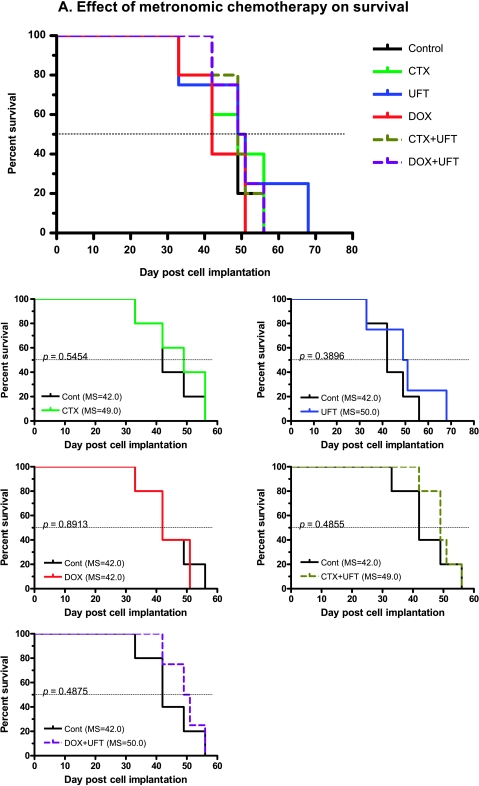
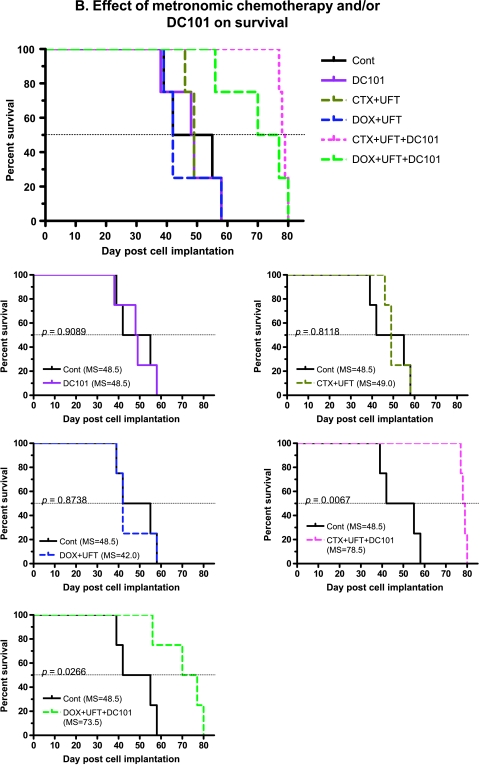
Therapeutic Impact of Metronomic Chemotherapy with or without a VEGF Pathway-Targeting Drug
We reported previously that certain low-dose metronomic chemotherapy regimens significantly prolonged the overall survival of mice with advanced metastatic human breast cancer or melanoma, especially the former [5,6], as well as advanced ovarian cancer [29]. We also reported, as have others, that the efficacy of metronomic chemotherapy can be significantly enhanced by combination with an antiangiogenic drug [21,22,29–31].We therefore decided to test metronomic chemotherapy in combination with an antiangiogenic drug in our model of advanced HCC. However, because of the toxicity associated with long-term sorafenib treatment in SCID mice, we decided to test various monotherapy or combination metronomic chemotherapy regimens (using UFT, CTX, and/or DOX) with or without the antiangiogenic agent DC101, the antimouse anti-VEGFR-2 monoclonal antibody, which we have used extensively in previous studies involving SCID mice [21,32]. Various single or combination metronomic chemotherapy regimens—without DC101—on established tumors (based on β-hCG levels) did not result in a statistically significant survival benefit in the Hep3B HCC model (Figure 5, A and B). There was also no prolongation of survival using CTX ± DC101 in the PLC HCC model or the Hep3B model (data not shown). However, combining metronomic chemotherapy using UFT and CTX or UFT + DOX with DC101 produced a statistically significant benefit in overall survival times (Figure 5B). The statistical significance values of overall survival in metronomic chemotherapy treatments are listed in Table 1. The most effective metronomic chemotherapy regimen when combined with DC101 (based on the median survival) was the two-drug CTX + UFT chemotherapy combination (78.5 days, P = .0067; Figure 5B and Table 1). As a means of visualizing the results of Figure 5 in a more comprehensive and comparative way (given the large number of groups), we have provided an expanded version showing individual survival curves of each treatment group compared with the control group.
