Abstract
Background
Valspodar, a non-immunosuppressive analog of cylosporine, is a potent P-glycoprotein (MDR1) inhibitor. As MDR1-mediated efflux of chemotherapeutic agents from leukemic blasts may contribute to drug resistance, a phase 1 study of valspodar combined with mitoxantrone and etoposide in pediatric patients with relapsed or refractory leukemias was performed.
Procedure
Patients received a valspodar loading dose (2 mg/kg) followed by a five-day continuous valspodar infusion (8, 10, 12.5 or 15 mg/kg/day) combined with lower than standard doses of mitoxantrone and etoposide. The valspodar dose was escalated using a standard 3 + 3 phase I design.
Results
21 patients were evaluable for toxicity and 20 for response. The maximum tolerated dose (MTD) of valspodar was 12.5 mg/kg/day, combined with 50% dose-reduced mitoxantrone and etoposide. The clearance of mitoxantrone and etoposide was decreased by 64% and 60%, respectively, when combined with valspodar. Dose-limiting toxicities included stomatitis, ataxia, and bone marrow aplasia. Three of 11 patients with acute lymphoblastic leukemia (ALL) had complete responses while no patient with acute myeloid leukemia (AML) had an objective response. In vitro studies demonstrated P-glycoprotein expression on the blasts of five of 14 patients, although only one had inhibition of rhodamine efflux by valspodar.
Conclusions
While this regimen was tolerable, responses in this heavily pretreated population were limited to a subset of patients with ALL.
Keywords: leukemia, MDR1, multidrug resistance, P-glycoprotein, PSC-833, valspodar
INTRODUCTION
Despite intensive chemotherapy and the selective use of bone marrow transplantation, approximately 50% of children with acute myeloid leukemia (AML) and 20% of those with acute lymphoblastic leukemia (ALL) will die from their disease [1]. For these children, outcomes are poor due in part to resistance to chemotherapeutic drugs [2,3]. The MDR1 (ABCB1) gene encodes P-glycoprotein, an ATPase-dependent multidrug transporter responsible for the efflux of multiple chemotherapeutic agents used in leukemia therapy, including vinca alkaloids, anthracyclines, and epipodophyllotoxins [4,5]. In the hope of improving the efficacy of MDR1-substrate chemotherapeutic drugs, pharmacologic agents that block MDR1-mediated drug efflux have been developed, including valspodar (PSC-833, Novartis) [6,7].
Valspodar is a non-immunosuppressive analog of cyclosporine (CsA) with more potent P-glycoprotein inhibition and without significant immunosuppression or nephrotoxicity [8]. Toxicities associated with valspodar and concomitant chemotherapy (mitoxantrone, etoposide, cytarabine) in adults with leukemia include stomatitis, cerebellar ataxia, and reversible hyperbilirubinemia, with a maximum tolerated dose (MTD) of 10 mg/kg/day [9]. In the Pediatric Oncology Group (POG) phase II protocol 9222, CsA was added to a five-day induction regimen of mitoxantrone and etoposide in pediatric patients with relapsed or refractory AML with a 50% response rate (complete plus partial) [10]. Based on these results and the correlation of P-glycoprotein expression with outcomes in pediatric leukemias [11,12], we initiated a phase I trial (POG 9423) of valspodar with the reduced-dose mitoxantrone and etoposide regimen from POG 9222 in children with recurrent or refractory leukemia.
METHODS
Patient Eligibility
Patients with relapsed or refractory leukemia < 22 years of age at the time of diagnosis were eligible. Patients with AML were eligible if they met one of the following criteria: (1) duration of first bone marrow remission < 6 months, (2) refractory to standard AML induction, (3) secondary AML or AML evolving from myelodysplastic syndrome, or (4) bone marrow relapse following bone marrow transplantation (BMT). Patients with ALL were eligible if they had: (1) second or subsequent bone marrow relapse or (2) bone marrow relapse following BMT. All patients with prior BMT were required to have had trilineage engraftment and be at least six months from transplant.
Other eligibility criteria included: (1) recovery from prior therapy; (2) no therapy, with the exception of hydroxyurea, in the 4 weeks prior to enrollment; (3) adequate performance status (Lansky >40% if < 12 years or Karnofsky ≥50% or ECOG ≤ 2); (4) life expectancy > 8 weeks; (5) adequate renal (normal creatinine or glomerular filtration rate for age) and hepatic function [bilirubin < 1.5x normal, alanine aminotransferase (ALT) < 2x normal, albumin > 3 g/dL]; and (6) cardiac ejection fraction >50% or shortening fraction ≥27% with less than 360 mg/m2 cumulative prior anthracycline exposure. Exclusion criteria included history of etoposide allergy, pregnancy or breast feeding, use of anticonvulsant medication, uncontrolled infection, and isolated central nervous system or extramedullary relapse.
Local institutional review board approval was obtained by all participating institutions, and all patients or their legal guardians provided written informed consent in accordance with federal and institutional requirements.
Study Design
Dose escalation of valspodar (PSC 833, Novartis Pharmaceutical Corporation, East Hanover, NJ) followed a traditional 3 + 3 phase I design in which cohorts of three patients at a time were treated at a dose level. If no dose limiting toxicity (DLT) was observed, the dose for the next cohort was escalated to the next level. If one of three patients experienced a DLT, three additional patients were enrolled at that dose level. If two of six patients experienced a DLT, then the maximum tolerated dose (MTD) was exceeded. If two of three patients experienced a DLT, then three additional patients were enrolled at the next lower dose level, if six patients had not already been enrolled. The MTD was defined as the dose immediately below the level at which two patients in a cohort of three to six experienced a DLT.
