Abstract
Increasing evidence indicates that early-life glucocorticoid exposure, either involving stress or the therapy of preterm labor, contributes to metabolic and cardiovascular disorders in adulthood. We investigated cellular mechanisms underlying these effects by administering dexamethasone (DEX) to neonatal rats on postnatal (PN) days 1–3 or 7–9, using doses spanning the threshold for somatic growth impairment: 0.05, 0.2 and 0.8 mg/kg. In adulthood, we assessed the effects on hepatic and cardiac cell function mediated through the adenylyl cyclase (AC) signaling cascade, which controls neuronal and hormonal inputs that regulate hepatic glucose metabolism and cardiac contractility. Treatment on PN1-3 produced heterologous sensitization of hepatic signaling, with upregulation of AC itself leading to parallel increases in the responses to β-adrenergic or glucagon receptor stimulation, or to activation of G-proteins by fluoride. The effects were seen at the lowest dose but increasing DEX past the point of somatic growth impairment led to loss of the effect in females. Nonmonotonic effects were also present in the heart, where males showed AC sensitization at the lowest dose, with decreasing effects as the dose was raised; females showed progressive deficits of cardiac AC activity. Shifting the exposure to PN7-9 still elicited AC sensitization but with a greater offsetting contribution at the higher doses. Our findings show that, in contrast to growth restriction, the glucocorticoids associated with stress or the therapy of preterm labor are more sensitive and more important contributors to the cellular abnormalities underlying subsequent metabolic and cardiovascular dysfunction.
Keywords: Adenylyl Cyclase, β-Adrenergic receptor, Cyclic AMP, Dexamethasone, Glucocorticoids, Heart development, Liver development, Muscarinic receptor
INTRODUCTION
Adverse events during fetal development contribute to subsequent metabolic and cardiovascular disease in adulthood. Barker linked low birth weight per se to disease outcomes [3], and excess glucocorticoid exposure associated with prenatal stress is thought to play a mechanistic role in this connection [4,9,31]. The societal impact of prenatal glucocorticoid exposure is increasingly important because of the expanded use of these agents in preterm labor [13], a treatment currently involving 10% of all US pregnancies [26]. Although glucocorticoids enhance lung maturation and thus prevent neonatal respiratory distress syndrome, such treatments are now implicated in subsequent hypertension, hyperglycemia, hyperinsulinemia, altered behavior and neuroendocrine responses, all of which can emerge throughout the lifespan after periods of apparent normality [32,34].
Notwithstanding these epidemiological relationships, few studies have provided a mechanistic understanding of how early glucocorticoid exposure leads to metabolic or cardiovascular dysfunction. Although there are numerous reports of glucocorticoid effects in animal models, most of these involve relatively high doses that elicit persistent stunting of somatic growth, outright cerebral atrophy and endocrine disruption [6,10–12,14,24–28,41,42], as well as causing a loss of insulin response similar to that in diabetes [8,15,32,33,43]. This still leaves major uncertainties as to glucocorticoid effects relevant to stress, or at or below the threshold for typical therapeutic use in preterm infants. In a series of recent studies, we showed that, even at subtherapeutic doses, dexamethasone (DEX) exposure in fetal and neonatal rats compromises key aspects of brain development when given during specific critical periods corresponding to the selfsame developmental stages recommended for preterm infants [18–20,37]. Here, we have used the same approach to address cell signaling in peripheral tissues that are the likely targets for the emergence of metabolic and cardiovascular disorders, the liver and heart.
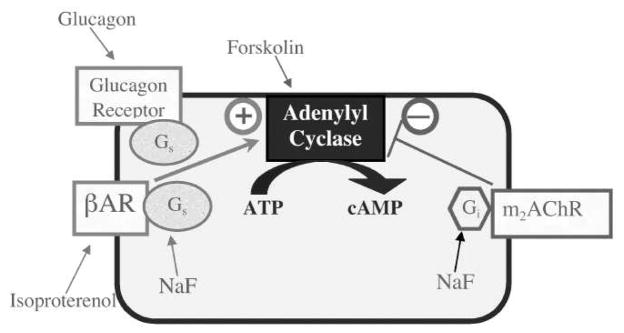
We focused on the key role played by the adenylyl cyclase (AC) cascade, which governs the formation of cyclic AMP, the second messenger that controls hepatic gluconeogenesis and glycogenolysis, that modulates insulin function, and that regulates heart rate and contractility. In the liver, β-adrenergic receptors (βARs) and glucagon receptors act through the stimulatory G-protein, Gs, to activate AC, thus eliciting gluconeogenesis and lipolysis. The importance of this pathway was recently emphasized by studies showing that neonatal exposures to organophosphate pesticides produce sensitization of hepatic AC to these receptor inputs, leading ultimately to metabolic abnormalities resembling those in prediabetes [22,29,36,39]. Similarly prediabetic abnormalities and altered susceptibility to development of diabetes have been identified in humans with AC gene polymorphisms [30]. Accordingly, we evaluated the effects of DEX exposure of neonatal rats in two treatment periods, postnatal days (PN) 1–3 and 7–9, bracketing stages of development in the rat that are equivalent to those in second- to early-third trimester human fetuses, the stage in which glucocorticoid use is recommended for preterm infants [13]; we focused on doses within the therapeutic range (0.2 or 0.8 mg/kg) as well as a much lower dose (0.05 mg/kg) likely to be more representative of stress-related glucocorticoid actions. The three-day regimens were chosen to correspond to multiple glucocorticoid courses, as used in approximately 85% of all cases of preterm delivery [7]. We then evaluated the impact on the AC signaling cascade in adulthood (PN75), focusing on each individual step in the pathway (Figure 1); the studies were modeled after our earlier work on organophosphates [2,29]. In addition to assessing the effects on basal AC activity, we evaluated the response to βARs and glucagon receptors, both of which stimulate AC through via activation of the stimulatory G-protein, Gs. We also determined the effect of fluoride, which evokes maximal activation of both Gs and the corresponding inhibitory protein, Gi. We then measured the maximal activation of AC itself by forskolin, which acts directly on the enzyme by binding to the catalytic core [17]. Finally, we measured ligand binding for βARs and for the inhibitory m2-muscarinic acetylcholine receptors (m2AChRs).