Figure 5.
Combination of various low-dose metronomic (LDM) chemotherapy regimens/drugs with or without DC101 in the orthotopic Hep3B HCC model (n = 5 per group). (A) Survival curves observed using single and combination LDM chemotherapy treatments, without DC101. (B) Survival curves using combination LDMchemotherapy treatments with or without DC101. The smaller lower five graphs shown in panels A and B are taken from the larger figure at the top of panels A and B. Together, these smaller graphs comprise the larger composite graph and are shown to more clearly illustrate the differences or similarities between each treatment group and the control untreated group. Note the prolongation of survival when metronomic UFT + CTX or UFT + DOX is combined with DC101. UFT = tegafur + uracil, a 5-FU prodrug. See Table 1 for median survival times and statistical significance.
Table 1.
Median Survival and Statistical Significance of Metronomic Chemotherapy Treatments without or with DC101.
Discussion
HCC is one of the five most common malignancies in the world with a particularly high incidence in Asian countries [33–35]. However, the incidence of the disease in North America has been rising rapidly in recent years, likely owing to increased cases of hepatitis C and B virus infection [33–35]. HCC is a highly malignant tumor characterized by active neovascularization [36].With respect to therapy, surgical resection is a potentially curative therapy. The current surgical mortality of hepatectomy for HCC is approximately 5% or less in major centers with surgical expertise and careful patient selection [37]. However, tumor resection is limited to patients with good liver function (i.e., Child A cirrhosis with no or only minimal portal hypertension) [37,38]. Although long-term survival after resection of HCC has improved with a current overall 5-year survival rate of approximately 50% [37,38], there are many cases where surgery is not possible owing to the proximity of the tumor to the hepatic artery. Furthermore, recurrence and metastasis of HCC are major impediments compromising prolonged survival after surgical resection.Moreover, there is no effective neoadjuvant or adjuvant chemotherapy for HCC, thus the need for better systemic therapies is both obvious and urgent.
Developing improved therapies for HCC would be facilitated by the availability of preclinical rodent models that better reflect the malignant nature of HCC. Few, if any, such models presently exist. Instead, most models consist of subcutaneous (ectopic) tumors of human HCC cells grown in immune deficient mice. This has been a standard methodology for decades for a number of reasons: the procedure is easy to perform and is minimally invasive and changes in tumor size are readily monitored by caliper measurements. Unfortunately, such models do not accurately reflect certain aspects of the respective clinical disease because the tumors are grown and treated in an ectopic and thus possibly inappropriate microenvironment. Indeed, previous studies involving comparisons between orthotopic and ectopic subcutaneous tumors have shown differences in invasiveness, angiogenesis, ability to metastasize, and response to therapy [39,40]. Advanced orthotopic HCC models, possibly in conjunction with distant metastases, would thus represent a potentially significant improvement for studying both the biology and treatment of HCC. Although some orthotopic HCC models have been developed, they have not taken into consideration the serial monitoring of tumor burden, which is a crucial experimental factor in the timing of initiation of therapy as well as the detection of recurrent disease or metastases in vivo [41,42]. In the case of HCC, orthotopic models suffer from the handicap of not being able to accurately monitor tumor size in the liver. To circumvent this problem, we have transfected the β-hCG gene into human PLC and Hep3B HCC cell lines, which can be used as an independent quantitative surrogate marker of tumor burden. The detection of secreted β-hCG by transfected cell lines has previously been used by us as a means of noninvasively monitoring tumor growth over long periods, including metastases [8,9,32]. Indeed, a linear relationship existed between detected β-hCG and tumor size, although this did not quite reach statistical significance (Figure 2B). However, it seems that β-hCG levels accurately reflected relative tumor burden—indicated by increasing levels of β-hCG, whereas low tumor burden induced by therapy was accompanied by barely detectable levels of β-hCG (Figure 3B). Therefore, β-hCG is a valid surrogate marker of tumor burden and response. Also of interest is the fact that the expression of β-hCG by the HCC cells allowed us to use anti-β-hCG antibodies for immunohistochemical staining of tumor cells in vivo, as shown in Figure 4.