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (CTC) version 2.0. Any nonhematologic grade 3 or greater toxicity that was possibly, probably, or definitely attributable to valspodar was considered a DLT with specific exclusions. Due to expected toxicities from mitoxantrone and etoposide, grade 3 nausea, infection, and stomatitis were not considered DLTs. As reversible hyperbilirubinemia was expected with valspodar, grade 3 hyperbilirubinemia that resolved following completion of the valspodar infusion was not considered a DLT. Myelosuppression was not considered a DLT except for prolonged bone marrow aplasia greater than 6 weeks. A daily neurologic examination was required throughout the valspodar infusion and grade 3 cerebellar ataxia (markedly abnormal gait defined as inability to walk without assistance from another person or walker) was considered a DLT. In the event of a single fatal toxicity, study registration would be suspended and the study reviewed with the Phase I Cooperative Agreement Scientific Principal Investigator. Six years following study completion, hard copies of all study documentation for each patient including flow sheets and laboratory values were obtained from the POG archives and retrospectively reviewed to ascertain toxicities which were not reported at the time of the study.
Drug Administration
Each valspodar-ME chemotherapy course lasted five days. On day 1 each patient received etoposide [100 mg/m2 intravenously (IV) over 1 hour] followed by mitoxantrone (10 mg/m2 IV over 15 minutes) without valspodar. The valspodar loading dose (2 mg/kg/hr IV over 2 hours) was then administered between hours 22 and 24 of the first day of therapy at the same time that a continuous valspodar infusion for 122 hours was initiated at the assigned dose level. This loading dose was administered in an attempt to rapidly achieve a steady-state valspodar level greater then 1 μM. The valspodar continuous infusion dose for the first cohort was 8 mg/kg/day over 122 hours based on the MTD in adults of 10 mg/kg/day, with subsequent escalations of the infusion dose to 10, 12.5, 15, 17.5 and 20 mg/kg/day over 122 hours. Following the valspodar loading dose, each patient received etoposide and mitoxantrone daily for 4 days (days 2-5) at 50% of the day 1 dose due to known interactions with MDR1 inhibitors and based on prior experience with cyclosporine plus mitoxantrone and etoposide [13]. Patients with concurrent CNS leukemia received intrathecal therapy with methotrexate, hydrocortisone, and cytarabine weekly x four doses.
Patient Evaluation
Clinical and laboratory studies prior to treatment included the following: history and physical examination with baseline cerebellar evaluation, performance status, bone marrow aspiration and biopsy, chest x-ray, echocardiogram, complete blood count (CBC), electrolytes, creatinine, and liver function tests. In addition, a bone marrow sample was obtained for P-glycoprotein studies. Evaluations during therapy included weekly physical examinations, twice weekly CBC, electrolytes and creatinine. Bilirubin was monitored daily during the valspodar infusion.
Response Assessment
Disease status was evaluated by bone marrow aspirate and biopsy at day 14 of the first cycle and prior to the next cycle. If there was evidence of progressive disease the patient was removed from the study. If the day 14 marrow was hypocellular and definite blasts were absent, no additional therapy was given until count recovery at which time the bone marrow aspirate was repeated to document response status. If the day 14 marrow was hypocellular with <25% leukemic blasts, the patient was eligible for a second course of valspodar-ME once any toxicities had resolved.
Response was defined as: (1) complete remission [CR, M1 marrow (<5% blasts) with peripheral blood absolute neutrophil count (ANC) > 500/ul and platelets (transfusion independent) > 100,000/ul]; (2) partial response [PR, M2 marrow (5-25% blasts) with > 50% reduction in bone marrow blast percentage]; or (3) progressive disease [PD, patients who either had no response by day 14 bone marrow aspirate or whose bone marrow became hypocellular in response to therapy but had leukemia recurrence (M3 marrow; >25% blasts) upon count recovery].
Pharmacokinetic Analyses
Peripheral blood was sampled for pharmacokinetic studies of valspodar, etoposide, and mitoxantrone. For valspodar, 1 mL of blood was obtained in EDTA-containing tubes prior to and at 24, 72, 85, 120, 122, 126, and 144 hours following the start of the infusion. Samples were kept frozen at less than −20°C until processing. Valspodar concentrations were determined by radioimmunoassay (ANAWA Laboratories, Zürich, Switzerland) by Novartis Pharmaceuticals Corporation (East Hanover, NJ) as previously described [14]. The lower limit of quantification was 75 ng/mL.
Etoposide and mitoxantrone concentrations were determined with and without concomitant valspodar. Blood (2.5 to 5 mL) was collected in heparinzed tubes prior to the chemotherapy infusion and at 0, 0.5, 5, 12, and 21 hours after the infusion on days 1 (without valspodar) and 4 (with valspodar) of cycle 1. Mitoxantrone plasma samples were stabilized with the addition of 0.3 ml of 5% ascorbate and stored at −80°C prior to analysis. Total plasma mitoxantrone was analyzed as previously described using a reverse-phase HPLC method using ametantrone (a gift provided by Wyeth-Ayerst Research, Philadelphia, PA, USA) as an internal standard [15]. Total plasma etoposide was analyzed by reverse-phase HPLC using a previously described method [15,16].
Pharmacokinetic Modeling
A linear two-compartment model with the intravenous infusion modeled as a zero order input was used to characterize mitoxantrone and etoposide pharmacokinetics. A hierarchical model was used to account for inter-individual and intra-individual variability [17]. Inter-individual variability in CL (clearance from the central compartment), V1 (volume of the central compartment), and V2 (volume in the peripheral compartment) were modeled assuming a lognormal distribution. Intra-individual residual variability was described using a proportional component.
