Summary
Objective
The purpose of this study was to measure the local electrical field or potential gradient, measured with a catheter-based system, required to terminate long duration electrically or ischaemically induced ventricular fibrillation (VF). We hypothesized that prolonged ischaemic VF would be more difficult to terminate when compared to electrically induced VF of similar duration.
Methods
Thirty anesthetized and instrumented swine were randomized to electrically induced VF or spontaneous, ischemically induced VF, produced by balloon occlusion of the left anterior descending coronary artery. After 7 min of VF, chest compressions were initiated and rescue shocks were attempted 1 min later. The potential gradient for each shock was measured and the mean values required for defibrillation compared for the VF all.
Results
The number of shocks and the shock strength required for termination of VF were not significantly different for the all. The potential gradient of the first successful defibrillating shock was significantly greater in the spontaneous, occlusion induced VF group (12.80 ± 2.82 vs 9.60 ± 2.48 V/cm, p = 0.002). The number of refibrillations was greater in the ischaemic group than in the non-ischaemic electrical group (6 ± 4 versus 1 ± 1, p <0.001). The number of animals requiring a shock at 360 joules was 2.5 times greater for the ischaemic group.
Conclusions
Defibrillation of prolonged VF produced by acute myocardial ischaemia requires a significantly greater potential gradient to terminate than prolonged VF induced by electrical stimulation of the right ventricular endocardium. The VF duration used in this study approximates that occurring in victims of out-of-hospital cardiac arrest. Our findings may be of clinical importance in the management of such patients.
Keywords: ischaemia, cardiac arrest, ventricular fibrillation, defibrillation
INTRODUCTION
It has been estimated that >300,000 people experience out-of-hospital cardiac arrest each year.1 Although spontaneous circulation is restored in 40–50%, the majority of patients initially resuscitated will subsequently die before leaving the hospital, resulting in a hospital survival rate of approximately 5% in major cities nationwide.2 The initial rhythm encountered in cardiac arrest victims has been shown to be a major determinant of outcome. Ventricular fibrillation (VF) has the greatest likelihood of yielding a positive resuscitation outcome and survival rates >40% have been reported.3 Because of its favorable prognosis if properly managed, research efforts in the field of sudden cardiac death and resuscitation have largely focused on this rhythm. Such research would include the development and dissemination of automated external defibrillators (AED), development and testing of new defibrillation waveforms, evaluation of shock strengths (low vs standard dose), single shocks vs rapidly administered escalating-energy shocks, and the current practice of performing CPR before defibrillation and minimizing interruptions in CPR chest compressions prior to rescue shocks.
Laboratory investigations of cardiac arrest due to VF have used animal models in which VF is induced electrically, typically using AC current delivered directly to the endocardium or by transthoracic AC shock. More recently, animal models in which VF is induced by acute myocardial ischemia have received renewed interest because of its clinical relevance.4 Several studies have demonstrated differences between the two VF models.5,6 Among these differences would be the greater number and higher shock strengths necessary to terminate ischaemically induced VF.7–10 A limitation of such investigations has been the brief duration of ischaemic VF after induction by coronary occlusion.
The purpose of this study was to measure the local electrical field or potential gradient, measured with a catheter-based system, required to terminate long duration ischaemically induced VF. We hypothesized that prolonged ischaemic VF would be more difficult to terminate when compared to electrically induced VF of similar duration.
METHODS
This investigation was approved by the Animal Care and Use Committee of our institution and conformed to the position of the National Institutes of Health on research animal use.
Domestic swine (n = 30, mean weight 40 ± 4 kg, range 34–47 kg) of both sexes were premedicated with ketamine (20 mg/kg) and xylazine (2 mg/kg). General anesthesia was induced with isoflurane via nose cone, and following endotracheal intubation, maintained with inhaled isoflurane (MAC 1.0–2.5%) and nitrous oxide in a 1 to 1 mixture with oxygen. End-tidal CO2 was continuously monitored and minute ventilation was adjusted to maintain a value of 35–45 mm Hg. Standard lead II of the surface ECG was monitored during instrumentation and throughout the study protocol. The ECG was recorded using an Electronics for Medicine VR6 (Honeywell, Inc) amplifier. Low and high frequency filters were set at 1 and 2000 Hz The sampling rate was 400–1000 Hz.