Figure 1.
Mechanisms controlling AC activity, showing probes for each step in the pathway: isoproterenol for the βAR, glucagon for the glucagon receptor, NaF for the G-proteins, and forskolin for AC itself. Both βARs and glucagon receptors enhance AC activity through the stimulatory G-protein, Gs, whereas m2AChRs inhibit AC through mediation of the inhibitory protein, Gi.
MATERIALS AND METHODS
Animal treatments
All experiments were carried out humanely and with due regard for alleviation of suffering, with protocols approved by the Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague-Dawley rats were housed in breeding cages, with a 12-hr light/dark cycle and free access to food and water. On the day of birth, all pups were randomized and redistributed to the dams with a litter size of 10 to maintain a standard nutritional status. Pups received daily subcutaneous injections of DEX phosphate (0.05, 0.2, or 0.8 mg/kg) on PN1-3 or PN7-9, whereas controls received equivalent volumes (1 ml/kg) of isotonic saline vehicle. On each day of treatment and at intervals of several days thereafter, pups were re-randomized within their respective treatment groups and in addition, dams were rotated among litters to distribute any maternal caretaking differences randomly across litters and treatment groups. Offspring were weaned on PN21. On PN75, one male and one female from each of the finally-assigned litters were decapitated and the heart and one liver lobe were dissected, blotted, frozen in liquid nitrogen and maintained at −45° C.
Assays
Tissues were thawed and homogenized (Polytron; Brinkmann Instruments, Westbury, NY) in buffer containing 145 mM sodium chloride, 2 mM magnesium chloride, and 20 mM Tris (pH 7.5), strained through several layers of cheesecloth to remove connective tissue, and the homogenates were then sedimented at 40,000 × g for 15 min. The pellets were washed twice and then resuspended in 250 mM sucrose, 2 mM MgCl2, and 50 mM Tris. For determinations of AC activity, aliquots of the membrane preparation were incubated for 30 min at 30°C with final concentrations of 100 mM Tris-HCl (pH 7.4), 10 mM theophylline, 1 mM ATP, 2 mM MgCl2, 10 μM GTP, 1 mg/ml bovine serum albumin, and a creatine phosphokinase–ATP–regenerating system consisting of 10 mM sodium phosphocreatine and 8 IU/ml phosphocreatine kinase. The enzymatic reaction was stopped by heating and sedimentation, and the supernatant solution was then assayed for cyclic AMP using commercial radioimmunoassay or immunoassay kits; the two types of kits gave equivalent results. In addition to assessing basal AC activity, we evaluated responses to 100 μM isoproterenol, 3 μM glucagon, 10 mM NaF and 100 μM forskolin. These concentrations produce maximal responses to each stimulant as assessed in earlier studies [2,44,45].
For the ligand binding determinations, there were technical limitations imposed by the large number of membrane preparations that had to be examined. The overall strategy was to determine binding at a single, subsaturating ligand concentration to enable the detection of changes that originate either in altered Kd or Bmax. To evaluate βAR binding, aliquots of the same membrane preparation were incubated with 67 pM [125I]-iodopindolol in 145 mM NaCl, 2 mM MgCl2, 1 mM sodium ascorbate, 20 mM Tris (pH 7.5), for 20 min at room temperature; samples were evaluated with and without 100 μM isoproterenol to displace specific binding. Incubations were stopped by addition of 3 ml ice-cold buffer, and the labeled membranes were trapped by rapid vacuum filtration onto glass fiber filters, which were washed with additional buffer and counted by liquid scintillation spectrometry. For cardiac m2AChR binding, the membrane suspension was reconstituted in 10 mM sodium-potassium phosphate buffer (pH 7.4) and incubated with 1 nM [3H] AFDX384, with or without 1 μM atropine to displace specific binding; determinations were not done in the liver, since this tissue is sparse in m2AChRs and lacks sufficient AC response to m2AChR agonists.
