Abstract
Accumulation of LDL-derived cholesterol by artery wall macrophages triggers atherosclerosis, the leading cause of cardiovascular disease. Conversely, HDL retards atherosclerosis by promoting cholesterol efflux from macrophages by the membrane-associated ATP-binding cassette transporter A1 (ABCA1) pathway. HDL has been proposed to lose its cardioprotective effects in subjects with atherosclerosis, but the underlying mechanisms are poorly understood. One potential pathway involves oxidative damage by myeloperoxidase (MPO), a heme enzyme secreted by human artery wall macrophages. We used mass spectrometry to demonstrate that HDL isolated from patients with established cardiovascular disease contains elevated levels of 3-chlorotyrosine and 3-nitrotyrosine, two characteristic products of MPO. When apolipoprotein A-I (apoA-I), the major HDL protein, was oxidized by MPO, its ability to promote cellular cholesterol efflux by ABCA1 was impaired. Moreover, oxidized apoA-I was unable to activate lecithin:cholesterol acyltransferase (LCAT), which rapidly converts free cholesterol to cholesteryl ester, a critical step in HDL maturation. Biochemical studies implicated tyrosine chlorination and methionine oxygenation in the loss of ABCA1 and LCAT activity by oxidized apoA-I. Oxidation of specific residues in apoA-I inhibited two key steps in cholesterol efflux from macrophages, raising the possibility that MPO initiates a pathway for generating dysfunctional HDL in humans.
Keywords: reverse cholesterol transport, inflammation, hypochlorous acid, peroxynitrite, reactive nitrogen species, foam cell
Atherosclerosis is the leading cause of death in industrialized societies. One important risk factor is an elevated level of low-density lipoprotein (LDL), the major cholesterol carrier in humans (1). Unregulated delivery of cholesterol by LDL converts macrophages into cholesteryl ester-laden foam cells, the pathological hallmark of the atherosclerotic lesion (2).
1. Myeloperoxidase is a source of oxidative stress in the human artery wall
High levels of LDL alone may not be sufficient to trigger atherosclerosis, however; oxidative stress is implicated in the pathogenesis of inflammatory diseases (3–5). Thus, in vitro and in vivo studies suggest that LDL must be modified, particularly by oxidative reactions, to promote the cholesterol uptake by macrophage scavenger receptors that trigger atherogenesis (4).
Macrophages use reactive oxygen species to kill invading pathogens (6). One oxidative pathway involves myeloperoxidase (MPO), a secreted heme protein expressed at high levels in human atherosclerotic lesions, where it co-localizes in part with macrophages (7). The enzyme uses hydrogen peroxide (H2O2) to execute oxidative reactions in the phagolysosome and extracellular milieu. The major end product at plasma concentrations of chloride ion is generally thought to be hypochlorous acid (HOCl), a potent anti-microbial agent.
Hypochlorous acid generated by MPO also has the potential to damage host tissue. For example, HOCl produced by the enzyme converts free and protein-bound tyrosine residues to 3-chlorotyrosine (8, 9). Studies of mice deficient in MPO demonstrated that the enzyme normally generates 3-chlorotyrosine in a model of acute inflammation and that this abnormal amino acid is a specific marker for MPO activity (10). Mass spectrometric (MS) analyses have detected high levels of 3-chlorotyrosine in LDL isolated from human atherosclerotic tissue (11), strongly suggesting that MPO is one pathway for oxidative damage in the human artery wall.
Another pathway for oxidizing artery wall proteins involves nitric oxide (NO), which is generated by vascular wall cells (12, 13). NO reacts rapidly with superoxide (O2•−) to form peroxynitrite (ONOO−), a reactive nitrogen species (14). Macrophages are a rich source of both O2•− and NO, suggesting that ONOO−may be an important source of reactive nitrogen species in vivo. Furthermore, oxidation of NO produces nitrite (NO2−), which MPO and H2O2 convert to nitrogen dioxide radical (NO2•), a potent nitrating intermediate (15, 16).
Both ONOO− and NO2• generate 3-nitrotyrosine when they react with tyrosine residues. Such reactive nitrogen species might promote inflammation by nitrating lipoproteins and other artery wall proteins. MS analyses have detected elevated levels of 3-nitrotyrosine in LDL isolated from human atherosclerotic tissue (17), and tyrosine nitration is impaired when mice are deficient in MPO (10). Thus, the MPO pathway might both nitrate and chlorinate lipoproteins in vivo.
2. Dysfunctional HDL may promote atherogenesis
In contrast to LDL, high-density lipoprotein (HDL)—the good form of cholesterol—is normally cardioprotective. It protects the artery wall from atherosclerosis in part by its ability to remove cholesterol from macrophage foam cells (18, 19). Two distinct pathways are involved. One involves a membrane-associated protein termed the ATP-binding cassette transporter A1 (ABCA1) (20). Lipid-free or lipid-poor apolipoprotein A-I (apoA-I), the major HDL protein (21), promotes sterol efflux by ABCA1. The second pathway involves another ABC transporter, ABCG1, and is mediated by the holo-HDL particle (22). Lecithin:cholesterol acyltransferase (LCAT) plays a key role in both pathways by rapidly converting free cholesterol to cholesteryl ester, which is sufficiently hydrophobic to be sequestered in the core of HDL particles (23, 24).
Proteins carried by HDL have been proposed to exert anti-inflammatory actions that also contribute to the lipoprotein’s ability to retard or reverse atherosclerosis (25). However, shotgun proteomics has suggested that, in coronary artery disease, HDL carries a unique cargo of proteins that might make previously unsuspected contributions to its pro- and anti-inflammatory properties (26, 27). Recent studies have suggested that HDL can become dysfunctional and lose its cardioprotective effects (25, 28). The underlying mechanisms are poorly understood, but may involve changes in protein composition and/or loss of the ability to deplete macrophages of cholesterol. One important pathway could involve oxidative damage to HDL (29–31). Oxidative and compositional changes could affect the ability of HDL particles to remove cellular cholesterol by the ABCG1 pathway or to regenerate lipid-free apolipoproteins that interact with ABCA1.