Under fluoroscopic guidance, high fidelity, micro-manometer tipped catheters (Millar Instruments, Houston, TX) were positioned in the ascending aorta via a femoral artery and in the right atrium (RA) via a jugular vein. A multi-lumen catheter with thermistor (Edwards Lifesciences, Irvine, CA) was positioned in a branch of the pulmonary artery for thermodilution cardiac output determinations. A multi-electrode catheter for measurement of local electrical field or potential gradient was positioned in the mid-portion of the coronary sinus via a jugular vein. This catheter based system for the measurement of potential gradients has been described in detail.11,12 Standard adhesive defibrillation electrode patches with an active surface area of approximately 115 cm2 were applied to the left and right lateral aspects of the shaved thorax. Transthoracic impedance was measured using a tetrapolar constant current impedance measuring system (THRIM®, Morro Bay, CA). A small value non-inductive resistor (30Ω) was then placed in series with the impedance compensating, truncated exponential biphasic defibrillation waveform defibrillator (LifePak 12, Medtronic Emergency Response Systems, Redmond, WA). Following instrumentation, heart rate, systolic and diastolic aortic pressure, mean RA pressure, and CO were recorded and arterial blood was analyzed (I-Stat EG7+, I-Stat Corp, Princeton, NJ). Hemodynamic data was recorded and stored on a laptop computer using PowerLab Chart v. 5.2 (ADInstruments, Colorado Springs, CO).
Animals were then randomized by permuted block design to undergo electrically induced VF or ischemically induced VF. VF was induced only once in all animals. In swine randomized to electrically induced VF, a standard 7F bipolar pacing catheter was introduced into a jugular vein and positioned in the apex of the right ventricle (RV) in contact with the RV endocardium. VF was induced by passing 60 Hz AC current for approximately 0.5 sec through the electrodes of the RV bipolar catheter. In those animals randomized to ischemically induced VF, a standard PTCA catheter and balloon (4 mm × 20 mm) was introduced into a guiding catheter inserted via a carotid artery and positioned in the left anterior descending (LAD) coronary artery just distal to the first septal perforator branch. The site of coronary occlusion and the occurrence of complete cessation of coronary flow after balloon inflation (8 to 10 atmospheres) were confirmed with manual contrast injections.
After 7 min of untreated VF, manual closed-chest compressions were begun with the animal in the supine position and were administered by a single investigator at a rate of approximately 100/min with force sufficient to depress the sternum 1.5 to 2.0 inches. Depth of compression was determined by visual inspection. In the ischemic VF group, the occluding balloon remained inflated throughout resuscitative efforts. One minute after starting chest compressions, a transthoracic countershock at 200 J was given. For the purpose of these experiments, successful defibrillation was defined as termination of VF, regardless of the postshock cardiac rhythm or hemodynamic outcome, e.g., spontaneous QRS complexes with or without associated arterial pressure pulses, determined 5 sec after a defibrillation shock.13 The continuous recording of the aortic pressure allowed detection of postshock arterial pressure pulses produced by a spontaneous QRS complex prior to amplifier recovery.10 If VF persisted, additional shocks in an escalating energy sequence (300, 360J) were administered. Chest compressions were performed between shocks and positive pressure ventilations (FiO2=1.00) were performed at a rate of 10 ventilations/min. If VF persisted after the initial three shocks, epinephrine, 0.01 mg/kg, was administered and CPR continued for one to three minutes before additional shocks at 360 J were given. If necessary, additional epinephrine at doses of 0.5 mg, were given at 4–5 min intervals, CPR continued, and shocks repeated until VF was terminated. The potential gradient for all shocks was measured and recorded. All shocks were administered at end-expiration during the chest compression relaxation phase.
The shock strength (joules) and potential gradient (V/cm) recorded for the first successful defibrillation in animals in both VF all were compared using the Student t test or the Mann-Whitney rank sum test. Probability of success versus potential gradient curves were plotted for electrically and ischemically induced VF. Data are presented as the mean ± SD unless otherwise stated. For all comparisons, p <0.05 was considered statistically significant.
RESULTS
Prearrest variables for the two VF all are presented in Table 1. Significant differences were not observed. All animals developed spontaneous, ischemically induced VF. Mean time to VF occurrence after occlusion of the LAD was 1110 ± 394 sec.
Table 1.
