Abstract
Mucopolysaccharidosis I (MPS I) and MPS VII are due to loss-of-function mutations within the genes that encode the lysosomal enzymes α-L-iduronidase and β-glucuronidase, respectively, and result in accumulation of glycosaminoglycans and multisystemic disease. Both disorders are associated with elastin fragmentation and dilatation of the aorta. Here, the pathogenesis and effect of gene therapy on aortic disease in canine models of MPS was evaluated. We found that cathepsin S is upregulated at the mRNA and enzyme activity level, while matrix metalloproteinase 12 (MMP-12) is upregulated at the mRNA level, in aortas from untreated MPS I and MPS VII dogs. Both of these proteases can degrade elastin. In addition, mRNA levels for the interleukin 6-like cytokine oncostatin M were increased in MPS I and MPS VII dog aortas, while mRNA for tumor necrosis factor α and toll-like receptor 4 were increased in MPS VII dog aortas. These cytokines could contribute to upregulation of the elastases. Neonatal intravenous injection of a retroviral vector expressing β-glucuronidase to MPS VII dogs reduced RNA levels of cathepsin S and MMP-12 and aortic dilatation was delayed, albeit dilatation developed at late times after gene therapy. A post-mortem aorta from a patient with MPS VII also exhibited elastin fragmentation. We conclude that aortic dilatation in MPS I and MPS VII dogs is likely due to degradation of elastin by cathepsin S and/or MMP-12. Inhibitors of these enzymes or these cytokine-induced signal transduction pathways might reduce aortic disease in patients with MPS.
Keywords: Mucopolysaccharidosis, canine, lysosomal storage disease, cathepsin S, elastin, aorta, gene therapy
Introduction
Mucopolysaccharidosis I [MPS I1; Online Mendelian Inheritance in Man (OMIM) #607014] and MPS VII (OMIM #253220) are autosomal recessive lysosomal storage diseases with an incidence of 1:100,000 and <1:1,000,000, respectively [1–2]. MPS I is due to α-L-iduronidase (IDUA) deficiency and results in accumulation of the glycosaminoglycans (GAGs) dermatan and heparan sulfates, whereas MPS VII is due to β-glucuronidase (GUSB) deficiency, and results in accumulation of dermatan, heparan, and chondroitin sulfates. Hematopoietic stem cell transplantation (HSCT) can reduce clinical manifestations of MPS, as hematopoietic cells migrate into different tissues and secrete mannose 6-phosphate (M6P)-modified enzyme that can be taken up by nearby cells [3–4]. Enzyme replacement therapy (ERT), which involves intravenous (IV) injection of M6P-modified enzyme that can diffuse to other organs and be taken up via the M6P receptor, can also reduce disease manifestations [5–6]. Gene therapy is also being tested in animal models [7]. One approach involves neonatal IV injection of a γ-retroviral vector (RV) expressing the appropriate enzyme, which results in transduction of liver cells and secretion of enzyme into blood [8–15].
Both MPS I and MPS VII can result in cardiovascular disease, which can include aortic dilatation and cardiac valve stenosis or insufficiency. Cardiac valves are thickened due to accumulation of GAGs, resulting in stenosis and regurgitation in all types of MPS [16–19] that has not been prevented with HSCT or ERT [4–6]. Although aortic disease has received less attention than valve disease in humans, aortic disease is substantial and will likely be a source of morbidity as patients live longer due to HSCT and ERT. In patients with attenuated MPS I, the aorta diameter was 122% of normal [20], and elasticity was reduced [21]. Furthermore, one patient with MPS VII had narrowing and friability of the aorta and required an aortic graft [18]. Mice with MPS I [9–12, 22] and MPS VII [23] have dilated aortas, while MPS I mice have reduced aortic elasticity [12]. Feline models of MPS I and MPS VI [24] and canine models of MPS I [14, 25] and MPS VII [26–28] also have dilatation of the aorta.
Our study focused on the pathogenesis of aortic dilatation in MPS I and MPS VII dogs. The major extracellular matrix (ECM) proteins of the aorta are elastin and collagen, which represent 30% [29–30] and 35% [30–31] of its dry weight, respectively. Tropoelastin monomers are secreted and then crosslinked into elastic fibers in a process that involves elastin binding protein (EBP), ECM microfibrils, and crosslinking enzymes [32]. For fibrillar collagens, 3 chains assembled into triple helices within the cell are secreted into the ECM and cross-linked to form collagen fibrils. Elastin was fragmented in the ascending aorta of humans, mice, and dogs with MPS I, and in dogs with MPS VII [14, 22, 33–35], which likely contributed to aortic dilatation and reduced elasticity. Hinek et al. demonstrated that exogenous administration of dermatan sulfate reduced EBP levels and inhibited elastin assembly in vitro, and proposed that reduced assembly was responsible for the elastin defects in MPS I [36]. Alternatively, we reported [22] that elastin fragmentation was temporally associated with increases in RNA and enzyme activity for two elastin-degrading enzymes, cathepsin S and matrix metalloproteinase 12 (MMP-12), which can contribute to aortic aneurisms [37–40], and proposed that degradation was the major factor responsible for elastin fragmentation. In MPS I, collagen structure was loosely arrayed in the aortic valve in humans and mice [35], which may be due to degradation, as fragmented collagen fibrils were observed in lysosomes of mitral valve fibroblasts [34].
HSCT and ERT have not prevented aortic disease in animal models of MPS. HSCT has reduced, but not prevented, accumulation of GAGs, elastin fragmentation, and/or dilatation of the aorta in MPS VII mice [41] and dogs [26], in MPS VI rats [42], and in MPS I dogs [25] and cats [43]. ERT has had little effect on the accumulation of lysosomal storage material in aortic smooth muscle cells (SMC) in MPS VI cats [44].
Complete correction of aortic disease has also been problematic with neonatal gene therapy. Although neonatal administration of a dose of an RV that resulted in very high serum IDUA activity prevented aortic dilatation and elastin fragmentation in MPS I mice, small amounts of lysosomal storage were still present in the aorta. Furthermore, mice with lower expression of IDUA after gene therapy to newborns or adults continued to have some aortic disease [9–12]. Similarly, when neonatal gene therapy to MPS I dogs resulted in very high serum IDUA activity, there was near-complete correction of histopathology, although gene therapy-treated MPS I dogs with lower serum IDUA activity had lysosomal storage and elastin fragmentation, although they did not develop aortic dilatation. Aortic dilatation, lysosomal storage, and elastin fragmentation were mitigated at 2 years after neonatal gene therapy in MPS VII dogs [27], but longer evaluation was not performed. The goal of our study was to further evaluate the ability of neonatal gene therapy to prevent aortic disease, and to identify the pathogenesis of this process in MPS I and MPS VII dogs.
Materials and methods
Reagents were from Sigma-Aldrich Chemical (St. Louis, MO) unless otherwise stated.
Animals
National Institutes of Health (NIH) and United States Department of Agriculture guidelines for the care and use of animals in research were followed in the animal colony of the School of Veterinary Medicine, University of Pennsylvania. Some MPS I and MPS VII dogs were injected IV with 0.3 to 1×1010 transducing units (TU)/kg of the γ-RV designated hAAT-cIDUA-WPRE or hAAT-cGUSB-WPRE, respectively, at 2 to 3 days after birth. All RV-treated MPS I dogs were reported previously [14]. Some RV-treated MPS VII dogs were reported previously [13], while others received a similar gene therapy protocol. Echocardiograms were performed using a Phillips Sonos 7500 echocardiographic machine.