A potential weakness and limitation of our advanced HCC model from the standpoint of undertaking therapy experiments and their potential clinical relevance is the absence of liver cirrhosis. This is frequently a comorbid condition observed in HCC patients and is a significant factor in limiting the use of conventional chemotherapy for the treatment of HCC [18,19]. HCC are secondary to either a viral hepatitis infection or cirrhosis, but most cases of HCC are caused by viral hepatitis infection. In fact, the cell lines we used are hepatitis B virus-integrated. However, with respect to chemotherapy, our major therapeutic interest is the use of very low nontoxic or minimally toxic doses of chemotherapy administered in close, regular intervals with no prolonged breaks (i.e., metronomic chemotherapy), used alone or in combination with a targeted antiangiogenic drug, such as an anti-VEGFR-2 antibody [21]. Such low-dose metronomic chemotherapy dosing and administration schedules are thought to inhibit tumor growth primarily by antiangiogenic mechanisms including the direct targeting of endothelial cells in the tumor neovasculature and circulating bone marrow-derived endothelial progenitor cells [5,21,22,30,43], although additional mechanisms are possible as well. Thus, the presence or absence of liver cirrhosis may not significantly impact the relative therapeutic outcomes of HCC therapy experiments using metronomic chemotherapy and/or targeted antiangiogenic drugs. Indeed, a recent study [44] showed that a metronomic CTX regimen could be used to safely and successfully treat a spontaneous rat hepatoma induced by chemical carcinogenesis where tumors were accompanied by liver cirrhosis [44]. By comparison, a maximum tolerated dose control arm was much more toxic and not as effective as the metronomic regimen [44]. In this regard, clinical trials testing metronomic UFT chemotherapy in combination with sorafenib have been initiated in advanced HCC patients (http://www.clinicaltrials.gov). Conversely, because UFTand CTX are both prodrugs that are activated by liver enzymes, it is possible that encouraging preclinical results may not be obtained in the clinic, at least in some cases, as a result of the presence of liver cirrhosis. In this regard, the aforementioned results by Park et al. [44] are reassuring with respect to the use of metronomic chemotherapy using a prodrug where liver cirrhosis is present. Nevertheless, additional studies using the metronomic chemotherapy protocols we have used in mice with liver cirrhosis will be required to determine whether the treatments are not overtly toxic and also maintain their efficacy.
In our studies, we tested several metronomic chemotherapy regimens: metronomic UFT alone, metronomic CTX alone, or both together; metronomic DOX alone, or with UFT, or CTX. We also tested DC101 alone or with the aforementioned metronomic chemotherapy regimens. The major finding is that no single or doublet metronomic chemotherapy regimen tested, or DC101 alone, had any demonstrable benefit in prolonging survival, when treatment was initiated on advanced orthotopic human HCC. In contrast, almost all combinations of concurrent combination metronomic chemotherapy regimens plus DC101 meaningfully prolonged survival and did so with minimal overt toxicity. As such, our results highlight the possibility that even if metronomic chemotherapy per se fails to cause a clinical benefit, combination with a targeted antiangiogenic drug could yield results superior to the antiangiogenic drug used alone. This possibility should now be considered for the use of drugs such as UFT when used in the adjuvant treatment setting. Indeed, UFT has shown a clinical benefit when administered postsurgically for the adjuvant treatment of a number of early stage cancers such as non-small cell lung carcinoma [25], breast cancer [45], and gastric cancer [46], but not necessarily HCC [47,48], although this is controversial [49]. However, a combination of antiangiogenic drugs with metronomic UFT may provide a clinical benefit exceeding either therapy used alone. In any case, the results of the therapy studies using our newly developed HCC model suggest that metronomic CTX plus UFT combined with a VEGF pathway-targeting antibody drug may be a potentially interesting and promising new therapeutic strategy for the treatment of this disease. It will be of interest to test metronomic UFT monochemotherapy with an antiangiogenic drug to determine whether it has an activity comparable to UFT + CTX or UFT + DOX.