To examine and quantify the drug interactions with valspodar (VAL) we modeled the change in clearance of mitoxantrone or etoposide as follows: CLMitox/etop= CL0+(CL1-CL0) * VAL/(VAL+EC50) where CL0 is the baseline clearance, CL1 is the asymptotic value of CL for large concentrations of valspodar (when VAL→∞), and EC50 is the concentration of valspodar when the clearance of the chemotherapy agent is 50% of the baseline clearance (CL0). Model selection was based on a comparison of the −2 log likelihood and visual inspection of goodness of fit diagnostic plots. Variances are expressed as percent coefficients of variation (%CV). All data analysis was carried out using NONMEM V (GloboMax LLC, Hanover, MD) [18]. Graphical output was generated with S-Plus [19].
P-glycoprotein expression
At enrollment, bone marrow (or peripheral blood if blast concentration was greater than 15%) was obtained for P-glycoprotein cell surface expression and function. Samples were diluted in an equal volume of RPMI-1640 medium and transported at room temperature by overnight courier to the central MDR1/drug-resistance reference laboratory (Y.R.; Wayne State University, Detroit, MI). Mononuclear cells were separated by centrifugation on Ficoll-Hypaque gradients and resuspended in RPMI-1640 medium containing 20% dialyzed fetal bovine serum and insulin, transferrin, and selenite. Surface expression of P-glycoprotein was measured by flow cytometry at room temperature using the phycoerythrin-conjugated monoclonal antibody 4E3 [20,21]. Staining intensity was evaluated by comparing 4E3-PE binding with that of its matched isotype control with the Kolmogorov-Smirnov statistic, expressed as a D-value ranging from 0 (identical distribution histograms) to 1.0 (no overlap in distribution histograms) [22]. A D-value of ≥0.15 is considered positive. P-glycoprotein function was measured by rhodamine123 (R123) efflux in the presence or absence of CsA or valspodar, as previously described [23]. Cell lines with and without P-glycoprotein expression (CEM, S226, CEM-VBL, S226-Dox) were used as controls. R123 efflux was quantified as the log-shift of the peak of the fluorescence curve with the addition of CsA or valspodar. A shift of 0.5 log or greater was considered evidence of inhibition of R123 efflux.
RESULTS
Patients
Twenty-three patients were enrolled on POG 9421 between November 1997 and June 2001. One patient was ineligible due to pre-existing mucositis requiring intravenous narcotics at the time of enrollment. Table I shows the demographic and clinical characteristics for the 22 eligible patients. One patient (#21) was initially enrolled but developed hyperbilirubinemia from cholelithiasis and biliary obstruction prior to starting treatment; he did not receive the study drug and was not evaluable for toxicity or response. One patient (#13) died at day 17 due to infection and was therefore not evaluable for response. Thus, 21 patients were evaluable for toxicity and 20 for response.
TABLE I.
Characteristics of eligible patients (n = 22)
| Patient ID |
Valspodar dose level |
Diagnosis | Age (years) |
Gender | Prior induction attempts |
Clinical history | Response |
|---|---|---|---|---|---|---|---|
| 1 | 1 | DS-AML | 2.3 | Male | 1 | On-therapy relapse after CR | PD |
| 2 | 1 | AML | 15.8 | Male | 1 | Off-therapy relapse | PD |
| 3 | 1 | T-cell ALL (post B-precursor ALL) |
18.2 | Female | 3 | Third bone marrow relapse | CR |
| 4 | 2 | AML | 5.2 | Male | 1 | Refractory to induction | PD |
| 6 | 2 | Biphenotypic | 6.9 | Female | 3 | On-therapy relapse after CR | PD |
| 7 | 2 | B-precursor ALL | 20.3 | Male | 5 | Initial disease refractory to chemotherapy, relapsed post-BMT |
PD |
| 8 | 2 | AML | 11.2 | Male | 2 | Refractory to induction | PD |
| 9 | 2 | B-precursor ALL | 13.2 | Female | 3 | Second off-therapy relapse; refractory to reinduction |
CR |
| 10 | 3 | AML | 10 | Male | 1 | On-therapy relapse after CR | PD |
| 11 | 3 | AML | 17.6 | Male | 1 | Off-therapy relapse | PD |
| 12 | 3 | AML | 6.7 | Male | 1 | Refractory to induction | PD |
| 13 | 3 | B-precursor ALL | 8.5 | Male | 3 | Second off-therapy relapse; s/p HSCT |
Not evaluable; death at day 17 from sepsis |
| 14 | 3 | AML | 9.3 | Female | 3 | Second off-therapy relapse; s/p HSCT |
PD |
| 15 | 3 | B-precursor ALL | 9.4 | Male | 2 | Second off-therapy relapse; s/p HSCT |
CR |
| 16 | 4 | B-precursor ALL | 11.1 | Male | 2 | Refractory to induction | PD |
| 17 | 4 | AML | 10.9 | Male | 1 | Off-therapy relapse; s/p HSCT | PD |
| 18 | 4 | AML | 12.6 | Male | 4 | Refractory to induction | PR |
| 19 | 4 | B-precursor ALL | 7.4 | Female | 2 | On-therapy relapse after CR | PD |
| 20 | 4 | B-precursor ALL | 4.6 | Male | 6 | On-therapy relapse followed by HSCT; relapse after HSCT, refractory to reinduction |
PD |
| 21 | 4 | AML | 3.7 | Male | 1 | On-therapy relapse after CR | Not evaluable; study drug not given due to high bilirubin |
| 22 | 4 | tAML (post JMML) |
13 | Male | 1 | N/A | PR |
| 23 | 4 | B-precursor ALL | 4.3 | Male | 2 | On-therapy relapse after CR; refractory to reinduction |
PD |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CR, complete response; DS, Down syndrome; HSCT, hematopoeitic stem cell transplant; JMML, juvenile myelomonocytic leukemia; PR, partial response; PD, progressive disease; tAML, therapy-related AML
Dose Escalation and Toxicities
Table II shows the grade 3 and 4 toxicities at the administered valspodar dose levels. Twenty-one patients were evaluable for toxicity. No dose-limiting toxicities were initially reported for the three patients treated at dose level 1 (8 mg/kg/day). However, after subsequent patients had been treated at dose level 2 (10 mg/kg/day), it was discovered that the first patient at dose level 1 had developed grade 4 stomatitis in the setting of Down syndrome (DS). Given the known sensitivity of patients with DS to cytotoxic chemotherapy, and the fact the subsequent non-DS patients treated at dose levels 1 and 2 had not had significant stomatitis, this was not deemed to be a DLT. While the study was not formally amended to exclude patients with DS, no further patients with DS were enrolled.