Histopathology
Ascending aortas obtained from a position that was 1 to 2 cm from the aortic valve were fixed with buffered formalin or with 4% paraformaldhedyde and 2% glutaraldehyde in phosphate buffered saline (PBS) for as briefly as 1 week, to as long as 7 years; the conditions of fixation had no obvious effect upon the staining characteristics. Aortas were embedded in paraffin and 6 µm sections were stained with Verhoeff’s Van Gieson (VVG) or picrosirius red stains. The computer program Corel PHOTO-PAINT X3 (Corel Inc., Mountain View, CA) was used to identify the dark-staining elastin using the Magic Wand Mask Tool, and the percent of the elastin-containing area was determined for 3 to 5 different regions for each animal.
RNA analysis
A piece of frozen ascending aorta that weighed 50 to 100 mg was homogenized for 30 seconds with a Braun Mikro-Dismembrator (Braun Biotech International, Melsungen, Germany), and 1 ml of Trizol was added and RNA was isolated. Reverse transcription (RT) was performed on 1 µg of DNase I-treated RNA with an oligo (dT) 20 primer using a Superscript III kit from Invitrogen Corp. (Carlsbad, CA) in a 20 µl volume, followed by real-time PCR on 0.4 µl of each cDNA sample per well using SYBR green reagents from Applied Biosystems (Foster City, CA). The primers are listed in Supplementary Table 1 of the supplementary methods section. The percent of a test RNA to that of β-actin was calculated by subtracting the cycle to reach the threshold (CT) for a gene from the CT for a separate real-time assay using β-actin primers to determine the ΔCT, and the formula: Percent β-actin = (100) × 2ΔCT. To determine the ratio of the gene in MPS animals to that in normal animals, the percent β-actin for MPS animals was divided by the percent β-actin in normal animals.
Cathepsin and MMP-12 assays
For the cathepsin S assay, frozen aortas were homogenized with a hand-held homogenizer in 100 mM sodium acetate pH 5.5 containing 2.5 mM ethylenediaminetetraacetic acid (EDTA), 0.01% Triton X-100, and 2.5 mM dithiothreitol (DTT), and centrifuged at 10,000 g for 5 minutes at 4°C. Approximately ~0.3 µg of the supernatant was incubated with 100 µM benzyloxycarbonyl-L-phenylalanyl-L-arginine-7-amido-4-methylcoumarin (Z-Phe-Arg-AMC) from Anaspec (San Jose, CA) at pH 7.5 in 100 mM sodium acetate with 2.5 mM EDTA, 0.01% Triton X-100, and 2.5 mM DTT in a microtiter plate at 37°C [22, 45]. The amount of product was determined by excitation at 355 nm and emission at 460 nm using kinetic readings and comparison with 7-Amino-4-methylcoumarin standards from Anaspec. One unit (U) of enzyme released 1 nmoles of the product per hour at 37°C. The protein concentration was determined with the Bradford assay (BioRad Laboratories, Hercules CA). The cathepsin B assay was performed using the same extracts and the substrate Z-Arg-Arg-AMC at pH 6.0, while the total cathepsin assay used the substrate Z-Phe-Arg-AMC at pH 5.5. Cathepsin inhibitors were from Calbiochem (San Diego, CA) and included the cathepsin S inhibitor Z-FL-COCHO (Product #219393), the cathepsin K inhibitor I [1,3-Bis(N-carbobenzoyloxy-L-leucyl)amino acetone; Product #219377], and the cathepsin L inhibitor VI [N-(4-Biphenylacetyl)-S-methylcysteine-(D)-Arg-Phe-b-phenethylamide; Product #219495]. Samples were incubated with the inhibitor for 10 minutes prior to starting the assay. An MMP-12 assay kit (EnzolyteTM 490 MMP-12) was obtained from Anaspec for which the substrate can also be cleaved by MMP-1, 2, 3, 8, and 13 and was performed as described previously [22].
Statistics
The Student’s t test compared values between 2 groups, and ANOVA with Tukey post-hoc analysis compared values between 3 groups using Sigma Stat software (Systat Software, Inc., Point Richmond, CA).
Results
Aortic dilatation in MPS VII dogs
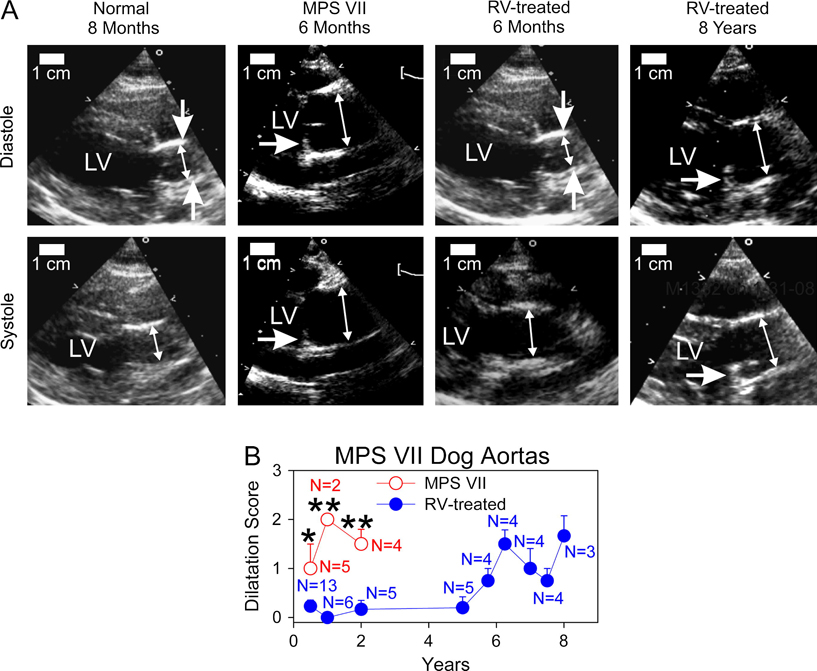
The progression of aortic dilatation was evaluated by echocardiogram for untreated MPS VII dogs and for MPS VII dogs that received IV injection of the RV designated hAAT-cGUSB-WPRE at 2 to 3 days of age (RV-treated). The RV-treatment resulted in transduction of liver cells, which secreted M6P-modified GUSB into blood, which could circulate to other organs and be taken up via the M6P receptor and reduce the accumulation of GAGs. All RV-treated animals that were evaluated here had stable GUSB activity in serum for the duration of evaluation, which was as long as 8 years for some dogs. As previously reported, the aorta GUSB activity averaged 16 U/mg (17.5% of normal) at 6 months of age for 2 RV-treated MPS VII dogs with an average of 241 U/ml (113% normal) of serum GUSB activity [27]. The relatively low activity in the aorta as compared with serum likely reflects poor diffusion of GUSB into the aorta. Fig. 1A shows representative images of echocardiograms in diastole and systole, while Fig. 1B quantifies the degree of aortic dilatation using a scale from 0 (normal) to +4 (severely dilated). A normal ascending aorta, that was evaluated at 8 months, had a discrete narrowing at the sinotubular junction (vertical arrows) during diastole, and showed expansion to a more tubular shape during systole. The aorta from an untreated MPS VII dog, that was evaluated at 6 months, did not have sinotubular narrowing during diastole, and was dilated throughout the cardiac cycle. In addition, the aortic valve was thickened in the MPS VII dog and had reduced motion during systole, although there was no evidence of aortic stenosis. The aorta from a 6 month-old RV-treated MPS VII dog with 317 U/ml (148% normal) of serum GUSB activity appeared normal. However, the aorta from an 8 year-old RV-treated dog with 111 U/ml (52% normal) of serum GUSB activity was dilated throughout the cardiac cycle and lost the narrowing at the sinotubular junction during diastole, while the aortic valve showed thickening and a reduced range of motion.