Pre-arrest control variables
| Group | Weight (kg) | Heart Rate | MAP (mm Hg) | LVEDP (mm Hg) |
|---|---|---|---|---|
| Electrical VF | 41 ± 5 | 109 ± 22 | 91 ± 11 | 5 ± 2 |
| Ischaemic VF | 40 ± 4 | 114 ± 14 | 98 ± 11 | 5 ± 3 |
LVEDP = left ventricular end-diastolic pressure
MAP = mean arterial pressure
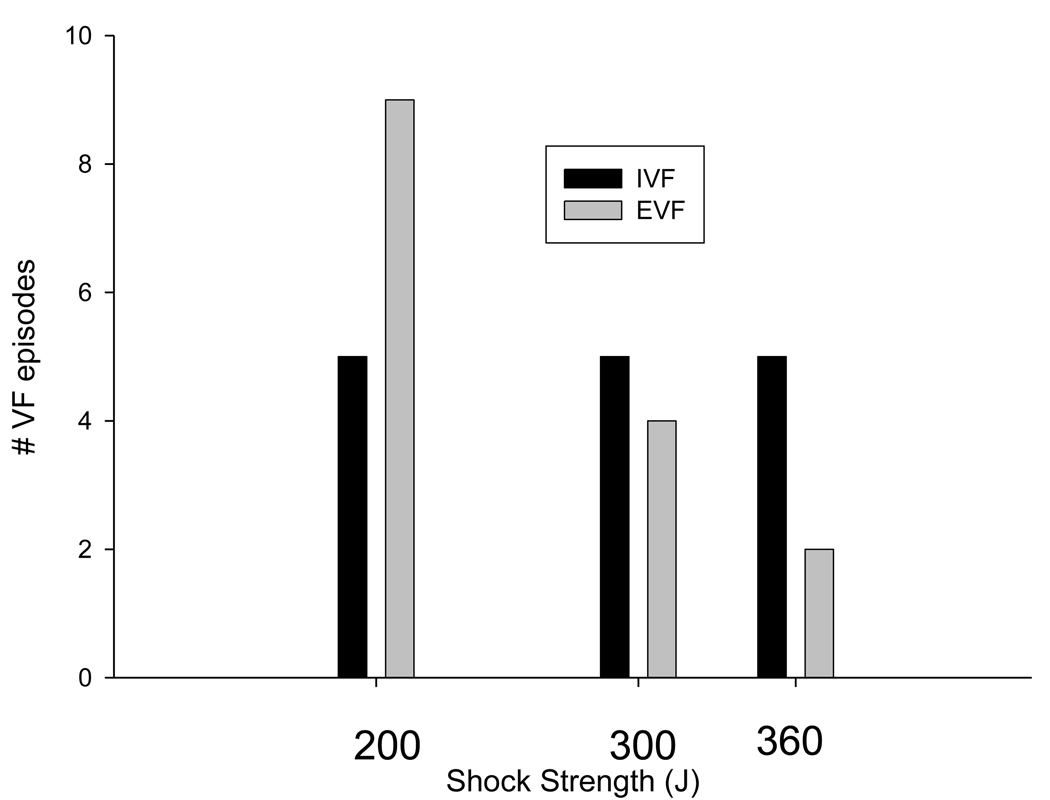
Defibrillation shock variables are shown in Table 2. Although the number of shocks and the shock strength required for termination of VF in the ischemic VF group were greater, differences failed to achieve statistical significance. The recorded potential gradient or local electrical field of the first successful defibrillating shock was significantly greater in the spontaneous, occlusion induced VF group. Additionally, the number of refibrillations was greater in the ischaemic group than in the non-ischaemic electrical group (6 ± 4 versus 1 ± 1, p <0.001). Shock strengths for successful termination of VF for the two all are shown in Figure 1. The number of animals requiring a shock at 360 joules was 2.5 times greater for the ischaemic group.
Table 2.
Defibrillation Shock Variables
| Impedance (Ω) |
Shocks to Defibrillation |
Defibrillation Shock Strength (J) |
Potential Gradient (V/cm) |
|
|---|---|---|---|---|
| Electrical VF | 71 ± 4 | 1.9 ± 1.8 | 248 ± 64 | 9.579± 2.476 |
| Ischaemic VF | 71 ± 5 | 2.8 ± 2.2 | 287 ± 68 | 12.804 ± 2.815 * |
p = 0.002 vs electrical VF
Figure 1. Shock strength for first successful defibrillation.
