Abstract
Transplantation of olfactory bulb-derived olfactory ensheathing glia (OEG) combined with step training improves hindlimb locomotion in adult rats with a complete spinal cord transection. Spinal cord injury studies use the presence of noradrenergic (NA) axons caudal to the injury site as evidence of axonal regeneration and we previously found more NA axons just caudal to the transection in OEG- than media-injected spinal rats. We therefore hypothesized that OEG transplantation promotes descending coeruleospinal regeneration that contributes to the recovery of hindlimb locomotion. Now we report that NA axons are present throughout the caudal stump of both media- and OEG-injected spinal rats and they enter the spinal cord from the periphery via dorsal and ventral roots and along large penetrating blood vessels. These results indicate that the presence of NA fibers in the caudal spinal cord is not a reliable indicator of coeruleospinal regeneration. We then asked if NA axons appose cholinergic neurons associated with motor functions, i.e., central canal cluster and partition cells (active during fictive locomotion) and somatic motor neurons (SMNs). We found more NA varicosities adjacent to central canal cluster cells, partition cells, and SMNs in the lumbar enlargement of OEG- than media-injected rats. As non-synaptic release of NA is common in the spinal cord, more associations between NA varicosities and motor-associated cholinergic neurons in the lumbar spinal cord may contribute to the improved treadmill stepping observed in OEG-injected spinal rats. This effect could be mediated through direct association with SMNs and/or indirectly via cholinergic interneurons.
Keywords: OEC, spinal cord injury, norepinephrine, ChAT, cholinergic neurons, locomotion
INTRODUCTION
The noradrenergic (NA) system can activate locomotor pattern generation in the absence of supraspinal innvervation. For example, administration of noradrenaline, its precursor dopamine, and its α1- and α2-receptor agonists such as clonidine and methoxamine induce and/or modulate hindlimb locomotion in acute complete spinal transected (i.e., spinal) adult cats (Barbeau et al., 1993; Chau et al., 1998; Giroux et al., 1998), chronically transected adult spinal rodents (Guertin, 2004), and in severely injured humans (Barbeau and Norman, 2003). Administration of both noradrenaline and its agonists also elicit and maintain fictive locomotion in the neonatal spinal cord in vitro (Kiehn et al., 1999; Sqalli-Houssaini and Cazalets, 2000). Furthermore, transplantation of embryonic locus coeruleus tissue into the spinal cord caudal to the lesion, i.e., caudal stump, in adult spinal rats reinnervates previous targets (Gimenez y Ribotta et al., 1996) and leads to improved hindlimb stepping (Yakovleff et al., 1989; Gimenez y Ribotta et al., 1998a; Gimenez y Ribotta et al., 1998b) and recovery of withdrawal reflexes (Moorman et al., 1990). NA α1- and α2-receptors are expressed broadly throughout the gray mater in an intact spinal cord and are up-regulated in the lumbar segments after a complete spinal cord transection (Roudet et al., 1994; Roudet et al., 1996). These observations indicate that the spinal cord caudal to the lesion remains responsive to noradrenaline after the loss of coeruleospinal innervation and, with proper stimulation, can contribute to locomotor activity of spinal animals.
When OEG transplantation is combined with long-term treadmill stepping in adult spinal rats, we found that step training alone did not improve stepping, while OEG transplantation alone improved plantar step performance. When OEG transplantation and step training were combined, however, locomotor ability improved over time and step frequency and trajectory were not significantly different from sham rats (Kubasak et al., 2008). The mechanisms by which OEG transplantation and treadmill training contribute to this recovery remain unclear. Based on previous pharmacological evidence, one possibility is that OEG promote regeneration of coeruleospinal axons that then contribute to locomotor recovery. After a complete spinal cord transection, several studies interpreted the presence of NA axons in the caudal stump as evidence of spinal cord regeneration (Chen et al., 1996; Ramon-Cueto et al., 2000; Lopez-Vales et al., 2006b). Kubasak et al. (2008), however, reported that dopamine β-hydroxylase (DBH; a marker for noradrenergic axons)-positive axons penetrate the glial scar and enter the spinal cord caudal to the transection in both media- and OEG-injected rats. The density of DBH-positive axons immediately caudal to the lesion was higher in OEG- than media-injected rats, suggesting that regenerating DBH-positive axons may cross the glial scar and contribute to the improved hindlimb stepping seen in OEG-injected rats.
Alternatively, OEG may promote the reorganization of spinal circuits within the caudal stump that leads to the recovery of hindlimb locomotion. For example, OEG transplantation influences the frequency of interactions between serotonergic axons and motor-associated cholinergic neurons in the caudal stump (Takeoka et al., 2009), and DBH-positive axons may undergo a similar reorganization. Two groups of spinal cholinergic interneurons, the central canal cluster and partition cells (Barber et al., 1984; Phelps et al., 1984), project directly to ipsilateral and contralateral somatic motor neurons (SMNs; Houser et al., 1983; Barber et al., 1984; Phelps et al., 1984) and influence their excitability (Miles et al., 2007), and are active during fictive locomotion (Carr et al., 1995; Huang et al., 2000). As noradrenaline modulates the output of SMNs (Harvey et al., 2006) and utilizes non-synaptic as well as synaptic transmission (Beaudet and Descarries, 1978; Ridet et al., 1993; Hentall et al., 2003), axonal varicosities located within a few microns to a millimeter from motor-associated cholinergic neurons could potentially modify their activity and contribute to hindlimb stepping ability.
In this study we examined the caudal stump of a cohort of spinal rats that demonstrated differential functional recovery between media- and OEG-injected spinal rats (Kubasak et al., 2008). We sought to determine 1) if there are more DBH-axons distributed throughout the caudal stump in OEG- than media-injected spinal rats; 2) the source of DBH-positive axons in the caudal stump of adult spinal rats; and 3) if OEG transplantation influences the density of DBH-positive axons apposing motor-associated cholinergic neurons.
MATERIAL AND METHODS
Animal procedures
All procedures followed the NIH guidelines and were approved by the Chancellor’s Animal Research Committee at UCLA. Olfactory bulb-derived OEG were dissected and immunopurified as reported in Kubasak et al. (2008). Briefly, we dissected OEG from olfactory bulbs of 8–10 week old Wistar Hannover rats (Harlan Laboratories, Indianapolis IN), used the p75 nerve growth factor receptor antibody (NGFR, 1:5, Chandler et al., 1984) to immunopurify OEG from the primary culture on day 7–8, and harvested cells after 17–19 days in vitro. Female Wistar Hannover rats (Harlan Laboratories, Indianapolis, IN), 10–12 weeks old, were anesthetized deeply with 2% isoflurane and received a complete spinal cord transection at ~T9. Media with or without 400,000 OEG were injected 1 mm rostral and 1 mm caudal to the transection. At 1 mo post-injury 50% of the rats in both the media- and OEG-injected groups began intensive manual hindlimb step training for 20 min a day, 5 days/week for 6 months (more than 50 hr/rat over a 6 mo period; Kubasak et al., 2008). In the present study we analyzed the entire caudal stump (i.e., from below the transection to the sacral levels) of 12 rats chosen for our previous analyses of the transection site. Three rats in each of four experimental groups (media-untrained, media-trained, OEG-untrained, and OEG-trained) represented a range of stepping abilities at 7 months (see Table 1 in Kubasak et al., 2008). Two female adult sham rats were tested for stepping kinematics (Fig. 2 in Kubasak et al., 2008) and their spinal cords were studied as positive immunohistochemical controls. To identify the association of NA axons with platelet endothelial cell adhesion molecule (PECAM)-labeled blood vessels, one additional adult OEG-injected spinal rat was perfused at both 1 and 7 mo post-transection. We analyzed an additional 6 media- and 6 OEG-injected rats with another blood vessel marker, lycopersicon esulentum (tomato) lectin and observed similar relationships between the blood vessels and NA axons.
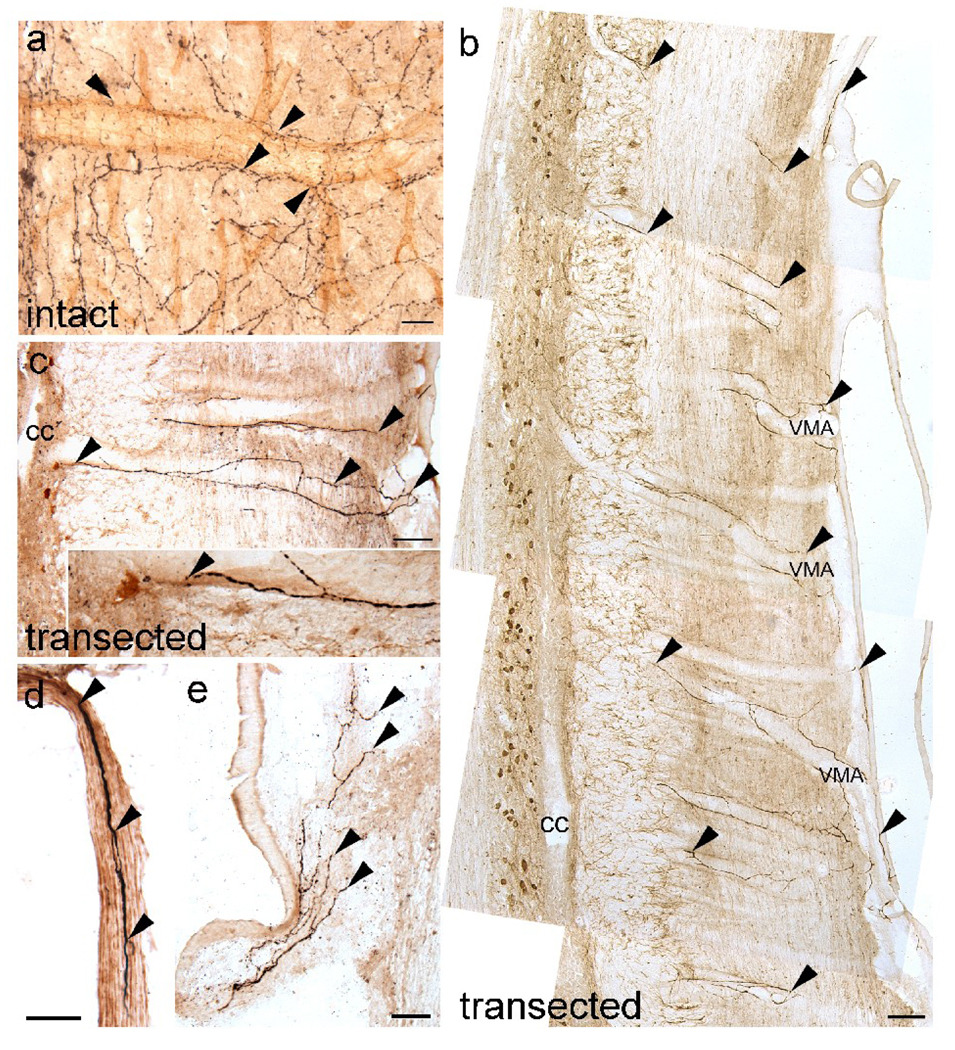
Fig. 2.
