Abstract
Using a rat model, we investigated the effects of circulating factors in pregnancy on cerebrovascular and systemic vascular function by comparing myogenic reactivity, tone, and endothelial vasodilator production of the posterior cerebral artery (PCA) and mesenteric artery (MA) of nonpregnant animals perfused with nonpregnant and pregnant human plasma. Arteries from late pregnant (LP) animals were then perfused similarly to evaluate a potential adaptive effect of pregnancy on vessel function. A three-hour exposure to pregnant plasma caused increased myogenic reactivity and tone in vessels from NP animals and produced a decreased endothelium-derived hyperpolarizing factor response in nonpregnant PCAs, findings that were not seen with MAs. The increased reactivity and tone noted in nonpregnant vessels was abolished when pregnant plasma was perfused through LP arteries, suggesting these vessels adapt during pregnancy to the vasoconstricting influence of pregnant plasma.
Keywords: circulating factors, plasma, cerebrovasculature, vasodilator
Introduction
Maternal cardiovascular adaptation to gestation is an important process necessary for maintenance of normal pregnancy and has been studied at length in several vascular beds, including renal, mesenteric, and uterine 1-3. The physiologic changes accompanying normal pregnancy are thought to occur due to a combination of circulating hormonal factors and a corresponding adaptation of the vasculature to these factors 4-7. For example, angiotensin II (Ang II) levels rise significantly during pregnancy, but the vasculature adapts such that the pregnant state is refractory to the vasoconstricting actions of this hormone 8-11. Similar to other vascular beds, the cerebral circulation also appears to adapt to pregnancy. For example, cerebral vessels from late pregnant (LP) rats exhibit significantly different reactivity to increases in intravascular pressure than vessels from nonpregnant (NP) rats, losing the ability to maintain myogenic reactivity at higher intravascular pressures 12. In addition, endothelium-dependant vasodilator production is different in cerebral vessels from LP rats than from NP rats 12. These studies suggest that cerebral vessels adapt to pregnancy, but the effect of circulating factors is currently unknown. Understanding the impact of normal pregnancy and the influence of circulating factors on cerebral blood flow and cerebrovascular resistance is important as alterations in cerebral perfusion are linked to pathologic processes such as eclampsia 13.
In the current study, we used a combination of an animal model and human plasma to investigate the effect of circulating factors in the plasma of pregnant women on cerebral artery reactivity and endothelium-dependant vasodilator production by perfusing arteries from NP rats with plasma from nonpregnant women and normotensive pregnant women. In addition, the effect of pregnancy on cerebral vessels was investigated by comparing arteries from NP and LP rats with a common pregnant plasma perfusate. Finally, in order to determine if effects of pregnant plasma were specific to the cerebrovasculature or represented a systemic effect, paired experiments examining the change in function of the posterior cerebral artery (PCA) vs. the mesenteric artery (MA) were compared from each animal.
Materials and Methods
Patients and Plasma Samples
Blood samples were collected from patients enrolled in a simultaneous ongoing Institutional Review Board (IRB) approved study at the University of Vermont. IRB exemption was granted to use these previously frozen plasma samples. Plasma was pooled from two groups of women: a group of nonpregnant women with no known medical problems (control) and normotensive pregnant women with uncomplicated pregnancies. The control group (n=6) had an average age of 31.5 (range 23 - 42) and were not taking any medication. Twelve healthy pregnant women with no history of hypertension, diabetes, or infection were included in the normal pregnant group. Their average age was 30.3 years (range 20 – 41), and average gestational age at venipuncture was 33.4 weeks (range 31.1 – 36.1). Blood samples were collected from patients into vacutainer tubes containing either ethylenediaminetetraacetic acid or lithium heparin. Blood was centrifuged at 1400-1600 g, the plasma removed, aliquoted, and the pooled samples frozen at -80 °C until experimentation.
Animals
All procedures were approved by the University of Vermont Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. To determine if vascular adaptation to pregnancy influenced the reactivity of resistance arteries to pregnant plasma, the reactivity of arteries from NP and LP animals were compared. Either female virgin NP (body wt: 245 – 295g), or LP (day 19, body wt: 390-402g) Sprague-Dawley rats were used. The animals were housed in the Animal Care Facility, an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility. Animals had access to food and water ad libitum and maintained a 12 hour light/dark cycle.
PCAs and MAs from NP animals were compared to determine the effect of pregnant plasma on vessel function. To assess this, vessels from NP animals only were perfused intraluminally with either control plasma (n=9 for PCA, n=6 for MA) or pregnant plasma (n=6 for PCA, n=7 for MA). Separately, we tested the hypothesis that pregnancy would cause vessels to have an adaptive effect, attenuating or modifying the influence of pregnant plasma. This was performed by comparing vessels from LP animals and vessels from NP animals using a common pregnant plasma perfusate (n=6 for PCA, n=6 for MA).
Preparation of arterial segments and arteriograph system
After being anesthetized with isofluorane, animals were quickly decapitated. The brain and a portion of mesentery were quickly removed and placed in cold physiologic saline solution (HEPES buffer) at pH 7.4 ± 0.05. A pressurized arteriograph system (Living Systems Instrumentation Inc., Burlington, VT) was used for all isolated vessel experiments as previously described 12. To determine the effect of pregnant plasma on vascular reactivity and vasodilator production, vessels were perfused intraluminally with either 20% or 40% plasma in HEPES from control or pregnant women. The suffusate consisted of HEPES solution only. A third-order branch of the PCA or a third-order branch of the MA from NP or LP rats was perfused with control or pregnant plasma. The proximal cannula was attached to a servomechanism and inline pressure transducer that controlled intravascular pressure. No flow was present through the vessels as the distal cannula was closed off. The entire chamber was transferred to an inverted microscope with a video camera and a video dimension analyzer (VDA), allowing for measurement of lumen diameter and vessel wall thickness. Output from the VDA was sent to a computer with data-acquisition software (WINDAQ) for continuous recording of vessel diameter and intravascular pressure.