3. MPO oxidizes HDL in humans with cardiovascular disease
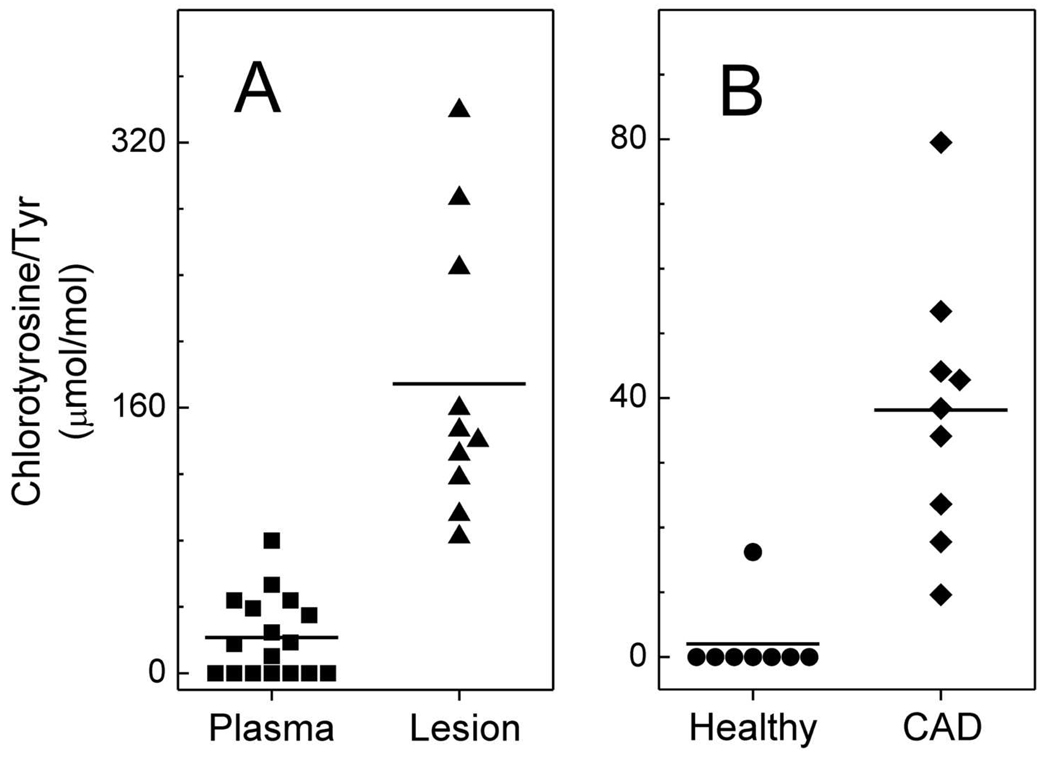
Remarkably little is known about the potential involvement of HDL oxidation in macrophage foam cell formation. To explore this issue, we first used isotope dilution MS to determine whether MPO oxidizes HDL in the human artery wall. We found that levels of both 3-chlorotyrosine and 3-nitrotyrosine were higher in HDL isolated from atherosclerotic lesions than in plasma HDL (Fig. 1A, ref. (29, 30)), and similar results were reported by other investigators (31). We also detected MPO protein in HDL isolated from atherosclerotic lesions (29). Moreover, we used antibodies to 3-nitrotyrosine and to proteins modified by HOCl to demonstrate that chlorinated and nitrated adducts co-localize with macrophages in human atherosclerotic lesions (29, 30). Because MPO is the only known source of chlorinating intermediates in humans and is a potent source of reactive nitrogen species in a mouse model of acute inflammation (10, 16), our observations strongly support the hypothesis that this enzyme oxidizes HDL in human atherosclerotic lesions.
Figure 1. HDL isolated from human atherosclerotic lesions and humans with established cardiovascular disease contained elevated levels of 3-chlorotyrosine.
Human atherosclerotic tissue was obtained at surgery from subjects undergoing carotid endarterectomy. Plasma was obtained from healthy humans and humans with established coronary artery disease. HDL was isolated by sequential ultracentrifugation. Oxidized amino acids isolated from hydrolyzed HDL proteins were quantified by isotope dilution GC/MS with selected ion monitoring. Reproduced with permission from (29).
We next investigated the possibility that oxidized HDL in plasma might serve as a marker for coronary artery disease (13). After isolating HDL from plasma of subjects with established CAD and control subjects, we quantified levels of protein-bound 3-chlorotyrosine and 3-nitrotyrosine. The levels were markedly higher in circulating HDL isolated from the CAD patients than in HDL isolated from the controls (Fig. 1B, ref. (29, 30)). In striking contrast, both chlorotyrosine and nitrotyrosine were undetectable in LDL or total plasma proteins of both control and CAD subjects. These observations suggest that circulating HDL in subjects with established CAD, but not in control subjects, is selectively targeted for oxidation by MPO. Because we failed to detect MPO oxidation products in LDL or total plasma proteins, it is unlikely that HDL was oxidized in the circulation. One possibility is that it was damaged in a microenvironment rich in MPO and depleted of antioxidants before entering the circulation. One likely location for such a reaction is the inflamed atherosclerotic lesion.
4.1 ApoA-I damaged by MPO loses its ability to promote cholesterol transport by ABCA1
Lipid-free apoA-I interacts with membrane-associated ABCA1 to remove excess cholesterol from artery wall macrophages (20, 22, 32). Because MPO is secreted into the pericellular environment and because the membrane-associated NADPH oxidase of macrophages is an important source of H2O2 (which the enzyme needs for its oxidative reactions), we determined whether MPO-dependent oxidation prevented apoA-I from promoting cholesterol efflux from cells (29). H2O2 alone had no effect on apoA-I’s ability to remove cellular cholesterol. In striking contrast, chlorination of apoA-I by the complete MPO-H2O2 chlorinating system or HOCl alone impaired the protein’s ability to promote cholesterol efflux (29, 33).
Thus, oxidation of apoA-I by HOCl or MPO impairs the protein’s ability to remove cholesterol from cells by the ABCA1 pathway. Together with our detection of oxidized HDL in humans, these data suggest that HOCl generated by MPO could be a mechanism for generating dysfunctional HDL in the human artery wall.
4.2 MPO impairs ABCA1-dependent cholesterol efflux by damaging specific residues in apoA-I
Tandem mass spectrometric analysis (MS/MS) can unequivocally determine the nature and locations of posttranslational modifications to proteins (34). We developed liquid chromatography–tandem mass spectrometry (LC-MS/MS) approaches for identifying and quantifying oxidized residues in proteins, using the ion current of each precursor and product peptide in the same sample to determine yields (35). This method does not require proteins or peptides to be labeled with an isotope. It is also well-suited to quantifying multiple site-specific modifications that occur when MPO oxidizes proteins (35).
Amino acid analysis has shown that tyrosine, methionine, and phenylalanine residues are the major targets when HOCl oxidizes apoA-I in vitro (36–38). However, those studies did not determine whether residues at specific locations are especially vulnerable to chlorination. Using LC-ESI-MS/MS, we identified a single tyrosine residue, Tyr192, as the major chlorination site when HOCl oxidizes apoA-I (39). Moreover, we noted a strong linear association between the extent of Tyr192 chlorination and loss of ABCA1 transport activity (33). Thus, Tyr oxidation might be important for impairing cholesterol transport.