Data analysis
Data are presented as means and standard errors obtained from 6 animals in each treatment group for each sex and treatment regimen. To establish treatment differences, a global analysis of variance (ANOVA; data log transformed because of heterogeneous variance across tissues and AC stimulants) was first conducted for all variables: the in vivo treatment groups (control vs. DEX doses), sex, tissue and the stimulant condition under which the measurement was made (basal AC, isoproterenol-stimulated AC, glucagon-stimulated AC, fluoride-stimulated AC, forskolin-stimulated AC); the latter was considered to be a repeated measure because the same membrane preparation was used for each of the multiple assay conditions. As justified by significant interactions of treatment with the other variables, data were then subdivided to permit testing of individual treatments and AC stimulant responses that differed from control values; these were conducted by lower order ANOVAs, followed, where appropriate, by Fisher’s Protected Least Significant Difference Test to identify individual values for which the DEX groups differed from the corresponding control. For all tests, significance for main treatment effects was assumed at p < 0.05. However, for interactions at p < 0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables [40]. The criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive variables requiring subdivision for lower-order tests of main effects of DEX, the variable of chief interest. Where treatment effects were not interactive with other variables, we report only the main treatment effects without performing lower-order analyses of individual values.
To enable ready visualization of treatment effects across different tissues, treatment regimens and stimulants, the results are given as the percent change from control values, but statistical procedures were always conducted on the original data. For reference, the corresponding control values are detailed in Table 1. For the liver, there were two control cohorts (those receiving vehicle injections on PN1-3 and PN7-9) and the values shown in Table 1 were normalized and combined across the two groups; however, the effects of DEX were compared only to the appropriately matched control cohort.
Table 1.
Adenylyl Cyclase Activities and Receptor Binding in Controls
| Liver | Heart | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Basal ACa | 4.1 ± 0.1 | 4.1 ± 0.1 | 6.4 ± 0.3 | 7.7 ± 0.2* |
| Isoproterenol-Stimulated ACa | 6.3 ± 0.3 | 6.7 ± 0.3 | 27 ± 1 | 35 ± 2* |
| Glucagon-Stimulated ACa | 43 ± 2 | 45 ± 2 | 14 ± 1 | 20 ± 1* |
| NaF-Stimulated ACa | 22 ± 1 | 24 ± 1 | 48 ± 3 | 49 ± 1 |
| Forskolin-Stimulated ACa | 121 ± 6 | 123 ± 6 | 205 ± 11 | 246 ± 8* |
| βAR Bindingb | 2.8 ± 0.1 | 3.6 ± 0.1* | 6.2 ± 0.2 | 6.0 ± 0.2 |
| m2AChR Bindingb | — | — | 119 ± 2 | 141 ± 9* |
| Tissue weight | 6.2 ± 0.3c | 3.9 ± 0.1c* | 1.22 ± 0.03c | 0.81 ± 0.03c* |
Body weights (grams): male 453 ± 7; female 280 ± 6*
pmol/min per mg protein
fmol/mg protein
grams; liver weight is for one lobe only
significant difference between males and females
Materials
Animals were purchased from Charles River (Raleigh, NC). [125I]Iodopindolol (specific activity, 2200 Ci/mmol) and [3H]AFDX384 (115 Ci/mmol) both came from PerkinElmer Life Sciences (Boston, MA), and cyclic AMP radioimmunoassay and enzyme immunoassay kits were purchased from GE Healthcare Biosciences (Piscataway, NJ). All other chemicals were bought from Sigma Chemical Company (St. Louis, MO).
RESULTS
In control rats (Table 1), both liver and heart AC activities showed robust responses to stimulants (p < 0.0001 for the main effect of each stimulant compared to basal activity). However, the overall pattern of AC activity differed between liver and heart, reflected disparities in the relative effects of the various AC stimulants. In the liver, glucagon produced a much larger response than did isoproterenol, whereas the opposite was true for the heart; this reflects the relatively greater physiologic importance of glucagon signals in the liver as compared to βAR signals in the heart. Similarly, in the liver, glucagon produced a greater stimulatory response than did fluoride, reflecting the mixed involvement of both stimulation (Gs-related) and inhibition (Gi-related) for the latter agent; in the heart, isoproterenol produced a smaller signal than did fluoride. Superimposed on these basic patterns, the heart showed generally higher AC activities and m2AChR binding in females as compared to males, whereas the liver showed sex-related differences only for βAR binding.
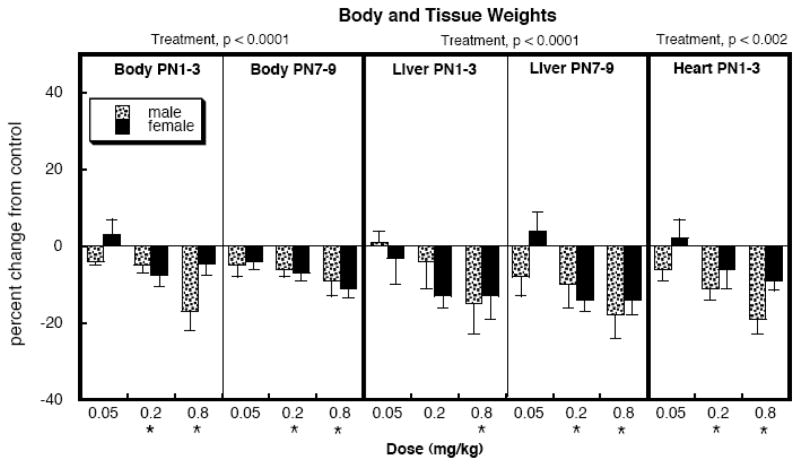
At the lowest dose, neonatal DEX treatment for either the PN1-3 or PN7-9 regimen did not have significant effects on body weights at PN75 but DEX did evoke statistically significant reductions at the two higher doses with either regimen (Figure 2). Liver weights were taken from a single lobe, making sure to take the same lobe from each animal. In general, liver weights were reduced with the same pattern as body weights, as were heart weights.