TABLE II.
Valspodar dose levels and non-bilirubin, non-hematologic grade 3 or 4 toxicity
| Dose Level |
Valspodar Dose (mg/kg/day) |
Number of Patients Treated at Dose Level |
Number of Patients with Grade 3 or 4 Toxicity in First Course |
Specific Toxicity | Toxicity Grade |
|---|---|---|---|---|---|
| 1 | 8 | 3 | 1 | Stomatitis$ | Grade 4 |
| 2 | 10 | 5 | 1 | Stomatitis | Grade 3 |
| 3 | 12.5 | 6 | 1 | Cerebellar | Grade 3* |
| 1 | Infection | Grade 5 (death) | |||
| 1 | Stomatitis | Grade 3 | |||
| 1 | Hyperglycemia | Grade 3# | |||
| 2 | Hypokalemia | Grade 3# | |||
| 4 | 15 | 7 | 3 | Stomatitis | Grade 4* |
| 1 | Bone marrow aplasia > 6 weeks |
Grade 4* | |||
| 1 | Hypokalemia | Grade 4* | |||
| 2 | Hypertension | Grade 3# |
Occurred in patient with Down syndrome
Dose-limiting toxicity
noted during retrospective chart review after study completion, not reported during therapy
Five eligible patients were treated at dose level 2 (10 mg/kg/day), without a reported DLT. This cohort was expanded to more than three patients due to the occurrence of significant stomatitis in one patient, who was subsequently found to be ineligible due to presence of pre-existing grade 3 stomatitis from prior cytotoxic chemotherapy at the time of enrollment. At dose level 3 (12.5 mg/kg/day), one patient experienced a DLT (grade 3 cerebellar toxicity) and the cohort was expanded to six patients. The fourth patient treated at dose level 3 developed bacterial sepsis while neutropenic at day 17 and died. The study was temporarily suspended for review, and it was decided that this event was not considered related to valspodar but rather to expected myelosuppression from the conventional chemotherapy and therefore was not considered a DLT in the absence of prolonged marrow aplasia. At dose level 4 (15 mg/kg/day), three of seven patients experienced grade 4 stomatitis, one with culture-positive herpes simplex virus infection. One patient at this dose level had prolonged aplasia with <10% bone marrow cellularity for 7 weeks at which point the bone marrow recovered with > 25% blasts. One patient also had grade 4 hypokalemia. Therefore, for this regimen, the maximum tolerated dose of valspodar in combination with mitoxantrone and etoposide (at 50% dosing) was 12.5 mg/kg/day (dose level 3).
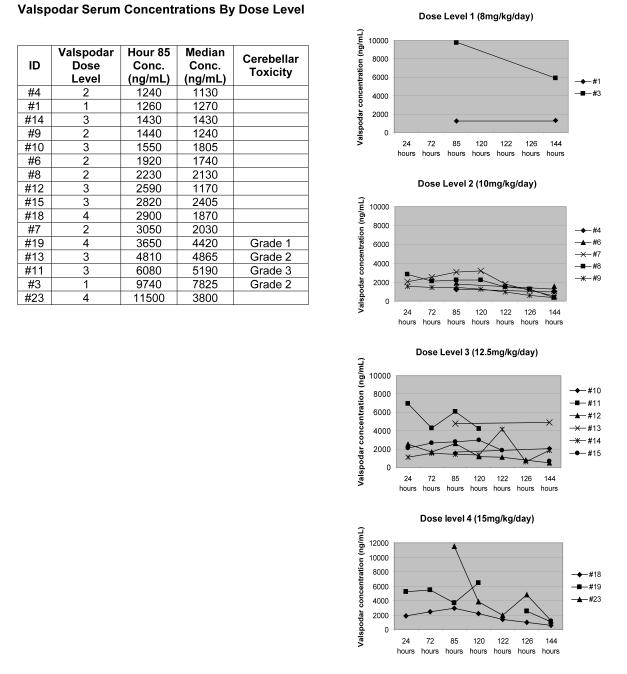
Cerebellar toxicity, manifested by ataxia or dizziness, was reported in five patients (one grade 3, two grade 2, two grade 1). Valspodar concentrations were measured in four of these patients (#3,11,13,19); in each case the median valspodar concentration over the course of the continuous infusion and the valspodar concentration at hour 85 (mid-infusion) were in the uppermost tertile, regardless of assigned dose level (Figure 1). Symptoms resolved following completion of the valspodar infusion in four of five patients, including the patient with grade 3 toxicity. The patient with persistent symptoms initially reported mild dizziness (grade 1) on day 4 of the valspodar infusion, which resolved within 48 hours. However, 20 days later she developed dizziness with movement diagnosed as vertigo after full neurologic evaluation. Symptoms improved with meclizine.