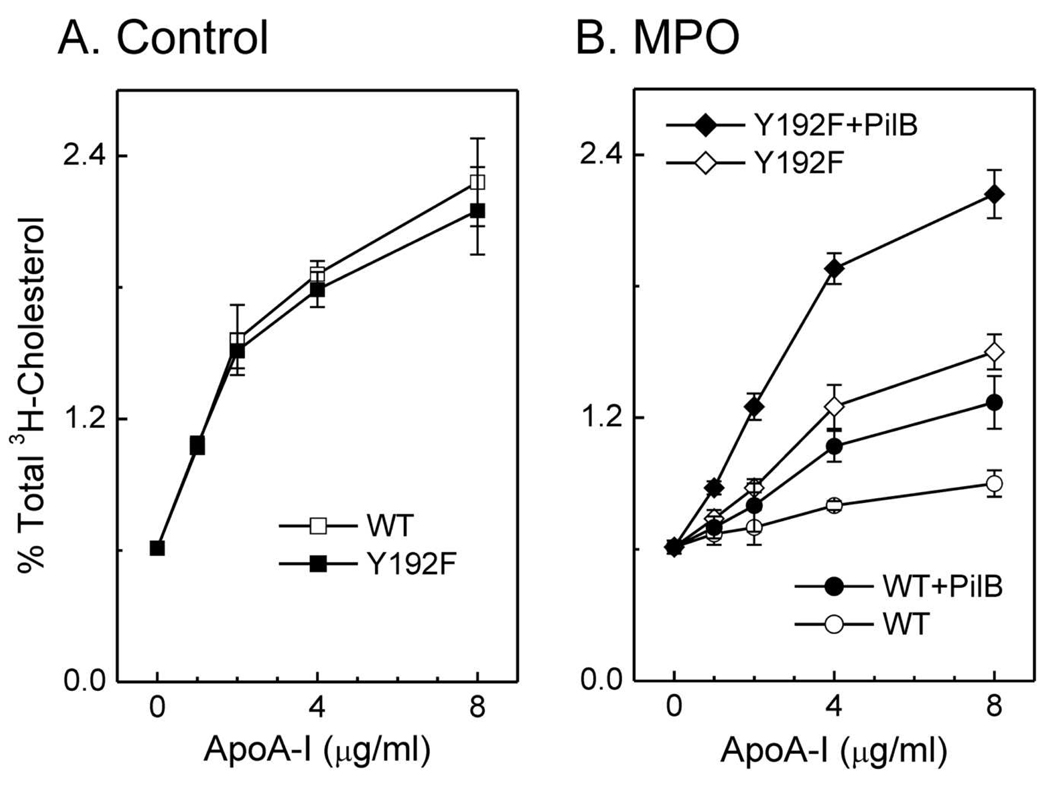
Other investigators replaced all seven tyrosine residues in apoA-I with phenylalanine, which is resistant to chlorination. As with wild-type apoA-I, this mutant protein was unable to promote ABCA1-dependent cholesterol efflux after being oxidized by MPO (40). To investigate the apparent discrepancy between these results and ours, we replaced only Tyr192 (Tyr192Phe) in apoA-I, and monitored that mutant protein’s ABCA1 activity. The concentration dependence for ABCA1-dependent cholesterol efflux of the mutant and wild-type proteins were virtually identical (Fig. 2A), strongly suggesting that the Tyr192Phe mutation had no major impact on the structure of lipid-free apoA-I. When this mutant protein was exposed to either HOCl or the MPO chlorinating system, the substitution offered a small but significant protection against inactivation (41). The differences between the proteins may reflect our use of transfected cells that express high levels of ABCA1, which may be more sensitive to subtle changes in apoA-I’s activity.
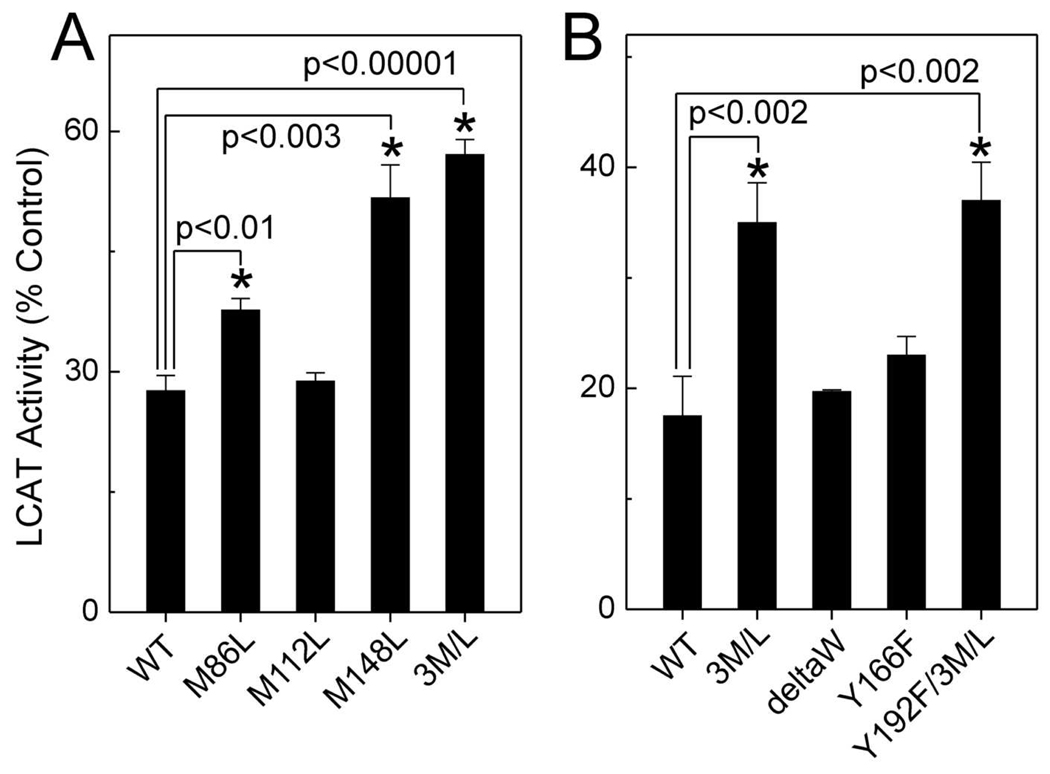
Figure 2. Chlorination of Tyr192 and Met oxidation are necessary for depriving apoA-I of its cholesterol efflux activity.