Experimental Protocol
After vessels were perfused with either control or pregnant plasma, intravascular pressure was set to 25 mm Hg for a one-hour equilibration. This resulted in a total exposure time of the vessel to plasma for approximately 3 hours. Active myogenic responses were determined by increasing intravascular pressure to 150 mm Hg in 25 mm Hg increments. Lumen diameter and wall thickness were measured at each pressure once stable. Intravascular pressure was set to 75 mm Hg for the remainder of the experiment. After myogenic responses were determined, a single concentration of Nω´-nitro-L-arginine (L-NNA, 0.1 mM), a nitric oxide synthase (NOS) inhibitor, was added to the vessel bath and lumen diameter and wall thickness measured. The resulting vasoconstriction was used as a measure of basal NO production. A single concentration of indomethacin (10-5 M) was then added to the vessel bath to inhibit cyclooxygenase (COX), and any change of vessel diameter was used as a measure of basal prostacyclin production. In the presence of NOS and COX inhibition, increasing concentrations of calcium ionophore (A23187) were cumulatively added to the bath (10-5 to 10-7 M) and lumen diameter at each concentration was measured to determine endothelium-derived hyperpolarizing factor (EDHF) response. At the end of the experiment, a single concentration of papaverine (0.1 mM) was added to the vessel bath to inactivate vascular smooth muscle and obtain fully relaxed passive vessel diameters at intravascular pressures between 25 mm Hg and 150 mm Hg.
After completion of the experiment with the PCA, a third-order branch of the MA was mounted in an identical fashion. The above steps of the experiment were repeated with the following exceptions: cumulative concentrations of acetylcholine (10-8 to 10-6 M) were used to assess EDHF responsiveness. Additionally, a single concentration of phenylephrine (10-7 M) was given prior to acetylcholine to precontract the vessels as MAs develop little basal tone.
A separate set of experiments was done to determine the effect of 20% vs. 40% pregnant plasma on vessel reactivity, tone, and endothelial vasodilator production. Experiments were repeated in identical fashion as listed above for PCAs from NP and LP animals with the exception that they were perfused with 40% control plasma (n=4 for NP vessels, n=6 for LP vessels) instead of 20%. MAs were also evaluated using 40% perfusate for myogenic reactivity, tone, and basal NO and prostacyclin production.
Data Calculations
Vessel tone was calculated assessing passive and active diameters at each pressure using the equation [1 − (φactive / φpapaverine) × 100%], where φactive and φpapaverine are the vessel's active diameter and the diameter in the presence of papaverine, respectively.
Constriction in response to L-NNA was measured as a percent decrease of vessel diameter from its baseline diameter at 75 mm Hg.
Vessel response to indomethacin was calculated using the equation [1 − (φindomthacin / φL-NNA) × 100%] where φindomthacin and φL-NNA represent vessel diameter after the addition of indomethacin and L-NNA to the vessel bath, respectively.
Percent dilation to 1.0 μM A23187 was calculated using the equation [(φ1.0μM - φstart) / φstart × 100%] where φ1.0μM represents vessel diameter at a A23187 concentration of 1 μM.
Drugs and Solutions
HEPES and indomethacin were purchased from Fisher Scientific (Hampton, NH); papaverine, L-NNA, phenylephrine, calcium ionophore and acetylcholine were purchased from Sigma (St. Louis, MO). HEPES physiologic salt solution was made fresh daily and consisted of (mM): 142.0 NaCl, 4.7 KCl, 1.71 MgSO4, 0.50 EDTA, 2.8 CaCl2, 10.0 HEPES, 1.2 KH2PO4 and 5.0 dextrose. L-NNA, indomethacin, phenylephrine, acetylcholine and papaverine were made fresh weekly at 10-2 M or 10-3 M stock solutions and stored at 4°C.
Statistical analysis
All data are presented as mean ± SE. Differences between control and pregnant plasma and between different vessels with the same plasma were determined using Student's t-test or ANOVA with a posthoc analysis for multiple comparisons. Differences were considered significant at p < 0.05. Differences between PCAs and MAs were also analyzed and determined using unpaired t-test.
Results
Effect of circulating factors in pregnant plasma on myogenic activity and tone in NP vessels
Arteries from NP animals were perfused with either control or pregnant plasma in order to determine the effect of plasma from pregnant women on vascular function. PCAs perfused with both types of plasma demonstrated considerable myogenic activity as demonstrated by the amount of vasoconstriction measured in response to increased intravascular pressure. The active response of PCAs to stepwise increases of intravascular pressure along with passive pressure curves are shown in Figure 1A. NP vessels perfused with control plasma dilated at pressures below the myogenic pressure range, from 25 – 50 mm Hg, then constricted and exhibited increased myogenic tone as pressure was increased to 75 mm Hg (Figure 1B). This level of active constriction was maintained up to 150 mm Hg. In contrast, NP vessels perfused with pregnant plasma constricted significantly more than vessels perfused with NP plasma, and developed greater myogenic tone at lower pressures. The difference in active diameters was not due to vessel size as both groups had similar passive diameters (also shown in Figure 1A).
Figure 1.