FIGURE 1.
Individual patient valspodar serum concentrations were measured during (hours 24-122) and after (hours 126-144) the valspodar continuous infusion in 16 patients (2 patients at dose level 1, 5 patients at dose level 2, 6 patients at dose level 3, 3 patients at dose level 4). Hour 85 is the midpoint of the continuous infusion. The median valspodar concentration over the course of the infusion was determined for each patient. Conc = concentration.
As anticipated, all patients experienced profound myelosuppression and required hematologic support with packed red blood cell and platelet transfusions. All patients experienced febrile neutropenia and were hospitalized for broad-spectrum antimicrobial therapy. Infections were common, including varicella zoster (n=1), sinusitis (n=1), central line cellulitis (n=1), pneumonia (n=3), and bacteremia (n=5). Only one fungal infection was reported (Candida sp. pneumonia). Transient hyperbilirubinemia (median maximum 2 mg/dl, range 0.8-6.6 mg/dl) that resolved after completion of valspodar infusion occurred in 16 of 21 patients. Four patients also had valspodar infusion-related transaminase elevations (one grade 1, two grade 2, one grade 3), which self-resolved.
Pharmacokinetic Analysis
Valspodar concentrations were measured in 16 of 20 patients and are summarized in Figure 1 by assigned dose level. Ten of 16 patient had the majority of required samples obtained while six had adequate samples submitted at only hour 85 (mid-infusion) and hour 144 (24 hours following the completion of the infusion). Valspodar concentrations were highly variable and did not correlate with assigned dose level; higher levels did correlate with cerebellar symptoms. At the mid-infusion sampling time (hour 85), measured valspodar levels ranged from 1,240 ng/mL to 11,500 ng/mL (median 2,590 ng/mL). Thus, at some point during the infusion, all patients had valspodar levels greater than those which achieve MDR1 reversal in vitro (1,000 ng/mL) [24]. Median valspodar concentrations for each patient over the course of the infusion ranged from 1,130 ng/mL to 7,825 ng/mL (overall median 2,030 ng/mL).
Valspodar, mitoxantrone, and etoposide levels were concurrently measured in 14 patients; levels were fit to the 2-compartment structural model as described in the methods section. The population pharmacokinetic parameters for mitoxantrone and etoposide are summarized in Table III. The population estimate of the day 1 mitoxantrone clearance (pre-valspodar) was 16.4 L/h, which decreased by 64% to 5.88 L/h on day 4 in the presence of valspodar. The population estimate of the day 1 etoposide clearance (pre-valspodar) was 1.52 L/h with a 60% decrease to 0.61 L/h with concomitant valspodar. Because the achieved valspodar concentrations exceeded the estimated EC50 by greater than 2 to 3-fold in the majority of patients (mean BSA 1.3 m2), the CL1 values were used to estimate the area under the curve (AUC) of mitoxantrone and etoposide. During valspodar treatment, the estimated mean AUC value for mitoxantrone increased 33% (1.11 versus 0.79 mg*h/L) and for etoposide, 23% (106 versus 85.5 mg*h/L). Thus, the empiric 50% dose reduction of chemotherapy during valspodar infusion maintained exposure in the typical patient.
TABLE III.
Population pharmacokinetic parameters for mitoxantrone and etoposide in presence and absence of valspodar
| MITOXANTRONE | ||||
|---|---|---|---|---|
| Parameter | Estimate | % CVa | Interindividual variability estimateb |
%CVc |
| CL0 (L/h) | 16.4 | 17.9 | 48.1 | 69 |
| CL1 (L/h) | 5.88 | 30.8 | ||
| V1 (L) | 23.2 | 19.3 | 64.4 | 68.5 |
| V2 (L) | 102 | 19.9 | - | - |
| Q (L/h) | 8.13 | 23.7 | - | - |
| EC50mitox (mg/L) | 0.525 | 121 | - | - |
| Residual errord | 60.6% | 17.8 | - | - |
| ETOPOSIDE | ||||
|---|---|---|---|---|
| PK parameter | Estimate | % CVa | Interindividual variability estimateb |
%CVc |
| CL0 (L/h) | 1.52 | 0.36 | 61.56 | 5.83 |
| CL1 (L/h) | 0.611 | 0.37 | ||
| V1 (L) | 3.58 | 0.18 | 98.5 | 4.56 |
| V2 (L) | 3.82 | 0.35 | - | - |
| Q (L/h) | 2.38 | 0.428 | - | - |
| EC50eto (mg/L) | 0.271 | 0.53 | - | - |
| Residual errord | 32.1% | 0.43 | - | - |
V1 = volume of the central compartment, V2 = volume in the peripheral compartment, Q = inter-compartmental clearance, CL = clearance from the central compartment, CL0 is the baseline clearance and CL1 is the asymptotic value of CL for large concentrations of valspodar (when valspodar →∞), EC50mitox= the concentration of valspodar required to reduce mitoxantrone clearance by 50%, EC50eto= the concentration of valspodar required to reduce etoposide clearance by 50%.