(A) [3H]cholesterol efflux was measured in ABCA1-transfected BHK cells incubated with the indicated concentrations of wild-type (WT) or Tyr192Phe mutant apoA-I. (B) WT or Tyr192Phe mutant apoA-I was oxidized by the MPO-H2O2-chloride system (25:1, mol/mol, H2O2/apoA-I). Where indicated, apoA-I was incubated with the methionine sulfoxide reductase PilB. Reproduced with permission from (41).
The alkylated thiol of methionine is much more reactive with HOCl than Tyr residues (42). Moreover, methionine sulfoxide [Met(O)] has been detected in circulating HDL (43). However, the role of methionine oxidation in apoA-I’s cholesterol efflux activity was unclear. Using tandem mass spectrometry, we first confirmed that HOCl or the complete MPO system quantitatively oxidizes all three methionine residues in apoA-I to Met(O) (41). Following methionine oxidation, Tyr192 of apoA-I was chlorinated in parallel with loss of ABCA1 activity. To determine whether Met(O) contributed to that loss, we used a bacterial methionine sulfoxide reductase that converts both the R- and S-forms of Met(O) residues back to methionine (44). Methionine sulfoxide reductase completely reversed methionine oxidation in apoA-I that had been exposed to MPO (41). However, the protein’s cholesterol efflux activity was only partly restored (Fig. 2B), implying that oxidation of Met residues alone is insufficient to eliminate that apoA-I function.
We next determined if Tyr chlorination together with Met oxidation contributes to the loss of ABCA1 activity when apoA-I is exposed to MPO. When Tyr192Phe apoA-I was exposed to HOCl or the MPO system, it lost most of its ability to promote cholesterol efflux by the ABCA1 pathway (Fig. 2B). Remarkably, subsequent treatment with methionine sulfoxide reductase almost completely restored this ability (Fig. 2B, ref. (41)). These observations indicate that neither Tyr192 chlorination (41) nor methionine oxidation (43) alone deprives apoA-I of its cholesterol efflux activity. However, a combination of the two—perhaps together with other structural changes—almost completely destroys that activity (41).
HOCl can also oxidize tryptophan residues in apoA-I (35, 38). However, oxidation failed to prevent a mutant apoA-I, in which all four Trp residues were replaced with Phe, from activating ABCA1 (45). Analysis by circular dichroism suggested that the α-helical content of lipid-free control protein was 56% while that of the mutant protein was 71% (45), which matches that (~70%) of lipid-associated native apoA-I ((36); Shao and Heinecke, unpublished). These observations indicate that the tryptophan substitutions significantly alter the tertiary structure of lipid-free apoA-I. Thus, the mutant protein’s oxidation resistance might result from structural alterations.
4.3 The YXXK motif directs protein chlorination by MPO
Most studies of protein oxidation have focused on the vulnerability of individual amino acid side chains. Remarkably little is known about the influence of nearby residues or specific motifs. As noted above, we showed that a single tyrosine residue, Tyr192, is the major target for chlorination by HOCl or the MPO system (29, 33, 39). However, the mechanism by which MPO oxidizes Tyr192 is controversial (31).
Tyr192 is located two residues away from Lys195 in the primary sequence of apoA-I. Therefore, it would be adjacent to the lysine residue if that region of the protein were α-helical (39). HOCl reacts rapidly with the ε-amino group of lysine to form long-lived chloramines, which have been proposed to promote tyrosine chlorination (8). To test that proposal, we used synthetic peptides to demonstrate that lysine residues can direct the chlorination of nearby tyrosine residues by a pathway involving chloramine formation (39). Based on these observations, we proposed that the YXXK motif can direct the regiospecific chlorination of tyrosine in α-helical proteins.
Using hydrogen-deuterium exchange, Zheng et al. showed that MPO interacts with the region of apoA-I containing Tyr192 (31). Based on these results, those investigators proposed an alternative model in which MPO promotes site-specific chlorination only if it binds directly to the region of apoA-I that contains Tyr192.
To distinguish between these two models, we used site-directed mutagenesis to engineer a series of mutations in the cDNA of human apoA-I (41). Studies with those mutations provided strong evidence that YXXK can direct the regiospecific chlorination of tyrosine residues by reagent HOCl, a system that clearly cannot involve direct interaction of MPO with apoA-I. For example, chlorination of Tyr192 was blocked when Lys195 was mutated to arginine. Also, tyrosine residues that normally resist chlorination were chlorinated in high yield when we introduced the KXXY motif into that region of the protein. Virtually identical results were observed with the complete MPO chlorinating system (41). These observations strongly support our proposed role for the YXXK motif, and argue against the hypothesis that MPO must interact directly with apoA-I to selectively chlorinate Tyr192.