Shock strength required for successful defibrillation are shown for the electrically induced and ischaemically induced VF all. One-third of VF episodes in the ischemic group required maximum output for termination of VF.
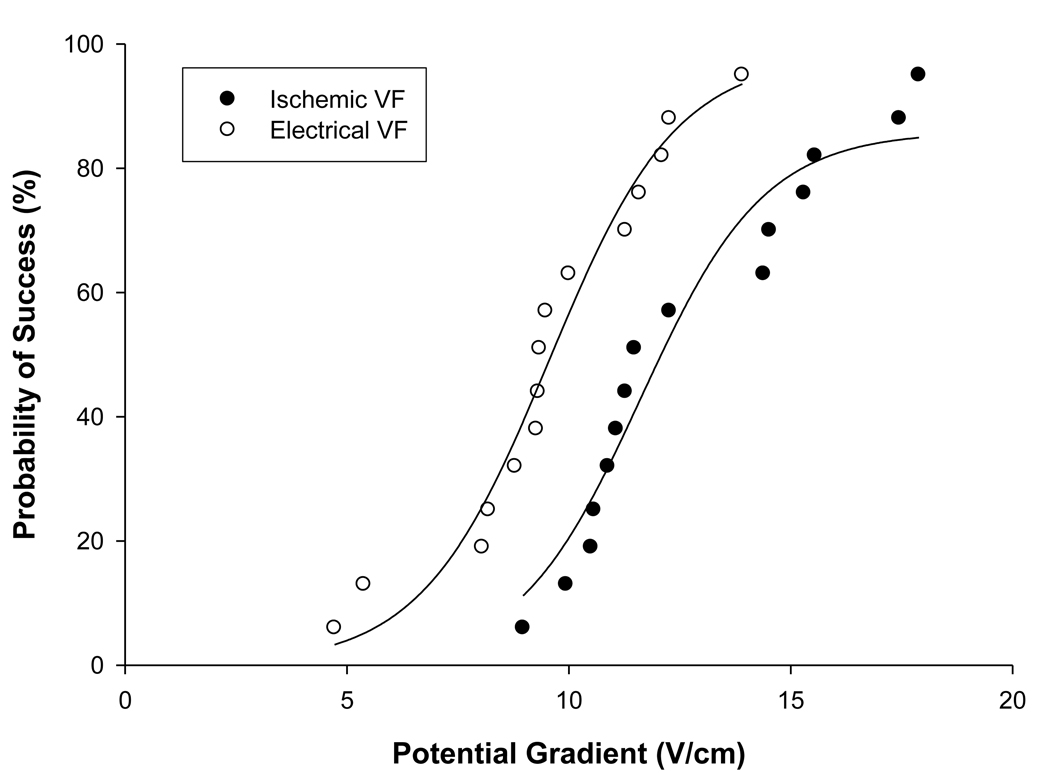
Probability of success curves for the two VF all are shown in figure 2. The curve for the spontaneous VF group is shifted rightward with a greater potential gradient observed for 50% probability of success defibrillation.
Figure 2. Probability of success curves for electrical and spontaneous VF.
The curve for ischaemically induced VF is shifted rightward and the mean potential gradient was significantly greater in the occlusion induced group.
DISCUSSION
This study suggests that prolonged spontaneous VF is more “difficult” to defibrillate than electrically induced VF. In a closed chest porcine model in which thoracic impedance was adjusted to approximate that observed in the typical human, the local electrical field required for successful defibrillation of prolonged spontaneous, ischaemically induced VF of 8 min duration is significantly greater than that for electrically induced VF of similar duration. Differences in shock strengths (joules) for successful VF termination were greater for spontaneous, ischaemic VF animals, but the difference was not statistically significant. However, one-third of ischaemic VF animals required the maximum energy output of the defibrillator (360 J) for successful defibrillation.