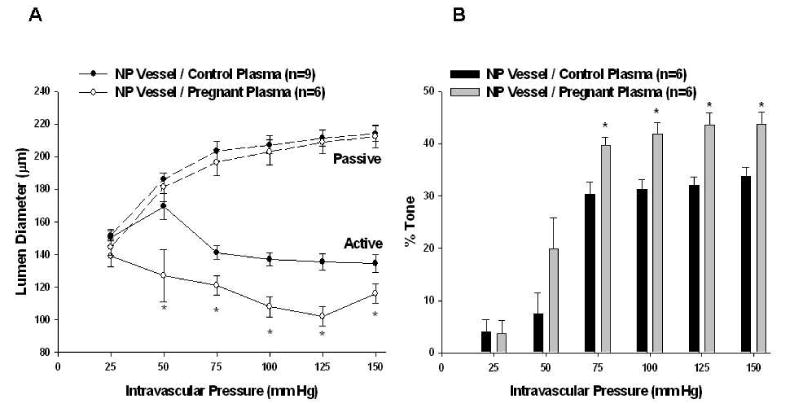
Effect of 20% pregnant plasma on myogenic reactivity and tone in posterior cerebral arteries from nonpregnant (NP) animals. A, Active and passive pressure vs. diameter curves. Pregnant plasma produced greater myogenic reactivity than control plasma in NP vessels (p < 0.05 using t-test at 50 – 150 mm Hg). Passive vessel diameters were similar (seen in dotted lines). B, Percent tone achieved. Pregnant plasma produced greater myogenic tone than control plasma when perfused through NP vessels (p < 0.05 using t-test at 75 – 150 mm Hg).
Unlike PCAs, MAs demonstrated considerably less myogenic reactivity, as demonstrated by the increase in lumen diameters with increased intravascular pressure between 25 - 100 mm Hg (Figure 2A). The amount of tone obtained by MAs was significantly less than obtained by the PCAs (p < 0.001 at intravascular pressures 75 mm Hg – 150 mm Hg), but there was no difference in the amount of tone in NP vessels perfused with either type of plasma (Figure 2B).
Figure 2.
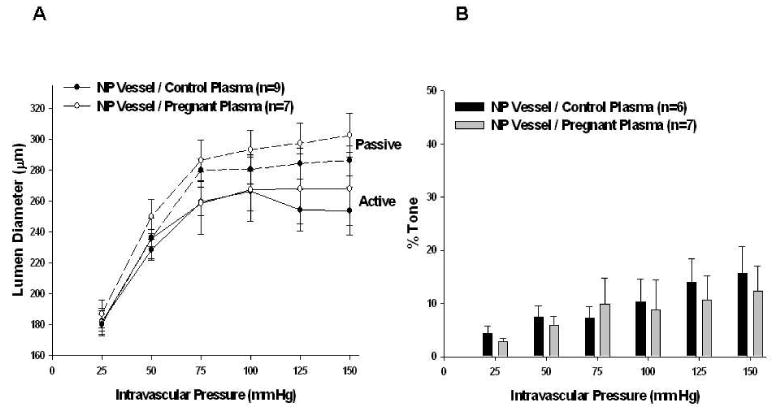
Effect of 20% pregnant plasma on myogenic reactivity and tone in mesenteric arteries (MAs) from nonpregnant (NP) animals. A, Active and passive pressure vs. diameter curves. MAs demonstrated little myogenic reactivity regardless of the type of plasma perfused through nonpregnant vessels. Passive vessel diameters were similar (seen in dotted lines). B, Percent tone achieved. MAs achieved significantly less tone than PCAs. There was no difference in tone between the two groups of plasma perfusate in NP vessels.
Adaptation of vessels from LP animals to pregnant plasma perfusate
To determine if vascular adaptation to pregnancy could influence the reactivity of vessels to pregnant plasma, the response to pregnant plasma was compared in arteries from NP and LP animals. PCAs from NP animals constricted and developed myogenic reactivity and tone that was maintained at intravascular pressures up to 150 mm Hg (Figure 3A and 3B). PCAs from LP animals constricted less than PCAs from NP animals. There was no significant difference in tone between LP and NP vessels. In fact, myogenic reactivity and tone in LP vessels perfused with pregnant plasma were similar to what was noted with NP vessels perfused with control plasma, suggesting these vessels adapt during pregnancy to the vasoconstricting influence of pregnant plasma.
Figure 3.
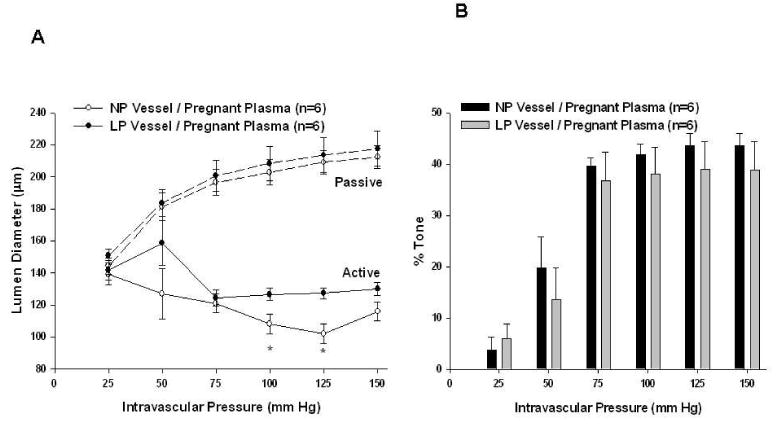
Adaptation of posterior cerebral arteries to pregnancy. A, Active and passive pressure vs. diameter curves with a common pregnant plasma perfusate. The increased myogenic reactivity seen when pregnant plasma (p < 0.05 at 100 and 125 mm Hg using t-test) was perfused through nonpregnant vessels is abolished when pregnant plasma is perfused through vessels from late pregnant animals, suggesting that pregnancy promoted vessel adaptation to adapt to the vasoconstricting influence of pregnant plasma. Passive vessel diameters were similar (seen in dotted lines). B, Percent tone achieved by different vessel types with common pregnant plasma perfusate. There was no significant difference between the amount of myogenic tone achieved by different vessel types.