Fig. 1. Aorta diameters in MPS VII dogs.
Echocardiograms were performed for normal dogs, untreated MPS VII dogs, and MPS VII dogs that received IV injection of 0.3 to 1×1010 TU/kg of the RV designated hAAT-cGUSB-WPRE at 2 to 3 days after birth (RV-treated). The latter achieved stable expression of GUSB activity in serum for the duration of evaluation. A. Representative images of aortas. Images are shown for one normal dog at 6 months (aortic dilatation score 0), one of 5 untreated MPS VII dog at 6 months (score +2), one of 13 RV-treated MPS VII dog at 6 months (score 0; dog M2420 with 317 U/ml of serum GUSB activity), and one of three RV-treated MPS VII dog that was evaluated at 8 years (score +2; M1332 with 111 U/ml of serum GUSB activity). The double-headed arrows indicate the inner diameters of the aorta at the position where dilatation was evaluated during diastole (top) and systole (bottom), and the left ventricle (LV) is indicated. For some images, the vertical single-headed arrows identify the narrowing of the aorta seen at the sinotubular junction in diastole, which is a normal finding. For other images, the horizontal single-headed arrow identifies a thickened base of the aortic valve that shows little movement during systole. The size marker represents 1 cm. B. Average aortic dilatation scores. Aortas were scored from 0 (normal) to +4 (severe dilatation) for the indicated (N) number of untreated MPS VII and RV-treated MPS VII dogs as shown in panel A, and the average +/− standard error of the mean (SEM) plotted. Normal dogs are not shown but generally have scores of 0. Untreated MPS VII dogs do not survive beyond 2 years. The Student’s t-test was used to compare values in the 2 groups at each time point where MPS VII dogs were evaluated, and * indicates a p value of 0.01 to 0.05, and ** indicates a p value <0.01.
Quantitative analysis showed that aortas from 5 untreated MPS VII dogs were moderately dilated at 6 months with a dilatation score of 1 +/− 0.5 [standard error of the mean (SEM)], and remained dilated to a similar extent at 1 and 2 years with scores of 2 +/− 0 (N=2) and 1.5 +/− 0.3 (N=4), respectively. Aortas from RV-treated MPS VII dogs were relatively normal for the first 5 years after neonatal gene therapy, and were significantly better than in untreated MPS VII dogs (p<0.03 vs. normal at 0.5 to 2 years). However, the aortas of RV-treated dogs were dilated thereafter, with a severity score of 1.7 +/− 0.4 at 8 years. Since all RV-treated dogs that were evaluated at late times had stable serum GUSB activity for the duration of evaluation, aortic dilatation at late ages was likely due to an incomplete effect of the gene therapy rather than a loss of expression at late times. We conclude that neonatal gene therapy can reduce and delay the onset of aortic dilatation in MPS VII dogs, but dilatation does occur at late times.
Elastin in MPS VII aortas
To evaluate the time course of development of abnormalities in ECM proteins, aortas were obtained at various ages from normal dogs, untreated MPS VII dogs, and MPS VII dogs that received neonatal gene therapy. Aortic valves from untreated MPS VII dogs that were 1 year or older, or from RV-treated MPS VII dogs that were 4 years or older, were difficult to cut, which was likely due to the calcification noted on radiographs (data not shown). This was consistent with the poor movement of the aortic valves in such dogs.
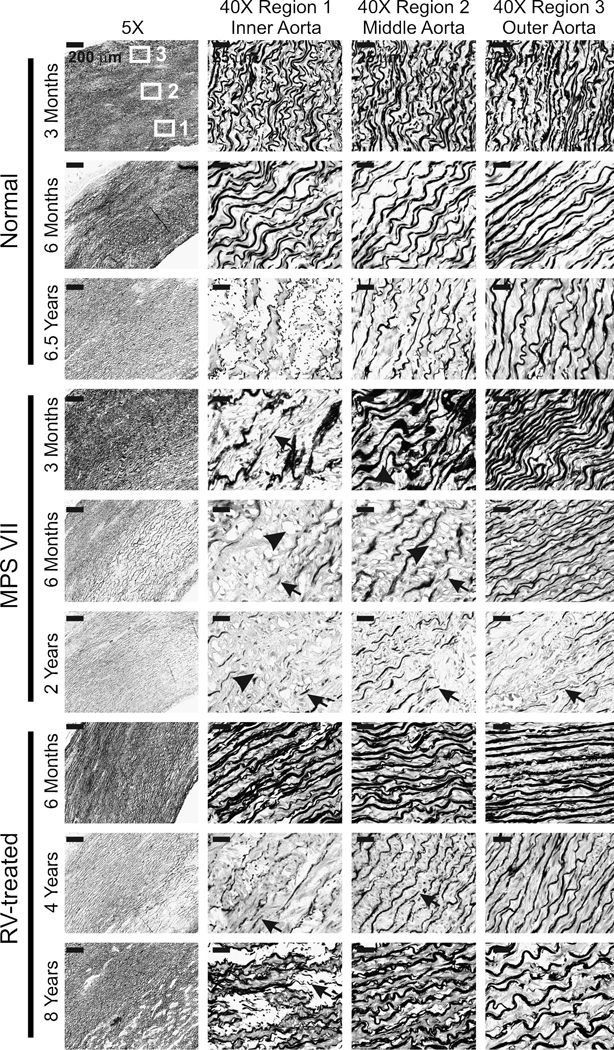
Paraffin-embedded sections of aortas were evaluated with a VVG stain to identify elastin as dark fibers, as shown in Fig. 2. Normal aortas had intact elastin fibers throughout the thickness of the aorta at 3 months (Fig. 2) and at most other ages that were evaluated (data not shown), although mild fragmentation was observed in the inner region of the aortic media at 6.5 years (Fig. 2). Fig. 3 quantifies the amount of elastin in the inner region of the aortic media by using computer software to determine the percent of the area that stained very dark with VVG. For normal dogs, 24 +/− 2% (SEM) of Region 1 of the aortic media (see upper left panel of Fig. 2 for the location of different regions) was elastin at 3 months, and 21 +/− 1% of Region 1 was elastin at 6 months or older.
Fig. 2. Elastin stain in MPS VII dog aortas.
Aortas were obtained from normal, untreated MPS VII, or RV-treated MPS VII dogs. Sections were stained with VVG, which stains elastin dark, and representative examples are shown here, which are quantified for all animals that were evaluated in Fig. 3. The left panels show low power images, where the intima is located at the lower right and the adventitia is at the upper left, and the scale bar is 200 µm. The boxes identify Region 1 (inner media), Region 2 (middle media), and Region 3 (outer media) where high power images were obtained, which are shown in the 3 columns to the right, where the scale bar represents 25 µm. In some panels, the black arrows identify fragmented elastin fibers and black arrowheads identify the clear-appearing lysosomal storage. For RV-treated dogs, M2420 (317 U/ml of serum GUSB activity), M1653 (88 U/ml), and M1337 (281 U/ml) were evaluated at 6 months, years, and 8 years, respectively.