Relative standard error of estimate (SE estimate/estimate) expressed as a percentage
Estimates of variability, expressed as % (100×√variance)
Percent square root of the relative standard error of the coefficient of variation()
Residual intrasubject variability
Treatment Response
Twenty patients were evaluable for response. One patient with pre-B ALL who died of Citrobacter freundii sepsis at day 17 was not evaluable for response. Bone marrow biopsy at autopsy was hypocellular with no evidence of blasts. Three patients, one with T-ALL and two with pre-B ALL, experienced complete remission (CR). Both pre-B ALL patients entered the study in their third bone marrow relapse. One was treated at dose level 2 with median valspodar level of 1,240 ng/mL (Figure 1, #9) and the other received two courses of valspodar-ME at dose level 3 with median valspodar level in the first course of 2,405 ng/mL (Figure 1, #15); both proceeded to bone marrow transplant. The T-ALL patient was treated at dose level 1, but had the highest measured median valspodar level (7,825 ng/mL) of any patient for whom levels were obtained (Figure 1, #3). Fifteen patients had progressive disease (9 of 11 AML, 5 of 7 pre-B ALL, and one of one biphenotypic leukemia). One patient with therapy-related AML ten years after successful treatment for juvenile myelomonocytic leukemia (JMML) received two cycles of valspodar-ME and had ANC recovery > 500/uL. Bone marrow biopsy at that time showed only 20% cellularity with 3% blasts consistent with a partial response. The patient was taken off study to pursue matched unrelated bone marrow transplantation.
P-glycoprotein expression
P-glycoprotein surface expression and function were measured in 14 patients (Table IV). Three patients had D-values and histograms consistent with p-glycoprotein expression on the majority of the blast population, while two had expression on a subpopulation of blasts, and nine had no evidence of p-glycoprotein expression. Rhodamine efflux studies demonstrated blockade of efflux in four samples. Patient #4 had evidence of efflux blockade with CsA but no P-glycoprotein expression by flow cytometry with the 4E3 antibody, suggesting the presence of a non-MDR1 transporter. The three other patients with evidence of efflux blockade (#5, #7, #9) had P-glycoprotein expression on the majority of blasts. However, only one (#9) had significant efflux blockade with valspodar. This patient did achieve a CR following one cycle of valspodar-ME.
TABLE IV.
P-glycoprotein (MDR1) surface expression and function in patient leukemic blasts
| Patient ID |
Diagnosis | Response |
MDR1 P-glycoprotein expression |
Rhodamine Efflux (log shift) | |||
|---|---|---|---|---|---|---|---|
| D-value | Interpretation^ | CsA | Valspodar | Interpretation# | |||
| #2 | AML | PD | 0.04 | Negative | 0.3 | 0.1 | No inhibition |
| #3 | T-cell ALL | CR | 0.08 | Negative | 0 | 0 | No inhibition |
| #4 | AML | PD | 0.12 | Negative | 0.7 | 0.3 | Inhibition with CsA |
| #5 | t-AML | PD | 0.47 | Entire population with expression |
1 | 0.1 | Inhibition with CsA |
| #6 | Biphenotypic leukemia |
PD | 0.35 | Subpopulation with expression |
0.1 | 0 | No inhibition |
| #7 | Pre-B ALL | PD | 0.65 | Entire population with expression |
0.7 | 0.4 | Inhibition with CsA |
| #9 | Pre-B ALL | CR | 0.26 | Entire population with expression |
0.5 | 0.9 | Inhibition with valspodar >CsA |
| #10 | AML | PD | 0.32 | Subpopulation with expression |
0 | 0 | No inhibition |
| #11 | AML | PD | 0.03 | Negative | 0.2 | 0.1 | No inhibition |
| #17 | AML | PD | 0.02 | Negative | 0 | ND | No inhibition |
| #18 | AML | PD | 0.09 | Negative | 0.2 | ND | No inhibition |
| #19 | Pre-B ALL | PD | 0.13 | Negative | 0.2 | ND | No inhibition |
| #20 | Pre-B ALL | PD | 0.14 | Negative | 0.4 | ND | No inhibition |
| #22 | t-AML | PR | 0.05 | Negative | 0.3 | ND | No inhibition |
P-glycoprotein expression interpretation based on combination of D-value (≥0.15 considered positive) as well as visual inspection of flow cytometry data to determine if expression present on entire blast population or a subset of blasts
Rhodamine efflux interpretation based on quantification of shift in peak of fluorescence intensity curve on log scale. Shift of ≥ 0.5 log considered evidence of inhibition of rhodamine efflux from blasts.
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; t-AML, therapy-related AML; CR, complete response; PD, progressive disease; PR, partial response; CsA, cyclosporine A; ND, not done
DISCUSSION
We found that the second-generation P-glycoprotein modulator valspodar can be administered in combination with 50% dose-reduced mitoxantrone and etoposide in children with relapsed leukemia with moderate toxicity. Adequate valspodar concentrations for P-glycoprotein inhibition (>1,000 ng/mL) were achieved in all patients. The MTD of valspodar for this regimen is 12.5 mg/kg/day continuous intravenous infusion, with stomatitis as the most common DLT. Cerebellar symptoms such as dizziness and ataxia were generally mild and reversible and were not associated with administered dose, but rather with higher serum valspodar concentrations. Retrospective chart review identified additional toxicities not reported during the study, including grade 3 hypertension, grade 3 and 4 hypokalemia, and grade 3 hyperglycemia. The relationship of these toxicities to valspodar is difficult to ascertain due to concurrent infection, pain, and treatment with multiple supportive care medications and parenteral nutrition. All of these toxicities were controlled or corrected with medications or with electrolyte supplementation.
Pharmacokinetic studies of mitoxantrone and etoposide demonstrated that the 50% dose reduction was necessary because clearance of both agents was reduced by approximately 60% in the presence of valspodar. This may be due to inhibition of both the cytochrome P450 system (3A4) and biliary excretion. The utility of second-generation P-glycoprotein inhibitors, including valspodar, is limited by these drug interactions. Third-generation inhibitors [tariquidar (XR-9576, Xenova) and zosuquidar (LY-335979, Kanisa Pharmaceuticals)] have high P-glycoprotein affinity and unlike valspodar, do not affect cytochrome P450 (3A4) at clinically relevant concentrations. Clinical trials of these drugs in adults have demonstrated only modest alterations in the pharmacokinetics of coadministered chemotherapy drugs [25-27]. However, dosing, efficacy, and pharmacokinetic interactions between chemotherapeutic drugs and these MDR1 modulators in children remain to be determined.
