Abstract
Iron deficiency is the most prevalent micronutrient deficiency worldwide. Whereas dietary calcium is known to reduce the bioavailability of iron, the molecular basis of this interaction is not understood. We tested the hypothesis that divalent metal-ion transporter-1 (DMT1—the principal or only mechanism by which nonheme iron is taken up at the intestinal brush border—is shared also by calcium. We expressed human DMT1 in RNA-injected Xenopus oocytes and examined its activity using radiotracer assays and the voltage clamp. DMT1 did not mediate 45Ca2+ uptake. Instead, we found that Ca2+ blocked the Fe2+-evoked currents and inhibited 55Fe2+ uptake in a noncompetitive manner (Ki ≈ 20 mM). The mechanism of inhibition was independent of voltage and did not involve intracellular Ca2+ signaling. The alkaline earth metal ions Ba2+, Sr2+ and Mg2+ also inhibited DMT1-mediated iron-transport activity. We conclude that Ca2+ is a low-affinity noncompetitive inhibitor—but not a transported substrate—of DMT1, explaining in part the effect of high dietary calcium on iron bioavailability.
Keywords: Anemia, Calcium transport, Iron deficiency, Iron transport, Nutrition, trace metals, SLC11A2
Introduction
Despite recent progress in our understanding of iron absorption, iron deficiency remains the most prevalent micronutrient deficiency worldwide. Whereas dietary calcium is known to reduce the bioavailability of iron [1,2,7,11-13], the molecular basis of this interaction is not understood. Divalent metal-ion transporter-1 (DMT1) (Ref. [5,10]) is the principal or only mechanism by which nonheme iron is taken up at the intestinal brush border [9], reviewed [15]. This widely-expressed transporter is also critical for the recovery of iron from recycling endosomes during transferrin receptor-mediated cellular iron uptake in erythroid precursor cells [4,5,9].
Since DMT1 is a proton-coupled ferrous-ion (Fe2+) transporter that is shared by certain other divalent transition metal ions [6,10,15-17,23], we considered whether calcium could also interact with DMT1. The literature contains at least two reports that suggest an association between Ca2+ transport and DMT1. For example, a DMT homolog from the scallop is reported to operate as a calcium transporter [24] and a glycine-to-arginine substitution (at residue 185 of mouse DMT1)—which disrupts DMT1-mediated iron transport and results in microcytic anemia in the mk mouse [5,22]—is thought to expose a calcium-entry pathway [25]. Therefore, we tested in vitro the hypothesis that Ca2+ is a transported substrate of human DMT1. We also assessed Ca2+ inhibition of DMT1-mediated Fe2+ and H+ transport activities, and explored whether the other group 2 alkaline earth metal ions (Mg2+, Sr2+, Ba2+) mimic the effects of Ca2+.
Materials and Methods
Expression of human DMT1 in Xenopus oocytes
We performed laparotomy and ovariectomy on adult female Xenopus laevis frogs (Nasco, Fort Atkinson, WI) under 2-aminoethylbenzoate methanesulfonate anesthesia (0.1% in 1:1 water/ice, by immersion) following a protocol approved by the University of Cincinnati Institutional Animal Care and Use Committee. Ovarian tissue was isolated and treated with collagenase A (Roche Diagnostics Corp., Indianapolis, IN), and oocytes were isolated and stored at 17 °C in modified Barths’ medium as described [14]. We expressed in Xenopus oocytes the 1A/IRE(+) isoform of DMT1, the product of the human SLC11A2 gene. We linearized the pOX(+) plasmid containing 1A/IRE(+) DMT1 cDNA under the SP6 promoter (as described [16]) using SnaBI (New England Biolabs Inc, Ipswich, MA) and synthesized RNA in vitro using the mMESSAGE mMACHINE / SP6 RNA polymerase kit (Applied Biosystems / Ambion, Austin, TX) according to the manufacturers’ protocols. Defolliculate stage V-VI oocytes were injected with approximately 50 ng of DMT1 RNA and incubated 6 to 7 days before being used in functional assays.