Previous work has demonstrated that the defibrillation threshold for brief duration electrically induced VF (15 or 20 sec) is significantly less that that for electrically induced VF of longer duration (5 min or 7 min) when shocks are delivered transthoracically.14 This difference can be mitigated somewhat by the defibrillation waveform. Three prior investigations have compared defibrillation parameters for brief duration VF induced either with 60-Hz delivered to the ventricular endocardium or occlusion of an epicardial coronary artery.7–9 In each study, significantly greater energy was needed to terminate brief VF, measured in seconds, due to acute ischaemia. Our study evaluated defibrillation shock strengths, potential gradients, and shock outcomes for electrically and ischaemically induced VF of a duration approximating that encountered in the treatment of patients experiencing out-of hospital cardiac arrest. In this population, defibrillation is typically delayed for greater than 5 minutes after onset. The findings of our study in a clinically relevant porcine model extend the previous work of Walcott and Qin in which transthoracic defibrillation of brief acute ischaemic VF was performed and defibrillation threshold determined.8,9
It is unclear why spontaneous VF following acute coronary occlusion is more difficult to terminate than VF after electrical stimulation of the endocardium. Focal mechanisms for the initiation and perpetuation of VF are likely to be additive or primary in causing a greater energy requirement for termination of VF due to acute, focal myocardial ischemia.8 One such focal mechanism may be regional hyperkalemia accompanying occlusion-induced ischemia prior to the onset of VF. Regional hyperkalemia has been shown to increase the energy required for defibrillation.15 However, it is not likely to occur with the abrupt onset VF which characterizes electrically induced VF.
Prolonged VF is accompanied by prolonged global myocardial ischemia which is believed to make successful defibrillation unlikely, prompting the current practice of performing chest compressions before rescue shocks are attempted.16 Chest compressions provide limited coronary perfusion and yet may facilitate successful defibrillation for unclear reasons. It has been suggested that pre-shock chest compressions may restore depleted high energy phosphate stores, minimize intracellular calcium accumulation or “wash out” toxic metabolites. These theories have recently been questioned.17 Of note, experimental studies supporting the use of chest compressions before defibrillation have all utilized electrically induced VF in canine or porcine cardiac arrest models with an unobstructed coronary vasculature. In our study, chest compressions were performed before the first rescue shock and between repeated shocks if multiple defibrillation attempts were required as is done in clinical care. Despite this intervention, the potential gradient required for termination of VF in the setting of ischemia was significantly greater than that observed for electrically induced VF. Chest compressions producing limited coronary flow in open coronary arteries, typically observed with coronary perfusion pressures > 15 mm Hg in animals, are unlikely to produce any flow in an occluded artery. Any benefits from chest compressions prior to countershock of prolonged VF are likely to be less dramatic in models of occlusion-induced VF.
Our study has several limitations. We measured the potential gradient at only one site and very high or low potential gradients at other sites would not be detected. However, with defibrillation electrodes located on the chest wall, it is likely that the electrical field produced by a shock was more uniform than if shocks had been applied to the epicardium.18 Due to the extended duration of VF, particularly in the ischaemic group in which restoration of spontaneous circulation was uncommon, we were unable to determine defibrillation thresholds in the conventional manner. Shock strength was increased after failed defibrillation in accordance with clinical practice, but did not conform to a conventional step-up protocol. It is possible that some animals might have been defibrillated at shock strengths between the selected levels. Despite these limitations, the shock strength (J) and potential gradients required for defibrillation of prolonged electrically induced VF are within the range reported by others for termination of VF of approximately the same duration.19 Animals in the ischaemic VF group received epinephrine if the third shock did not terminate VF. We were unable to determine if epinephrine affected defibrillation outcome.
CONCLUSIONS
Defibrillation of prolonged VF produced by acute myocardial ischaemia requires a significantly greater potential gradient to terminate than prolonged VF induced by electrical stimulation of the right ventricular endocardium. The VF duration used in this study approximates that occurring in victims of out-of-hospital cardiac arrest. Our findings may be of clinical importance in the management of such patients. Pre-shock chest compressions may not be as effective in facilitating defibrillation in the setting of acute coronary occlusion. Additionally, greater shock strengths may be required as the duration of VF is extended.
Acknowledgements
This study was funded, in part, by a grant from the National Institutes of Health, NHLBI R01 HL076671.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
REFERENCES
- 1.American Heart Association Heart Disease and Stroke Statistics. 2009 Update at-a-glance. http://www.americanheart.org.