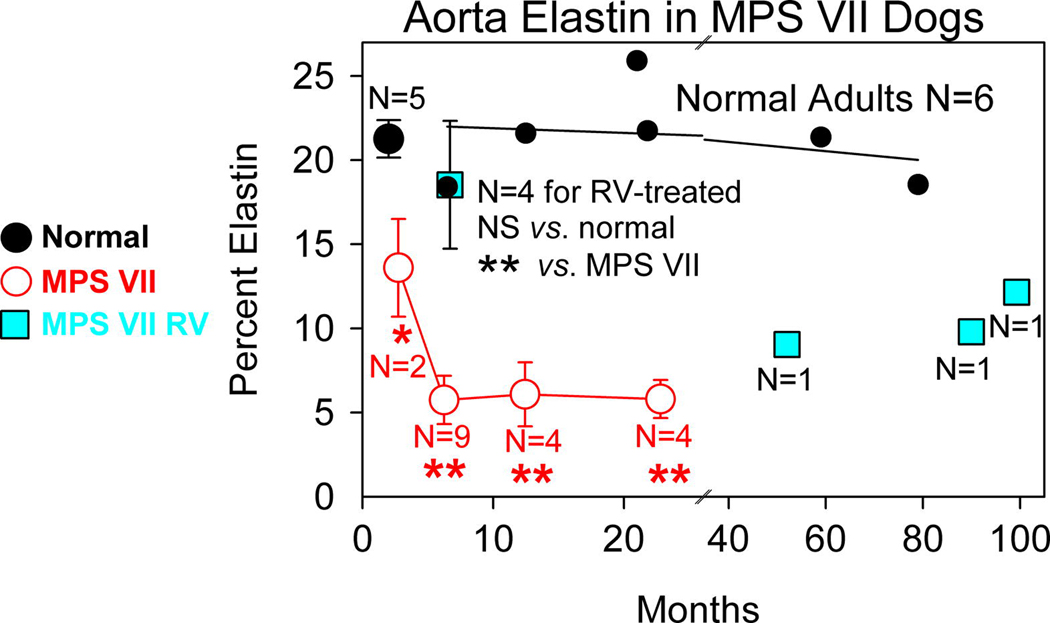
Fig. 3. Quantitation of elastin in MPS VII dog aortas.
Computer software was used to determine the percent of the inner region of the aorta that consisted of elastin , as detailed in the methods section, and as shown for representative examples in Fig. 2. For normal dogs, 5 animals were evaluated at 3 months and are shown as the black circle that shows the average percentage elastin +/− SEM , while 6 adult dogs are shown as individual black circles, with the linear regression line shown for those values. The indicated number of MPS VII dogs were evaluated at the indicated age; values for two 3 month-old MPS VII dogs compared with those from five 3 month-old normal dogs, and values from the nine 6 month-old, four 12 month-old, and four 24 month-old MPS VII dogs were compared with the values in six adult normal dogs using the Student’s t test. Four RV-treated MPS VII dogs were evaluated at 6 months (M1312, M2420, M2427, and M2428 with 281, 317, 1227, and 752 U/ml of serum GUSB activity, respectively) and values were compared with 6 month-old MPS VII dogs and with adult normal dogs. Three additional RV-treated MPS VII dogs were evaluated at older ages (M1653 at 4 years with 88 U/ml, M1328 at 7.7 years with 503 U/ml, and M1337 at 8.3 years with 281 U/ml) and are shown as individual data points.
MPS VII aortas had modest elastin fragmentation (arrow) at 3 months of age that was limited to the inner third of the media, where 14+/−3% of the aorta was elastin, which was significantly lower than in normal dogs at the same age (p=0.04). Elastin fragmentation in MPS VII dogs was severe in the inner third at 6 months, where elastin represented just 6+/−1% of the total area (p<0.001 vs. normal; N=4). In addition, elastin fragmentation extended to involve the middle region of the aortic media (Region 2) at 6 months, although there was substantial variation in the severity for individual animals. Lysosomal storage was also visible as clear regions (arrowheads) in aortic SMC in the inner and middle regions of the media at 6 months. At 2 years, elastin fragmentation remained severe in the inner region of the aortic media, where elastin represented 6 +/− 1% of the aorta (p<0.001 vs. normal), and fragmentation generally extended throughout the entire thickness, although variation among individual animals was again observed. Older dogs were not evaluated, as untreated MPS VII dogs do not survive beyond 2 years.
RV-treated dogs were first evaluated at 6 months of age, when elastin fragmentation was very mild and limited to the inner fifth of the aorta, and elastin represented 19 +/− 8% of the area of the inner aortic media (not significant vs. normal; p=0.002 vs. untreated MPS VII). Three additional RV-treated dogs were evaluated at 52, 90, and 99 months (individual symbols in Fig. 3), at which time all animals had some elastin fragmentation in the inner region of the aortic media. Elastin fragmentation was somewhat worse in a 4 year-old RV-treated MPS VII dog with relative low serum GUSB activity of 88 U/ml (41% normal; M1653), than in an 8 year-old RV-treated MPS VII dog with somewhat higher serum GUSB activity of 281 U/ml (131% normal; M1337). The average percent of the inner media of aorta that was elastin in the older RV-treated dogs was 10+/−1% at an average age of 6.7 years, which was significantly lower than in normal adult dogs (p=0.001), but was significantly higher than in untreated MPS VII dogs at 2 years (p=0.03). In sum, untreated MPS VII dogs had modest elastin fragmentation that was restricted to the inner region of the media at 3 months. Increasing age resulted in an increase in the severity of fragmentation and an extension of the region with elastin fragmentation to the middle and outer regions of the aorta. RV-treated dogs had reduced elastin fragmentation compared with untreated MPS VII dogs, but some abnormalities still developed in the inner regions of the aortas.
Collagen
Another major ECM protein of the aorta is collagen. On picrosirius red-stained sections, collagen appeared red with regular light, and yellow, red, or green with polarized light (Supplementary Figure 1). The red and the green light signal above background was quantified, which demonstrated that the amount of collagen was similar for normal, untreated MPS VII, and RV-treated MPS VII dogs at all ages of evaluation (data not shown).
RNA analysis in MPS VII dogs
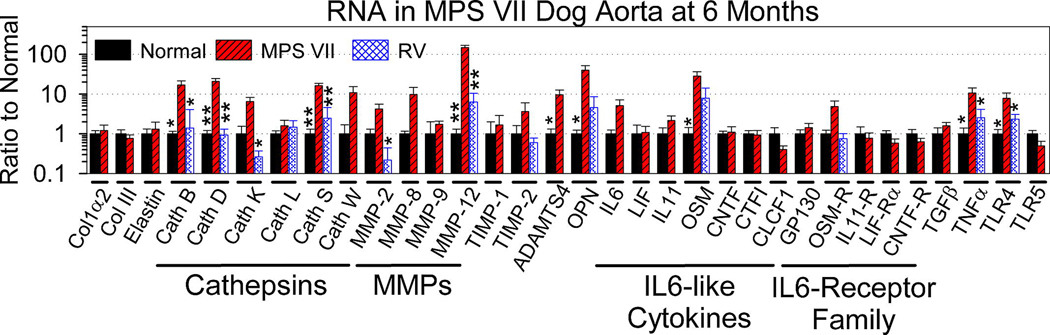
To try to identify the etiology of aortic dilatation in MPS VII dogs, transmural pieces of frozen ascending aortas were homogenized, and RNA was tested for levels of a variety of genes that play a role in elastin or collagen biogenesis or degradation using reverse transcriptase real-time PCR. Most genes with mRNA levels that were elevated in MPS VII dogs were also tested in RV-treated dogs to determine if treatment could prevent the abnormalities from occurring. Supplementary Table 1 shows the primers that were used and quantifies expression levels as a percentage of the β-actin levels, which gives a measure of the abundance of the mRNA. Fig. 4 shows the levels of each gene in untreated MPS VII and in some cases RV-treated MPS VII dogs relative to that in normal dogs. Levels of elastin, collagen Iα2, and collagen III mRNA were similar in normal and MPS VII dogs, suggesting that expression of these genes was not affected by the disease.