MAs had considerably less myogenic reactivity (i.e. constriction in response to increased pressure) and significantly less tone than PCAs (p < 0.01 at intravascular pressures 75 mm Hg – 150 mm Hg). Myogenic reactivity and tone were unaffected by the type of plasma perfused through either vessels from NP or LP animals (Figures 4A and 4B).
Figure 4.
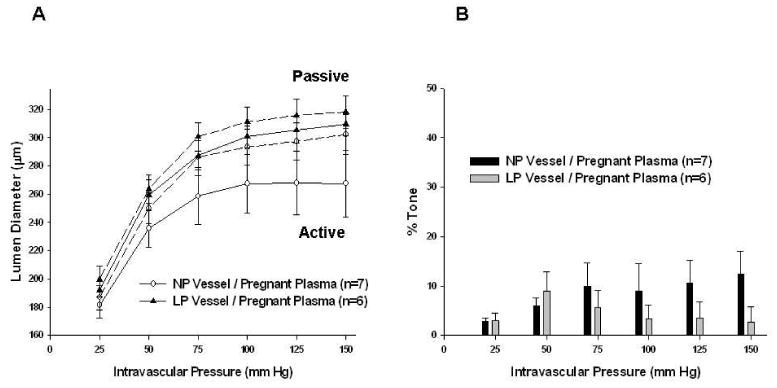
Adaptation of mesenteric arteries (MAs) to pregnancy. A, Active and passive pressure vs. diameter curves with a common pregnant plasma perfusate. There was less myogenic reactivity demonstrated by MAs vs. posterior cerebral arteries (PCAs). Vessel type did not influence active or passive vessel diameters (seen in dotted lines). B, Percent tone achieved by different vessel types with common pregnant plasma perfusate. MAs again had significantly less tone than PCAs. There was no significant difference between the amount of myogenic tone achieved by different vessel types.
Basal NO and prostacyclin production
Figure 5 shows the contractile response of both PCAs and MAs to NOS and COX inhibition. PCAs and MAs from NP and LP animals constricted in response to NOS inhibition with L-NNA, indicating basal NO production. There was no difference in the amount of constriction between arteries from NP or LP animals or with plasma type, suggesting the effect of circulating factors in pregnant plasma on tone was not due to a decrease in basal NO production. The addition of indomethacin to inhibit COX caused minimal changes to vessel diameters that were not different between the groups (Figure 5A and 5B).
Figure 5.
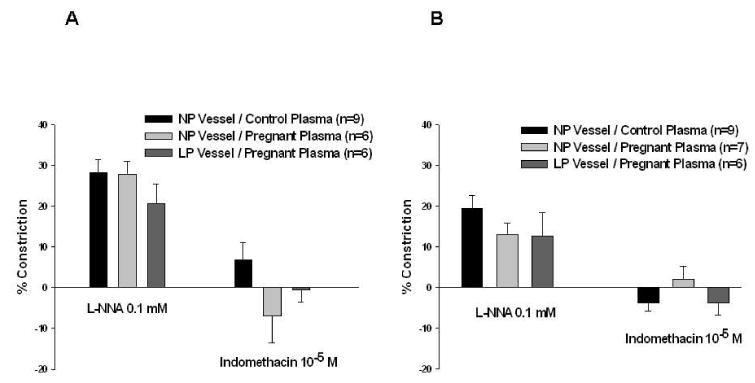
Endothelial vasodilator production. Percent constriction of posterior cerebral arteries (A) and mesenteric arteries (B) with the addition of L-NNA and indomethacin to inhibit nitric oxide synthase and cyclooxygenase, respectively. There was constriction of all vessel / plasma combinations with the addition of L-NNA, indicating basal endothelial nitric oxide production. The addition of indomethacin caused minimal changes in vessel diameter. Neither the type of vessel nor plasma perfusate affected the amount constriction produced by L-NNA or indomethacin, suggesting that varying amounts of NO or prostacyclin production are not responsible for the vasoconstricting effects of pregnant plasma on nonpregnant vessels.
Effect of circulating factors in pregnant plasma on EDHF activity in NP vessels
Addition of A23187 in the presence of NOS and COX inhibition was used to assess EDHF response in PCAs from NP animals perfused with control or pregnant plasma. This is a commonly used approach to assess EDHF responsiveness 14, 15. A23187 caused dilation in both groups; however, EDHF dilation was diminished in NP vessels exposed to pregnant plasma, suggesting circulating factors during normal pregnancy inhibit stimulated EDHF (Figure 6A). The percent dilation to 1.0 μM A23187 in PCAs perfused with control vs. pregnant plasma was 111 ± 17% vs. 35 ± 11%; p<0.01.
Figure 6.