Figure 2.
Effects of neonatal DEX treatment on body and tissue weights. Data represent means and standard errors obtained from 6 animals in each group, presented as the percent change from the corresponding control values (Table 1). ANOVA appears at the top and asterisks denote values that differ from the control.
Because we made assessments for two treatment regimens (PN1-3, PN7-9) in the liver but only one regimen (PN1-3) in the heart, we initially performed two multivariate ANOVAs to evaluate the effects of DEX treatment. In the liver, the global test indicated a significant main effect of DEX treatment reflecting overall increases in AC activity (p < 0.0002) that depended upon regimen and sex (treatment × regimen × sex, p < 0.02). Accordingly, we separated the data by regimen (PN1-3 and PN7-9) and sex, and then performed lower-order tests to reexamine the results for treatment effects and interactions with the remaining variable of AC stimulant. In the second multivariate ANOVA, we assessed effects on liver and heart AC for the PN1-3 regimen and identified a main treatment effect of DEX (p < 0.0001) that was interactive with tissue (p < 0.0001) and sex (p < 0.002); accordingly, we separated the values for the heart and liver, and for males and females for the lower-order tests.
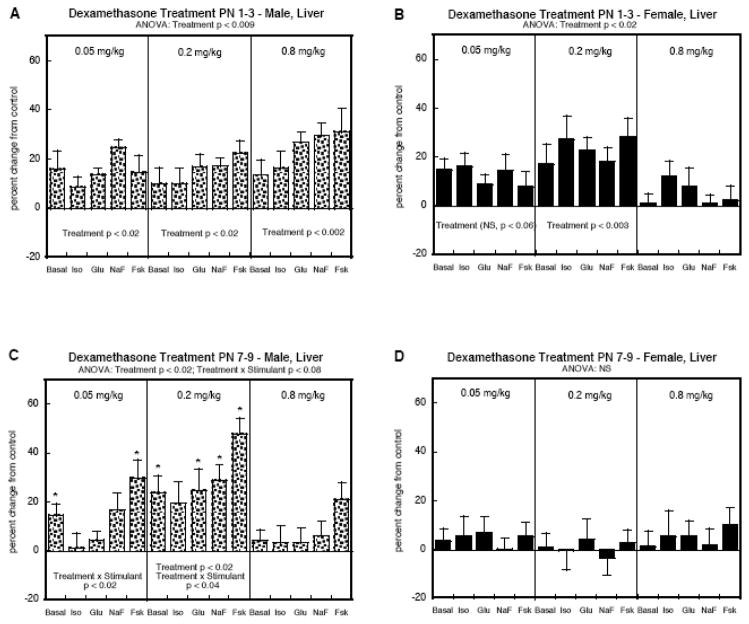
Liver AC
In the liver, PN1-3 DEX treatment elicited a significant overall increase in AC activity in both males (Figure 3A) and females (Figure 3B), without distinction among the various AC stimulants (no treatment × stimulant interaction). Importantly, both sexes showed significant effects even at the lowest dose (0.05 mg/kg), which did not cause any growth restriction. However, there was a sex difference in that males showed significant increases at each of the three different DEX doses whereas females showed a nonmonotonic dose-effect relationship, with no significant increases at the highest dose.
Figure 3.
Liver AC activity in animals given DEX on PN1-3 (A, B) or PN7-9 (C, D). Data represent means and standard errors obtained from 6 animals in each group, presented as the percent change from the corresponding control values (Table 1). ANOVA appears at the top of each panel and lower-order tests are shown within the panels. Where there was a significant treatment × stimulant interaction, asterisks denote specific responses that differ from the control. Abbreviations: Iso, isoproterenol; Glu, glucagon; NaF, sodium fluoride; Fsk, forskolin; NS, not significant.
Sex disparities became more evident when the treatment window was shifted later, to PN7-9. In males (Figure 3C), we still saw significant overall upregulation of AC activity (main treatment effect, p < 0.02) whereas now, there were no significant effects in females (Figure 3D). For the males, the dose-effect relationship and stimulant response pattern differed from that obtained with the PN1-3 regimen. Exposure on PN7-9 produced significant increases in AC responses even at the lower dose, but the dose-effect relationship was nonmonotonic, showing loss of effect at the highest dose. Furthermore, DEX augmented the response to forskolin significantly more than for the other stimulants (significant treatment × stimulant interactions, Figure 3C). In the group given 0.05 mg/kg DEX, there was no significant increase for isoproterenol-stimulated AC or glucagon-stimulated AC, and the response to fluoride showed a small, nonsignificant effect. Raising the dose to 0.2 mg/kg did produce significant increases for glucagon- and fluoride-stimulated AC but the response to forskolin was still enhanced to a greater extent.
