Abstract
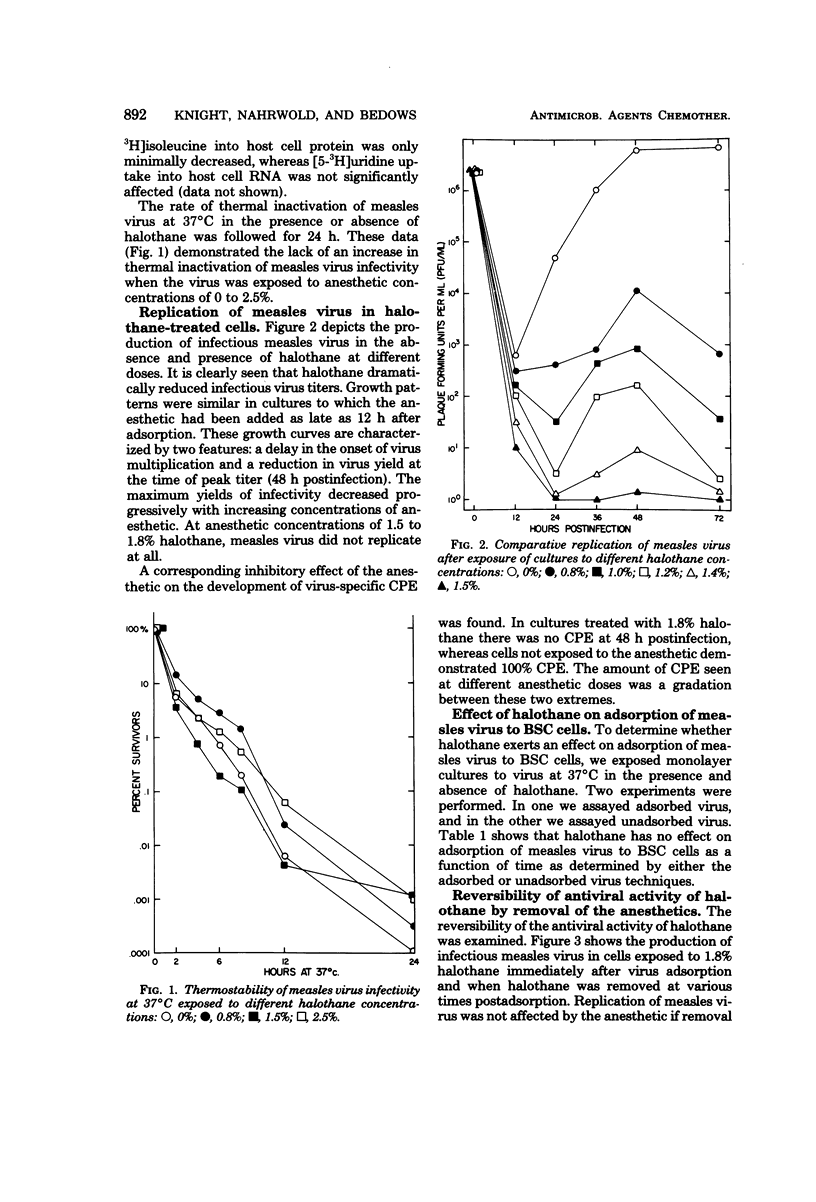
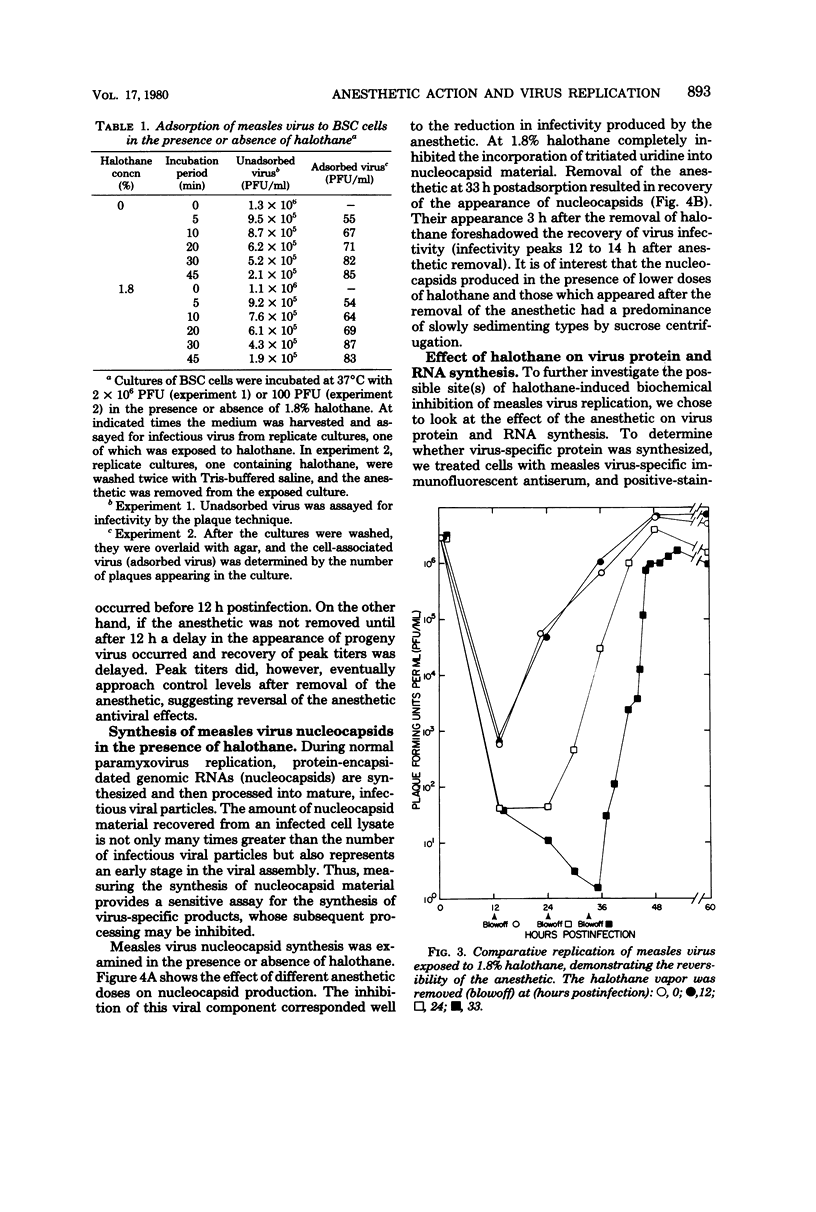
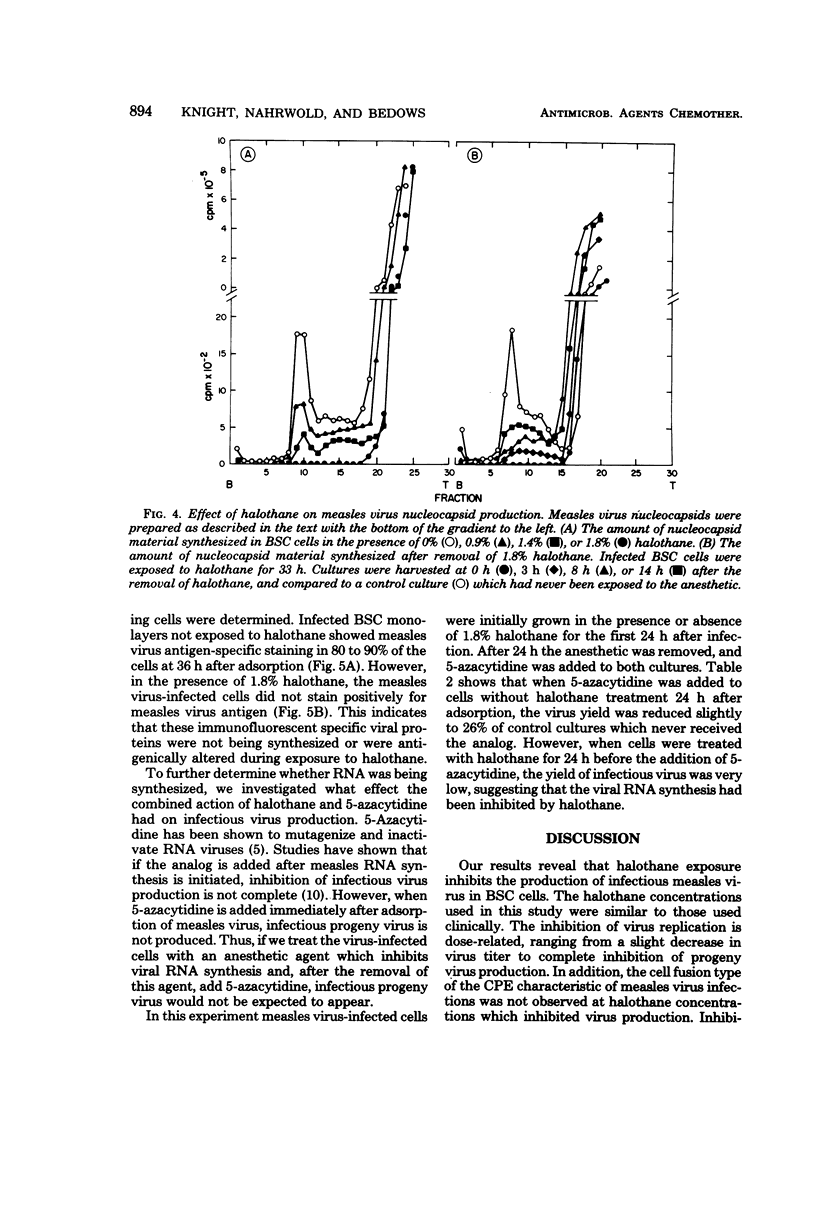
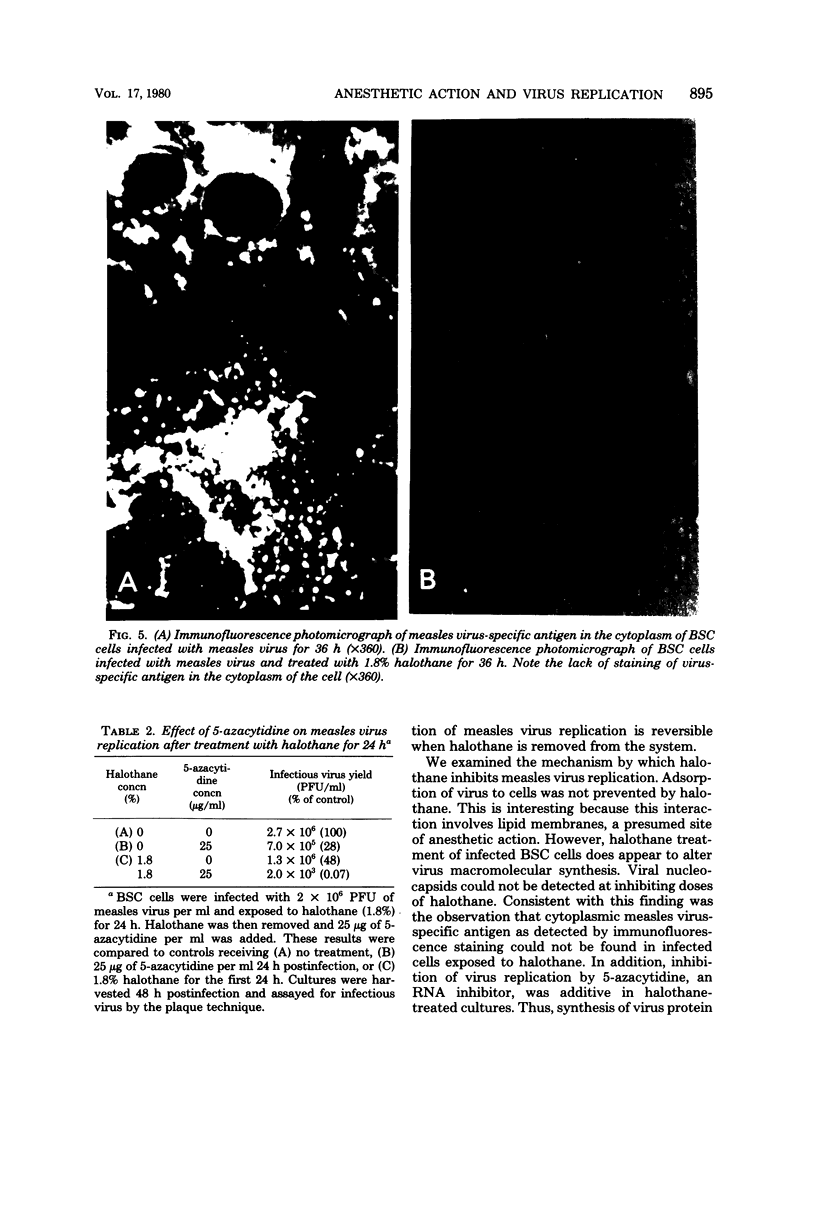
Replication of measles virus in BSC cells was studied in the presence of halothane, a commonly used volatile anesthetic. At clinical concentrations of the anesthetic, appearance of progeny virus was decreased in a dose-related manner. This inhibition was reversible as the removal of halothane allowed virus replication to be resumed. Studies attempting to elucidate the mechanism of action of the anesthetic inhibition of virus replication revealed that halothane did not directly inactivate the virus particle or prevent viral adsorption to the cell. Infectious virus and nucleocapsid production were decreased or stopped, depending on the anesthetic dosage used. Direct