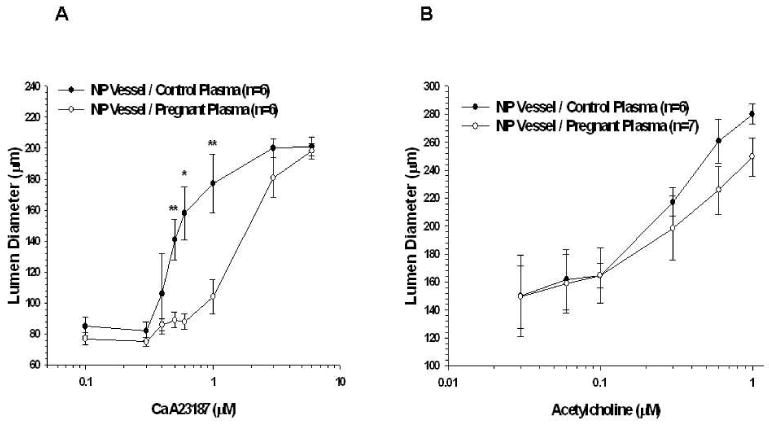
Stimulated endothelium-derived hyperpolarizing factor (EDHF) response in vessels from nonpregnant animals. A, Percent dilation of posterior cerebral arteries with the addition of cumulative doses of A23187 in the presence of nitric oxide synthase (NOS) and cyclooxygenase (COX) inhibition. Pregnant plasma produced a significantly attenuated EDHF response. B, Percent dilation of mesenteric arteries with the addition of cumulative doses of acetylcholine in the presence of NOS and COX inhibition. There was no difference in EDHF response in vessels from nonpregnant animals perfused with either control or pregnant plasma.
EDHF response in MAs was elicited by adding acetylcholine to the vessel bath in the presence of NOS and COX inhibition. Acetylcholine caused dilation in vessels from NP animals perfused with either control or pregnant plasma (Figure 6B). There was no significant difference in EDHF dilation between plasma groups.
Adaptation EDHF response in vessels from LP animal to pregnant plasma perfusate
In order to determine if the vascular adaptation to pregnancy affects EDHF responsiveness to pregnant plasma, EDHF activity in vessels from LP animals perfused with pregnant plasma was compared to vessels from NP animals perfused with the same type of plasma (Figure 7A and 7B). There was no significant difference between PCAs and MAs from LP animals and NP animals perfused with pregnant plasma.
Figure 7.
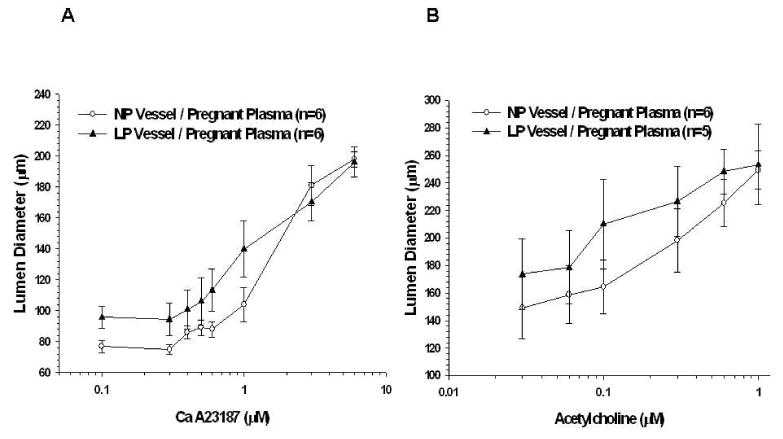
Stimulated endothelium-derived hyperpolarizing factor (EDHF) response in different vessel types with a common pregnant plasma perfusate. A, Percent dilation of posterior cerebral arteries with the addition of cumulative doses of A23187 in the presence of nitric oxide synthase (NOS) and cyclooxygenase (COX) inhibition. Pregnant plasma no longer produced an attenuated EDHF response when perfused through late pregnant (LP) vessels. B, Percent dilation of mesenteric arteries with the addition of cumulative doses of acetylcholine in the presence of NOS and COX inhibition. There was no significant difference in EDHF response between vessels from nonpregnant and LP animals perfused with pregnant plasma.
Effect of 40% plasma perfusate on vascular reactivity and endothelial vasodilator production
A separate set of experiments with NP and LP animals was completed to compare the effect of 20% vs. 40% pregnant plasma on vessel function. There was no difference between myogenic reactivity, tone or endothelial vasodilator production in PCAs from NP or LP animals with 40% perfusate vs. 20% perfusate (data not shown). Consistent with findings utilizing 20% pregnant plasma, PCAs from LP animals perfused with 40% plasma had greater active lumen diameters and decreased myogenic tone compared to vessels from NP animals perfused with 40% plasma, again demonstrating an adaptation to pregnancy that counteracts the vasoconstricting effects of pregnant plasma (data not shown). Endothelial vasodilator activity was similar in all cerebral vessels. MAs from NP animals perfused with 40% pregnant plasma exhibited similar myogenic reactivity and tone to those perfused with 20% plasma. MAs from NP animals perfused with 40% pregnant plasma had similar constriction to L-NNA compared with MAs from LP animals, suggesting basal NO production was similar in both groups. Reactivity to indomethacin was also similar with both concentrations of plasma perfusate.
Discussion
Results from this study suggest the presence of circulating factors in plasma of normal pregnant women that selectively affects reactivity and tone of cerebral arteries vs. systemic arteries. This was demonstrated by the finding that pregnant plasma elicited a significant vasoconstricting effect on nonpregnant cerebral vessels, increasing the level of basal tone. However, pregnancy produced a vascular adaptation that counteracted the vasoconstricting properties of pregnant plasma such that cerebral arteries from LP animals no longer constricted to pregnant plasma but had reactivity and tone similar to vessels from NP animals perfused with control plasma. Together, these results suggest that both circulating factors and vessel adaptation may play a role in maintaining cerebrovascular function in pregnancy.