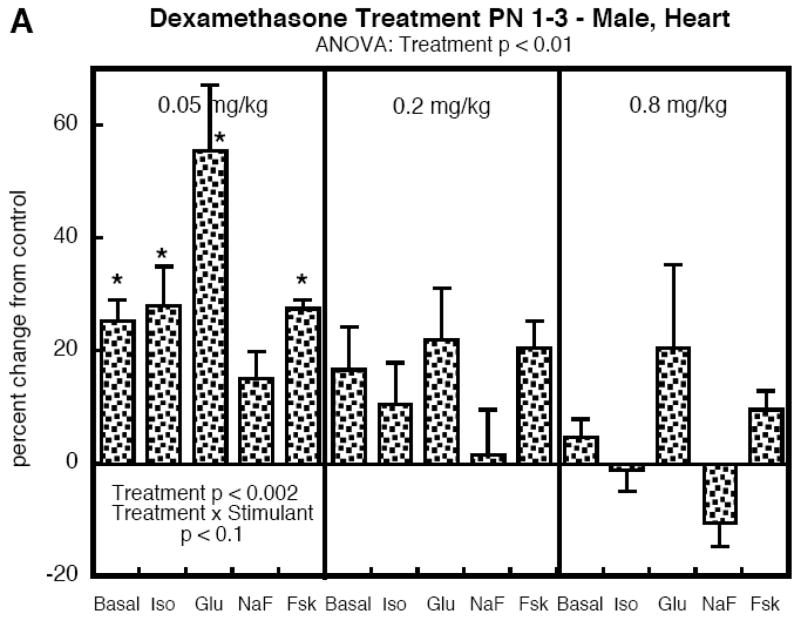
Heart AC
To determine the selectivity of the effects of DEX on AC signaling, we compared the effects to those seen in the heart, focusing on PN1-3, the regimen which evoked increases in both sexes. Males exposed to DEX again displayed significant overall increases in AC activity (Figure 4A). However, the dose-effect relationship was distinctly different from that seen in the liver, with a large increase obtained at the lowest dose and a progressive loss of effect as the dose was raised. Further, the response to glucagon showed the greatest increase, a pattern not seen in the liver. In females, the differences between heart and liver were even more stark (Figure 4B). There were no significant effects at 0.05 mg/kg, and increasing the dose to 0.2 and then 0.8 mg/kg produced deficits in AC responses, at first involving glucagon and then encompassing all the AC stimulants, with the largest effect still exerted toward the glucagon response.
Figure 4.
Heart AC activity in animals given DEX on PN1-3. Data represent means and standard errors obtained from 6 animals in each group, presented as the percent change from the corresponding control values (Table 1). ANOVA appears at the top of each panel and lower-order tests are shown within the panels. Where there was a significant treatment × stimulant interaction, asterisks denote specific responses that differ from the control. Abbreviations: Iso, isoproterenol; Glu, glucagon; NaF, sodium fluoride; Fsk, forskolin; NS, not significant.
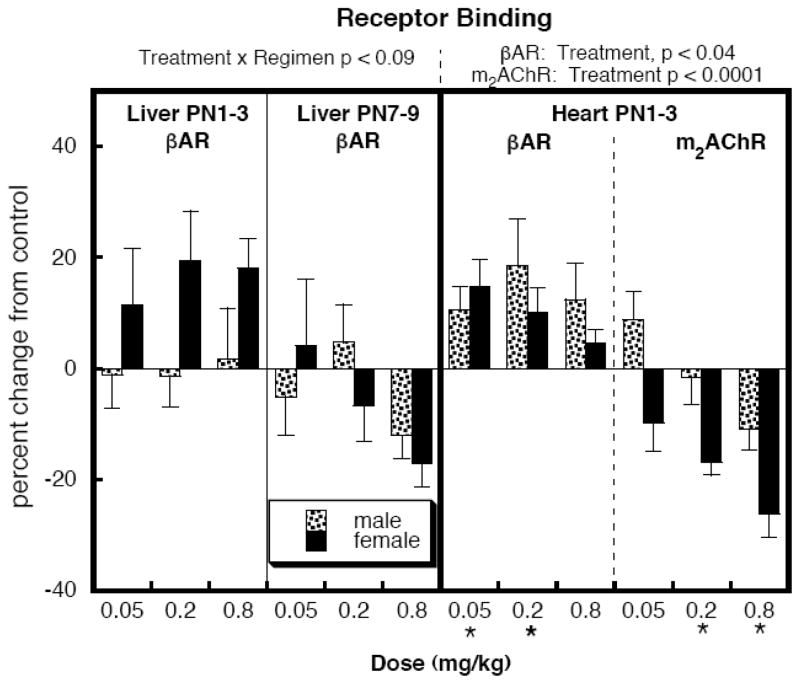
Receptor binding (Figure 5)
Figure 5.
Effects of neonatal DEX treatment on liver βAR binding and on heart βARs and m2AChRs. Data represent means and standard errors obtained from 6 animals in each group, presented as the percent change from the corresponding control values (Table 1). ANOVA appears at the top and asterisks denote values that differ from the control.
For liver βARs, multivariate ANOVA identified a weak treatment × regimen (p < 0.09); therefore, we separated the values for both treatment regimens and examined lower-order main treatment effects and interactions of treatment with other variables, but no significant differences were seen after the subdivision. There was a trend toward elevated βAR values for females with PN1-3 DEX exposure, an effect that did not correspond to any selective increase in the AC response to isoproterenol. In the heart, there was significant upregulation of βARs and downregulation of m2AChRs.