4.4 Biophysical studies suggest why oxidizing apoA-I with MPO might impair cholesterol efflux activity
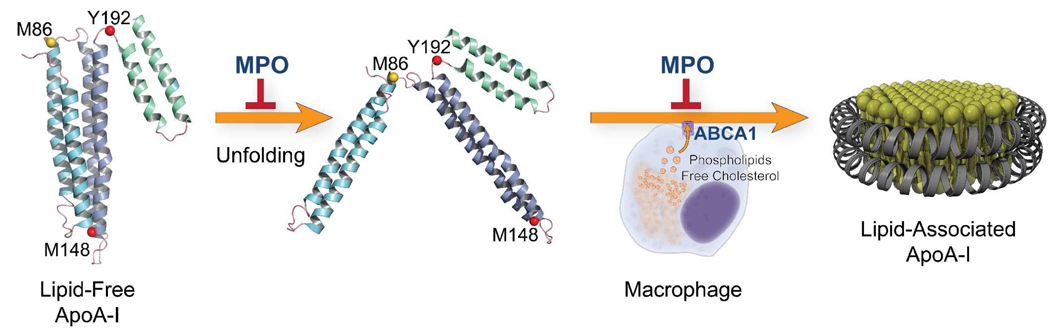
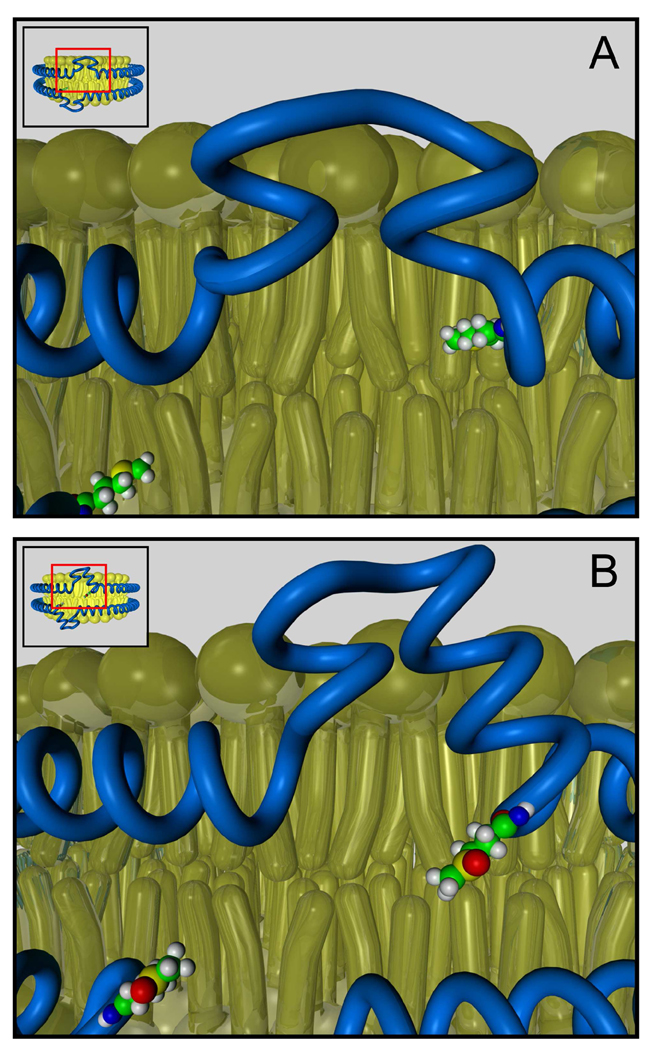
Biophysical, chemical, and biochemical studies strongly suggest that a major structural component of lipid-free apoA-I is a four-helix bundle with hairpin loops between the helices (21, 46–48). ApoA-I is a metastable molecule that transitions from the compact four-helix lipid-free conformation to an extended ellipitical “belt” conformation upon lipid association (21, 46–50). We have proposed a mechanism for the role of amino acid oxidation in blocking the conversion of lipid-free apoA-I into an ABCA1 and/or lipid-associated form (28). In this model, apoA-I remodels into an intermediate that subsequently converts to an open elliptical form. A key feature of the model is that the helical bundles in lipid-free apoA-I undergo a series of unfolding events centered on rearrangement of non-helical regions of the protein. Thus chemical modifications that tip the balance of apoA-I’s energetic state will affect the protein’s ability to conformationally adapt to lipid. One region that may be important in this transition is an 18-residue random-coil loop (188–205; ref. (51)) near the C-terminus that becomes alpha-helical when the protein associates with lipid. Thus, we have proposed that this random coil to alpha-helix transition energetically drives apoA-I’s conformational adaptation to the presence of lipid (51). Importantly, Tyr192 resides near the middle of the 18-residue region, suggesting that its chlorination might alter the energetics of protein remodeling to favor retention of the conformation of lipid-free apoA-I.
Two of the three methionine residues of lipid-free apoA-I are in or near proposed hairpin loops in the four-helix bundle (21, 46, 48). These loops likely serve as hinges that unfold when the protein remodels to associate with lipid. All three amino acids are readily oxidized by MPO. Therefore, our observations suggest a potential mechanism for oxidative inhibition of apoA-I’s ABCA1 activity (Fig. 3). Modification of key residues near the C-terminus disrupts the random coil to alpha-helical transition of this region of the proteins, while alterations near hinge domains disrupts remodeling of the four helical bundle, and the structurally abnormal apoA-I cannot interact productively with ABCA1.
Figure 3. Oxidation may alter the remodeling pathway of apoA-I.
The conversion of lipid-free apoA-I to a lipid-associated form has been proposed to involve remodeling around the protein’s hairpin loops (21, 46). Based on this model, we suggest that MPO could inactivate the ABCA1 activity of apoA-I by oxidizing residues in or near its loop regions, which serve as hinges when the protein unfolds and refolds.
5.1 Oxidation of a single methionine residue of apoA-I impairs LCAT activation
ApoA-I acquires both free cholesterol and phospholipid when it is lipidated by ABCA1 to form a nascent, discoidal HDL particle (21). ApoA-I is also the major physiological activator of lecithin:cholesterol acyltransferase (LCAT), which plays a critical role in HDL maturation. LCAT converts free cholesterol to cholesteryl ester, which migrates into the core of the particle, forming the mature, spherical form of HDL (23, 24).
Reactive intermediates have been proposed to inhibit apoA-I’s ability to activate LCAT (52–55). We hypothesized that this inhibition might occur if MPO modified lipid-associated apoA-I. Indeed, when we exposed HDL3, the dense subfraction of HDL, to increasing concentrations of HOCl or H2O2 in the MPO-chlorinating system, the oxidized HDL progressively lost its ability to activate LCAT (56).
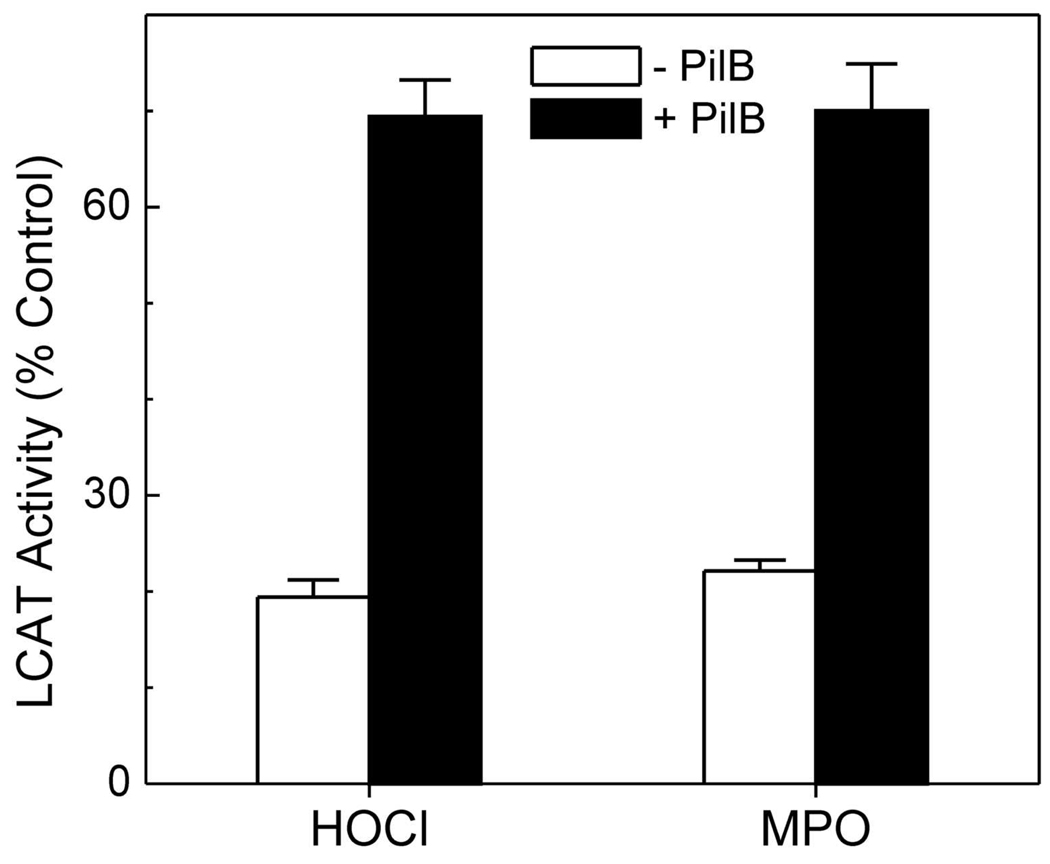
Previous studies have shown that residues 143–164 of apoA-I are crucial for LCAT activation (57), and a single Met residue (Met148) lies in that domain. Because Met residues are extremely susceptible to oxidation by HOCl, we determined whether Met oxidation promotes the loss of LCAT activity that occurs when MPO oxidizes lipid-associated apoA-I (56). Treating oxidized HDL3 with methionine reductase to convert Met(O) back to Met increased the lipoprotein’s LCAT activity from 20% to 70% (Fig. 4, ref. (56)). This observation strongly suggests that Met oxidation plays a major role in the loss of LCAT activity that is observed when apoA-I is exposed to MPO.