Reagents and media
Reagents were obtained from Sigma-Aldrich Corp. (St. Louis, MO) or Research Products International Corp. (Prospect, IL) unless otherwise indicated. Oocytes were superfused or incubated at room temperature (22-24 °C) in transport media containing 100 mM NaCl, 1 mM KCl, 1 mM l-ascorbic acid, buffered using 0-5 mM 2-(N-morpholino)ethanesulfonic acid (MES) and 0-5 mM N′,N′-diethylpiperazine (DEPP) (GFS Chemicals, Columbus, OH) to obtain pH 5.5-7.5 as indicated. Where indicated, we added up to 50 mM Ca2+, or up to 20 mM Mg2+, Sr2+ or Ba2+ by equimolar replacement of Na+.
Radiotracer assays
We obtained radiochemicals from Perkin-Elmer Life Science Products (Boston, MA). We used 55Fe at final specific activity 0.31-1.99 GBq.mg−1 (added as FeCl3) and 45Ca at 75-263 MBq.mg−1 (added as CaCl2). Radiotracer metal-ion uptake was measured over 10 min, within the linear portion of the timecourse of 55Fe2+ uptake [16]. We terminated radiotracer uptake by rapidly washing the oocytes three times in ice-cold pH 5.5 transport medium containing 1 mM l-ascorbic acid. Oocytes were then solubilized using 5% (w/v) sodium dodecylsulfate and radiotracer content assayed by liquid-scintillation counting using Scintisafe-30% liquid-scintillation cocktail (Fisher Scientific, Pittsburgh, PA). Uptake data for 0.38-38 μM 55Fe2+ in the absence of Ca2+ or in the presence of 20 mM Ca2+ were fit by a modified Hill function (equation 1) for which Vmax is the maximal velocity of 55Fe2+ uptake (V), S is the concentration of substrate S (Fe2+), K0.5S is the concentration of S at which V is half-maximal, and nH is the Hill coefficient.
| (Eq. 1) |
Saturation kinetics parameters derived using equation 1 were used to estimate KiCa, the Ca2+ concentration resulting in half-maximal inhibition of 55Fe2+ transport, according to equation 2 (see Ref. [19]) for which Vmax and Vmaxi are respectively the maximal velocities in the absence and presence of inhibitor, [I] is the inhibitor concentration (in this case, 20 mM Ca2+).
| (Eq. 2) |
Voltage-clamp experiments
We used the two-microelectrode voltage clamp (Dagan CA-1B amplifier) to measure currents in control oocytes and oocytes expressing DMT1. Microelectrodes (resistance 3-6 MΩ) were filled with 3 M KCl. Voltage-clamp experiments comprised two protocols: (i) Continuous current recordings were made at a membrane potential (Vm) of −70 mV, digitized at 10 Hz and low-pass filtered at 1 Hz. (ii) Oocytes were clamped at Vm = −50 mV, and step-changes in Vm were applied from +50 mV to −150 mV (in 20-mV increments) each for a duration of 200 ms, before and after the addition of metal-ion substrate. Current was digitized at 5 kHz and low-pass filtered at 500 Hz. Steady-state data were obtained by averaging the points over the final 16.7 ms at each Vm step. Data from protocol (i) exploring the inhibition of the H+ leak current by Ca2+ (see Fig. 3B) were fit by equation 3 for which I is the Ca2+-induced shift in current, Imax the derived current maximum, S the concentration of Ca2+, and KiCa the Ca2+ concentration resulting in half-maximal inhibition of the H+ leak current.
Fig. 3.