Noradrenergic (NA) axons enter the spinal cord in association with blood vessels via ventral roots and meninges regardless of transplantation status. (a) Lumbar spinal cord of an intact rat shows numerous dopamine β-hydroxylase (DBH)-positive axons (black; arrowheads) aligned along a penetrating blood vessel identified with platelet endothelial cell adhesion molecule (PECAM; brown). (b, c) DBH-positive axons (black; arrowheads) course along the wall of large ventralmedium arteries (VMA) and extend toward lamina X that contains ChAT-positive central canal cluster cells (amber brown; c is enlarged in inset). cc, central canal (d) A ventral root contains DBH-positive axons (arrowheads) in adult spinal rats. (e) DBH-labeled axons (arrowheads) often are detected along the meninges. Scale a = 25 µm, Scale d, e = 50 µm, Scale b, c = 100 µm.
Tissue preparation
Rats were anesthetized deeply with ketamine (0.9 µl/g) and Anased (0.5 µl/g), perfused with 4% paraformaldehyde, and post-fixed overnight for analyses of NA axons or for 4 hrs for blood vessel analyses. Spinal cords were dissected, cryoprotected, frozen on dry ice, and stored at 80°C. We sectioned all spinal cords sagittally at 25 µm and mounted the sections, so that each of the 16 slides contained every 16th section. Alternate slides were double-labeled for DBH and choline acetyltransferase (ChAT; current study). The other 50% of the sections were used to examine the distribution of serotonin and ChAT in the caudal stump (Takeoka et al., 2009).
Immunocytochemical procedures
To identify DBH-labeled axons, we used a mouse anti-rat DBH (1:1,000, Chemicon, Temecula, CA) raised against purified bovine DBH that recognizes both the soluble (70 kD) and membrane bound (75 kD) forms of the enzyme. For ChAT immunolabeling, a polyclonal anti-ChAT antiserum (AB144P, 1:500, Chemicon, Temecula, CA) raised against human placental ChAT was used. For tyrosine hydroxylase (TH) immunolabeling, both monoclonal (TH-16, 1:1,000, Sigma-Aldrich, St Louis, MO) and rabbit polyclonal (ab6211, 1:8,000, Abcam Inc, Cambridge, MA) antibodies were used. To most effectively identify blood vessels, we used purified mouse anti-rat CD31 (PECAM-1; 1:150, BD Biosciences, San Jose, CA).
To localize NA axons, sections were initially washed with 0.1M Tris buffer containing 1.4% NaCl and 0.1% bovine serum albumin (TBS) followed by a 30 min pre-soak in 0.3% H2O2 and 0.1% NaN3. After a 1% Triton pre-soak for 15 min, sections were incubated in 1.5% normal horse serum with 0.3% Triton and an additional blocking step to reduce non-specific avidin/biotin binding (Vector Laboratories; Burlingame, CA). Sections were incubated in mouse anti-rat DBH antibody (1:1,000) overnight at room temperature. The following day, sections were rinsed in TBS, anti-mouse biotinylated IgG (1:200, Elite Standard kit, Vector laboratories) for one hour, followed by avidin-biotin complex (1:100) for one hour. Tissue was rinsed in 0.1M acetate buffer for 10 min before being developed with 0.06% 3,3-diaminobenzidine (DAB) with Nickel glucose oxidase, producing a black immunoproduct.
For ChAT immunolabeling we followed the protocol reported in Takeoka et al. (2009). We rinsed the sections followed by the normal serum and avidin-biotin blocking steps before incubating with the goat anti-ChAT antiserum (1:500) overnight at room temperature. Sections were incubated with Vector rabbit anti-goat IgG (1:200, Elite Goat kit, Vector Laboratories, Burlingame, CA) and ABC (1:100) for one hour each for signal amplification. Sections were developed in 0.02 M imidazole-DAB producing an amber brown product. The TH and PECAM protocols were identical to that reported for ChAT. The tissues were processed with sections from intact spinal cords as positive controls.
Quantification
DBH-positive axons were quantified using methods identical to those reported by Takeoka et al. (2009) and similar to those of (Fouad et al., 2005)and Kubasak et al. (2008). We counted all DBH-immunopositive axons longer than 25 µm in alternate sections, i.e., 50% of the lower thoracic to sacral spinal cord. For arborized processes, we first traced and counted the longest single fiber and then other isolated immunopositive fibers. We measured the tissue volume processed for DBH-immunoreactivity using Openlab (Improvision Inc., Lexington, MA) software and calculated the DBH-fiber density. NA varicosities adjacent to cholinergic neurons were quantified as reported in Takeoka et al. (2009) and similar to that used by (Miller and Salvatierra, 1998; Mullner et al., 2008). The quantification method is based on the assumption that NA is released both synaptically and non-synaptically and does not distinguish between these transmission modes. We counted the number of DBH-positive varicosities directly adjacent (maximum of 1–2 µm away) to the cholinergic somata or processes at high magnification with the light microscope and normalized these counts per unit volume (mm3).
Statistical analyses
All statistical analyses were carried out using the resampling method (Efron and Tibshirani, 1991) that computationally simulates the null hypothesis with minimal assumptions about the data distribution or estimators (i.e., the means in this study) and, therefore, is suitable for small samples. Scripts for individual analyses were written using Resampling stats in MATLAB software package (Resampling Stats, Inc, Arlington, VA; MATLAB 7.0, Mathworks, Inc, Natick, MA). Statistical significance of mean differences was set at P< 0.05.
RESULTS
Media- and OEG-injected adult spinal rats have NA axons throughout the caudal stump
DBH-positive axons densely innervate the dorsal horn, laminae VII, VIII, and X of an intact spinal cord, and concentrate along the sympathetic preganglionic neurons in lamina VII (Fig. 1a; Aramant et al., 1986; Fritschy et al., 1987; Proudfit and Clark, 1991; Rajaofetra et al., 1992a; Rajaofetra et al., 1992b). The descending coeruleospinal tract originates mainly from the ventral caudal locus coeruleus and the A5 and A7 cell groups in the pontine lateral tegmentum (Satoh et al., 1977; Moore and Bloom, 1979; Westlund et al., 1981, 1983) and degenerates within several weeks after a complete spinal cord transection (Commissiong, 1985). Surprisingly, however, we detected a small number of NA axons immediately caudal to the transection site in both media- and OEG-injected spinal rats (Kubasak et al., 2008). Although 25% of spinal rats analyzed contained no DBH-positive axons just below the lesion (Kubasak et al., 2008), we now report that all rats in the same cohort of spinal rats had DBH-positive axons in the entire caudal stump, regardless of transplantation status. These DBH-immunoreactive axons were often present within lamina X (Fig. 1b), laminae I-III (Fig. 1d, e) and most commonly coursing rostrocaudally within or along the edge of the white matter (Fig. 1c). Although OEG-injected rats contained more DBH-labeled axons in the glial fibrillary acidic protein (GFAP)-positive region immediately below the transection than media-injected rats (Kubasak et al., 2008), there were no differences in axonal density between media- and OEG-injected rats within the remainder of the caudal stump (Fig. 1f; thoracic: P = 0.34, lumbar: P = 0.49, sacral: P = 0.40).