Fig. 4. mRNA levels of genes involved in extracellular matrix and signal transduction in MPS VII dog aortas.
Real-time RT-PCR was used to determine the amount of each mRNA in the aorta for samples from 4 normal and 9 untreated MPS VII dogs that were isolated at 6 months of age, and the ratio of expression to that in normal dogs was determined. For genes whose expression was elevated in MPS VII dogs and was sufficiently abundant for accurate evaluation, levels were also evaluated in 7 MPS VII dogs that received neonatal gene therapy with an RV; 3 were sacrificed at 6 months of age (M2420, M2427, and M2428 with 317, 1227, and 752 U/ml of serum GUS activity, respectively), and 1 dog each was sacrificed at 24 months (M2065 with 2089 U/ml), 52 months (M1653 with 88 U/ml), 92 months (M1328 with 503 U/ml), and 99 months (M1337 with 281 U/ml). Since there were no major differences for samples isolated at different ages, these values were pooled. When only 2 groups were evaluated, statistical comparisons were between values from normal and untreated MPS VII dogs using the student’s T test and are indicated with the number of asterisks about the bar for normal mice, where * indicates a p value of 0.01 to 0.05, and ** indicates a p value <0.01. When 3 groups were evaluated, ANOVA with Tukey post-hoc analysis was used to compare values between normal dogs and RV-treated dogs with those in untreated MPS VII dogs. Abbreviations are collagen 1α2 (Col1α2), collagen III (Col III), cathepsin (Cath), and a disintegrin and metalloproteinase with a thrombospondin type 1 motif (ADAMTS).
Several cathepsins were tested for mRNA levels. Cathepsin S mRNA was increased to 16 +/− 2-fold (SEM) normal in MPS VII dogs (p=0.001 vs. normal), and was quite abundant in MPS VII dogs at 25% of the levels of β-actin. Cathepsin S is a cysteine protease with elastin-degrading activity that maintains reasonable stability at neutral pH. mRNA levels for cathepsin B were markedly elevated at 17 +/− 4-fold normal (p=0.02 vs. normal) in MPS VII dogs and were abundant at 169% of the levels of β-actin, although this cathepsin is not stable at neutral pH. Levels of mRNA for the cysteine proteases cathepsin K (6 +/− 2-fold normal) and cathepsin W (11 +/− 5-fold normal) appeared to be elevated in aortas in MPS VII dogs, but neither of these was significantly different from values in normal dogs, and the absolute levels of the mRNAs were relatively low in MPS VII dogs at <1% of β-actin. Levels of the cysteine protease cathepsin L mRNA were ~2-fold normal (not significant vs. normal) and were relatively low at ~3% of β-actin levels in MPS VII dogs (Fig. 4), while levels of cathepsin V mRNA were too low to quantify, but did not appear to be increased in MPS VII dogs (Supplemental Table 1). mRNA levels for cystatin C, a protein inhibitor of cysteine cathepsins, were low in both normal and untreated MPS VII dogs. Cathepsin D is a lysosomal aspartic protease that can activate other cathepsins and has a low pH optimum. Cathepsin D mRNA was markedly increased at 21 +/− 4-fold normal, and was abundant at 12% of β-actin (p=0.001 vs. normal). Levels of cathepsins B, D, K, and S were significantly reduced in RV-treated MPS VII dogs compared with untreated MPS VII dogs (p<0.02).
MMP mRNA levels were also determined, as shown in Fig. 4 and Table 1. mRNA for MMP-12, which has potent elastase activity, was increased to 148 +/− 21-fold normal and was abundant at 10% of the level of β-actin in aortas in untreated MPS VII dogs (p<0.001 vs. normal). Levels of MMP-2 were 4 +/− 1-fold normal and were abundant in untreated MPS VII dogs at 35% of β-actin, although this was not significantly higher than the values in normal animals. mRNA for MMP-3, MMP-8, MMP-9, and MMP-13 were all relatively low in MPS VII dogs at <0.065% of β-actin, and were not significantly different from values in normal dogs, while mRNA for MMP-1 was undetectable in both normal and untreated MPS VII dogs. Levels of mRNA for natural inhibitors of MMPs known as tissue inhibitor of metalloproteinase 1 (TIMP1) and TIMP2 were not significantly different from the values in normal dogs. mRNA for osteopontin (OPN), a protein that can activate some MMPs in a non-proteolytic fashion [4646], was markedly increased in MPS VII dogs to 40 +/− 12-fold normal (p=0.04) and was abundant at 87% of β-actin. Enzymes of the ADAMTS family are metalloproteinases that can degrade ECM proteins. ADAMTS4 was increased to 10 +/− 7-fold normal (p=0.05 vs. normal) and was present at 0.9% of β-actin levels in MPS VII dogs, while ADAMTS5 levels were too low to quantify in both groups. RV-treated MPS VII dogs had a significant reduction in MMP-12 mRNA levels to 6 +/− 4-fold normal (p<0.001vs. untreated MPS VII dogs; not significant vs. normal). OPN mRNA was reduced to 5 +/− 4-fold normal in RV-treated MPS VII dogs, although this was not significantly different from the values in untreated MPS VII or normal dogs.
RNA levels of signal transduction genes
We previously demonstrated that aortas from MPS I mice had increased phosphorylation of the transcription factors known as signal transducer and activator of transcription 1 (STAT1) and STAT3 [22]. Since one mechanism for activation of STATs involves binding of members of the interleukin (IL)-6 family of cytokines to the IL-6 receptor or related receptors, we tested mRNA levels for these family members in MPS VII dog aortas. mRNA for the IL-6-like cytokine oncostatin M (OSM) was increased in MPS VII dog aortas to 28 +/− 8-fold normal (p=0.04), and was present in MPS VII dogs at 0.16% of the level of β-actin. IL-6 was 5 +/− 2%-fold normal (0.073% of β-actin) in MPS VII dog aortas, although this was not significantly different from the values in normal dogs. mRNA levels of other IL-6-like cytokines such as IL-11, leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), cardiotrophin 1 (CTF1), and cardiotrophin-like cytokine 1 (CLCF1) were similar for samples from MPS VII and normal dogs. mRNA levels for cytokine receptors were also determined. mRNA for the OSM receptor (OSM-R) represented 1% of β-actin levels for the MPS VII dogs and was 5 +/− 2-fold normal in MPS VII dogs, although this was not significantly different from the value in normal dogs. Levels of glycoprotein 130 (GP130; 1.4-fold normal), the IL-11 receptor (IL-11 R; 0.8-fold normal), the LIF receptor α (LIF-Rα; 0.8-fold normal), and the CNTF receptor (CNTF-R; 0.6-fold normal) were not elevated in MPS VII dogs. In RV-treated MPS VII dogs, OSM mRNA levels were reduced to 9 +/− 6-fold normal, although these values were not significantly different from those in untreated MPS VII dogs, while the reduction in OSM-R mRNA levels to 0.7 +/− 0.3-fold normal was similarly not significant when compared with values in untreated MPS VII dogs.