The mechanism by which circulating factors in pregnant plasma increase myogenic tone is unclear, but is unlikely related to NO or prostacyclin production. There were no differences in constriction to L-NNA or indomethacin in vessels from NP animals perfused with control or pregnant plasma, suggesting basal NO and prostacyclin production were not affected. Despite our finding of decreased EDHF responsiveness in PCAs from NP animals exposed to pregnant plasma, this is unlikely related to the increased tone noted in PCAs from NP animals perfused with pregnant plasma because there needs to be basal EDHF production for this vasodilator to affect myogenic tone. While basal EDHF activity has been shown in brain parenchymal arterioles, our lab has found that PCAs do not exhibit basal EDHF (unpublished results) 16. It is therefore unlikely that inhibition of EDHF production by pregnant plasma was responsible for the increase in basal tone.
Another possible explanation for the increase of myogenic reactivity and tone in PCAs from NP animals exposed to pregnant plasma may be the presence of a vasoconstrictor in the pregnant plasma, such as thromboxane, endothelin-1 (ET-1) or Ang II. Thromboxane is a potent vasoconstrictor that increases as pregnancy progresses and opposes the actions of prostacyclin 17, 18. Walsh et al. demonstrated preeclamptic placentas produced more than three times the amount of thromboxane and less than one half the amount of prostacyclin as normal placentas, thought to contribute to a net vasoconstricted state throughout the body 19. However, thromboxane is less likely to be causing increased reactivity and tone in vessels from NP animals exposed to pregnant plasma seen in our experiments as the amount of constriction to COX inhibition with indomethacin was similar between vessel and plasma types. Another vasoconstrictor that may be present in the pregnant plasma used in our experiments is ET-1. It has been postulated that increased levels of ET-1 may be capable of counteracting the vasodilatory effect on NO 20, 21. Perhaps the most likely candidate for a vasoconstrictor enabling pregnant plasma used in our study to elicit increased myogenic reactivity and tone in NP arteries is Ang II. Ang II is a potent vasoactive compound that elicits action through two receptor subtypes: AT1 receptor activation results in vasoconstriction while the AT2 receptor mediates vasodilation 22, 23. Multiple studies have shown there to be increased circulating levels of Ang II with pregnancy 8, 9, 24. In addition, Ang II has been found to induce constriction in cerebral arteries of nonpregnant female and male rats or mice by acting on the AT1 receptor subtype 25-28. Though experiments have yet to be performed on pregnant rats, and less constriction has been seen in female animals compared with male, it is plausible that the human pregnant plasma used in our study contains increased levels of Ang II, causing an increase in myogenic reactivity and tone27, 28.
While vessels from NP animals perfused with pregnant plasma exhibited greater myogenic reactivity and tone than arteries perfused with control plasma, constriction was abolished when pregnant plasma was perfused through vessels from LP animals. This finding suggests that the cerebrovasculature adapts to the vasoconstricting effect of pregnant plasma, resulting in a more relaxed state. The mechanism of this vascular adaptation is unclear, however, one possibility involves the vasodilatory effects of AT2 receptors. Studies have shown that Ang II activates AT2 receptors, and may induce the release of vasodilators such as NO and EDHF 23, 29-31. AT2 receptors are heavily expressed in placental and fetal tissue, and vascular AT2 receptors in rats, including in the aorta and mesenteric artery, increases with both estrogen exposure as well as pregnancy 23, 29, 32-35. It is plausible that pregnancy and its resulting increase in estrogen increases cerebrovascular expression of AT2 receptors such that vessels are able to normalize the vasoconstricting effects of pregnant plasma perfusate.
The specific effect of pregnant plasma on vascular tone in cerebral arteries vs. MAs is not clear but may be due to the unique properties of cerebral arteries. Cerebral pial arteries are known to demonstrate myogenic tone and contribute to segmental vascular resistance in the brain 36, 37. Though MAs in our experiments demonstrated little tone, the MAs used were third-order branches of the mesenteric artery with average intraluminal diameters that were significantly larger than the third-order branches of the PCAs used. It is possible that smaller resistance-sized MAs would develop myogenic reactivity and tone, and would react more similarly to PCAs. Our findings contrast those from Merchant et al,, who found that MAs from virgin NP mice developed increased tone when exposed to plasma pooled from nonpregnant women compared to pregnant women 38. In that study, vessels were exposed extraluminally to 2% plasma for approximately one hour. This is in contrast to the current study in which vessels were exposed intraluminally to a higher, more physiological level of plasma 38. It is likely that extraluminal incubation in plasma in the previous study directly affected vascular smooth muscle that is not normally exposed to plasma and did not allow a higher concentration to be used.
There are several limitations of this study that are worth noting. First, our study examined the effects of plasma on isolated cerebral vessels using an in vitro approach. Because the entire brain vasculature is not intact, upstream and downstream influences such as shear stress and flow are not accounted for. Second, we examined cerebral vascular function in a rat model of pregnancy perfused with human plasma, which may facilitate an inter-species interaction between human plasma and rat vasculature. Myers et al. found the impaired endothelium-dependent relaxation demonstrated in isolated human myometrial arteries exposed to human preeclamptic plasma was not reproducible using mouse mesenteric and uterine arteries 39. However, these experiments were performed with wire myography, a technique significantly different than the pressurized arteriograph used in our experiments. Another limitation to our study is the acute exposure the cerebral and MAs have to plasma of approximately three hours. The function of the PCAs and MAs may differ with chronic exposure to plasma. Another limitation is the discrepancy between the concentrations of plasma we used (20% and 40%) as a perfusate compared to physiologic values of about 60 - 65%. The concentrations used in the study were secondary to limitations of plasma availability. However, the current study attempted to mimic a more physiologic state, exposing vessels to plasma intraluminally and studying vessels that are pressurized. Prior studies investigating the effects of plasma on vessel function have involved comparing human omental and myometrial arteries from nonpregnant and normotensive pregnant women that were incubated (intra and abluminally) in 2% plasma from normal pregnant or preeclamptic women for either one or 16 hour periods 39-43. In addition, while other studies have investigated the effect of pregnant plasma on mesenteric arteries, this is the first study we are aware of investigating the effects of circulating factors in normal pregnancy on cerebral artery function.