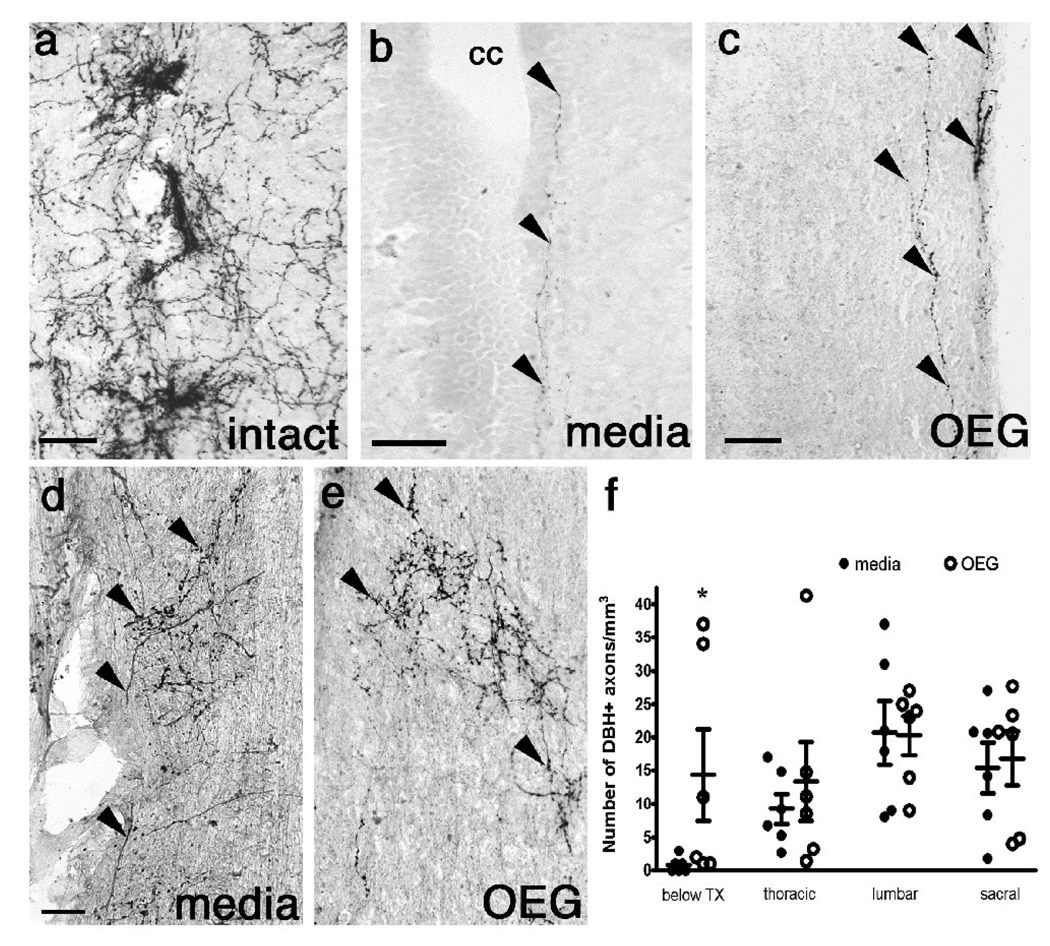
Fig. 1.
Noradrenergic (NA) axons in sagittal sections of lower thoracic spinal cord processed for dopamine β-hydroxylase (DBH) from an intact rat (a), and media- (b, d), and OEG-injected (c, e) rats 7 months after transection. (a–c) Many DBH-labeled axons are found around presumed sympathetic preganglionic neurons in lamina VII in an intact (a) rat. Only a few DBH immunopositive axons (arrowheads) are detected in lamina X along the central canal (cc; b) or at the edge of the white matter (c) in spinal rats. (d, e) DBH-positive axons (arrowheads) arborize extensively in the dorsal horn. (f) The density of DBH-labeled fibers is greater in OEG- than media-injected rats just below the transection site (data from Kubasak et al., 2008), but not significantly different in the remainder of the caudal spinal cord. Bars in (f) represent the mean ± SEM for 6 media-and 6 OEG-injected rats. Scale a–e = 50 µm.
As the administration of noradrenaline improves the stepping performance of spinal animals, we asked if the overall density of NA axons below the lesion would correlate with the better hindlimb stepping performance observed in the OEG-trained rats compared to the other groups (Kubasak et al., 2008). When we compared the density of NA axons in the OEG-trained group to each of the other three groups, none were statistically different. We then asked if step training influenced the density of DBH-positive axons present in the caudal stump. Neither training groups combined (OEG- and media-trained vs. OEG- and media-untrained; P = 0.25) nor comparison of training status within the same transplantation status (OEG-trained vs. OEG-untrained or media-trained vs. media-untrained; P = 0.22 and P= 0.23, respectively) affected the DBH fiber density. Thus the overall density of DBH-positive axons below the lesion did not correlate with the stepping ability of spinal rats.
Peripheral NA axons innervate the spinal cord caudal to the transection
Amenta et al. (1987) reported that NA axons associated with the spinal cord blood vessels could originate from peripheral sympathetic neurons. Other studies demonstrated that NA axons coursed along meningeal blood vessels and entered the spinal cord or associated with blood vessels found within the ventral and dorsal roots (McNicholas et al., 1980; Karlsson and Hildebrand, 1993; Hildebrand et al., 1997). In intact rats, DBH-positive axons coursed near PECAM-positive large and small diameter blood vessels (Fig. 2a), but could not be distinguished from the abundant coeruleospinal innervation. In spinal rats, however, the association of DBH-positive axons with blood vessels was clearly evident along the large diameter ventralmedian spinal arteries (Fig. 2b; VMA). These DBH-labeled fibers course along the endothelial wall of the VMA within the white matter and extend toward lamina X (Fig. 2c). We also found DBH-positive axons within the ventral (Fig. 2d), and less frequently the dorsal (data not shown) roots as reported in various species (Stevens et al., 1983; Hara and Kobayashi, 1987; Kummer, 1994). Most commonly, we detected branches of DBH-positive axons within the meninges (Fig. 2e). We also verified that NA axons entering the caudal spinal cord from the periphery expressed TH, the rate limiting enzyme for the synthesis of catecholamines (data not shown).
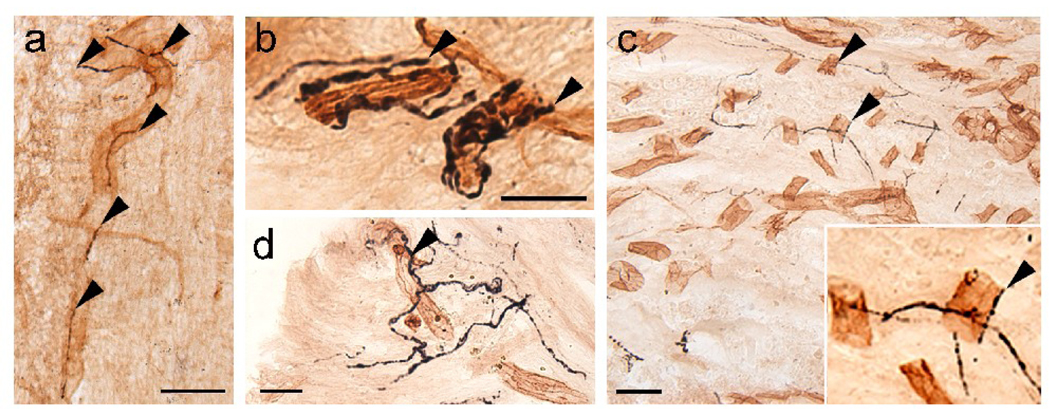
To determine the relationship between DBH-positive axons and blood vessels in the caudal stump, we identified the vessels with anti-PECAM. We often detected DBH-positive axons associated with large diameter (25 to 50 µm) blood vessels in the white matter, within the ventral roots (data not shown), and the gray matter (Fig. 3a). Less frequently, we found DBH-labeled axons along small blood vessels (5–15 µm diameter) in the gray matter (Fig. 3b). DBH-positive axons readily penetrated the GFAP-negative scar formed around the lesion in both media- and OEG-injected rats (Kubasak et al., 2008). Therefore, in addition to supraspinal reinnervation, the association of sympathetic axons with blood vessels contributed to the DBH-labeled axons detected in the GFAP-negative zone at 1 (Fig. 3c) or 7 months (Fig. 3d) post-transection. Labeled axons course along PECAM-positive blood vessels within the transection site and often associate with blood vessels that penetrate the GFAP-negative zone from the periphery (data not shown). Together, our data suggest that NA axons within or below the transection site may be sympathetic axons and once these axons enter the spinal cord, they cannot be distinguished from coeruleospinal regeneration.
Fig. 3.
Noradrenergic (NA) axons labeled with dopamine β-hydroxylase (DBH) associate with blood vessels caudal to (a, b) and in the (c, d) injury site in adult spinal rats. (a, b) DBH-positive axons (black; arrowheads) in the caudal stump of spinal rats associate with large (a; 35 µm) and small (b; 10 µm) blood vessels identified with the platelet endothelial cell adhesion molecule (PECAM, brown). (c, d) DBH-positive axons (arrowheads) associate with PECAM-labeled blood vessels within the astroglial scar. Inset in c depicts the close association of NA axons along vessels. Scale a, c, d = 50 µm, Scale b = 25 µm.
Intrinsic DBH-positive somata are present in the cervical spinal cord but not below the transection
We asked if there are other sources that could contribute to NA axons in the caudal stump of spinal rats. Mouchet et al. (1986) first reported the presence of intrinsic spinal NA neurons in adult rats but found them only at cervical levels, whereas TH-positive dopaminergic interneurons were present at both the cervical and sacral levels. In contrast, (Cassam et al., 1997) detected DBH-positive interneurons throughout the spinal cord of intact rats and reported that their number increased in the spinal cord caudal to a complete mid-thoracic transection. Therefore, we asked if the DBH-labeled axons in the caudal stump are derived from NA interneurons. We rarely detected DBH-immunolabeled somata and found them only in the cervical dorsal and lateral funiculi (Fig. 4a). The DBH-positive somata were 20–35 µm in diameter with bipolar or multipolar processes. We also found a few TH-positive somata that were 20–25 µm in diameter in the upper cervical gray matter (Fig. 4b) that resembled those reported by Mouchet et al. (1986) and Cassam et al. (1997). We did not detect any DBH-positive interneurons between the lower thoracic and sacral segments of our spinal rats but found some TH-positive somata (0–15 somata per rat) in the sacral lateral white matter (Fig. 4c). Sacral TH-positive interneurons were typically bipolar and smaller (20–25 µm) than those at the cervical level, and embedded in the lateral white matter or occasionally in the lateral gray matter.