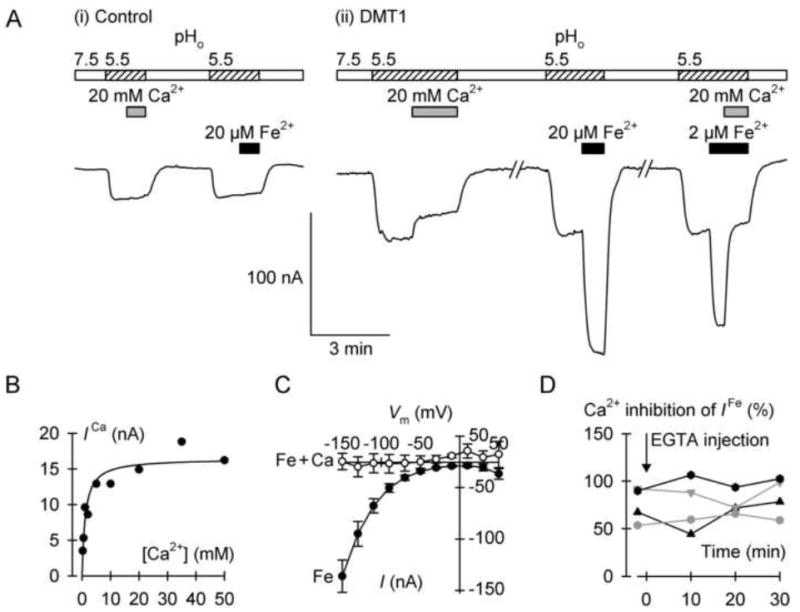
Calcium inhibition of the DMT1-mediated H+ leak and Fe2+-evoked currents. (A) Typical continuous current recordings at membrane potential (Vm) of 70mV in a control oocyte (i) and in an oocyte expressing DMT1 (ii). Oocytes were superfused with Ca2+-free medium at pH 7.5 for the periods shown by the empty bars, or with Ca2+-free medium at pH 5.5 (hatched bars). We presented 20 mM Ca2+ as indicated by the gray bars and 20 μM or 2 μM Fe2+ (black bars). (B) Ca2+-induced current (ICa) in the absence of Fe2+ metal ion as a function of Ca2+ concentration. Data were fit by equation 3 to describe the Ca2+ inhibition of the DMT1-mediated H+ leak current: Imax = 16.4 ± 1.0 nA, KiCa = 1.1 ± 0.3 mM (adjusted r2 = 0.88, P < 0.001). (C) Current-voltage relationships of the currents induced by 2 μM Fe2+ alone (Fe, filled symbols) or 2 μM Fe2+ plus 20 mM Ca2+ (Fe + Ca, empty symbols) (n = 4, paired). (D) Effect of intracellular Ca2+ chelation on the Ca2+ inhibition of the DMT1-mediated Fe2+-evoked current. We voltage-clamped at −70 mV four oocytes expressing DMT1, and measured currents evoked by 2 μM Fe2+ alone or 2 μM Fe2+ plus 20 mM Ca2+ before (−2 min) and after (10, 20, 30 min) microinjection with EGTA. Each symbol shape represents an individual oocyte. The mean Fe2+-evoked current at −2 min was −88 (SD 64) nA and did not change with time (one-way repeated measures ANOVA, P = 0.83). We obtained percent inhibition by Ca2+ and found no effects over time (one-way repeated measures ANOVA, P = 0.18).
| (Eq. 3) |
Intracellular calcium chelation
To chelate intracellular calcium in oocytes expressing DMT1 (see Fig. 3D), we microinjected oocytes with 23 nl of a 50-mM solution of ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), adjusted to pH 7.5 using KOH, to obtain a nominal final intracellular EGTA concentration of 1 mM.