Other cytokine genes were also evaluated for mRNA levels. Tumor necrosis factor α (TNFα) mRNA levels were elevated at 11 +/− 4-fold normal in MPS VII dogs (p=0.05 vs. normal), although transforming growth factor β (TGFβ) levels were not elevated. Toll-like receptor 4 (TLR4) has been proposed to serve as a receptor for GAGs that can activate signaling pathways in MPS [4747]. mRNA levels for TLR-4 were 8 +/− 3-fold normal in MPS VII dogs (p=0.05), while levels of TLR-5 were too low to quantify. In RV-treated MPS VII dogs, TNFα mRNA was reduced to 3 +/− 2-fold normal, while TLR4 mRNA was reduced to 2 +/− 1-fold normal (p=0.05 vs. untreated MPS VII, not significant vs. normal for both genes). mRNA for interferon γ (IFN-γ) and IL-1β were undetectable in both normal and untreated MPS VII dogs (Table 1). We conclude that upregulation of cytokines might contribute to the increased expression of cathepsin S and MMP-12 in MPS VII dogs, and that neonatal gene therapy can reduce levels of some of these cytokines. RV mRNA sequences were not detected in the aorta of RV-treated MPS VII dogs (Table 1), suggesting that improvements in the aorta were due to diffusion of enzyme from blood rather than transduction of the aorta itself.
Cathepsin S activity
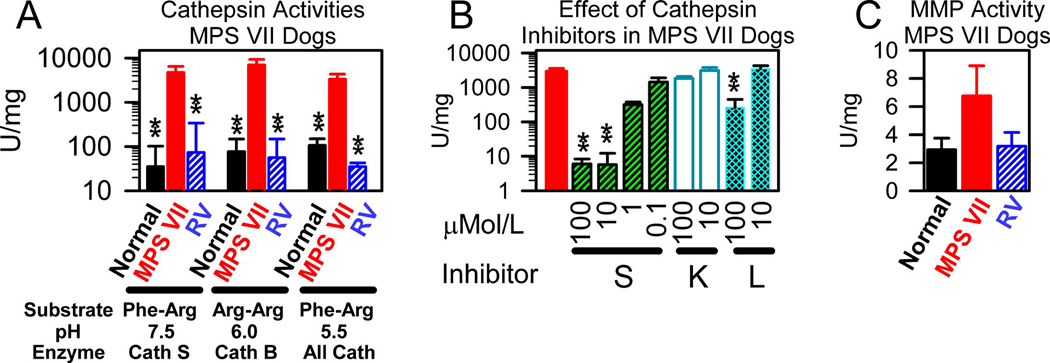
Since mRNAs for several cathepsins of the cysteine protease class were markedly elevated in aortas from MPS VII dogs, extracts were tested for cathepsin activity using 3 assays, as shown in Fig. 5A. The cathepsin S assay used the substrate Z-Phe-Arg-AMC, which is cleaved by many cathepsins, but was performed at pH 7.5, where human cathepsin S maintains 64% of its peak activity at 1 hour [48], but other cathepsins are unstable. The cathepsin B assay used the substrate Z-Arg-Arg-AMC, which is specifically cleaved by cathepsin B. The assay for total cathepsin activity used the substrate Z-Phe-Arg-AMC at pH 5.5, where most cathepsins are stable and active. Cathepsin S activity was markedly elevated in untreated MPS VII dogs at 4733+/−1781 U/mg, which was 99-fold as high as the value in normal dogs of 48+/−21 (p<0.001). Cathepsin B activity was also markedly elevated in untreated MPS VII dogs at 7013+/−2367 U/mg, which was 64-fold the value in normal dogs of 109+/−63 U/mg (p<0.001 vs. normal), while total cathepsin activity was increased in MPS VII dogs at 3342+/−1014 (31-fold normal, p=0.005 vs. normal). Although the nmoles of substrate produced per hour, and hence the U of enzyme activity, may vary with different substrates and pH values and, thus, activities are not necessarily additive, these data suggest that cathepsin B and S might account for most of the elevation in the activities of cathepsins of the cysteine protease class. RV-treated MPS VII dogs had reductions in all cathepsin activity assays to near-normal (p<0.001 vs. untreated MPS VII, and not significant vs. normal).
Fig. 5. Cathepsin and MMP activities in MPS VII dogs.
Ascending aortas were isolated at 6 months after birth from 6 normal and 5 untreated MPS VII dogs, and from 2 RV-treated MPS VII dogs at 25 months (M2065; 2090 U/ml of serum GUSB activity) and at 52 months (M1653; 88 U/ml) of age and homogenenates were prepared. A. Cathepsin activity. Cathepsin assays were performed with Z-Phe-Arg-AMC (Phe-Arg) or Z-Arg-Arg-AMC (Arg-Arg) at the indicated pH to measure primarily activities for cathepsin S (Cath S), cathepsin B (Cath B), or several cathepsins (All Cath) as indicated, and the mean activity +/− SEM is shown. Statistics were performed with ANOVA with Tukey post-hoc analysis, with ** indicating a p value <0.01 for comparison of values from the indicated group with those in untreated MPS VII dogs. B. Effect of cathepsin inhibitors. Only samples from 3 untreated MPS VII dogs were analyzed for the effect of specific cathepsin inhibitors at the indicated final concentrations upon enzyme activity using the substrate Z-Phe-Arg-AMC at pH 7.5, which primarily measures cathepsin S activity. Values with the inhibitor were compared with values without the inhibitor using the Student’s t-test. C. MMP-12 activity. Average MMP-12 activity ± SEM was determined.
The identity of the cathepsin(s) with activity at pH 7.5 is of particular importance, as elastin is located in the ECM and would be expected to be at a neutral pH. To further evaluate the identity of the cathepsin with activity at pH 7.5, cathepsin inhibitors were used to determine the effect upon activity in extracts from MPS VII dogs, as shown in Fig. 5B. The activity was almost completely inhibited with 10 µM or more of the cathepsin S inhibitor, while 1 µM of the cathepsin S inhibitor reduced activity in MPS VII dogs to 11+/−2% of the activity found with no inhibitor. In contrast, a cathepsin K inhibitor had no effect on the cathepsin activity at pH 7.5, while a cathepsin L inhibitor had no effect at 10 µM, although 100 µM did reduce activity to 7+/−5% of the value with no inhibitor. These data support the hypothesis that the cathepsin with activity at pH 7.5 represents cathepsin S.
MMP-12 activity
Ascending aorta extracts from MPS VII dogs were tested for MMP activity using a substrate that can be cleaved by MMP-12 to release a fluorescent molecule. MMP-12 activity was only 2.3-fold normal in MPS VII dogs at 6 months, which was not significant vs. normal, as shown in Fig. 5C. This result seems discordant with the fact that MMP-12 mRNA levels were 148 +/− 21-fold normal in MPS VII dogs. Since MMP-12 requires activation by proteolysis or non-proteolytic mechanisms, it is possible that such activation did not occur. Alternatively, it is feasible that the canine version of MMP-12 has a different substrate preference than human MMP-12.