In summary, the current study demonstrates the presence of circulating factors in the plasma of pregnant women that increases cerebral artery reactivity and tone, and decreases EDHF production. This alteration in vessel function was specific to cerebral vessels as the response in MAs was not different with the different plasma. However, it appears that pregnancy itself produces an adaptive affect, such that pregnant cerebral vessels counteract the vasoconstricting effects of pregnant plasma. This adaptive effect may contribute to the ability of the cerebrovasculature to maintain relatively constant CBF during normal pregnancy 44.
Acknowledgments
The Preeclampsia Foundation Vision Grant (OAA) NINDS NS045940 and AHA EI 0540082 (to MJC) NIH RO1 HL 71944 (IMB)
Contributor Information
Ödül A. Amburgey, Email: odul.amburgey@vtmednet.org.
Shane A. Reeves, Email: shane.reeves@vtmednet.org.
Ira M. Bernstein, Email: ira.bernstein@vtmednet.org.
References
- 1.Gerber RT, Anwar MA, Poston L. Enhanced acetylcholine induced relaxation in small mesenteric arteries from pregnant rats: an important role for endothelium-derived hyperpolarizing factor (EDHF) Br J Pharmacol. 1998;125(3):455–460. doi: 10.1038/sj.bjp.0702099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cipolla MJ, Binder ND, Osol G. Myoendometrial vs. placental uterine arteries: structural, mechanical, and functional differences in late-pregnant rabbits. Am J Obstet Gynecol. 1997;177(1):215–21. doi: 10.1016/s0002-9378(97)70464-2. [DOI] [PubMed] [Google Scholar]
- 3.Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18(2):152–161. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- 4.Edouard DA, Pannier BM, London GM, Cuche JL, Safar ME. Venous and arterial behavior during normal pregnancy. Am J Physiol. 1998;274(5 Pt 2):H1605–1612. doi: 10.1152/ajpheart.1998.274.5.H1605. [DOI] [PubMed] [Google Scholar]
- 5.Weiner CP, Thompson LP. Nitric oxide and pregnancy. Semin Perinatol. 1997;21(5):367–380. doi: 10.1016/s0146-0005(97)80003-1. [DOI] [PubMed] [Google Scholar]
- 6.Elsheikh A, Creatsas G, Mastorakos G, et al. The renin-aldosterone system during normal and hypertensive pregnancy. Arch Gynecol Obstet. 2001;264(4):182–185. doi: 10.1007/s004040000104. [DOI] [PubMed] [Google Scholar]
- 7.Gant NF, Worley RJ, Everett RB, MacDonald PC. Control of vascular responsiveness during human pregnancy. Kidney Int. 1980;18(2):253–258. doi: 10.1038/ki.1980.133. [DOI] [PubMed] [Google Scholar]
- 8.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52(11):2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magness RR, Cox K, Rosenfeld CR, Gant NF. Angiotensin II metabolic clearance rate and pressor responses in nonpregnant and pregnant women. Am J Obstet Gynecol. 1994;171(3):668–679. doi: 10.1016/0002-9378(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 10.Talledo OE. Renin-angiotensin system in normal and toxemic pregnancies. I. Angiotensin infusion test. Am J Obstet Gynecol. 1966;96(1):141–143. doi: 10.1016/s0002-9378(16)34655-5. [DOI] [PubMed] [Google Scholar]
- 11.Massani ZM, Sanguinetti R, Gallegos R, Raimondi D. Angiotensin blood levels in normal and toxemic pregnancies. Am J Obstet Gynecol. 1967;99(3):313–317. doi: 10.1016/s0002-9378(16)34536-7. [DOI] [PubMed] [Google Scholar]
- 12.Cipolla MJ, Vitullo L, McKinnon J. Cerebral artery reactivity changes during pregnancy and the postpartum period: a role in eclampsia? Am J Physiol Heart Circ Physiol. 2004;286(6):H2127–2132. doi: 10.1152/ajpheart.01154.2003. [DOI] [PubMed] [Google Scholar]
- 13.Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50(1):14–24. doi: 10.1161/HYPERTENSIONAHA.106.079442. [DOI] [PubMed] [Google Scholar]
- 14.Gillham JC, Kenny LC, Baker PN. An overview of endothelium-derived hyperpolarising factor (EDHF) in normal and compromised pregnancies. Eur J Obstet Gynecol Reprod Biol. 2003;109(1):2–7. doi: 10.1016/s0301-2115(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 15.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann Med. 2007;39(7):495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- 16.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40(4):1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2004;70(2):223–232. doi: 10.1016/j.plefa.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Ylikorkala O, Viinikka L. Thromboxane A2 in pregnancy and puerperium. Br Med J. 1980;281(6255):1601–1602. doi: 10.1136/bmj.281.6255.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152(3):335–340. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 20.Khedun SM, Naicker T, Moodley J. Endothelin-1 activity in pregnancy. J Obstet Gynaecol. 2002;22(6):590–593. doi: 10.1080/0144361021000020321. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71(6):1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 22.Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45(2):205–251. [PubMed] [Google Scholar]