Response with this regimen appears unrelated to valspodar. The patient with T-ALL had a short-lived CR and the highest measured valspodar concentrations. However, her blasts had no detectable P-glycoprotein expression or inhibition of rhodamine efflux (Table IV, patient #3), suggesting that her response was unrelated to valspodar inhibition of P-glycoprotein. Each of three pre-B ALL patients with CR (2) or hypocellular marrow without blasts at the time of death from sepsis (1) had previously achieved CR twice and each time had relapsed after being off therapy at least one year. In contrast, the five patients with pre-B ALL who failed to achieve remission with valspodar-ME therapy either were refractory to all prior therapies (2) or had relapsed while on therapy (3). Therefore, the responders likely had more chemotherapy-sensitive disease and response was unrelated to P-glycoprotein inhibition.
Of the 14 patients with cells available for P-glycoprotein expression and efflux analysis, five had P-glycoprotein expression on at least a subset of blasts. Of these five, only one had evidence of blockade of rhodamine efflux with valspodar. This patient did have a CR with this regimen. Interestingly, three samples had evidence of rhodamine efflux blockade with CsA but not with valspodar, raising the possibility that drug efflux in these samples is occurring via alternative transporters, which are blocked by CsA but not valspodar. Preclinical data has demonstrated that CsA modulates the MRP-1, BCRP, and LRP transporters in addition to MDR1 P-glycoprotein, while valspodar modulation is limited to MDR1 [28]. As different transporters may be clinically relevant in different patients with leukemia, a more broad-spectrum inhibitor may be more effective.
Despite the theoretical advantages of multiple-transporter inhibition with CsA, the results of POG 9421, in which P-glycoprotein modulation with CsA during consolidation chemotherapy did not improve outcomes in children with de novo AML, suggest that there is little future role for P-glycoprotein inhibitors in de novo pediatric leukemia [29,30]. The results of this study suggest that P-glycoprotein inhibition may only be clinically effective in patients with relapsed disease and high P-glycoprotein expression in combination with specific therapeutic agents, such as gemtuzumab ozogamicin (Mylotarg, Wyeth Pharmaceuticals), that are targeted to the leukemic cell and substrates of P-glycoprotein-mediated drug efflux [31]. In adults, P-glycoprotein drug efflux activity predicts both in vitro resistance and clinical response to gemtuzumab ozogamicin monotherapy even when adjusting for CD33 expression [32-34]. In a phase I study of gemtuzumab ozogamicin monotherapy in relapsed and refractory pediatric AML patients, the mean MDR1-mediated drug efflux was significantly lower in the 30% of patients who achieved CR compared with nonresponders [35].
Modulation of drug resistance in pediatric leukemia requires a multifaceted approach, as demonstrated by this cohort of children with multiply relapsed or refractory leukemia. Only a subset of patients had detectable P-glycoprotein expression on their blasts, and in some cases, expression was heterogeneous, indicating that mechanisms other than P-glycoprotein-mediated drug efflux significantly contribute to clinical drug resistance. For children with high-risk or relapsed leukemias, improved understanding of the biology of resistance mechanisms and novel targeted strategies will be needed to impact clinical outcomes.
ACKNOWLEDGEMENTS
This study was supported in part by NIH grants: R01 52168 (B.I.S.), M01 RR 00070 (General Clinical Research Center, Stanford University School of Medicine; N.J.L. - Clinical Associate Physician Award), U10 CA98543 (COG Chair’s Grant) and U10 CA98413 (COG Statistics and Data Center Grant). A complete listing of grant support for research conducted by the Children’s Cancer Group and Pediatric Oncology Group before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.” The work was in part supported by an endowment to Robert J. Arceci for the King Fahd Chair in Pediatric Oncology.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pui CH, Schrappe M, Ribeiro R, Niemeyer CM. Childhood and adolescent lymphoid and myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2004:118–145. doi: 10.1182/asheducation-2004.1.118. [DOI] [PubMed] [Google Scholar]
- 2.Chessells JM, Veys P, Kempski H, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukemia. Br J Haematol. 2003;123:396–405. doi: 10.1046/j.1365-2141.2003.04584.x. [DOI] [PubMed] [Google Scholar]
- 3.Aladjidi N, Auvrignon A, Leblanc T, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. J Clin Oncol. 2003;21:4377–4385. doi: 10.1200/JCO.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Gill DR, Hyde SC, Higgins CF, et al. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992;71:23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- 5.Arceci RJ. Clinical significance of P-glycoprotein in multidrug resistance malignancies. Blood. 1993;81:2215–2222. [PubMed] [Google Scholar]
- 6.Sikic BI. Pharmacologic approaches to reversing multidrug resistance. Semin Hematol. 1997;34:40–47. [PubMed] [Google Scholar]
- 7.Jonsson B, Nilsson K, Nygren P, Larsson R. SDZ PSC-833--a novel potent in vitro chemosensitizer in multiple myeloma. Anticancer Drugs. 1992;3:641–646. doi: 10.1097/00001813-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Visani G, Milligan D, Leoni F, et al. Combined action of PSC 833 (Valspodar), a novel MDR reversing agent, with mitoxantrone, etoposide and cytarabine in poor-prognosis acute myeloid leukemia. Leukemia. 2001;15:764–771. doi: 10.1038/sj.leu.2402117. [DOI] [PubMed] [Google Scholar]