With respect to antiangiogenic drugs, the proven benefit of small molecule with oral antiangiogenic RTKIs such as sorafenib in treating advanced human HCC would make them obvious promising candidates for combination with metronomic chemotherapy. This would provide an oral combination therapy when using drugs such as UFT and CTX for the metronomic chemotherapy. In addition, because the toxicities associated with drugs such as sorafenib sometimes require frequent interruptions in the otherwise daily therapy schedule [50–52], treatment with the minimally toxic metronomic chemotherapy regimens could be continued during such interruptions, thereby minimizing the possible drawbacks associated with interrupting RTKI antiangiogenic therapy such as rapid “rebound” of tumor growth [53,54].
Acknowledgments
The authors thank Cassandra Cheng for her excellent secretarial assistance. The authors also thank Scott Wilhelm, Bayer Pharmaceuticals, for providing the sorafenib, Larry Witte, ImClone Systems, for providing DC101, and Teiji Takechi, Taiho Pharmaceuticals, for providing UFT.
Footnotes
This work was supported by grants to RSK from the National Institutes of Health, USA (CA-41233), the Canadian Institutes for Health Research, the Canadian Cancer Society Research Institute, and a sponsored research agreement with ImClone System, Inc.
References
- 1.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived—but they can be improved. Cancer Biol Ther. 2003;2(4 Suppl 1):S134–S139. [PubMed] [Google Scholar]
- 2.Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007;26:737–747. doi: 10.1007/s10555-007-9087-6. [DOI] [PubMed] [Google Scholar]
- 3.Kamb A. What's wrong with our cancer models? Nat Rev Drug Discov. 2005;4(2):161–165. doi: 10.1038/nrd1635. [DOI] [PubMed] [Google Scholar]
- 4.Burchill SA. What do, can and should we learn from models to evaluate potential anticancer agents? Future Oncol. 2006;2(2):201–211. doi: 10.2217/14796694.2.2.201. [DOI] [PubMed] [Google Scholar]
- 5.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, Kerbel RS. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66(7):3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68(12):4500–4505. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Munoz W, Man S, Kerbel RS. Effective treatment of advanced human melanoma metastasis in immunodeficient mice using combination metronomic chemotherapy regimens. Clin Cancer Res. 2009;15(15):4867–4874. doi: 10.1158/1078-0432.CCR-08-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francia G, Man S, Lee CJ, Lee CR, Xu P, Mossoba ME, Emmenegger U, Medin JA, Kerbel RS. Comparative impact of trastuzumab and cyclophosphamide on HER-2-positive human breast cancer xenografts. Clin Cancer Res. 2009;15(20):6358–6366. doi: 10.1158/1078-0432.CCR-09-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francia G, Emmenegger U, Lee CR, Shaked Y, Folkins C, Massoba M, Medin JA, Man S, Zhu Z, Witte L, et al. Long-term progression and therapeutic response of visceral metastatic disease non-invasively monitored in mouse urine using β-human choriogonadotropin secreting tumor cell lines. Mol Cancer Ther. 2008;7(10):3452–3459. doi: 10.1158/1535-7163.MCT-08-0200. [DOI] [PubMed] [Google Scholar]
- 10.Shih IM, Torrance C, Sokoll LJ, Chan DW, Kinzler KW, Vogelstein B. Assessing tumors in living animals through measurement of urinary beta-human chorionic gonadotropin. Nat Med. 2000;6(6):711–714. doi: 10.1038/76299. [DOI] [PubMed] [Google Scholar]