- 2.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome. Epidemiology, pathophysiology, treatment, and prognostication. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 3.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta D, Curwin J, Gomes A, Fuster V. Sudden death in coronary artery disease. Acute ischemia versus myocardial substrate. Circulation. 1997;96:3215–3223. doi: 10.1161/01.cir.96.9.3215. [DOI] [PubMed] [Google Scholar]
- 5.Niemann JT, Rosborough UP, Youngquist S, Thomas J, Lewis RJ. Is all ventricular fibrillation the same? A comparison of ischemically induced with electrically induced ventricular fibrillation in a porcine cardiac arrest and resuscitation model. Crit Care Med. 2007;35:1356–1361. doi: 10.1097/01.CCM.0000261882.47616.7D. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Weil MH, Tang W, Chang Y, Huang L. A comparison of electrically induced cardiac arrest with cardiac arrest produced by coronary occlusion. Resuscitation. 2007;72:477–483. doi: 10.1016/j.resuscitation.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang P, Brinker JA, Bulkley BH, Jugdutt BI, Varghese PJ. Ischemic ventricular fibrillation: The importance of being spontaneous. Am J Cardiol. 1981;48:455–459. doi: 10.1016/0002-9149(81)90072-2. [DOI] [PubMed] [Google Scholar]
- 8.Walcott GP, Killingsworth CR, Smith WM, Ideker RE. Biphasic waveform external defibrillation thresholds for spontaneous ventricular fibrillation secondary to acute ischemia. J Am Coll Cardiol. 2002;39:359–365. doi: 10.1016/s0735-1097(01)01723-5. [DOI] [PubMed] [Google Scholar]
- 9.Qin H, Walcott GP, Killingsworth CR, Rollins DL, Smith WM, Ideker RE. Impact of myocardial ischemia and reperfusion on ventricular fibrillation patterns, energy requirements, and detection of recovery. Circulation. 2002;105:2537–2542. doi: 10.1161/01.cir.0000016702.86180.f6. [DOI] [PubMed] [Google Scholar]
- 10.Niemann JT, Rosborough JP, Walker RG. A model of ischemically induced ventricular fibrillation for comparison of fixed-dose and escalating-dose defibrillation strategies. Acad Emerg Med. 2004;11:619–624. [PubMed] [Google Scholar]
- 11.Niemann JT, Walker RG, Rosborough JP. Intracardiac voltage gradients during transthoracic defibrillation: Implications for postshock myocardial injury. Acad Emerg Med. 2005;12:99–105. doi: 10.1197/j.aem.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Rosborough JP, Deno C, Walker RG, Niemann JT. A percutaneous catheter-based system for the measurement of potential gradients applicable to the study of transthoracic defibrillation. PACE. 2007;30:166–174. doi: 10.1111/j.1540-8159.2007.00645.x. [DOI] [PubMed] [Google Scholar]
- 13.Gliner BR, Roger RD. Electrocardiographic evaluation of defibrillation shocks delivered to out-of-hospital sudden cardiac arrest patients. Resuscitation. 1999;41:133–144. doi: 10.1016/s0300-9572(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 14.Walcott GP, Melnick SB, Chapman FW, Jones JL, Smith WM, Ideker RE. Relative efficacy of monophasic and biphasic waveforms for transthoracic defibrillation after short and long durations of ventricular fibrillation. Circulation. 1998;98:2210–2215. doi: 10.1161/01.cir.98.20.2210. [DOI] [PubMed] [Google Scholar]
- 15.Sims JJ, Miller AW, Ujhelyi MR. Regional hyperkalemia increases ventricular fibrillation energy requirements: role of electrical heterogeneity in defibrillation. J Cardiovasc Electrophysiol. 2000;11:634–641. doi: 10.1111/j.1540-8167.2000.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 16.2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care, Part 5: Electrical therapies. Automated external defibrillators, defibrillation, cardioversion, and pacing. Circulation. 2005;112 suppl IV:IV-35–IV-46. doi: 10.1161/CIRCULATIONAHA.110.970954. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain D, Frenneaux M, Steen S, Smith A. Why do chest compressions aid delayed defibrillation? Resuscitation. 2008;77:10–15. doi: 10.1016/j.resuscitation.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, Wolf PD, Claydon FJ, et al. The potential gradient created by epicardial defibrillation in dogs. Circulation. 1986;74:626–636. doi: 10.1161/01.cir.74.3.626. [DOI] [PubMed] [Google Scholar]
- 19.Allred JD, Killingsworth CR, Allison S, et al. Transmural recording of shock potential gradient fields, early postshock activations, and refibrillation episodes associated with external defibrillation of long-duration ventricular fibrillation in swine. Heart Rhythm. 2008;5:1599–1606. doi: 10.1016/j.hrthm.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]