Figure 4. Methionine sulfoxide reductase restores the LCAT activity of oxidized HDL.
LCAT activity was determined in HDL3 exposed to a 25-fold molar ratio of the indicated oxidant or the same oxidized HDL preparation after incubation with PilB, a bacterial methionine sulfoxide reductase with activity on both epimers of Met(O). Reproduced with permission from (56).
Using 15N-labeled apoA-I as the internal standard, we developed an isotope dilution LC-MS/MS method to quantify the loss of precursor peptides after HDL was exposed to HOCl or the MPO system (56). This unbiased method revealed that all three peptides containing a Met residue were oxidized in near-quantitative yield. However, only oxidation of Met148 associated strongly with loss of LCAT activity.
We next mutated each of apoA-I’s Met residues to Leu (which resists oxidation), and then prepared reconstituted HDL (rHDL) particles with each mutant protein. When the Met148Leu mutant protein was exposed to a relatively high concentration of HOCl, it retained twice as much LCAT activity as the oxidized wild-type protein (Fig. 5A, ref. (56)). In contrast, when either of the other two Met residues was mutated to Leu, the LCAT activity of apoA-I was little protected against oxidative inactivation. When we mutated all three Met residues at the same time, the triple mutant showed about the same resistance to inactivation as the single Met148Leu mutant (Fig. 5A). Our observations provide strong evidence that oxidation of Met148 makes an important contribution to the loss of apoA-I’s LCAT activity.
Figure 5. Mutation of Met148 to Leu protects apoA-I from the oxidative loss of LCAT activity.
rHDL was prepared with wild-type (WT) or mutated apoA-I. (A) LCAT activity of Met-mutated rHDLs exposed to a 30-fold molar ratio of HOCl. (B) LCAT activity of Met-, Trp-, and Tyr-mutated rHDLs exposed to a 40-fold molar ratio of HOCl. DeltaW (ΔW), deletion all four Trp residues of apoA-I. 3M/L, Met86Leu/Met112Leu/Met148Leu mutant apoA-I. Reproduced with permission from (56).
5.2 Tyrosine chlorination in apoA-I does not contribute to LCAT inactivation
Tyr166 of apoA-I has been proposed to contribute to LCAT activation (58), because rHDL prepared with a Tyr166Phe mutant lost 70% of its ability to activate LCAT. Moreover, these investigators detected chlorination of Tyr166 when apoA-I was exposed to MPO, leading to the suggestion that this accounted for oxidized apoA-I’s inability to activate LCAT.
The conclusion that Tyr166 is a quantitatively important target for oxidation was apparently based on the 70% loss of LCAT activity in Tyr166Phe apoA-I (58). When we prepared the same mutant protein, however, we observed only a 20% loss (56). The discrepancy between the two results may reflect differences in the methods used to generate rHDL or in other experimental conditions. We then compared the effects of oxidation on the ability of control and Tyr-substituted apoA-I rHDL to activate LCAT. The Tyr166Phe substitution failed to protect apoA-I from inactivation by HOCl (Fig. 5B, ref. (56)), suggesting that oxidation of Tyr166 of apoA-I does not make a major contribution to the impaired LCAT activity that occurs when HDL is oxidized by MPO. Importantly, we found that <5% of Tyr166 was chlorinated, even when HDL was exposed to a high concentration of HOCl or H2O2 in the MPO system (56).Wu et al. (58) observed that only ~2% of Tyr166 was chlorinated when LCAT activity was inhibited by ~50%. Thus, chlorination of Tyr166 could not account quantitatively for the loss of LCAT activity.
5.3 Potential mechanisms by which MPO might impair LCAT activation
Based on structural studies of discoidal rHDL, we recently proposed that a loop centered on residues 133 and 146 of apoA-I is involved in LCAT activation (59). It is noteworthy that Met148 lies next to this loop. These observations suggest that oxidation of Met148 diminishes apoA-I’s ability to activate LCAT because it disrupts the loop domain (56). In this model, oxidation shifts Met148 from the protein’s hydrophobic face to its hydrophilic face, rotating the residue’s orientation to the solvent. This relocation unwinds the helix locally, perturbing the LCAT activation site (Fig. 6).
Figure 6. Model of Met148 oxidation in apoA-I in LCAT inactivation.
Oxidation of Met148 disrupts the central loop-like structure and affects the conformation of apoA-I. Modifying Met148 might disrupt the proper alignment of the hydrophobic face of repeat 6 in apoA-I, and then disrupt the maintenance and stability of the helix-bilayer and helix-helix interactions, which are key structural requirements for LCAT activation.
This hypothesis assumes that the central loop is more dynamic and hence more prone to structural perturbation than the rest of apoA-I, whose helices are stabilized by salt bridges to a second apoA-I molecule (21, 60) and by its association with phospholipids. Our observations may be of widespread importance because oxidation of Met residues in proteins could be a general mechanism for disrupting loop domains and the hydrophobic helix–lipid and helix–helix interactions that are key features of proteins.