- 11.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrist R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26(30):4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 13.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100(15):1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 15.Steeg PS, Anderson RL, Bar-Eli M, Chambers AF, Eccles SA, Hunter K, Itoh K, Kang Y, Matrisian LM, Sleeman JP, et al. Preclinical drug development must consider the impact on metastasis. Clin Cancer Res. 2009;15(12):4529–4530. doi: 10.1158/1078-0432.CCR-09-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 18.Gish RG, Baron A. Hepatocellular carcinoma (HCC): current and evolving therapies. IDrugs. 2008;11(3):198–203. [PubMed] [Google Scholar]
- 19.Rougier P, Mitry E, Barbare JC, Taieb J. Hepatocellular carcinoma (HCC): an update. Semin Oncol. 2007;34(2 Suppl 1):S12–S20. doi: 10.1053/j.seminoncol.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Voiculescu M, Winkler RE, Moscovici M, Neuman MG. Chemotherapies and targeted therapies in advanced hepatocellular carcinoma: from laboratory to clinic. J Gastrointestin Liver Dis. 2008;17(3):315–322. [PubMed] [Google Scholar]
- 21.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105(8):R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66(24):11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 24.Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, Bortolon E, Ichetovkin M, Chen C, McNabola A, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59(5):561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 25.Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350(17):1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 26.Yonekura K, Basaki Y, Chikahisa L, Okabe S, Hashimoto A, Miyadera K, Wierzba K, Yamada Y. UFT and its metabolites inhibit the angiogenesis induced by murine renal cell carcinoma, as determined by a dorsal air sac assay in mice. Clin Cancer Res. 1999;5(8):2185–2191. [PubMed] [Google Scholar]
- 27.Abraham SA, McKenzie C, Masin D, Ng R, Harasym TO, Mayer LD, Bally MB. In vitro and in vivo characterization of doxorubicin and vincristine coencapsulated within liposomes through use of transition metal ion complexation and pH gradient loading. Clin Cancer Res. 2004;10(2):728–738. doi: 10.1158/1078-0432.ccr-1131-03. [DOI] [PubMed] [Google Scholar]
- 28.Peterson SR, Kurimasa A, Oshimura M, Dynan WS, Bradbury EM, Chen DJ. Loss of the catalytic subunit of the DNA-dependent protein kinase in DNA double-strand-break-repair mutant mammalian cells. Proc Natl Acad Sci USA. 1995;92(8):3171–3174. doi: 10.1073/pnas.92.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto K, Man S, Xu P, Cruz-Munoz W, Tang T, Kumar R, Kerbel RS. Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer. Mol Cancer Ther. doi: 10.1158/1535-7163.MCT-09-0960. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60(7):1878–1886. [PubMed] [Google Scholar]
- 31.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 32.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62(10):2731–2735. [PubMed] [Google Scholar]
- 33.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9(2):191–211. doi: 10.1016/j.cld.2004.12.009. v. [DOI] [PubMed] [Google Scholar]
- 34.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25(2):143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 35.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5 Suppl 2):S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 36.Abou-Shady M, Baer HU, Friess H, Zimmermann A, Buchler MW. Molecular aspects of hepatocellular carcinoma. Swiss Surg. 1999;5(3):102–106. doi: 10.1024/1023-9332.5.3.102. [DOI] [PubMed] [Google Scholar]
- 37.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10(2 Suppl 1):S39–S45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 38.Sherman M, Takayama Y. Screening and treatment for hepatocellular carcinoma. Gastroenterol Clin North Am. 2004;33(3):671–691. doi: 10.1016/j.gtc.2004.04.012. xi. [DOI] [PubMed] [Google Scholar]
