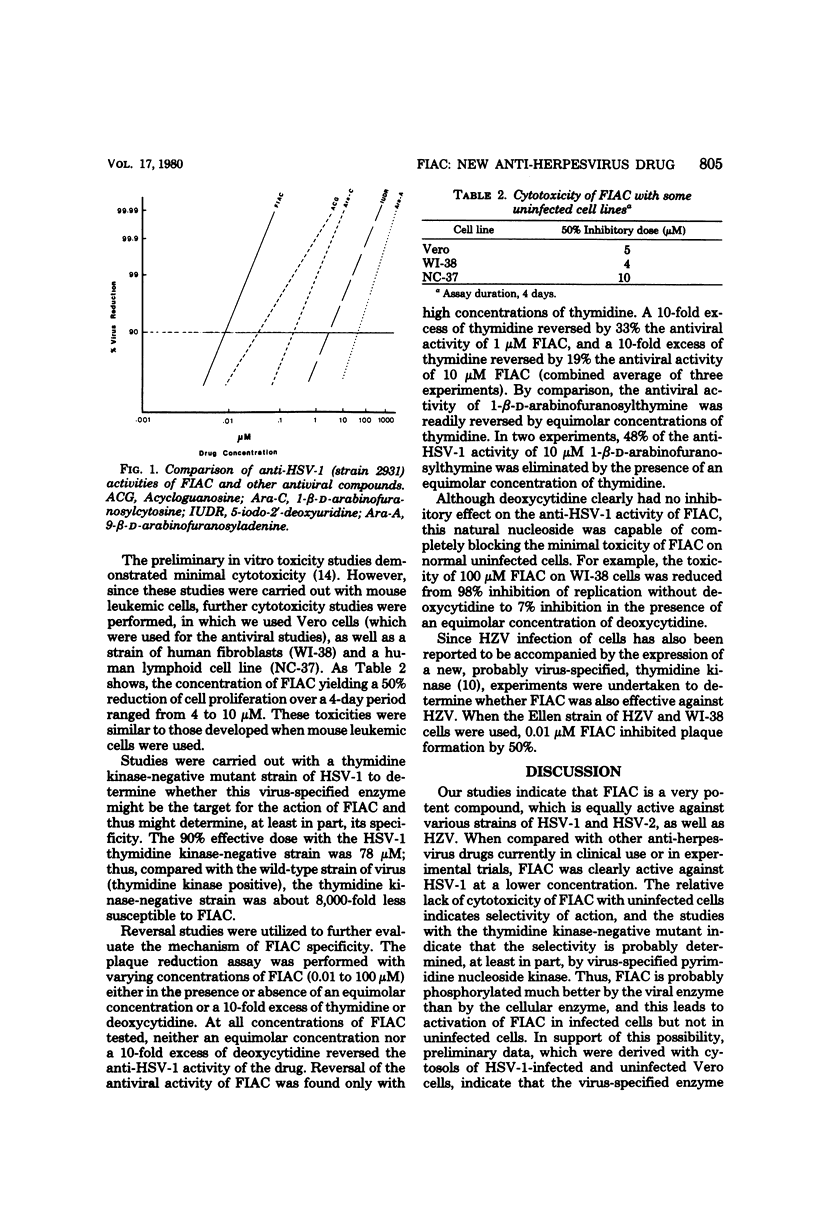
Abstract
A newly synthesized pyrimidine analog, 2'-fluoro-5-iodo-aracytosine (FIAC), suppressed by 90% the replication of various strains of herpes simplex virus types 1 and 2 at concentrations of 0.0025 to 0.0126 microM. Cytotoxicity was minimal, as determined by trypan blue dye exclusion with norman Vero, WI-38, and NC-37 cell proliferation; the 50% inhibitory dose was 4 to 10 microM in a 4-day assay. When compared with other antiviral drugs, FIAC was active at much lower concentrations than arabinosylcytosine, iododeoxyuridine, and arabinosyladenine. It was slightly more active against herpes simplex virus type 1 than acycloquanosine and slightly more toxic to normal cells. FIAC was about 8,000 times more active against the replication of wild-type herpes simplex virus type 1 than against a mutant strain lacking the expression of virus-specified thymidine kinase. Since FIAC appears to be preferentially phosphorylated by the viral enzyme, this is probably responsible, at least in part, for the selectivity of its antiviral actions. Although FIAC appears to be an arabinosylcytosine analog, its antiviral activity was not reversed by deoxycytidine. The minimal cytotoxicity exhibited by FIAC for normal cells, however, was reversed by equimolar concentrations of deoxycytidine. Thymidine, which reversed the antiviral activity, was effective only when used in great excess.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drach J. C., Shipman C., Jr The selective inhibition of viral DNA synthesis by chemotherapeutic agents: an indicator of clinical usefulness? Ann N Y Acad Sci. 1977 Mar 4;284:396–409. doi: 10.1111/j.1749-6632.1977.tb21976.x. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Anken M. Altered properties of thymidine kinase after infection of mouse fibroblast cells with herpes simplex virus. J Virol. 1967 Feb;1(1):238–240. doi: 10.1128/jvi.1.1.238-240.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Viral-induced thymidine kinase isozymes. Prog Med Virol. 1975;21:13–34. [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- Mar E. C., Huang Y. S., Huang E. S. Purification and characterization of varicella-zoster virus-induced DNA polymerase. J Virol. 1978 May;26(2):249–256. doi: 10.1128/jvi.26.2.249-256.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Otsuka T., Takahashi M. Induction of deoxypyrimidine kinase activity in human embryonic lung cells infected with varicella-zoster virus. J Virol. 1977 Mar;21(3):1232–1235. doi: 10.1128/jvi.21.3.1232-1235.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Lenoir G., Daillie J. Characterization of an Epstein-Barr virus-induced DNA polymerase. J Virol. 1979 Jan;29(1):1–10. doi: 10.1128/jvi.29.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purifoy D. J. Comparison of DNA polymerase activities induced by herpes simplex virus types 1 and 2. Intervirology. 1975;6(6):356–366. doi: 10.1159/000149492. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. Herpes simplex virus DNA polymerase as the site of phosphonoacetate sensitivity: temperature-sensitive mutants. J Virol. 1977 Nov;24(2):470–477. doi: 10.1128/jvi.24.2.470-477.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K. A., Reichman U., Hirota K., Lopez C., Fox J. J. Nucleosides. 110. Synthesis and antiherpes virus activity of some 2'-fluoro-2'-deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem. 1979 Jan;22(1):21–24. doi: 10.1021/jm00187a005. [DOI] [PubMed] [Google Scholar]