6. MPO is a pro-inflammatory enzyme that may generate dysfunctional HDL in humans
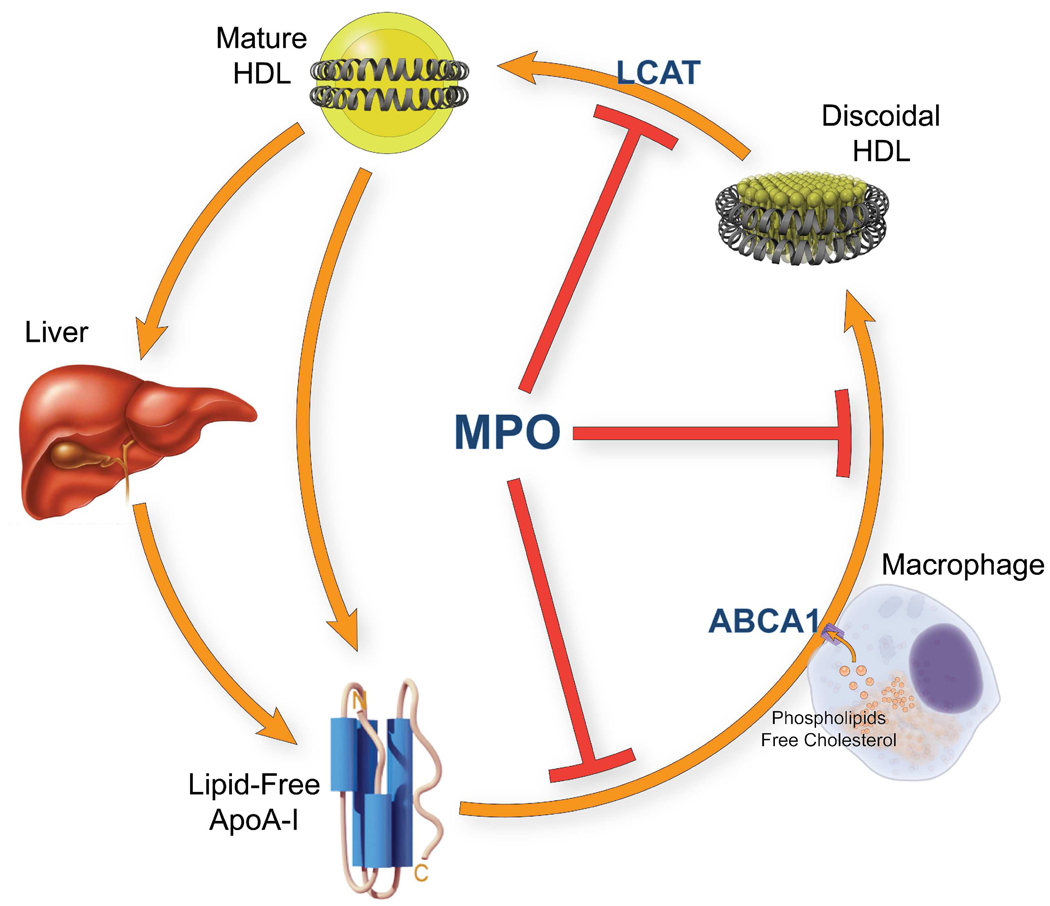
Our observations suggest the following model for generating dysfunctional HDL (Fig. 7). Activated macrophages use NADPH oxidase to produce high concentrations of H2O2 near the plasma membrane. MPO secreted by the cells then converts H2O2 to HOCl, which modifies specific Tyr (Tyr192) and Met residues in apoA-I. Oxidation of lipid-free apoA-I inhibits cholesterol efflux by the ABCA1 pathway, while damage to lipid-associated apoA-I impairs LCAT activation. Thus, MPO inhibits two key early steps in cholesterol efflux from macrophages by modifying specific Met or Tyr residues in apoA-I. This impairment of cholesterol removal could promote foam cell formation and atherogenesis.
Figure 7. Oxidation of apoA-I by MPO inhibits two steps of reverse cholesterol transport.
Oxidation of lipid-free apoA-I by MPO impairs the protein’s ability to transport cellular cholesterol by the ABCA1 pathway. Oxidation of lipid-associated apoA-I by MPO impairs its ability to activate LCAT.
HDL is anti-inflammatory and inhibits lipid oxidation in vivo (25). These properties may also contribute significantly to HDL’s ability to inhibit atherosclerosis. MPO is a key inflammatory mediator of macrophages and other leukocytes, and systemic inflammation has been proposed to convert HDL to a dysfunctional form that loses these antiatherogenic effects. Recent studies suggest that oxidation of apoA-I by MPO results in loss of HDL-mediated anti-apoptotic and anti-inflammatory activities (61). Moreover, recent proteomics studies have revealed that HDL carries proteins that might play a previously unsuspected role in its anti-inflammatory properties (26, 27). Loss of anti-inflammatory and antioxidant proteins, perhaps in concert with gain of pro-inflammatory proteins, may thus be another key component in making HDL dysfunctional.
Our observations also suggest that chlorinated and/or nitrated HDL might serve as a marker—and perhaps a mediator—of active cardiovascular disease in humans. If oxidation of HDL by MPO converts the cardioprotective lipoprotein into a dysfunctional form, the enzyme might be a suitable therapeutic target for preventing vascular disease.
ACKNOWLEDGEMENTS
Supported by grants from the National Institutes of Health (K99HL091055, P01 HL092969, P01 HL030086, R01 HL085437, R01 HL086798, R01 HL077268) and Tobacco Related Disease Research Program of California grant 16FT-0163.