MPS I dogs
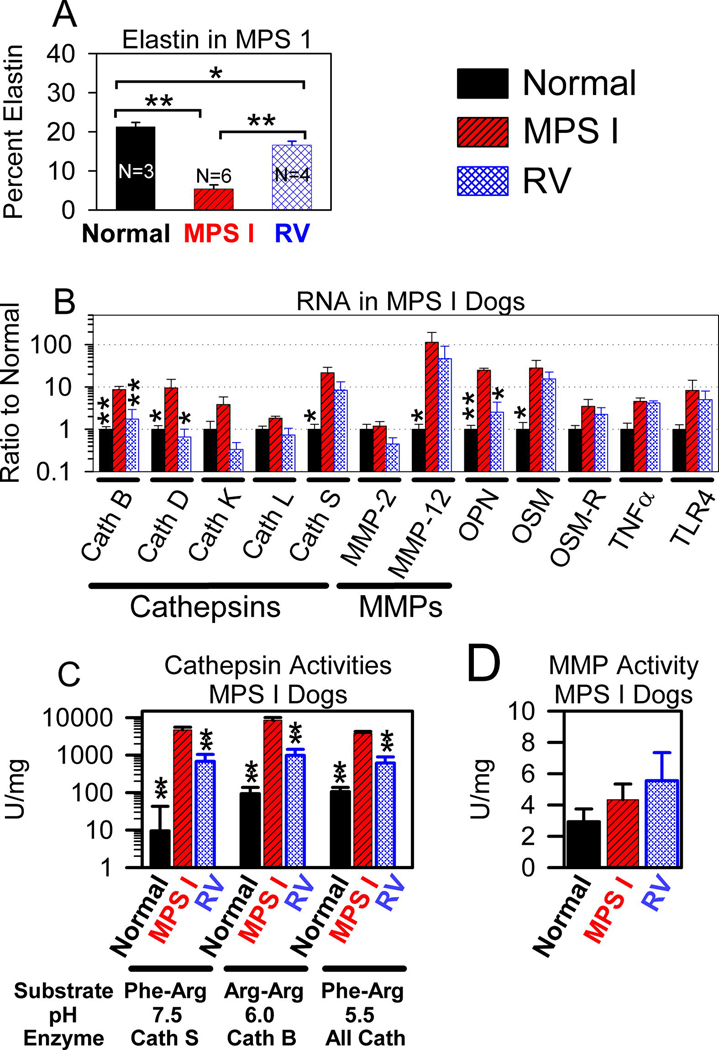
MPS I dogs were analyzed in a fashion analogous to that in MPS VII dogs. We previously reported that the aortic dilatation score for untreated MPS I dogs was 1.0 ± 0.8 on a scale of 0 (normal) to 4 (severely dilated) at 1 year of age, which was significantly higher than the value of 0±0 (P=0.015) in RV-treated MPS I dogs [14]. Furthermore, untreated MPS I dogs had substantial fragmentation of elastin fibers in the aorta at 1 to 1.75 years of age, which was eliminated in RV-treated dogs with very high serum IDUA activity (553 to 888 U/ml; 5-fold normal) and 3 U/mg of IDUA activity in the aorta (26% normal), and was only partly reduced in RV-treated dogs with lower serum IDUA activity (~25 U/ml; 2-fold normal) and 1 U/ml of IDUA activity in the aorta (9% normal) [14]. Fig. 6A shows quantitative analysis of VVG (elastin) stain of aortas from untreated MPS I dogs at 1 to 1.5 years, where only 5.4 +/− 1% of the aorta was elastin in the inner region of the aorta, which was significantly lower than the value in adult normal dogs of 21 +/− 1% (p<0.001), and was significantly lower than the value of 17 +/− 1% in RV-treated MPS I dogs (p<0.001). However, the percentage of the aorta that was elastin in the RV-treated MPS I dogs was significantly lower than in normal dogs (p=0.03).
Fig. 6. Evaluation of MPS I dog aortas.
Some MPS I dogs received neonatal IV injection of 1×1010 TU/kg of the RV hAAT-cIDUA-WPRE (RV-treated) as previously described [14], while other MPS I and normal dogs were untreated. Aortas were evaluated at 1 to 1.8 years of age. A. Elastin fragmentation. Elastin fragmentation was quantified in the inner region of the aorta (see Region 1 in Fig. 2) for the indicated number (N) of animals, as described in Fig. 3, and the average +/− SEM is shown. RV-treated MPS I dogs that were evaluated included two dogs with relatively low serum IDUA activity [I-99 with 23 U/ml (2-fold normal) serum IDUA activity and I-101 with 27 U/ml (2-fold normal)], and two dogs with relatively high serum IDUA activity [I-107 with 553 U/ml (43-fold normal), and I-140 with 888 U/ml (68-fold normal)]. The p value determined using ANOVA with Tukey post-hoc analysis for comparison of the groups joined with a bracket are shown. B. mRNA levels. mRNA levels for the indicated genes in aortas of 8 normal, 3 untreated MPS I, and 3 RV-treated MPS I dogs are shown as a ratio to the values found in normal dogs. The RV-treated dogs that were analyzed included two dogs with relatively low expression (I-99 and I-101) and one dog with relatively high expression (I-107). C and D. Cathepsin and MMP-12 enzyme activity. Cathepsin and MMP-12 assays were performed as described in Fig. 5A and 5C for the number of animals described in panel A.
Aortas were also tested for mRNA levels for some of the genes that were evaluated in dogs from the MPS VII colony, as shown in Fig. 6B. Untreated MPS I dogs had significant elevations in mRNA levels for cathepsin S (22 +/− 5-fold normal), MMP-12 (114 +/− 48-fold normal), cathepsin B (9 +/− 2-fold normal), cathepsin D (10 +/− 4-fold normal), OPN (21 +/− 3-fold normal), and the IL-6 like cytokine OSM (28 +/− 14-fold normal). In RV-treated MPS I dogs, mRNA levels for cathepsin B, D, and OPN were significantly reduced when compared with values in untreated MPS I dogs, but values for other genes were not significantly reduced. This may reflect the small number of samples that were evaluated for the MPS I colony, and that 2 of the 3 RV-treated dogs (I-99 and I-101) that were evaluated had relatively low serum IDUA activity and still had some elastin fragmentation [14].
Extracts of aortas from the MPS I colony were also evaluated for cathepsin and MMP enzyme activities, as shown in Figs. 6C and 6D, respectively. Untreated MPS I dogs had marked and significant elevations in cathepsin S, cathepsin B, and total cathepsin activities, and these were significantly reduced in RV-treated dogs. Values in RV-treated dogs were higher than in normal dogs, although this was not significant. As was the case for MPS VII dogs, MMP-12 activity was not significantly elevated in untreated MPS I dogs.