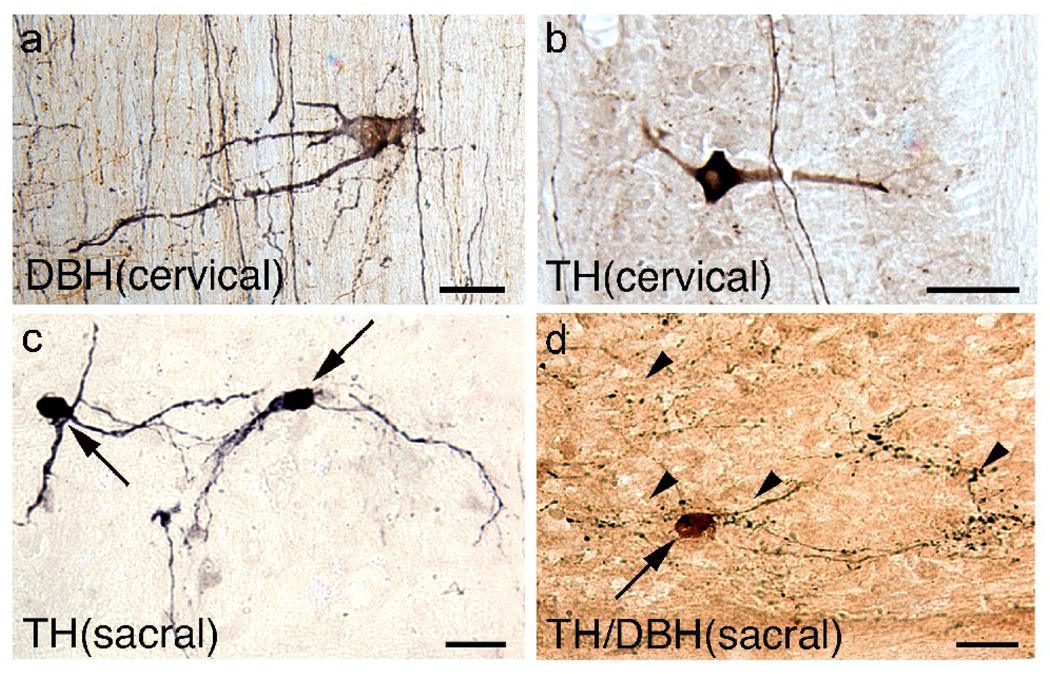
Fig 4.
Noradrenergic cells are present only at cervical levels while dopaminergic somata are distributed at cervical and sacral levels. (a, b) Rare NA (a; labeled with dopamine β-hydroxylase, DBH) and dopaminergic (b; labeled with tyrosine hydroxylase, TH) interneurons are found in the dorsal funiculi at upper cervical levels. (c, d) TH-positive only interneurons (c; arrows) at the sacral levels have smaller somata than those found at the cervical levels. Double-labeling experiments detected a TH-positive soma (d; amber brown, arrow) in the sacral spinal cord that was DBH-negative with DBH-labeled axons (black, arrowheads) coursing nearby. Scale a, b = 50 µm, Scale c, d = 25 µm.
To verify that the small number of TH-labeled neurons observed in the sacral cords were dopaminergic but not NA, we performed double-immunolabeling experiments. Although we detected a number of DBH-positive axons near TH-positive somata, we found no evidence of double-labeled somata (Fig. 4d). Thus, in the present study it appears that the catecholaminergic somata in the sacral cord are not a source of DBH-positive axons below the complete transection.
DBH-positive axons appose motor-associated cholinergic neurons in the caudal stump of spinal rats
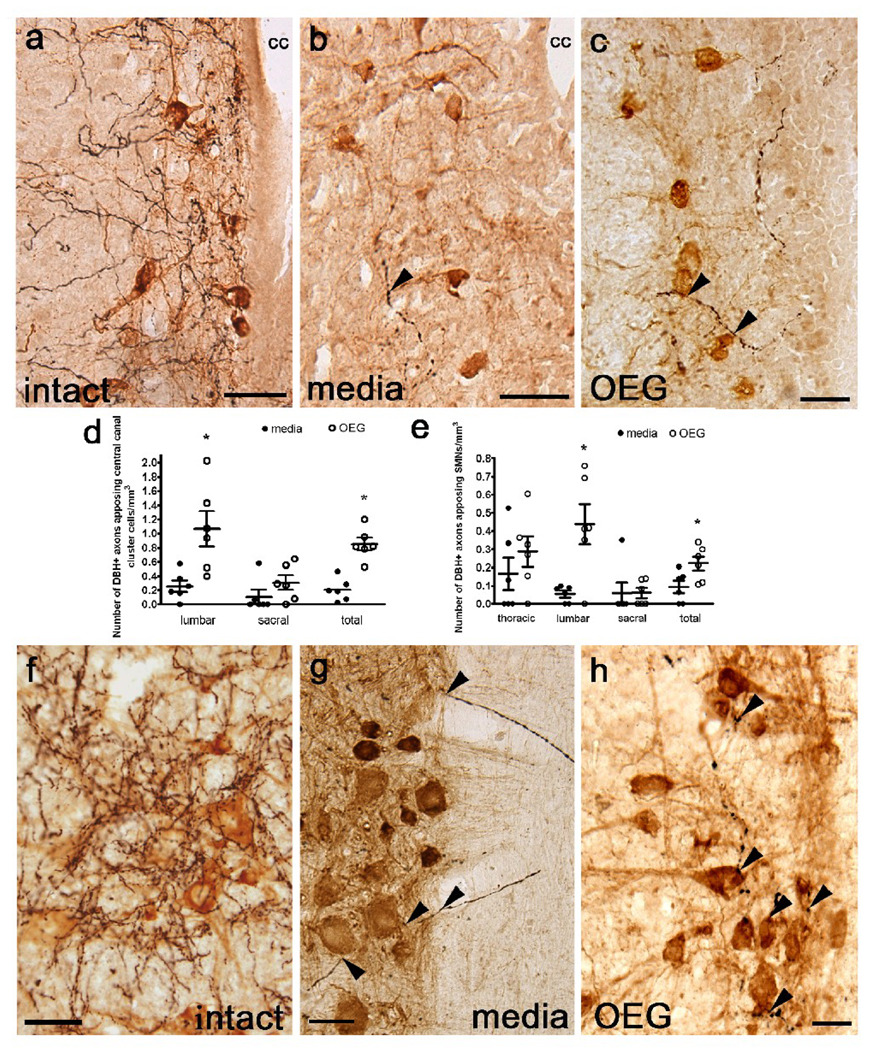
In intact rats, DBH-positive axons course near motor-associated cholinergic spinal cord neurons, i.e., central canal cluster cells (Fig. 5a), SMNs (Fig. 5f; Commissiong et al., 1978), and partition cells (Fig. 6a). As both media- and OEG-injected rats contain DBH-positive axons that associate with these cholinergic neurons, we asked if these appositions were more common in OEG- than media-injected rats (Fig. 5b, c). In the lower thoracic and upper lumbar segments both central canal cluster cells and centrally located sympathetic preganglionic neurons reside in dorsal lamina X (Barber et al., 1991) and are difficult to distinguish conclusively. Therefore we only evaluated the proximity of DBH-labeled varicosities to these cholinergic neurons in the lower lumbar and sacral segments. More DBH-positive varicosities apposed central canal cluster cells in lower lumbar segments of OEG- than media-injected spinal rats, but there were no differences found at the sacral levels. When the total number of appositions was combined, however, we detected significantly more DBH-positive axons apposed to central canal cluster cells in OEG- than in media-injected rats (Fig. 5d, lumbar: P = 0.006, sacral: P = 0.09, combined: P = 0.003). To determine if long-term treadmill step training affects the density of NA axons associated with central canal cluster cells, we compared the number of appositions relative to training status (i.e., OEG- and media-trained vs. OEG- and media-untrained). We found no training effect on DBH appositions along central canal cluster cells at either the lumbar or sacral levels or when the results from both levels are combined (lumbar: P = 0.31, sacral: P = 0.34, combined: P = 0.37; data not shown).
Fig. 5.
Noradrenergic (NA) axons labeled with dopamine β-hydroxylase (DBH) appose central canal cluster cells (a–c) and somatic motor neurons (f–h) in the lumbar spinal cord from intact, media-, and OEG-injected spinal rats. (a–c) Many DBH-positive fibers (black) course near central canal cluster cells (brown) in a sagittal section from an intact rat (a) with fewer fibers found in spinal rats (b, c; arrowheads). cc = central canal (d) OEG-injected rats contain significantly more appositions of DBH-positive fibers along central canal cluster cells than media-injected rats at lower lumbar (P = 0.006) but not at sacral (P = 0.09) levels. When data from both segments were combined, OEG-injected rats contained more DBH-positive varicosities apposing central canal cluster cells than media-injected rats (P = 0.003) (e) The number of DBH-labeled fibers apposing SMNs at the lumbar level was greater in OEG- than media-injected rats (P = 0.014). When data from all segmental levels were combined, OEG-injected rats contained more DBH-positive varicosities apposing SMNs than media-injected rats (P = 0.017). (f–h) Numerous NA axons appose SMNs in an intact rat (f). DBH-positive axons (arrowheads) associate with blood vessels that course within the white matter and then appose SMNs (brown) in a media-injected rat (g). Varicose NA axons are associated with SMNs in an OEG-injected spinal rat (h). Bars in (d, e) indicate mean ± SEM for 6 media-injected and 6 OEG-injected rats. *significant difference between media- and OEG-injected rats. Scale a–b, f–h = 50 µm, Scale c = 25 µm.