The abbreviations used are
- ABCA1
ATP-binding cassette transporter A1
- apoA-I
apolipoprotein A-I
- CAD
coronary artery disease
- HDL
high-density lipoproteins
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- LCAT
lecithin:cholesterol acyltransferase
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LDL
low-density lipoprotein
- Met(O)
methionine sulfoxide
- MPO
myeloperoxidase
- NO
nitric oxide
- NO2−
nitrite
- NO2•
nitrogen dioxide radical
- O2•−
superoxide
- ONOO−
peroxynitrite
- rHDL
reconstituted HDL
REFERENCES
- 1.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 3.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 5.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 6.Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol. 2000;20:1716–1723. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 7.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domigan NM, Charlton TS, Duncan MW, Winterbourn CC, Kettle AJ. Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J Biol Chem. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 9.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species. Trends Biochem Sci. 2002;27:489–492. doi: 10.1016/s0968-0004(02)02191-6. [DOI] [PubMed] [Google Scholar]
- 13.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 14.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 15.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 16.Gaut JP, Byun J, Tran HD, Lauber WM, Carroll JA, Hotchkiss RS, Belaaouaj A, Heinecke JW. Myeloperoxidase produces nitrating oxidants in vivo. J Clin Invest. 2002;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 18.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR The Framingham Study. High density lipoprotein as a protective factor against coronary heart disease. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 19.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 20.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: A cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 21.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 22.Tall AR, Costet P, Wang N. Regulation and mechanisms of macrophage cholesterol efflux. J Clin Invest. 2002;110:899–904. doi: 10.1172/JCI16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 24.Jonas A. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim Biophys Acta. 1991;1084:205–220. doi: 10.1016/0005-2760(91)90062-m. [DOI] [PubMed] [Google Scholar]
- 25.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 26.Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, Brunzell J, Knopp RH, Zhao XQ, Heinecke JW. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 2008;118:1259–1267. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr Opin Cardiol. 2006;21:322–328. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 29.Bergt C, Pennathur S, Fu X, Byun J, O'Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O'Brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao B, Bergt C, Fu X, Green P, Voss JC, Oda MN, Oram JF, Heinecke JW. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 34.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 35.Shao B, Heinecke JW. Using tandem mass spectrometry to quantify site-specific chlorination and nitration of proteins: model system studies with high-density lipoprotein oxidized by myeloperoxidase. Methods Enzymol. 2008;440:33–63. doi: 10.1016/S0076-6879(07)00803-8. [DOI] [PubMed] [Google Scholar]
- 36.Bergt C, Oettl K, Keller W, Andreae F, Leis HJ, Malle E, Sattler W. Reagent or myeloperoxidase-generated hypochlorite affects discrete regions in lipid-free and lipid-associated human apolipoprotein A-I. Biochem J. 2000;346(Pt 2):345–354. [PMC free article] [PubMed] [Google Scholar]
- 37.Francis GA, Mendez AJ, Bierman EL, Heinecke JW. Oxidative tyrosylation of high density lipoprotein by peroxidase enhances cholesterol removal from cultured fibroblasts and macrophage foam cells. Proc Natl Acad Sci U S A. 1993;90:6631–6635. doi: 10.1073/pnas.90.14.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panzenboeck U, Raitmayer S, Reicher H, Lindner H, Glatter O, Malle E, Sattler W. Effects of reagent and enzymatically generated hypochlorite on physicochemical and metabolic properties of high density lipoproteins. J Biol Chem. 1997;272:29711–29720. doi: 10.1074/jbc.272.47.29711. [DOI] [PubMed] [Google Scholar]
- 39.Bergt C, Fu X, Huq NP, Kao J, Heinecke JW. Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-I when hypochlorous acid oxidizes high density lipoprotein. J Biol Chem. 2004;279:7856–7866. doi: 10.1074/jbc.M309046200. [DOI] [PubMed] [Google Scholar]
- 40.Peng DQ, Wu Z, Brubaker G, Zheng L, Settle M, Gross E, Kinter M, Hazen SL, Smith JD. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J Biol Chem. 2005;280:33775–33784. doi: 10.1074/jbc.M504092200. [DOI] [PubMed] [Google Scholar]
- 41.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 42.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 43.Panzenbock U, Stocker R. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim Biophys Acta. 2005;1703:171–181. doi: 10.1016/j.bbapap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Peng DQ, Brubaker G, Wu Z, Zheng L, Willard B, Kinter M, Hazen SL, Smith JD. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler Thromb Vasc Biol. 2008;28:2063–2070. doi: 10.1161/ATVBAHA.108.173815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson WS, Silva RA. Apolipoprotein structural organization in high density lipoproteins: belts, bundles, hinges and hairpins. Curr Opin Lipidol. 2005;16:295–300. doi: 10.1097/01.mol.0000169349.38321.ad. [DOI] [PubMed] [Google Scholar]
- 47.Silva RA, Hilliard GM, Fang J, Macha S, Davidson WS. A three-dimensional molecular model of lipid-free apolipoprotein A-I determined by cross-linking/mass spectrometry and sequence threading. Biochemistry. 2005;44:2759–2769. doi: 10.1021/bi047717+. [DOI] [PubMed] [Google Scholar]
- 48.Thomas MJ, Bhat S, Sorci-Thomas MG. Three-dimensional models of HDL apoA-I: implications for its assembly and function. J Lipid Res. 2008;49:1875–1883. doi: 10.1194/jlr.R800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borhani DW, Rogers DP, Engler JA, Brouillette CG. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc Natl Acad Sci U S A. 1997;94:12291–12296. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- 51.Oda MN, Forte TM, Ryan RO, Voss JC. The C-terminal domain of apolipoprotein A-I contains a lipid-sensitive conformational trigger. Nat Struct Biol. 2003;10:455–460. doi: 10.1038/nsb931. [DOI] [PubMed] [Google Scholar]
- 52.Anantharamaiah GM, Hughes TA, Iqbal M, Gawish A, Neame PJ, Medley MF, Segrest JP. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988;29:309–318. [PubMed] [Google Scholar]
- 53.Bielicki JK, Forte TM. Evidence that lipid hydroperoxides inhibit plasma lecithin:cholesterol acyltransferase activity. J Lipid Res. 1999;40:948–954. [PubMed] [Google Scholar]
- 54.McCall MR, Carr AC, Forte TM, Frei B. Ldl modified by hypochlorous acid is a potent inhibitor of lecithin-cholesterol acyltransferase activity. Arterioscler Thromb Vasc Biol. 2001;21:1040–1045. doi: 10.1161/01.atv.21.6.1040. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Subbaiah PV. Importance of the free sulfhydryl groups of lecithin-cholesterol acyltransferase for its sensitivity to oxidative inactivation. Biochim Biophys Acta. 2000;1488:268–277. doi: 10.1016/s1388-1981(00)00130-x. [DOI] [PubMed] [Google Scholar]
- 56.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci U S A. 2008;105:12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorci-Thomas MG, Thomas MJ. The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc Med. 2002;12:121–128. doi: 10.1016/s1050-1738(01)00163-3. [DOI] [PubMed] [Google Scholar]
- 58.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, 3rd, Smith JD, Gogonea V, Hazen SL. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 59.Martin DD, Budamagunta MS, Ryan RO, Voss JC, Oda MN. Apolipoprotein A-I assumes a "looped belt" conformation on reconstituted high density lipoprotein. J Biol Chem. 2006;281:20418–20426. doi: 10.1074/jbc.M602077200. [DOI] [PubMed] [Google Scholar]
- 60.Jones MK, Catte A, Patterson JC, Gu F, Chen J, Li L, Segrest JP. Thermal stability of apolipoprotein A-I in high-density lipoproteins by molecular dynamics. Biophys J. 2009;96:354–371. doi: 10.1016/j.bpj.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]