- 23.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am J Physiol Endocrinol Metab. 2000;278(3):E357–374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- 24.Hanssens M, Keirse MJ, Spitz B, van Assche FA. Angiotensin II levels in hypertensive and normotensive pregnancies. Br J Obstet Gynaecol. 1991;98(2):155–161. doi: 10.1111/j.1471-0528.1991.tb13361.x. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JM, Kwan YW, Chan SL, et al. Constrictor and dilator effects of angiotensin II on cerebral arterioles. Stroke. 2005;36(12):2691–2695. doi: 10.1161/01.STR.0000190002.79052.bf. [DOI] [PubMed] [Google Scholar]
- 26.Naveri L, Stromberg C, Saavedra JM. Angiotensin II AT1 receptor mediated contraction of the perfused rat cerebral artery. Neuroreport. 1994;5(17):2278–2280. doi: 10.1097/00001756-199411000-00018. [DOI] [PubMed] [Google Scholar]
- 27.De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller AA. Gender influences cerebral vascular responses to angiotensin II through Nox2-derived reactive oxygen species. Stroke. 2009;40(4):1091–1097. doi: 10.1161/STROKEAHA.108.531707. [DOI] [PubMed] [Google Scholar]
- 28.Faraci FM, Lamping KG, Modrick ML, et al. Cerebral vascular effects of angiotensin II: new insights from genetic models. J Cereb Blood Flow Metab. 2006;26(4):449–455. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- 29.Stennett AK, Qiao X, Falone AE, Koledova VV, Khalil RA. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am J Physiol Heart Circ Physiol. 2009;296(3):H745–755. doi: 10.1152/ajpheart.00861.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haberl RL, Anneser F, Villringer A, Einhaupl KM. Angiotensin II induces endothelium-dependent vasodilation of rat cerebral arterioles. Am J Physiol. 1990;258(6 Pt 2):H1840–1846. doi: 10.1152/ajpheart.1990.258.6.H1840. [DOI] [PubMed] [Google Scholar]
- 31.Gwathmey TM, Shaltout HA, Pendergrass KD, et al. Nuclear Angiotensin II - Type 2 (AT2) Receptors Are Functionally Linked to Nitric Oxide Production. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88(3):921–933. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judson JP, Nadarajah VD, Bong YC, Subramaniam K, Sivalingam N. A preliminary finding: immunohistochemical localisation and distribution of placental angiotensin II receptor subtypes in normal and preeclamptic pregnancies. Med J Malaysia. 2006;61(2):173–180. [PubMed] [Google Scholar]
- 34.Nickenig G, Baumer AT, Grohe C, et al. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97(22):2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 35.Silva-Antonialli MM, Tostes RC, Fernandes L, et al. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62(3):587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Faraci FM, Mayhan WG, Heistad DD. Segmental vascular responses to acute hypertension in cerebrum and brain stem. Am J Physiol. 1987;252(4 Pt 2):H738–742. doi: 10.1152/ajpheart.1987.252.4.H738. [DOI] [PubMed] [Google Scholar]
- 37.Baumbach GL, Heistad DD. Regional, segmental, and temporal heterogeneity of cerebral vascular autoregulation. Ann Biomed Eng. 1985;13(34):303–310. doi: 10.1007/BF02584248. [DOI] [PubMed] [Google Scholar]
- 38.Merchant SJ, Davidge ST. The role of matrix metalloproteinases in vascular function: implications for normal pregnancy and pre-eclampsia. BJOG. 2004;111(9):931–939. doi: 10.1111/j.1471-0528.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 39.Myers J, Irvine R, Gillham J, et al. Altered endothelial function in isolated human myometrial vessels induced by plasma from women with pre-eclampsia is not reproducible in isolated mouse vessels. Clin Sci (Lond) 2005;108(5):457–462. doi: 10.1042/CS20040343. [DOI] [PubMed] [Google Scholar]
- 40.Hayman R, Warren A, Brockelsby J, Johnson I, Baker P. Plasma from women with pre-eclampsia induces an in vitro alteration in the endothelium-dependent behaviour of myometrial resistance arteries. BJOG. 2000;107(1):108–115. doi: 10.1111/j.1471-0528.2000.tb11586.x. [DOI] [PubMed] [Google Scholar]
- 41.Hayman R, Warren A, Johnson I, Baker P. Inducible change in the behavior of resistance arteries from circulating factor in preeclampsia: an effect specific to myometrial vessels from pregnant women. Am J Obstet Gynecol. 2001;184(3):420–426. doi: 10.1067/mob.2001.109733. [DOI] [PubMed] [Google Scholar]
- 42.Myers J, Mires G, Macleod M, Baker P. In preeclampsia, the circulating factors capable of altering in vitro endothelial function precede clinical disease. Hypertension. 2005;45(2):258–263. doi: 10.1161/01.HYP.0000153461.58298.a4. [DOI] [PubMed] [Google Scholar]
- 43.Myers JE, Hart S, Armstrong S, et al. Evidence for multiple circulating factors in preeclampsia. Am J Obstet Gynecol. 2007;196(3):266, e261–266. doi: 10.1016/j.ajog.2006.10.875. [DOI] [PubMed] [Google Scholar]
- 44.Cipolla MJ, Bullinger LV, Godfrey JA. Pregnancy and PPARγ Activation Cause Small Vessel Remodeling in the Maternal Brain and Diminished Cerebrovascular Resistance: a Role in Eclampsia? Reprod Sci. 2009;16(3 Supplement):91A–92A. [Google Scholar]