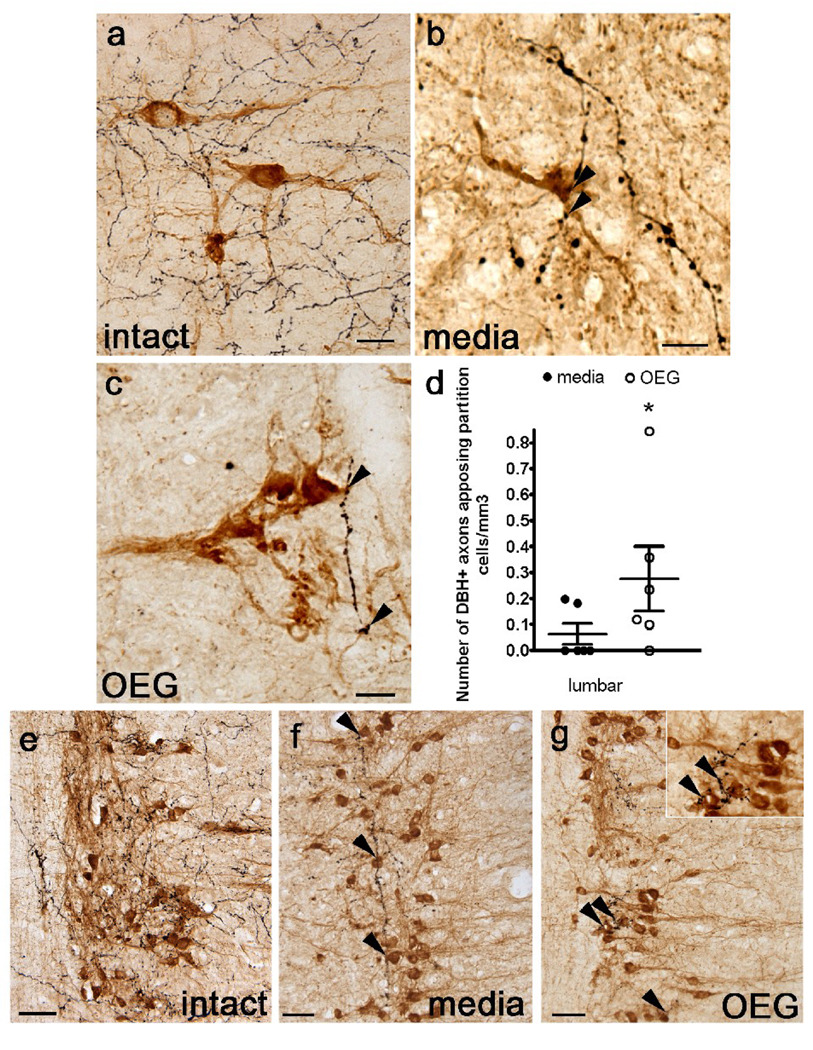
Fig. 6.
Dopamine β-hydroxylase (DBH)-labeled axons appose cholinergic partition cells in sagittally sectioned lower lumbar spinal cord and parasympathetic preganglionic neurons (PPNs) in the sacral cord of an intact rat, and in media- and OEG-injected spinal rats. (a–c) Many DBH-labeled axons (black) course near partition cells (amber-brown) in intact rats (a). DBH-positive axons (arrowheads) also course near partition cells in the caudal stump of media- (b) and OEG-injected (c) spinal rats. (d) The density of DBH-labeled axons that appose partition cells in the lumbar spinal cord is significantly higher in OEG- than media-injected rats (P = 0.004). (e–g) DBH-labeled axons, presumably from the locus coeruleus, densely innervate cholinergic PPNs (brown) in intact rats (e). DBH-positive axons (arrowheads) course among clusters of PPN in media- (f) and OEG-injected (g) spinal rats. Area in (g) marked by double arrowhead is enlarged (inset) to illustrate the proximity of DBH-labeled axons to PPNs. Bars in (d), mean ± SEM for 6 media- and 6 OEG-injected rats. *significant difference between media- and OEG-injected rats. Scale a–c, e–f = 50 µm.
Compared to the central canal cluster cells in lamina X, fewer DBH-positive axons course near SMNs in adult spinal rats (Fig. 5g, h). Although more DBH-positive axons apposed SMNs in OEG- than media-injected rats at the lumbar levels, other segmental levels did not differ (Fig. 5e, thoracic: P = 0.17, lumbar: P = 0.014, sacral: P = 0.095). When the total number of DBH varicosities near SMNs is combined and averaged for all three spinal segments, however, more appositions are detected in OEG-than media-injected rats (Fig. 5e, combined: P = 0.017). In contrast, step training did not affect the number of DBH appositions along SMNs at any spinal cord level caudal to the transection or when the results were combined (thoracic: P = 0.29; lumbar: P = 0.07, sacral: P = 0.31, combined: P = 0.06; data not shown). C-boutons are derived from cholinergic interneurons that terminate on SMNs (Houser et al., 1983; Nagy et al., 1993). Since we previously found evidence of these cholinergic terminals on SMNs in all spinal rats (Takeoka et al., 2009), we suggest that cholinergic interneurons, i.e., partition and central canal cluster cells, can modulate the SMN excitability even after a complete spinal cord transection.
We chose to analyze the partition cells only in the lumbar enlargement where they do not intermingle with the cholinergic sympathetic and parasympathetic preganglionic neurons and where most of the rhythmogenic centers in the spinal cord reside (Grillner and Zangger, 1979; Kjaerulff and Kiehn, 1996; Bonnot and Morin, 1998; Kiehn and Kjaerulff, 1998). In intact spinal cords, DBH-positive axons appear to appose partition cells (Fig. 6a). Occasional DBH-labeled axons also course near the somata and large dendrites of partition cells found in both media- and OEG-injected rats (Fig. 6b, c). Interestingly, we found more DBH-positive varicosities apposing partition cells in OEG-than media-injected rats (P = 0.004; Fig. 6d), while step training did not influence the number of associations (P = 0.15; data not shown).
NA axons appose parasympathetic preganglionic neurons in adult spinal rats
In intact spinal cords, DBH-positive axons densely innervate cholinergic sympathetic (Fig. 1a) and parasympathetic preganglionic neurons (PPN; Fig. 6e; Kohno et al., 1988). Although DBH appositions along PPNs in the spinal cord of media- and OEG-injected rats were less dense than in intact rats, they were frequent and extensive (Fig. 6f, g). DBH-positive axons often coursed within and between clusters of PPN somata. In addition, we occasionally detected DBH appositions to cholinergic sympathetic preganglionic neurons in the thoracic and upper lumbar segments (data not shown). Thus, the presence of DBH-positive axons along preganglionic neurons may influence autonomic control in adult spinal rats.
DISCUSSION
In this study we asked if the DBH-labeled axons observed caudal to the transection in adult spinal rats are derived from a source other than regeneration of coeruleospinal axons, vary in density depending on the transplantation or training status, and appose motor-associated cholinergic neurons. Coeruleospinal axons degenerate in the caudal stump after a complete spinal cord transection (Commissiong, 1984), yet a small amount of noradrenaline remains in the caudal stump of the spinal cord (Roudet et al., 1994). Our results suggest that this low level of noradrenaline is derived from peripheral NA axons that associate with spinal blood vessels. Based on current and previous results (McNicholas et al., 1980; Amenta et al., 1987; Hildebrand et al., 1997), we conclude that the presence of NA axons in the spinal cord caudal to the lesion is not conclusive evidence of coeruleospinal regeneration even after a complete spinal cord transection. Additionally, the overall density of NA axons in the entire caudal stump was comparable in media- and OEG-injected rats despite the better locomotor performance of OEG-injected spinal rats (Kubasak et al., 2008). Nevertheless, NA varicosities associated more often with partition cells, central canal cluster cells, and SMNs in OEG- than media-injected rats. These interactions may contribute to the greater recovery of locomotor ability reported previously in OEG- compared to media-injected rats (Kubasak et al., 2008).
NA axons enter the caudal stumps of the transected spinal cords along several pathways
Sympathetic postganglionic neurons synthesize noradrenaline and innervate various peripheral tissues, including the vasculature. In addition, DBH-immunoreactive axons course along cranial arteries and are abolished by a superior sympathetic ganglionectomy (Crutcher, 1981; Cohen et al., 1992). In the spinal cord, TH- and DBH-positive postganglionic sympathetic axons enter the ventral roots along blood vessels and also are found in the meninges (Fig. 2e; Hildebrand et al., 1997). Sympathetic fibers penetrate and sprout within the spinal cord (McNicholas et al., 1980, Commissiong, 1984) and the dorsal roots (Beattie et al., 1997; Brook et al., 2000) in response to injury. Therefore, NA axons associated with blood vessels or dorsal roots may have sprouted in response to injury in the present study. The central- and peripheral-derived NA axons differ in their morphology with the former being thin with regularly spaced varicosities, and the latter being thick and more uniform in diameter (Crutcher, 1982). With regeneration and reinnervation, however, targets seem to determine the morphology of the NA axons regardless of their origins (Bjorklund and Stenevi, 1971; Olson and Seiger, 1976; Bjorklund and Stenevi, 1977). Therefore we did not attempt to distinguish central-versus peripheral-derived NA axons based on axonal thickness. Our results suggest that future studies will need to perform tracing experiments to unambiguously distinguish regenerating coeruleospinal axons from the peripheral innervation of NA axons.