Statistical and regression analyses
We performed statistical analyses using SigmaPlot version 11 (Systat Software) with critical significance level α = 0.05. We have presented our data as mean and standard deviation (SD) for n independent observations and used parametric tests in their analysis since our data either (1) passed the Shapiro-Wilk test for normality, or (2) n ≥ 30 in each group, so normality was not tested. Data were fit by equations 1 and 3 using the least-squares method of nonlinear regression analysis, the results of which are expressed as the estimates of fit parameters ± standard error (SE); adjusted r2 is the adjusted regression coefficient and P describes the significance of the fit. CI is the 95% confidence interval. Between-group comparisons were made using one-way or two-way analysis of variance (ANOVA) followed by pairwise multiple comparisons by the Holm-Šidák test. Data in Fig. 3D were analyzed using repeated-measures one-way ANOVA.
Results
DMT1 does not mediate calcium transport
We expressed human DMT1 in Xenopus oocytes and assayed metal-ion transport activity. Under conditions that stimulated over 60-fold the uptake of 55Fe2+ compared with control (i.e. 2 μM metal ion at pH 5.5), the expression of DMT1 did not increase 45Ca2+ transport compared with control (Fig. 1A). We considered that DMT1 could perhaps exhibit much lower affinity for Ca2+ than for Fe2+, so we tested whether significant 45Ca2+ transport could be observed at a higher concentration. Expression of DMT1 resulted in a minute increase in the uptake of 45Ca2+ at 100 μM (Fig. 1B); the DMT1-induced 45Ca2+ uptake was only 1.2% ± 0.3% (SE) the DMT1-induced 55Fe2+ uptake observed at 1/50th the concentration. Moreover, we found this increase to be nonspecific (possibly due to increased ‘leakiness’ of the oocytes overexpressing an exogenous protein) since the DMT1-induced 45Ca2+ uptake was not inhibited by a saturating concentration (100 μM) of Fe2+. We therefore conclude that human DMT1 is not capable of transporting calcium.
Fig. 1.
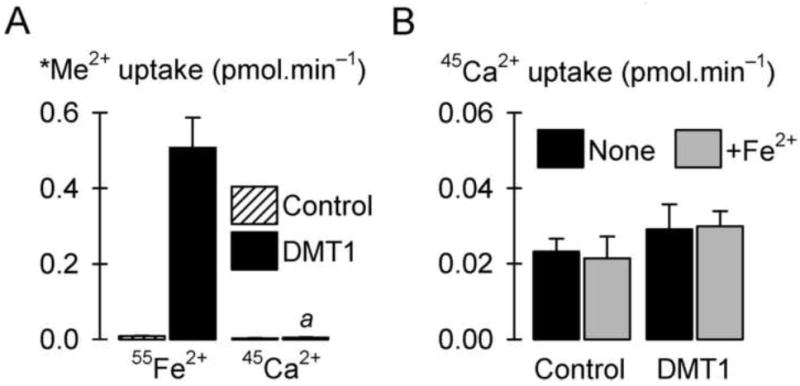
DMT1 does not mediate 45Ca2+ transport in Xenopus oocytes. (A) Radiotracer metal-ion (*Me2+) uptake (pH 5.5, 1 mM Mg2+) in control oocytes and oocytes expressing human DMT1 (n = 9-13). We compared uptake of 2 μM 55Fe2+ with that of 2 μM 45Ca2+. Two-way ANOVA revealed an interaction (P < 0.001); a within 45Ca2+ uptake, DMT1 did not differ from control (unadjusted P = 0.933). (B) Uptake of 100 μM 45Ca2+ (pH 5.5) in the absence (None) or presence (+Fe2+) of 100 μM Fe2+ in control oocytes and oocytes expressing human DMT1 (n = 22-32). (Note the y-axis scale is one tenth that in A.) Two-way ANOVA revealed a main effect of DMT1 (P < 0.001) but not of [Fe2+] (P = 0.99), and no interaction (P = 0.094).