DISCUSSION
There are four main findings of this study. First, DEX treatment in the developmental phases corresponding to those in which glucocorticoids are used in preterm infants, elicits global increases in hepatic AC cell signaling. Second, robust effects were seen even at 0.05 mg/kg, a dose well below those used therapeutically, and likely to be more representative of the actions of endogenous glucocorticoids released in stress. Third, the effects involved AC gain-of-function, thus producing heterologous sensitization, in which the responses to disparate inputs all showed the same augmented effect. Fourth, there were distinct sex differences as well as disparities in the effects on liver vs. heart, indicative of selective actions rather than ubiquitous increases in AC signaling.
The results for the hepatic effects of DEX treatment on PN1-3 provide the clearest example of heterologous sensitization of the AC pathway. At each of the three different doses, we found significant increases in AC regardless of whether the activity was assessed in the basal state, in response to receptor stimulants or G-protein activation, or with direct AC activation by forskolin. The parallel changes point to gain-of-function of AC itself as the underlying mechanism. Accordingly, there is an augmented response to any upstream signal, including the responses to activation of βARs or glucagon receptors, which together provide major inputs to glucose and lipid metabolism; further emphasizing the point that sensitization involves AC itself, we did not observe any significant βAR upregulation that would signify a selective, upstream effect at the receptor level. The results thus resemble those seen in our earlier work with developmental exposure to organophosphates [2,29]; in that case, we found that this change in hepatic cellular function leads to prediabetes [21,36], the same outcome as found for AC polymorphisms in humans [30]. Given the known connection between early-life glucocorticoid exposure and later emergence of similar metabolic disorders [8,15,32,33,43], our results thus provide one of the likely contributory mechanisms that connect DEX exposure to adult disease outcomes.
Superimposed on the heterologous activation of AC signaling, DEX treatment on PN1-3 showed selectivity for both sex and tissue. Although females, like males, showed hepatic AC sensitization, the dose-response curve was nonmonotonic, with loss of effect at the highest dose. A similar nonmonotonic relationship between dose and effect was seen in the heart. In males, cardiac AC was maximally sensitized by the lowest dose of DEX and then showed a progressive loss of effect as the dose was raised; females showed a dose-dependent reduction in AC instead of the increase that had been seen in the liver. Since high doses of DEX are known to disrupt neuroendocrine function and to produce general somatic deficits, it is likely that the loss of the promotional effect on AC signaling involves mechanisms other than a direct influence on the signaling cascade [6,10–12,14,24–28,41,42]. Nevertheless, just as for the metabolic outcomes related to the effects on hepatic cell signaling, the functional consequences for cardiac structure and function are known: outright cell loss [38], alterations in the expression of cardiac contractile proteins [5] and impaired heart rate control [16,23]. As seen here, the greater negative impact on the glucagon response may indicate a further deficit in cardiac glucose utilization [1]. Superimposed on these effects, DEX exposure impairs the development of cardiac sympathetic projections, which would augment any deficits in AC signaling downstream from βAR input [35]. Viewed in this light, the small βAR upregulation and m2AChR downregulation found here may represent partial compensation to offset the effects of the downstream changes in signaling; it would be worthwhile to pursue the balance of physiological responses (heart rate, contractility) to sympathetic and parasympathetic input to determine how these cellular changes influence physiological responses.
Shifting the DEX exposure to PN7-9 again produced heterologous hepatic AC sensitization but with notable differences from the effects of the PN1-3 regimen. In males, the dose-response curve became nonmonotonic, with loss of effect at the highest DEX dose; this is compatible with the greater deficits in somatic growth with the PN7-9 treatment, which would then be expected to offset the direct effects of DEX on signaling as shown above. However, in females, there was no significant effect at any of the doses; this points instead to the closing of the critical developmental period in which DEX evokes lasting changes in AC signaling. A similar conclusion was reached in our earlier work with neurodevelopmental indices [18,20,37]. Thus, the most sensitive period for reprogramming of cell signaling evoked by DEX exposure corresponds to the developmental phase in which glucocorticoids are most likely to be used in preterm labor [13]. The later treatment also showed selective effects on the responses to AC stimulants that were not seen with the PN1-3 regimen, characterized by smaller effects on the responses to receptor stimulants than on the direct AC response to forskolin. This indicates reductions in the efficiency of receptor coupling to cyclic AMP generation; future studies should address the issue of whether these targeted deficits are of functional significance over and above the heterologous effects exerted at the level of AC itself.
Our findings thus extend the Barker Hypothesis, which originally related prenatal stress and associated growth restriction to subsequent development of cardiovascular disease and diabetes [3]. Specifically, our finding that glucocorticoid exposures below the threshold for somatic growth impairment nevertheless lead to cell signaling changes that underlie metabolic and cardiac dysfunction points to the greater relative importance of stress as distinct from growth impairment in adverse outcomes. In turn, this means that a wider variety of prenatal stressors and chemical exposures that are insufficient to elicit growth impairment, may nevertheless contribute to the worldwide increase in the incidence of diabetes. Finally, our results indicate that the common use of glucocorticoids in preterm labor may ultimately contribute to these outcomes.