Dopaminergic, but not NA, interneurons are found in the caudal stump of adult spinal rats
Intrinsic spinal catecholaminergic neurons are a potential source of NA axons in spinal rats as they are frequently detected in lower vertebrates (Heathcote and Chen, 1993, 1994) and occasionally in mammals, including rodents (Mouchet et al., 1986; Cassam et al., 1997). We confirmed previous reports that TH-positive interneurons are found in restricted regions of the cervical and sacral spinal cord (Singhaniyom et al., 1983; Dietl et al., 1985; Mouchet et al., 1986; Cassam et al., 1997). We did not, however, find intraspinal NA neurons in the spinal cord caudal to the transection site of our Wistar Hannover spinal rats. Our results are similar to those of Mouchet et al. (1986), but differ from those of Cassam et al. (1997). Using Wistar rats, Cassam et al. (1997) detected DBH-positive cell bodies 14 days after a complete spinal cord transection, a significantly shorter time than our paradigm of 7 months. One possible explanation is that a transient up-regulation of NA interneurons may occur early after the injury. These differences also may be due to the rat strain or even sub-strain as these factors appear to influence spinal NA innervation (Clark et al., 1991; Clark and Proudfit, 1992). Nevertheless, our finding of dopaminergic, but not NA, neurons in the spinal cord below the lesion is consistent with biochemical assays indicating that there is a normal level of dopamine after a chronic complete spinal cord transection (Commissiong, 1985).
Noradrenaline in the caudal stump may contribute to locomotor recovery in adult spinal rats
While adult cats with a complete spinal cord transection can learn to plantar step bipedally with training (Lovely et al., 1986; Giroux et al., 2001), adult rodents with a complete spinal cord transection require interventions such as locus coeruleous grafts (Yakovleff et al., 1989; Gimenez y Ribotta et al., 1998a; Gimenez y Ribotta et al., 1998b), or pharmacological and/or electrical interventions (Guertin, 2004; Ichiyama et al., 2005; Lavrov et al., 2006; Musienko et al., 2008; Courtine et al., 2009) that likely activate central pattern generation in the lumbosacral cord to recover bipedal plantar stepping ability. Administration of noradrenaline depolarizes the membrane potential and reduces afterhyperpolarization of rat motoneurons in vitro (Elliott and Wallis, 1992; Fedirchuk and Dai, 2004). As noradrenaline utilizes non-synaptic release, i.e., volume transmission, it can diffuse from a few microns up to a millimeter in the extracellular space (Beaudet and Descarries, 1978; Ridet et al., 1993; Hentall et al., 2003). Therefore, NA varicosities near motor-associated cholinergic neurons in the caudal stump potentially could contribute to the recovery of stepping ability in OEG-injected rats. Lopez-Vales et al. (2006) reported a correlation between NA fiber density in the lumbar spinal cord and functional recovery and found NA axons apposed SMNs in OEG- but not media-injected adult spinal rats. In contrast, we did not find a correlation between NA fiber density in the caudal stump and hindlimb stepping ability, and observed NA axons apposing SMNs regardless of transplantation status. Nonetheless, we did detect significantly more DBH-positive varicosities associated with central canal cluster and partition cells, as well as SMNs in the lumbar spinal cord, in OEG- than media-injected rats. In addition, NA axons may also interact with other populations of locomotor pattern generating neurons (MacLean et al., 1995; Kjaerulff and Kiehn, 1996) that contribute to locomotor improvement in OEG-injected rats. While most in vivo studies transplanting OEG focus on how they promote axonal regeneration (Cheng and Olson, 1995; Chen et al., 1996; Ramon-Cueto et al., 1998; Ramon-Cueto et al., 2000; Lu et al., 2001; Lu et al., 2002; Fouad et al., 2005; Lopez-Vales et al., 2006a, 2007; Kubasak et al., 2008), our results indicate that they also promote the reorganization of NA axons that interact with the existing spinal locomotor networks in the caudal stump. Future pharmacological receptor blocking experiments will be important to examine the functional role of noradrenaline in the caudal stump during locomotion.
SMNs develop supersensitivity to noradrenaline after a complete spinal cord transection that can lead to muscle spasms, a form of hyper-reflexia (Rank et al., 2007). The supersensitivity to sensory input is partially mediated by a sodium channel persistent inward current (PIC) which is completely eliminated by the blockade of 5-HT2A, 5-HT2C and NAα1 receptors, suggesting that endogenous sources of noradrenaline and 5-HT below the level of the lesion are necessary for the induction of sodium PICs in spinal rats. (Harvey et al., 2006). Even a small amount of noradrenaline in the caudal stump could contribute to an improvement in locomotor ability since noradrenaline could modulate afferent input, a critical component for successful stepping, and could also directly excite SMNs. In fact, an early study of spinal cord injury suggested that plasma DOPA could be converted into catecholamines and potentially modulate locomotor activity of spinal rats (Andén et al., 1972).
While the hindlimb stepping ability of OEG-trained rats was significantly better than that of OEG-untrained spinal rats (Kubasak et al., 2008), we did not find any correlation between long-term training and the NA fiber density or NA varicosities associated with any motor-associated cholinergic neurons. It is possible that 20 min/day, 5 days/week for 6 months of hindlimb step training was not sufficient to induce activity-dependent sprouting and reorganization within the adult rat spinal cord caudal to the lesion. Alternatively, the training effect may be mediated by other mechanisms such as 1) the sprouting and reorganization of other neurotransmitter pathways; 2) changes in synaptic strength among the locomotor networks including motor-associated cholinergic neurons; 3) decreased inhibitory influences of GABAergic and glycinergic innervation on SMNs (de Leon et al., 1999; Tillakaratne et al., 2002); and/or 4) changes in the NA receptor expression levels that contribute to locomotor recovery.
Other possible functions of NA in the caudal stump of spinal rats
Since we detected NA axons in the superficial dorsal horn as well as intermediate laminae where autonomic preganglionic neurons are located, noradrenaline in the lower thoracic, lumbar, and sacral spinal cord may contribute to physiological functions related to nociception and autonomic control after complete coeruleospinal denervation. Descending coeruleospinal pathways, and pontine A5 and A7 cells also innervate the superficial dorsal horn (Schroder and Skagerberg, 1985; Fritschy et al., 1987; Kwiat and Basbaum, 1992) and modulate nociception (Yeomans et al., 1992; Lu and Perl, 2007) and therefore, extensive arborization of NA axons in the superficial dorsal horn may contribute to pain modulation after a spinal cord injury.
Noradrenaline normally contributes to the pontine micturition center, as the locus coeruleus and A5 cells project their axons bilaterally to PPN in the sacral cord (Vizzard et al., 1995; Nuding and Nadelhaft, 1998). In addition, NAα1 receptors are activated during bladder voiding reflexes and these functions are partially mediated by a rhythmic motor pattern that can elicit motor output in paralyzed and deafferented adult rats (McKenna et al., 1991; Carro-Juarez and Rodriguez-Manzo, 2000; Carro-Juarez et al., 2003).
CONCLUSIONS
In combination with previous reports, we demonstrate that the presence of NA axons in the spinal cord caudal to a complete spinal cord transection in adult rats cannot be interpreted as evidence for coeruleospinal regeneration. Whether from peripheral or supraspinal sources, however, these NA axons could contribute to the recovery of hindlimb stepping ability after a complete spinal cord transection. These NA axons may influence locomotion by activating SMNs directly or indirectly through the excitation of cholinergic or other interneurons that then terminate on SMNs. Regeneration of other descending motor pathways as well as reorganization of the locomotor circuitries within the caudal stump also could contribute to functional recovery (Edgerton et al., 2004).