Calcium is a noncompetitive inhibitor of DMT1-mediated iron transport
We further explored the interaction of calcium with DMT1 by testing the ability of calcium to inhibit DMT1-mediated transport activities. The addition of 20 mM Ca2+ inhibited by 52% ± 5% (SE) the uptake of 2 μM 55Fe2+ in oocytes expressing DMT1 (Fig. 2A). We examined the saturation kinetics of 55Fe2+ uptake in the absence and presence of calcium and found that 20 mM Ca2+ decreased the Vmax for DMT1-specific 55Fe2+ uptake by 48% ± 6% (SE, P < 0.001) without effect on K0.5 for Fe2+ (not significant, P = 0.12), indicating that calcium is a noncompetitive inhibitor of DMT1-mediated 55Fe2+ transport (Fig. 2B). In contrast, a competitive inhibitor is expected to increase the apparent K0.5 without effect on Vmax [19]. Using equation 2, we estimated KiCa to be 21.7 (CI 17.5, 25.9) mM in this preparation. In a second preparation (not shown), we obtained KiCa = 17.8 (CI 9.8, 25.8) mM. Therefore Ca2+ is a noncompetitive inhibitor of DMT1-mediated Fe2+ transport with KiCa ≈ 20 mM.
Fig. 2.
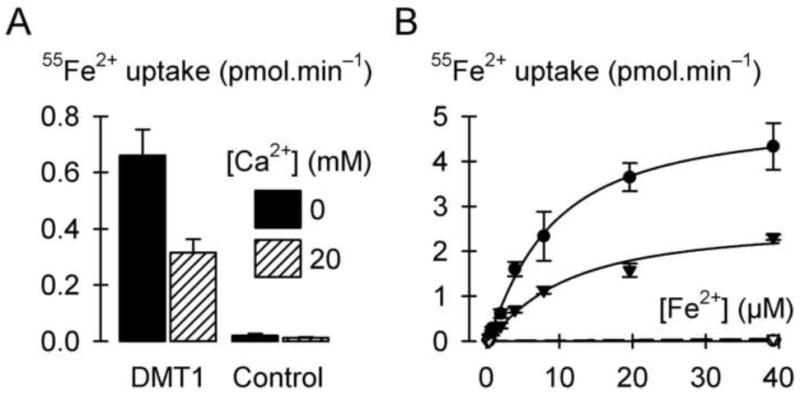
Ca2+ is a noncompetitive inhibitor of DMT1-mediated Fe2+ transport. (A) Uptake of 2 μM 55Fe2+ (pH 5.5, 1 mM Mg2+) in the absence or presence of 20 mM Ca2+ in control oocytes and oocytes expressing DMT1 (n = 10-12). Two-way ANOVA revealed an interaction (P < 0.001). (B) Saturation kinetics of 55Fe2+ uptake (pH 5.5, 1 mM Mg2+) in the absence (circles) or presence (triangles) of 20 mM Ca2+ (n = 10-14 per group at each concentration). We obtained the specific DMT1-mediated 55Fe2+ uptake by subtracting from the 55Fe2+ uptake in oocytes expressing DMT1 (filled symbols) the endogenous 55Fe2+ uptake derived from linear fits (dashed lines) of the 55Fe2+ uptake in control oocytes (empty symbols). Specific DMT1-mediated 55Fe2+ uptake data were fit (solid lines) by equation 1, yielding for [Ca2+] = 0 the parameters Vmax = 5.0 ± 0.3 pmol.min−1, nH = 1.2 ± 0.1, and K0.5 = 8.4 ± 1.0 μM (adjusted r2 = 0.96, P <0.001) and for [Ca2+] = 20 mM the parameters Vmax = 2.6 ± 0.1 pmol.min−1, nH = 1.2 ± 0.1, and K0.5 = 10.2 ± 1.2 μM (adjusted r2 = 0.97, P <0.001).