- 39.Fidler IJ. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 1991;10(3):229–243. doi: 10.1007/BF00050794. [DOI] [PubMed] [Google Scholar]
- 40.Manzotti C, Audisio RA, Pratesi G. Importance of orthotopic implantation for human tumors as model systems: relevance to metastasis and invasion. Clin Exp Metastasis. 1993;11(1):5–14. doi: 10.1007/BF00880061. [DOI] [PubMed] [Google Scholar]
- 41.Tang ZY, Sun FX, Tian J, Ye SL, Liu YK, Liu KD, Xue Q, Chen J, Xia JL, Qin LX. Metastatic human hepatocellular carcinoma models in nude mice and cell line with metastatic potential. World J Gastroenterol. 2001;7(5):597–601. doi: 10.3748/wjg.v7.i5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okubo H, Takei Y, Serizawa N, Enomoto N, Ikejima K, Sato N. Orthotopic hepatocellular carcinoma model with a controlled and reproducible tumorigenicity. J Gastroenterol Hepatol. 2007;22(3):423–428. doi: 10.1111/j.1440-1746.2006.04520.x. [DOI] [PubMed] [Google Scholar]
- 43.Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106(9):3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park ST, Jang JW, Kim GD, Park JA, Hur W, Woo HY, Kim JD, Kwon JH, Yoo CR, Bae SH, et al. Beneficial effect of metronomic chemotherapy on tumor suppression and survival in a rat model of hepatocellular carcinoma with liver cirrhosis. Cancer Chemother Pharmacol. 2009 doi: 10.1007/s00280-009-1108-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Watanabe T, Sano M, Takashima S, Kitaya T, Tokuda Y, Yoshimoto M, Kohno N, Nakagami K, Iwata H, Shimozuma K, et al. Oral uracil and tegafur compared with classic cyclophosphamide, methotrexate, fluorouracil as postoperative chemotherapy in patients with node-negative, high-risk breast cancer:National Surgical Adjuvant Study for Breast Cancer 01 Trial. J ClinOncol. 2009;27(9):1368–1374. doi: 10.1200/JCO.2008.18.3939. [DOI] [PubMed] [Google Scholar]
- 46.Surenkok S, Beyzadeoglu M, Oysul K, Ozyigit G, Ataergin S, Arpaci F, Ozet A. The management of gastric adenocarcinoma with postoperative chemoirradiation. A non-randomized comparison of oral UFTand 5-FU. Tumori. 2008;94(1):70–74. doi: 10.1177/030089160809400113. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda K, Saitoh S, Koida I, Tsubota A, Arase Y, Chayama K, Kumada H. A prospective randomized evaluation of a compound of tegafur and uracil as an adjuvant chemotherapy for hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Am J Clin Oncol. 1995;18(3):204–210. doi: 10.1097/00000421-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa K, Takayama T, Ijichi M, Matsuyama Y, Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology. 2006;44(4):891–895. doi: 10.1002/hep.21341. [DOI] [PubMed] [Google Scholar]
- 49.Ueda H, Tanaka H, Kida Y, Fukuchi H, Ichinose M. Adjuvant chemotherapy with tegafur/uracil administration after transcatheter arterial chemoembolization for advanced hepatocellular carcinoma. Oncol Rep. 2008;19(5):1355–1361. [PubMed] [Google Scholar]
- 50.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12(24):7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 51.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13(9):1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 52.Hutson TE, Figlin RA, Kuhn JG, Motzer RJ. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist. 2008;13(10):1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- 53.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26(11):1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 54.Desar IM, Mulder SF, Stillebroer AB, van Spronsen DJ, van der Graaf WT, Mulders PF, van Herpen CM. The reverse side of the victory: flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncol. 2009;48(6):927–931. doi: 10.1080/02841860902974167. [DOI] [PubMed] [Google Scholar]