ACKNOWLEDGMENTS
We thank Drs. V. Reggie Edgerton and Almudena Ramón-Cueto for assistance with many aspects of this study, Dr. Leif Havton for helpful comments on the manuscript and Kimberly McFarland for technical assistance. Funding from the Christopher Reeve Paralysis Foundation (PA-1-0102-2, PAC1-0102-2) and NINDS (R21NS42000-01, RO1NS54159).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
LITERATURE CITED
- Amenta F, Bronzetti E, Cavallotti C, Felici L. Quantitative image analysis of the density and pattern of adrenergic innervation of blood vessels of rat spinal cord. J Auton Nerv Syst. 1987;18:261–264. doi: 10.1016/0165-1838(87)90124-x. [DOI] [PubMed] [Google Scholar]
- Andén N-E, Engel J, Rubenson A. Central decarboxylation and uptake of L-DOPA. Naunyn Schmiedebergs Arch Pharmacol. 1972;273:11–26. doi: 10.1007/BF00508077. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Giron LT, Jr, Ziegler MG. Postnatal development of dopamine-beta-hydroxylase-immunoreactive fibers of the spinal cord of the rat. Brain Res. 1986;390:161–171. doi: 10.1016/s0006-8993(86)80224-4. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Chau C, Rossignol S. Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res Bull. 1993;30:387–393. doi: 10.1016/0361-9230(93)90270-l. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Norman KE. The effect of noradrenergic drugs on the recovery of walking after spinal cord injury. Spinal Cord. 2003;41:137–143. doi: 10.1038/sj.sc.3101374. [DOI] [PubMed] [Google Scholar]
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Barber RP, Phelps PE, Vaughn JE. Generation patterns of immunocytochemically identified cholinergic neurons at autonomic levels of the rat spinal cord. J Comp Neurol. 1991;311:509–519. doi: 10.1002/cne.903110406. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3:851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U. Growth of central catecholamine neurones into smooth muscle grafts in the rat mesencephalon. Brain Res. 1971;31:1–20. doi: 10.1016/0006-8993(71)90630-5. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U. Experimental reinnervation of the rat hippocampus by grafted sympathetic ganglia. I. Axonal regeneration along the hippocampal fimbria. Brain Res. 1977;138:259–270. doi: 10.1016/0006-8993(77)90744-2. [DOI] [PubMed] [Google Scholar]
- Bonnot A, Morin D. Hemisegmental localisation of rhythmic networks in the lumbosacral spinal cord of neonate mouse. Brain Res. 1998;793:136–148. doi: 10.1016/s0006-8993(98)00153-x. [DOI] [PubMed] [Google Scholar]
- Brook GA, Houweling DA, Gieling RG, Hermanns T, Joosten EA, Bar DP, Gispen WH, Schmitt AB, Leprince P, Noth J, Nacimiento W. Attempted endogenous tissue repair following experimental spinal cord injury in the rat: involvement of cell adhesion molecules L1 and NCAM? Eur J Neurosci. 2000;12:3224–3238. doi: 10.1046/j.1460-9568.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- Carr PA, Huang A, Noga BR, Jordan LM. Cytochemical characteristics of cat spinal neurons activated during fictive locomotion. Brain Res Bull. 1995;37(2):213–218. doi: 10.1016/0361-9230(94)00271-2. [DOI] [PubMed] [Google Scholar]
- Carro-Juarez M, Cruz SL, Rodriguez-Manzo G. Evidence for the involvement of a spinal pattern generator in the control of the genital motor pattern of ejaculation. Brain Res. 2003;975:222–228. doi: 10.1016/s0006-8993(03)02686-6. [DOI] [PubMed] [Google Scholar]
- Carro-Juarez M, Rodriguez-Manzo G. Sensory and motor aspects of the coital reflex in the spinal male rat. Behav Brain Res. 2000;108:97–103. doi: 10.1016/s0166-4328(99)00143-6. [DOI] [PubMed] [Google Scholar]
- Cassam AK, Llewellyn-Smith IJ, Weaver LC. Catecholamine enzymes and neuropeptides are expressed in fibres and somata in the intermediate gray matter in chronic spinal rats. Neuroscience. 1997;78:829–841. doi: 10.1016/s0306-4522(96)00599-4. [DOI] [PubMed] [Google Scholar]
- Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259:6882–6889. [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol. 1998;79:2941–2963. doi: 10.1152/jn.1998.79.6.2941. [DOI] [PubMed] [Google Scholar]
- Chen A, Xu XM, Kleitman N, Bunge MB. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp Neurol. 1996;138:261–276. doi: 10.1006/exnr.1996.0065. [DOI] [PubMed] [Google Scholar]
- Cheng H, Olson L. A new surgical technique that allows proximodistal regeneration of 5-HT fibers after complete transection of the rat spinal cord. Exp Neurol. 1995;136:149–161. doi: 10.1006/exnr.1995.1092. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. Anatomical evidence for genetic differences in the innervation of the rat spinal cord by noradrenergic locus coeruleus neurons. Brain Res. 1992;591:44–53. doi: 10.1016/0006-8993(92)90976-g. [DOI] [PubMed] [Google Scholar]
- Clark FM, Yeomans DC, Proudfit HK. The noradrenergic innervation of the spinal cord: differences between two substrains of Sprague-Dawley rats determined using retrograde tracers combined with immunocytochemistry. Neurosci Lett. 1991;125:155–158. doi: 10.1016/0304-3940(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Bovento G, Lacombe P, Seylaz J, MacKenzie ET, Hamel E. Cerebrovascular nerve fibers immunoreactive for tryptophan-5-hydroxylase in the rat: distribution, putative origin and comparison with sympathetic noradrenergic nerves. Brain Res. 1992;598:203–214. doi: 10.1016/0006-8993(92)90184-b. [DOI] [PubMed] [Google Scholar]
- Commissiong JW. Fetal locus coeruleus transplanted into the transected spinal cord of the adult rat: some observations and implications. Neuroscience. 1984;12:839–853. doi: 10.1016/0306-4522(84)90174-x. [DOI] [PubMed] [Google Scholar]
- Commissiong JW. The synthesis and metabolism of catecholamines in the spinal cord of the rat after acute and chronic transections. Brain Res. 1985;347:104–111. doi: 10.1016/0006-8993(85)90893-5. [DOI] [PubMed] [Google Scholar]
- Commissiong JW, Hellstrom SO, Neff NH. A new projection from locus coeruleus to the spinal ventral columns: histochemical and biochemical evidence. Brain Res. 1978;148:207–213. doi: 10.1016/0006-8993(78)90391-8. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher KA. Cholinergic denervation of rat neocortex results in sympathetic innervation. Exp Neurol. 1981;74:324–329. doi: 10.1016/0014-4886(81)90172-2. [DOI] [PubMed] [Google Scholar]
- Crutcher KA. Histochemical studies of sympathetic sprouting: fluorescence morphology of noradrenergic axons. Brain Res Bull. 1982;9:501–508. doi: 10.1016/0361-9230(82)90158-7. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Dietl M, Arluison M, Mouchet P, Feuerstein C, Manier M, Thibault J. Immunohistochemical demonstration of catecholaminergic cell bodies in the spinal cord of the rat. Preliminary note. Histochemistry. 1985;82:385–389. doi: 10.1007/BF00494068. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Statistical Data Analysis in the Computer Age. Science. 1991;253:390–395. doi: 10.1126/science.253.5018.390. [DOI] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and L-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience. 1992;47:533–544. doi: 10.1016/0306-4522(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–361. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Lyons WE, Mullen CA, Kosofsky BE, Molliver ME, Grzanna R. Distribution of locus coeruleus axons in the rat spinal cord: a combined anterograde transport and immunohistochemical study. Brain Res. 1987;437:176–180. doi: 10.1016/0006-8993(87)91541-1. [DOI] [PubMed] [Google Scholar]
- Gimenez y Ribotta MG, Roudet C, Sandillon F, Privat A. Transplantation of embryonic noradrenergic neurons in two models of adult rat spinal cord injury: ultrastructural immunocytochemical study. Brain Res. 1996;707:245–255. doi: 10.1016/0006-8993(95)01266-4. [DOI] [PubMed] [Google Scholar]
- Gimenez y Ribotta MG, Orsal D, Feraboli-Lohnherr D, Privat A. Recovery of locomotion following transplantation of monoaminergic neurons in the spinal cord of paraplegic rats. Ann N Y Acad Sci. 1998a;860:393–411. doi: 10.1111/j.1749-6632.1998.tb09064.x. [DOI] [PubMed] [Google Scholar]
- Gimenez y Ribotta MG, Orsal D, Feraboli-Lohnherr D, Privat A, Provencher J, Rossignol S. Kinematic analysis of recovered locomotor movements of the hindlimbs in paraplegic rats transplanted with monoaminergic embryonic neurons. Ann N Y Acad Sci. 1998b;860:521–523. doi: 10.1111/j.1749-6632.1998.tb09093.x. [DOI] [PubMed] [Google Scholar]
- Giroux N, Brustein E, Chau C, Barbeau H, Reader TA, Rossignol S. Differential effects of the noradrenergic agonist clonidine on the locomotion of intact, partially and completely spinalized adult cats. Ann N Y Acad Sci. 1998;860:517–520. doi: 10.1111/j.1749-6632.1998.tb09092.x. [DOI] [PubMed] [Google Scholar]
- Giroux N, Reader TA, Rossignol S. Comparison of the effect of intrathecal administration of clonidine and yohimbine on the locomotion of intact and spinal cats. J Neurophysiol. 2001;85:2516–2536. doi: 10.1152/jn.2001.85.6.2516. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Guertin PA. Synergistic activation of the central pattern generator for locomotion by l-beta-3,4-dihydroxyphenylalanine and quipazine in adult paraplegic mice. Neurosci Lett. 2004;358(2):71–74. doi: 10.1016/j.neulet.2003.12.120. [DOI] [PubMed] [Google Scholar]
- Hara H, Kobayashi S. Adrenergic innervation of the vasa nervorum in the cranial nerves and spinal roots in the subarachnoid space. Exp Neurol. 1987;98:673–676. doi: 10.1016/0014-4886(87)90275-5. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote RD, Chen A. A nonrandom interneuronal pattern in the developing frog spinal cord. J Comp Neurol. 1993;328:437–448. doi: 10.1002/cne.903280309. [DOI] [PubMed] [Google Scholar]
- Heathcote RD, Chen A. Morphogenesis of catecholaminergic interneurons in the frog spinal cord. J Comp Neurol. 1994;342:57–68. doi: 10.1002/cne.903420107. [DOI] [PubMed] [Google Scholar]