Calcium inhibition of DMT1-mediated H+ leak and Fe2+-evoked currents
We examined the effects of calcium on the currents associated with expression of human DMT1 in Xenopus oocytes (Fig. 3). In control oocytes voltage clamped at membrane potential (Vm) of −70 mV, we observed a small inward current upon switching the superfusion medium from pH 7.5 to 5.5; however, neither Ca2+ nor Fe2+ had any effect on current at pH 5.5 in control oocytes (Fig. 3A). Switching from pH 7.5 to pH 5.5 induced a more pronounced inward current in oocytes expressing DMT1; this current—in the absence of metal-ion—is attributed to a H+ leak pathway associated with DMT1 as previously described [10,16,17]. The DMT1-associated H+ leak current was inhibited in the presence of 20 mM Ca2+. The magnitude of the inhibition was dependent upon the Ca2+ concentration (Fig. 3B); using equation 3, we obtained KiCa = 1.1 ± 0.3 mM. That the KiCa for inhibition of the H+ leak was lower than the KiCa for inhibition of 55Fe2+ uptake most likely reflects a higher affinity for Ca2+ at Vm = −70 mV compared with that at depolarized potentials expected in nonclamped oocytes in the presence of 55Fe2+.
We found that 20 μM Fe2+ evoked large, reversible inward currents in oocytes expressing DMT1 (Fig. 3A), as previously observed [10,16,17]. The currents evoked by 2 μM Fe2+ were strongly inhibited by the addition of 20 mM Ca2+. Ca2+ inhibited the Fe2+-evoked currents at any given Vm between −150 mV and +50 mV (Fig. 3C), strengthening the conclusion that Ca2+ specifically interacts with DMT1. To test whether the effect of Ca2+ on the Fe2+-evoked currents was mediated via intracellular signaling, we measured in four oocytes expressing DMT1 the Fe2+-evoked current before and after microinjection with EGTA to chelate the intracellular Ca2+ (Fig. 3D). The inhibitory effect of Ca2+ persisted at least up to 30 min following EGTA injection, indicating that the Ca2+-inhibitory effect is not exerted via intracellular signaling.
Inhibition of DMT1-mediated iron transport by other alkaline earth metals
We explored whether other alkaline earth metal ions could also inhibit DMT1-mediated transport activity. Again, 20 mM Ca2+ inhibited the uptake of 2 μM 55Fe2+ by 53% ± 5% (SE) and Sr2+ inhibited 55Fe2+ transport to a similar extent (58% ± 5%, SE) (Fig. 4). Ba2+ resulted in stronger inhibition (73% ± 5%, SE) and Mg2+ more weakly inhibited 55Fe2+ uptake (28% ± 6%, SE).
Fig. 4.
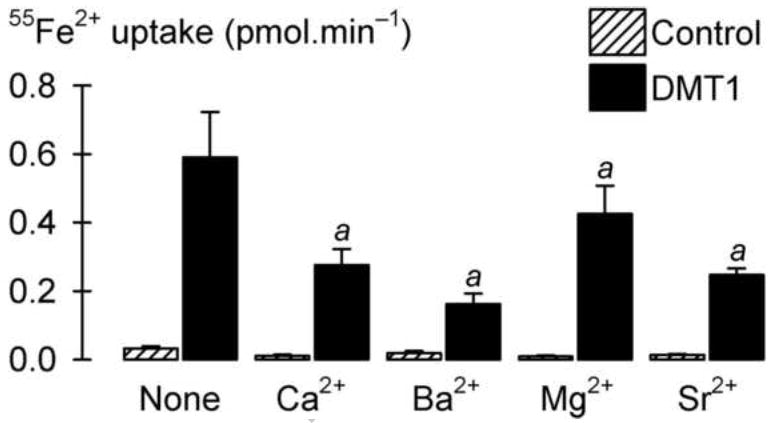
Inhibition of DMT1-mediated Fe2+ transport by the alkaline-earth metals. We measured uptake of 2 μM 55Fe2+ at pH 5.5 in standard transport medium (None) or in the presence of 20 mM Ca2+, Ba2+, Mg2+, or Sr2+ in control oocytes and oocytes expressing DMT1 (n = 12-16). Two-way ANOVA revealed an interaction (P < 0.001); a within DMT1, all treatments with alkaline-earth metals differed from no treatment (P < 0.001).