- 10.Dahl GV, Brophy N, Grier H, et al. Mitoxantrone, etoposide and Cyclosporine A therapy for relapsed and refractory acute myelogenous leukemia, a Pediatric Oncology Group (POG) phase II trial. Ann Hematol. 1994;68(Suppl 1):A32. [Google Scholar]
- 11.Dhooge C, De Moerloose B, Laureys G, et al. Expression of the multidrug transporter P-glycoprotein is highly correlated with clinical outcome in childhood acute lymphoblastic leukemia: results of a long-term prospective study. Leuk Lymphoma. 2002;43:309–314. doi: 10.1080/10428190290006080. [DOI] [PubMed] [Google Scholar]
- 12.Ivy SP, Randal S, Olshefski S, et al. Correlation of P-glycoprotein expression and function in childhood acute leukemia: A Children’s Oncology Group Study. Blood. 1996;88:309–318. [PubMed] [Google Scholar]
- 13.Lacayo NJ, Lum BL, Becton DL, et al. Pharmacokinetic interactions of cyclosporine with etoposide and mitoxantrone in children with acute myeloid leukemia. Leukemia. 2002;16:920–927. doi: 10.1038/sj.leu.2402455. [DOI] [PubMed] [Google Scholar]
- 14.Chico I, Kang MH, Bergan R, et al. Phase I study of infusional paclitaxel in combination with the P glycoprotein antagonist PSC 833. J Clin Oncol. 2001;19:832–842. doi: 10.1200/JCO.2001.19.3.832. [DOI] [PubMed] [Google Scholar]
- 15.Lum BL, Kaubisch S, Yahanda AM, et al. Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporine in a phase I trial to modulate multidrug resistance. J Clin Oncol. 1992;10:1635–1642. doi: 10.1200/JCO.1992.10.10.1635. [DOI] [PubMed] [Google Scholar]
- 16.Miller AA, Stewart CF, Tolley EA. Clinical pharmacodynamics of continuous infusion etoposide. Cancer Chemother Pharmacol. 1990;25:361–366. doi: 10.1007/BF00686238. [DOI] [PubMed] [Google Scholar]
- 17.Davidian M, Giltinan DM. Nonlinear Models for Repeated Measurement Data. Chapman and Hall; New York: 1995. [Google Scholar]
- 18.Boeckmann AJ, Beal SL, Sheiner LB. NONMEM V Users Guides. Technical Report of the Division of Clinical Pharmacology, University of California at San Francisco. 1999.
- 19.Insightful Corporation S-Plus. http://www.insightful.com/
- 20.Broxterman HJ, Sonneveld P, Feller N, et al. Quality control of multidrug resistance assays in adult acute leukemia: correlation between assays for P-glycoprotein expression and activity. Blood. 1996;87:4809–4816. [PubMed] [Google Scholar]
- 21.Beck WT, Grogan TM, Willman CL, et al. Methods to detect P-glycoprotein–associated multidrug resistance in patients’ tumors: consensus recommendations. Cancer Res. 1996;56:3010–3020. [PubMed] [Google Scholar]
- 22.Young IT. Proof without prejudice: use of the Kolmogorov-Smirnov test for the analysis of histograms from flow systems and other sources. J Histochem Cytochem. 1977;25:935–941. doi: 10.1177/25.7.894009. [DOI] [PubMed] [Google Scholar]
- 23.Kessel D, Beck WT, Kukuruga D, Schulz V. Characterization of multidrug resistance by fluorescent dyes. Cancer Res. 1991;51:4665–4670. [PubMed] [Google Scholar]
- 24.Sonneveld P, Lowemberg B, Vossebeld P, et al. Dose-finding study of valspodar (PSC 833) with daunorubicin and cytarabine to reverse multidrug resistance in elderly patients with previously untreated acute myeloid leukemia. Hematol J. 2000;1:411–21. doi: 10.1038/sj.thj.6200050. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859–1876. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 26.Morschhauser F, Zinzani PL, Burgess M, et al. Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride ( LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2007;48:708–715. doi: 10.1080/10428190701190169. [DOI] [PubMed] [Google Scholar]
- 27.Sandler A, Gordon M, De Alwis DP, et al. A phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride ( LY335979), administered intravenously in combination with doxorubicin in patients with advanced malignancy. Clin Cancer Res. 2004;10:3265–3272. doi: 10.1158/1078-0432.CCR-03-0644. [DOI] [PubMed] [Google Scholar]
- 28.Qadir M, O’Loughlin KL, Fricke SM, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–2326. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 29.Becton D, Dahl GV, Ravindranath Y, et al. Randomized use of cyclosporine (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood. 2006;107:1315–1324. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwaan CM, den Boer ML, Karin M, et al. Does modulation of P-glycoprotein have clinical relevance in pediatric acute myeloid leukemia? Blood. 2007;107:4975–4976. doi: 10.1182/blood-2006-01-0309. [DOI] [PubMed] [Google Scholar]
- 31.Linenberger ML. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19:176–182. doi: 10.1038/sj.leu.2403598. [DOI] [PubMed] [Google Scholar]
- 32.Linenberger ML, Hong T, Flowers D, et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood. 2001;98:988–994. doi: 10.1182/blood.v98.4.988. [DOI] [PubMed] [Google Scholar]
- 33.Naito K, Takeshita A, Shigeno K, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab ozogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000;14:1436–1443. doi: 10.1038/sj.leu.2401851. [DOI] [PubMed] [Google Scholar]
- 34.Walter RB, Gooley TA, Van der Velden VHJ, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109:4168–4170. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arceci RJ, Sande J, Lange B, et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood. 2005;106:1183–1188. doi: 10.1182/blood-2004-10-3821. [DOI] [PubMed] [Google Scholar]