immunofluorescent staining for measles virus antigen was negative in cells treated at the higher concentrations of halothane. Recovery of nucleocapsid production started within a few hours after removal of halothane. Furthermore, the combined inhibitory effects on viral ribonucleic acid synthesis of 5-azacytidine and halothane were additive. This evidence suggests that inhibition of measles virus replication occurs at or before ribonucleic acid synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce D. L. Halothane inhibition of rna and protein synthesis of PHA-treated human lymphocytes. Anesthesiology. 1975 Jan;42(1):11–14. doi: 10.1097/00000542-197501000-00003. [DOI] [PubMed] [Google Scholar]
- Donovan C. A. The influence of diethyl ether inhalation on experimental canine distemper. Vet Med Small Anim Clin. 1968 Apr;63(4):345–347. [PubMed] [Google Scholar]
- Donovan C. A. The use of diethyl ether inhalation in canine distemper. Vet Med Small Anim Clin. 1967 Jul;62(7):658–660. [PubMed] [Google Scholar]
- Halle S. 5-Azacytidine as a mutagen for arboviruses. J Virol. 1968 Oct;2(10):1228–1229. doi: 10.1128/jvi.2.10.1228-1229.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D. N., Corbascio A. N. Some metabolic effects of halothane on mammalian tissue culture cells in vitro. Anesthesiology. 1971 May;34(5):427–438. doi: 10.1097/00000542-197105000-00013. [DOI] [PubMed] [Google Scholar]
- Jackson S. H. The metabolic effects of halothane on mammalian hepatoma cells in vitro. II. Inhibition of DNA synthesis. Anesthesiology. 1973 Oct;39(4):405–409. doi: 10.1097/00000542-197310000-00013. [DOI] [PubMed] [Google Scholar]
- Kiley M. P., Gray R. H., Payne F. E. Replication of measles virus: distinct species of short nucleocapsids in cytoplasmic extracts of infected cells. J Virol. 1974 Mar;13(3):721–728. doi: 10.1128/jvi.13.3.721-728.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P., Duff R., Rapp F. Latency of human measles virus in hamster cells. J Virol. 1972 Nov;10(5):995–1001. doi: 10.1128/jvi.10.5.995-1001.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moudgil G. C. Proceedings: Influence of halothane on mortality from murine hepatitis virus (MHV3). Br J Anaesth. 1973 Dec;45(12):1236–1236. doi: 10.1093/bja/45.12.1236. [DOI] [PubMed] [Google Scholar]
- Telser A., Hinkley R. E. Cultured neuroblastoma cells and halothane: effects on cell growth and macromolecular synthesis. Anesthesiology. 1977 Feb;46(2):102–110. doi: 10.1097/00000542-197702000-00004. [DOI] [PubMed] [Google Scholar]