Discussion
We have expressed human DMT1 in RNA-injected Xenopus oocytes and found that DMT1 did not mediate 45Ca2+ uptake, in contrast to the scallop DMT homolog which transports calcium [24]. We found, however, that Ca2+ (i) blocked the DMT1-mediated H+ leak currents, with KiCa ≈ 1 mM, (ii) blocked the Fe2+-evoked currents, and (iii) inhibited 55Fe2+ uptake in a noncompetitive manner, with KiCa ≈ 20 mM. The other group 2 alkaline earth metals—Ba2+, Mg2+ and Sr2+—also inhibited iron transport. Since DMT1 interacts with Ca2+ at relatively low affinity, it is unlikely that Ca2+ would be a useful experimental tool with which to block DMT1 transport in vitro. Ba2+ afforded stronger inhibition at 20 mM; however, further investigation is needed to determine whether Ba2+ could serve as a sufficiently-specific blocker of DMT1 in vitro.
DMT1 is expressed in erythroid precursor cells where it transports iron from recycling endosomes to the cytoplasm following transferrin receptor-mediated cellular iron uptake. Intracellular Ca2+ is thought to regulate iron uptake in K562 cells, an erythroid-cell model, by accelerating transferrin/transferrin-receptor cycling [3]. Noting this observation, we explored whether intracellular Ca2+ may also regulate the activity of DMT1 expressed in the oocyte. We found, however, that chelation of intracellular Ca2+ (by EGTA injection) affected neither the magnitude of the Fe2+-evoked currents nor the magnitude of the Ca2+-inhibitory effect. We conclude that the Ca2+-inhibitory effect is not mediated via intracellular Ca2+ signaling and propose instead that Ca2+ acts at an extracellular site on DMT1.
The transferrin cycle also accounts for iron uptake in most other cell types. Since relatively large concentrations of Ca2+ are required to inhibit DMT1 transport activities (Kica = 1-20 mM) in the oocyte it is unlikely that Ca2+ should interfere with DMT1-mediated transport of Fe2+ in most cell types. One notable exception is the enterocyte, in which DMT1 is expressed at the apical (luminal) membrane, since intestinal luminal Ca2+ concentrations may periodically reach the millimolar range as a result of dietary calcium. For example, the free Ca2+ concentration of milk (of various sources) is estimated at 3.2-4.2 mM by the use of an ion-sensitive electrode [21]; total soluble Ca is about 12.5 mM and the total calcium concentration (including that bound by casein) in milk is around 30 mM [8]. Whereas the luminal contents will be diluted as a result of intestinal secretions, the concentration of calcium from dietary sources such as milk may be sufficient to inhibit iron absorption significantly [7,13] and calcium supplementation has been shown to reduce iron absorption [12,13,18]. Our data indicate that an interaction of calcium with DMT1 could, at least in part, provide the molecular basis to the observation that dietary calcium inhibits iron absorption, and calcium inhibition of DMT1 may be taken into consideration when developing strategies for improved nutrition, milk formulation and iron fortification.
Acknowledgments
We thank Eric J. Niespodzany, MS (University of Cincinnati) for his help in the laboratory. This study was supported by PHS grant R01 DK080047 (to B.M.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and by the University of Cincinnati (to B.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health.