Acknowledgments
Acknowledgments/disclaimers: Research was supported by NIH ES10356. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), Gutglass Erickson Bonville & Larson (Madison WI) and Pardieck Law (Seymour IN).
Abbreviations
- AC
adenylyl cyclase
- ANOVA
analysis of variance
- βAR
β-adrenergic receptor
- DEX
Dexamethasone
- m2AChR
m2-muscarinic acetylcholine receptor
- PN
postnatal day
Footnotes
Conflict of interest
A conflicting interest exists when professional judgement concerning a primary interest (such as patient’s welfare or the validity of research) may be influenced by a secondary interest (such as financial gain or personal rivalry). It may arise for the authors when they have financial interest that may influence their interpretation of their results or those of others. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anousis N, Carvalho RA, Zhao P, Malloy CR, Sherry AD. Compartmentation of glycolysis and glycogenolysis in the perfused rat heart. NMR Biomed. 2004:51–59. doi: 10.1002/nbm.860. [DOI] [PubMed] [Google Scholar]
- 2.Auman JT, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure targets multiple proteins governing the hepatic adenylyl cyclase signaling cascade: implications for neurotoxicity. Dev Brain Res. 2000;121:19–27. doi: 10.1016/s0165-3806(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJP. The developmental origins of adult disease. Eur J Epidemiol. 2003;18:733–736. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Ortiz L, Quan A. Fetal origins of cardiovascular disease. Curr Opin Pediatr. 2003;15:166–170. doi: 10.1097/00008480-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bian X, Briggs MM, Schachat FH, Seidler FJ, Slotkin TA. Glucocorticoids accelerate the ontogenetic transition of cardiac ventricular myosin heavy-chain isoform expression in the rat: promotion by prenatal exposure to a low dose of dexamethasone. J Dev Physiol. 1992;18:35–42. [PubMed] [Google Scholar]
- 6.Bohn MC. Glucocorticoid induced teratologies of the nervous system. In: Yanai J, editor. Neurobehavioral Teratology. Elsevier; Amsterdam: 1984. pp. 365–387. [Google Scholar]
- 7.Dammann O, Matthews SG. Repeated antenatal glucocorticoid exposure and the developing brain. Pediatr Res. 2001;50:563–564. doi: 10.1203/00006450-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 8.De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol. 2007;293:E75–E82. doi: 10.1152/ajpendo.00689.2006. [DOI] [PubMed] [Google Scholar]
- 9.Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- 10.Fuxe K, Cintra A, Chadi G, Gustafsson JÅ, Agnati LF. Central glucocorticoid receptors and neuronal plasticity. Meth Neurosci. 1994;22:372–382. [Google Scholar]
- 11.Fuxe K, Diaz R, Cintra A, Bhatnagar M, Tinner B, Gustafsson JA, Ogren SO, Agnati LF. On the role of glucocorticoid receptors in brain plasticity. Cell Mol Neurobiol. 1996;16:239–258. doi: 10.1007/BF02088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilad GM, Gilad VH, Eliyayev Y, Rabey JM. Developmental regulation of the brain polyamine-stress-response. Int J Dev Neurosci. 1998;16:271–278. doi: 10.1016/s0736-5748(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 13.Gilstrap LC, Christensen R, Clewell WH, D’Alton ME, Davidson EC, Escobedo MB, Gjerdingen DK, Goddard-Finegold J, Goldenberg RL, Grimes DA, et al. Effect of corticosteroids for fetal maturation on perinatal outcomes. J Am Med Assoc. 1995;273:413–418. [Google Scholar]
- 14.Gould E, Tanapat P, McEwen BS. Activation of the type 2 adrenal steroid receptor can rescue granule cells from death during development. Dev Brain Res. 1997;101:265–268. doi: 10.1016/s0165-3806(97)00023-0. [DOI] [PubMed] [Google Scholar]
- 15.Holness MJ, Sugden MC. Dexamethasone during late gestation exacerbates peripheral insulin resistance and selectively targets glucose-sensitive functions in β cell and liver. Endocrinology. 2001;142:3742–3748. doi: 10.1210/endo.142.9.8379. [DOI] [PubMed] [Google Scholar]
- 16.Hou QC, Slotkin TA. Effects of prenatal dexamethasone or terbutaline exposure on development of neural and intrinsic control of heart rate. Pediatr Res. 1989;26:554–557. doi: 10.1203/00006450-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Hurley JH. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem. 1999;274:7599–7602. doi: 10.1074/jbc.274.12.7599. [DOI] [PubMed] [Google Scholar]
- 18.Kreider ML, Aldridge JE, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Disruption of rat forebrain development by glucocorticoids: critical perinatal periods for effects on neural cell acquisition and on cell signaling cascades mediating noradrenergic and cholinergic neurotransmitter/neurotrophic responses. Neuropsychopharmacology. 2005;30:1841–1855. doi: 10.1038/sj.npp.1300743. [DOI] [PubMed] [Google Scholar]
- 19.Kreider ML, Levin ED, Seidler FJ, Slotkin TA. Gestational dexamethasone treatment elicits sex-dependent alterations in locomotor activity, reward-based memory and hippocampal cholinergic function in adolescent and adult rats. Neuropsychopharmacology. 2005;30:1617–1623. doi: 10.1038/sj.npp.1300716. [DOI] [PubMed] [Google Scholar]