- Hentall ID, Mesigil R, Pinzon A, Noga BR. Temporal and spatial profiles of pontine-evoked monoamine release in the rat's spinal cord. J Neurophysiol. 2003;89:2943–2951. doi: 10.1152/jn.00608.2002. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Karlsson M, Risling M. Ganglionic axons in motor roots and pia mater. Prog Neurobiol. 1997;51:89–128. doi: 10.1016/s0301-0082(96)00052-4. [DOI] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Barber RP, Salvaterra PM, Vaughn JE. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983;266:97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Huang A, Noga BR, Carr PA, Fedirchuk B, Jordan LM. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol. 2000;83:3537–3547. doi: 10.1152/jn.2000.83.6.3537. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Hildebrand C. Routes of putative afferent axons in rat lumbosacral ventral roots and pia mater. Brain Res. 1993;600:298–304. doi: 10.1016/0006-8993(93)91386-7. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci. 1998;860:110–129. doi: 10.1111/j.1749-6632.1998.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Sillar KT, Kjaerulff O, McDearmid JR. Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord. J Neurophysiol. 1999;82:741–746. doi: 10.1152/jn.1999.82.2.741. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno J, Shinoda K, Kawai Y, Peng Y, Ono K, Shiotani Y. Direct adrenergic inputs to sacral autonomic neurons: using a double-immunostaining method at the light and electron microscopic levels. Brain Res. 1988;461:158–162. doi: 10.1016/0006-8993(88)90734-2. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Munoz-Quiles C, Roy RR, Edgerton VR, Ramon-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W. Sensory ganglia as a target of autonomic and sensory nerve fibres in the guinea-pig. Neuroscience. 1994;59:739–754. doi: 10.1016/0306-4522(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens Mot Res. 1992;9:157–173. doi: 10.3109/08990229209144768. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Navarro X, Verdu E. Olfactory ensheathing glia graft in combination with FK506 administration promote repair after spinal cord injury. Neurobiol Dis. 2006a;24:443–454. doi: 10.1016/j.nbd.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Navarro X, Verdu E. Chronic transplantation of olfactory ensheathing cells promotes partial recovery after complete spinal cord transection in the rat. Glia. 2007;55:303–311. doi: 10.1002/glia.20457. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Verdu E, Navarro X. Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol Dis. 2006b;21:57–68. doi: 10.1016/j.nbd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lu J, Feron F, Ho SM, Mackay-Sim A, Waite PM. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 2001;889:344–357. doi: 10.1016/s0006-8993(00)03235-2. [DOI] [PubMed] [Google Scholar]
- Lu J, Feron F, Mackay-Sim A, Waite PM. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol. 2007;582:127–136. doi: 10.1113/jphysiol.2007.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Hochman S, Magnuson DS. Lamina VII neurons are rhythmically active during locomotor-like activity in the neonatal rat spinal cord. Neurosci Lett. 1995;197:9–12. doi: 10.1016/0304-3940(95)11882-w. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Chung SK, McVary KT. A model for the study of sexual function in anesthetized male and female rats. Am J Physiol. 1991;261:R1276–R1285. doi: 10.1152/ajpregu.1991.261.5.R1276. [DOI] [PubMed] [Google Scholar]
- McNicholas LF, Martin WR, Sloan JW, Nozaki M. Innervation of the spinal cord by sympathetic fibers. Exp Neurol. 1980;69:383–394. doi: 10.1016/0014-4886(80)90221-6. [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Salvatierra AT. Apposition of enkephalin- and neurotensin-immunoreactive neurons by serotonin-immunoreactive varicosities in the rat spinal cord. Neuroscience. 1998;85:837–846. doi: 10.1016/s0306-4522(97)00522-8. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Moorman SJ, Whalen LR, Nornes HO. A neurotransmitter specific functional recovery mediated by fetal implants in the lesioned spinal cord of the rat. Brain Res. 1990;508:194–198. doi: 10.1016/0006-8993(90)90396-s. [DOI] [PubMed] [Google Scholar]
- Mouchet P, Manier M, Dietl M, Feuerstein C, Berod A, Arluison M, Denoroy L, Thibault J. Immunohistochemical study of catecholaminergic cell bodies in the rat spinal cord. Brain Res Bull. 1986;16:341–353. doi: 10.1016/0361-9230(86)90055-9. [DOI] [PubMed] [Google Scholar]
- Mullner A, Gonzenbach RR, Weinmann O, Schnell L, Liebscher T, Schwab ME. Lamina-specific restoration of serotonergic projections after Nogo-A antibody treatment of spinal cord injury in rats. Eur J Neurosci. 2008;27:326–333. doi: 10.1111/j.1460-9568.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- Musienko P, Gerasimenko Y, Brand RVD, Roy RR, Zhong H, Edgerton VR, Courtine G. Monoaminergic modulation of locomotion facilitated by epidural stimulation (ES) in spinal rats. Soc Neurosci Abstr 573.2. 2008 [Google Scholar]
- Nagy JI, Yamamoto T, Jordan LM. Evidence for the cholinergic nature of C-terminals associated with subsurface cisterns in alpha-motoneurons of rat. Synapse. 1993;15:17–32. doi: 10.1002/syn.890150103. [DOI] [PubMed] [Google Scholar]
- Nuding SC, Nadelhaft I. Bilateral projections of the pontine micturition center to the sacral parasympathetic nucleus in the rat. Brain Res. 1998;785:185–194. doi: 10.1016/s0006-8993(97)01347-4. [DOI] [PubMed] [Google Scholar]
- Olson L, Seiger A. Locus coeruleus: fibre growth regulation in oculo. Med Biol. 1976;54:142–145. [PubMed] [Google Scholar]
- Phelps PE, Barber RP, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. Postnatal development of neurons containing choline acetyltransferase in rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:347–361. doi: 10.1002/cne.902290306. [DOI] [PubMed] [Google Scholar]
- Proudfit HK, Clark FM. The projections of locus coeruleus neurons to the spinal cord. Prog Brain Res. 1991;88:123–141. doi: 10.1016/s0079-6123(08)63803-0. [DOI] [PubMed] [Google Scholar]
- Rajaofetra N, Poulat P, Marlier L, Geffard M, Privat A. Pre- and postnatal development of noradrenergic projections to the rat spinal cord: an immunocytochemical study. Brain Res Dev Brain Res. 1992a;67:237–246. doi: 10.1016/0165-3806(92)90224-k. [DOI] [PubMed] [Google Scholar]
- Rajaofetra N, Ridet JL, Poulat P, Marlier L, Sandillon F, Geffard M, Privat A. Immunocytochemical mapping of noradrenergic projections to the rat spinal cord with an antiserum against noradrenaline. J Neurocytol. 1992b;21:481–494. doi: 10.1007/BF01186952. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol. 2007;97:3166–3180. doi: 10.1152/jn.01168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Rajaofetra N, Teilhac JR, Geffard M, Privat A. Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions. Neuroscience. 1993;52:143–157. doi: 10.1016/0306-4522(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Roudet C, Gimenez Ribotta M, Privat A, Feuerstein C, Savasta M. Regional study of spinal alpha 2-adrenoceptor densities after intraspinal noradrenergic-rich implants on adult rats bearing complete spinal cord transection or selective chemical noradrenergic denervation. Neurosci Lett. 1996;208:89–92. doi: 10.1016/0304-3940(96)12547-7. [DOI] [PubMed] [Google Scholar]
- Roudet C, Mouchet P, Feuerstein C, Savasta M. Normal distribution of alpha 2-adrenoceptors in the rat spinal cord and its modification after noradrenergic denervation: a quantitative autoradiographic study. J Neurosci Res. 1994;39:319–329. doi: 10.1002/jnr.490390309. [DOI] [PubMed] [Google Scholar]
- Satoh K, Tohyama M, Yamamoto K, Sakumoto T, Shimizu N. Noradrenaline innervation of the spinal cord studied by the horseradish peroxidase method combined with monoamine oxidase staining. Exp Brain Res. 1977;30:175–186. doi: 10.1007/BF00237249. [DOI] [PubMed] [Google Scholar]
- Schroder HD, Skagerberg G. Catecholamine innervation of the caudal spinal cord in the rat. J Comp Neurol. 1985;242:358–368. doi: 10.1002/cne.902420305. [DOI] [PubMed] [Google Scholar]
- Singhaniyom W, Wreford NG, Guldner FH. Asymmetric distribution of catecholamine-containing neuronal perikarya in the upper cervical spinal cord of rat. Neurosci Lett. 1983;41:91–97. doi: 10.1016/0304-3940(83)90228-8. [DOI] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR. Noradrenergic control of locomotor networks in the in vitro spinal cord of the neonatal rat. Brain Res. 2000;852:100–109. doi: 10.1016/s0006-8993(99)02219-2. [DOI] [PubMed] [Google Scholar]
- Stevens RT, Hodge CJ, Jr, Apkarian AV. Catecholamine varicosities in cat dorsal root ganglion and spinal ventral roots. Brain Res. 1983;261:151–154. doi: 10.1016/0006-8993(83)91295-7. [DOI] [PubMed] [Google Scholar]
- Takeoka A, Kubasak MD, Zhong H, Roy RR, Phelps PE. Serotonergic innervation of the caudal spinal stump in rats after complete spinal transection: Effect of olfactory ensheathing glia. J Comp Neurol. 2009;515:664–676. doi: 10.1002/cne.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol. 1995;355:629–640. doi: 10.1002/cne.903550411. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Origins of spinal noradrenergic pathways demonstrated by retrograde transport of antibody to dopamine-beta-hydroxylase. Neurosci Lett. 1981;25:243–249. doi: 10.1016/0304-3940(81)90399-2. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263:15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- Yakovleff A, Roby-Brami A, Guezard B, Mansour H, Bussel B, Privat A. Locomotion in rats transplanted with noradrenergic neurons. Brain Res Bull. 1989;22:115–121. doi: 10.1016/0361-9230(89)90135-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Clark FM, Paice JA, Proudfit HK. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain. 1992;48:449–461. doi: 10.1016/0304-3959(92)90098-V. [DOI] [PubMed] [Google Scholar]