Footnotes
This work was presented in part at Experimental Biology, Anaheim, California, 24-28 April, 2010 [20].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barton JC, Conrad ME, Parmley RT. Calcium inhibition of inorganic iron absorption in rats. Gastroenterology. 1983;84:90–101. [PubMed] [Google Scholar]
- 2.Benkhedda K, L’Abbé MR, Cockell KA. Effect of calcium on iron absorption in women with marginal iron status. [15 January 2010];Br J Nutr. 2009 doi: 10.1017/S0007114509992418. Published online by Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- 3.Ci W, Li W, Ke Y, Qian ZM, Shen X. Intracellular Ca2+ regulates the cellular iron uptake in K562 cells. Cell Calcium. 2003;33:257–266. doi: 10.1016/s0143-4160(02)00240-3. [DOI] [PubMed] [Google Scholar]
- 4.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 6.Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- 7.Gleerup A, Rossander-Hulthen L, Gramatkovski E, Hallberg L. Iron absorption from the whole diet: Comparison of the effect of two different distributions of daily calcium intake. Am J Clin Nutr. 1995;61:97–104. doi: 10.1093/ajcn/61.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Grosvenor MB, Smolin LA. Nutrient Composition of Foods. Wiley; Hoboken: 2010. [Google Scholar]
- 9.Gunshin H, Fujiwara Y, Custodio ÁO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 11.Hallberg L. Does calcium interfere with iron absorption? Am J Clin Nutr. 1998;68:3–4. doi: 10.1093/ajcn/68.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Hallberg L, Brune M, Erlandsson M, Sandberg AS, Rossander-Hulten L. Calcium: Effect of different amounts on nonheme- and heme-iron absorption in humans. Am J Clin Nutr. 1991;53:112–119. doi: 10.1093/ajcn/53.1.112. [DOI] [PubMed] [Google Scholar]
- 13.Hallberg L, Rossander-Hulten L, Brune M, Gleerup A. Bioavailability in man of iron in human milk and cow’s milk in relation to their calcium contents. Pediatr Res. 1992;31:524–527. doi: 10.1203/00006450-199205000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie B. Selected techniques in membrane transport. In: Van Winkle LJ, editor. Biomembrane Transport. Academic Press; San Diego: 1999. pp. 327–342. [Google Scholar]
- 15.Mackenzie B, Garrick MD. Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G981–G986. doi: 10.1152/ajpgi.00363.2005. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1) Biochem J. 2007;403:59–69. doi: 10.1042/BJ20061290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackenzie B, Ujwal ML, Chang M-H, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+-coupled Fe2+ transport and uncoupled fluxes. Pflügers Arch Eur J Physiol. 2006;451:544–558. doi: 10.1007/s00424-005-1494-3. [DOI] [PubMed] [Google Scholar]
- 18.Perales S, Barbera R, Lagarda MJ, Farre R. Fortification of milk with calcium: Effect on calcium bioavailability and interactions with iron and zinc. J Agric Food Chem. 2006;54:4901–4906. doi: 10.1021/jf0601214. [DOI] [PubMed] [Google Scholar]
- 19.Segel IH. Biochemical Calculations. 2. John Wiley & Sons: New York; 1975. [Google Scholar]
- 20.Shawki A, Mackenzie B. Calcium interactions with divalent metal-ion transporter-1 (DMT1) FASEB J. 2010;24 doi: 10.1016/j.bbrc.2010.02.025. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silanikove N, Shapiro F, Shamay A. Use of an ion-selective electrode to determine free Ca ion concentration in the milk of various mammals. J Dairy Res. 2003;70:241–243. doi: 10.1017/s0022029903006083. [DOI] [PubMed] [Google Scholar]
- 22.Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC. The G185R mutation disrupts function of the iron transporter Nramp2. Blood. 1998;92:2157–2163. [PubMed] [Google Scholar]
- 23.Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, Srai SK, Sharp P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J Biol Chem. 2000;275:1023–1029. doi: 10.1074/jbc.275.2.1023. [DOI] [PubMed] [Google Scholar]
- 24.Toyohara H, Yamamato S, Hosoi M, Takagi M, Hayashi I, Nakao K, Kaneko S. Scallop DMT functions as a Ca2+ transporter. FEBS Lett. 2005;579:2727–2730. doi: 10.1016/j.febslet.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Jin J, DeFelice LJ, Andrews NC, Clapham DE. A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway. PLoS Biol. 2004;2:E50. doi: 10.1371/journal.pbio.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]