- 20.Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- 21.Lassiter TL, Ryde IT, Levin ED, Seidler FJ, Slotkin TA. Neonatal exposure to parathion alters lipid metabolism in adulthood: interactions with dietary fat intake and implications for neurodevelopmental deficits; Brain Res Bull; 2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassiter TL, Ryde IT, MacKillop EA, Brown KK, Levin ED, Seidler FJ, Slotkin TA. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ Health Perspect. 2008;116:1456–1462. doi: 10.1289/ehp.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau C, Slotkin TA. Maturation of sympathetic neurotransmission in the rat heart. VII. Supression of sympathetic responses by dexamethasone. J Pharmacol Exp Ther. 1981;216:6–11. [PubMed] [Google Scholar]
- 24.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 25.Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47:291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Matthews SG, Owen D, Banjanin S, Andrews MH. Glucocorticoids, hypothalamo-pituitary-adrenal (HPA) development, and life after birth. Endocr Res. 2002;28:709–718. doi: 10.1081/erc-120016991. [DOI] [PubMed] [Google Scholar]
- 27.McEwen BS. Steroid hormones: effect on brain development and function. Hormone Res. 1992;37:1–10. doi: 10.1159/000182393. [DOI] [PubMed] [Google Scholar]
- 28.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 29.Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ Health Perspect. 2004;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordman S, Abulaiti A, Hilding A, Långberg EC, Humphreys K, Östenson CG, Efendic S, Gu HF. Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Intl J Obesity. 2008;32:407–412. doi: 10.1038/sj.ijo.0803742. [DOI] [PubMed] [Google Scholar]
- 31.Nyirenda MJ, Seckl JR. Intrauterine events and the programming of adulthood disease: the role of fetal glucocorticoid exposure. Int J Mol Med. 1998;2:607–614. doi: 10.3892/ijmm.2.5.607. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien K, Sekimoto H, Boney C, Malee M, O’Brien K, Sekimoto H, Boney C, Malee M. Effect of fetal dexamethasone exposure on the development of adult insulin sensitivity in a rat model. J Maternal Fetal Neonatal Med. 2008;21:623–628. doi: 10.1080/14767050802213073. [DOI] [PubMed] [Google Scholar]
- 33.O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- 34.Seckl JR. Glucocorticoid programming of the fetus: adult phenotypes and molecular mechanisms. Mol Cell Endocrinol. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 35.Slotkin TA, Barnes G, Lau C, Seidler FJ, Trepanier P, Weigel SJ, Whitmore WL. Development of polyamine and biogenic amine systems in brains and hearts of neonatal rats given dexamethasone: role of biochemical alterations in cellular maturation for producing deficits in ontogeny of neurotransmitter levels, uptake, storage and turnover. J Pharmacol Exp Ther. 1982;221:686–693. [PubMed] [Google Scholar]
- 36.Slotkin TA, Brown KK, Seidler FJ. Developmental exposure of rats to chlorpyrifos elicits sex-selective hyperlipidemia and hyperinsulinemia in adulthood. Environ Health Perspect. 2005;113:1291–1294. doi: 10.1289/ehp.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology. 2006;31:904–911. doi: 10.1038/sj.npp.1300892. [DOI] [PubMed] [Google Scholar]
- 38.Slotkin TA, Seidler FJ, Kavlock RJ, Bartolome JV. Fetal dexamethasone exposure impairs cellular development in neonatal rat heart and kidney: effects on DNA and protein in whole tissues. Teratology. 1991;43:301–306. doi: 10.1002/tera.1420430404. [DOI] [PubMed] [Google Scholar]
- 39.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Imbalances emerge in cardiac autonomic cell signaling after neonatal exposure to terbutaline or chlorpyrifos, alone or in combination. Dev Brain Res. 2005;260:219–230. doi: 10.1016/j.devbrainres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Snedecor GW, Cochran WG. Statistical Methods, Editoin Edition. Iowa State University Press; Ames, Iowa: 1967. [Google Scholar]
- 41.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 42.Welberg LAM, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 43.Wyrwoll CS, Mark PJ, Mori TA, Waddell BJ. Developmental programming of adult hyperinsulinemia, increased proinflammatory cytokine production, and altered skeletal muscle expression of SLC2A4 (GLUT4) and uncoupling protein 3. J Endocrinol. 2008;198:571–579. doi: 10.1677/JOE-08-0210. [DOI] [PubMed] [Google Scholar]
- 44.Zeiders JL, Seidler FJ, Iaccarino G, Koch WJ, Slotkin TA. Ontogeny of cardiac β-adrenoceptor desensitization mechanisms: agonist treatment enhances receptor/G-protein transduction rather than eliciting uncoupling. J Mol Cell Cardiol. 1999;31:413–423. doi: 10.1006/jmcc.1998.0875. [DOI] [PubMed] [Google Scholar]
- 45.Zeiders JL, Seidler FJ, Slotkin TA. Ontogeny of regulatory mechanisms for β-adrenoceptor control of rat cardiac adenylyl cyclase: targeting of G-proteins and the cyclase catalytic subunit. J Mol Cell Cardiol. 1997;29:603–615. doi: 10.1006/jmcc.1996.0303. [DOI] [PubMed] [Google Scholar]