Aortic dilatation and fragmentation in a patient with MPS VII
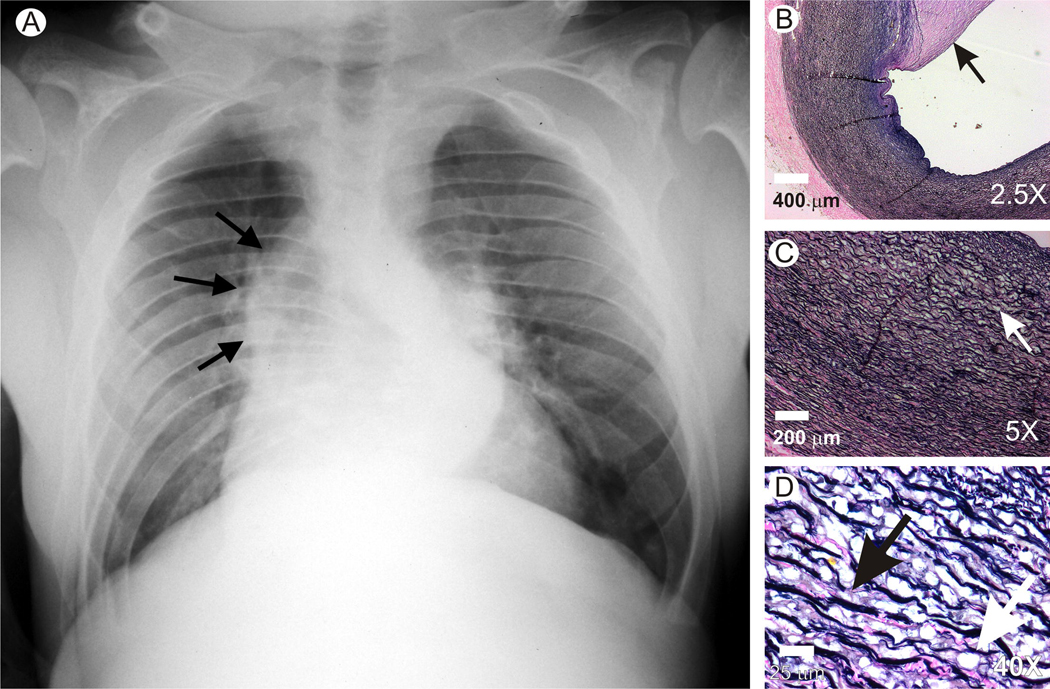
An aorta obtained post-mortem was evaluated from a young man with MPS VII who died suddenly at age 19 years and was found to have aspiration without evidence of aortic rupture or dissection, as reported previously by Vogler et al. in 1994 [19]. He was the first reported patient with MPS VII and was known to have ~1% of normal GUSB activity in organs [19] and ~2% of normal activity in white blood cells [49]. Aortic dilatation was visible on a chest radiograph, as shown in Fig. 7A. A large atheroma was present in the inner region of his aorta, as noted previously [19], and as shown in the VVG stain in Fig. 7B. Fig. 7C shows that some lysosomal storage was present in the inner half of the aortic media, as previously reported [19]. In addition, elastin fragmentation was present, which was better appreciated in the high power view of the inner region of the aorta in Fig. 7D. These observations demonstrated that elastin fragmentation and dilatation also occur in the aorta of patients with MPS VII.
Fig. 7. Evaluation of aorta from a patient with MPS VII.
This patient was the first to be diagnosed with MPS VII [19, 49]. A. Chest radiograph. At 18 years-of-age, a posterior-anterior radiograph of this patient demonstrates dilatation of the ascending aorta, as indicated by the black arrows. B to D. Elastin stain. The patient died at 19 years of age and an autopsy was performed. Sections of the aorta were stained with VVG. B. Atheroma. A low power view demonstrated a large atheroma (arrow) on the inner surface of the aorta. Scale bar = 400 µm. C. Lysosomal storage at low power. The white arrow identifies a region in the inner third of the aorta with lysosomal storage and elastin fragmentation. The intima is at the upper right and the adventitia is at the lower left. Scale bar = 200 µm. D. Lysosomal storage and elastin fragmentation at high power. A high power image was obtained from the inner aortic media. The black arrow identifies fragmented elastin while the white arrowhead identifies the clear-appearing lysosomal storage. Scale bar = 25 µm.
Discussion
Aortic dilatation in MPS is important, as it will likely result in aortic dissection and possibly death as patients live longer after treatment with HSCT or ERT. Determining the pathogenesis of the elastin fragmentation that is likely responsible for this dilatation might lead to the identification of a drug that could block this process. In addition, the natural history of aortic disease must be understood to determine if gene therapy can prevent the associated complications.
Time course and severity of aortic abnormalities in MPS dogs
Aortas from MPS VII dogs were dilated at 6 months of age, and appeared to be somewhat more dilated at 2 years, although values at late times were not significantly different from values at 6 months due to the small number of animals evaluated. It should be noted that the aortic dilatation scores reported here by MS in untreated MPS VII dogs were lower than the scores reported previously by a different cardiologist [26–28], which could reflect inter-observer variation or the presence of some gene(s) that ameliorates aortic disease in the recent studies in these outbred dogs. Elastin fragmentation was severe in the inner regions of the aortic media at 6 months, and extended to involve more of the aorta at later times in MPS VII dogs. In addition, some MPS VII dogs were evaluated histologically soon after birth, when elastin fragmentation was not apparent (data not shown), although the analysis was more difficult to perform as the inner region of the aorta from both normal and MPS VII dogs appeared more disrupted than in older animals, which may reflect incomplete cross-linking in newborns. There were no overt collagen abnormalities detected with picrosirius red stain with regular and polarized light (supplementary Fig. 1), or with Masson’s trichrome stain (data not shown). Evaluation of the time course for aortic dilatation and elastin fragmentation was not possible in MPS I dogs, as we had not maintained this colony for as long, and fewer samples were available. Although neither MPS VII nor MPS I dogs appear to die frequently of aortic disease or heart failure, they are usually sacrificed for humane reasons due to bone and joint disease, and may have developed these cardiovascular manifestations if they had been allowed to live longer.
Neonatal IV administration of an RV dramatically reduced aortic disease for a few years in MPS I and MPS VII dogs, as reported previously [13–14, 27–28]. However, aortic dilatation was present in RV-treated MPS VII dogs 6 years or later despite the continued stable expression of GUSB activity in serum, which demonstrated that this gene therapy approach was not fully effective in the aorta long-term. In addition, elastin fragmentation was visible in the aorta in RV-treated MPS VII dogs at late times, and was inversely related to the serum GUSB activity, although too few animals were evaluated to be certain of the relationship. Occurrence of aortic dilatation late in RV-treated MPS VII dogs calls for more-effective gene therapy or identification of ancillary treatments to prevent aortic disease. Similarly, HSCT and ERT have not prevented aortic disease in large animal models or children with MPS, as reviewed in the introduction.
The role of cathepsin S and MMP-12 in aortic elastin fragmentation
Elastin fiber fragmentation in MPS could be due to defects in elastin assembly or to elastin degradation. Hinek et al. proposed that elastin defects in MPS I were due to abnormalities in assembly, and suggested that accumulation of heparan sulfate was likely responsible [36]. However, although we cannot rule out a role for elastin assembly in the elastin defects in MPS I and MPS VII dogs, we favor the hypothesis that destruction is the major process. In fact, lysosomal storage and the appearance of elastin fragmentation were modest at the time of puberty (~ 6 months), and became progressively worse during young adulthood. Since elastin formation is believed to be largely completed by adulthood [32], it seems likely that degradation plays a more important role.
There are 11 lysosomal cysteine cathepsins, all of which are primarily destined for the lysosome but can also be secreted [50]. Cathepsin S is an excellent candidate for a cysteine protease that could contribute to elastin fragmentation in MPS dog aortas, as cathepsin S has elastase activity and is relatively stable at the neutral pH that is expected to be found in the extracellular space [48]. Indeed, cathepsin S mRNA levels were 17- and 22-fold normal for MPS VII and MPS I dogs, respectively. Furthermore, a cathepsin assay performed at neutral pH (that is likely specific for cathepsin S) demonstrated that activity was 99-fold normal for extracts from aortas of both MPS VII and MPS I dogs, and that this enzyme activity was inhibited for extracts from MPS VII dogs by a cathepsin S inhibitor. Upregulation of cathepsin S mRNA was also found in the aorta of MPS I mice [22], in the aorta of MPS VII mice (J. Metcalf and K. Ponder, unpublished data), and in the brain of MPS I mice [51]. Although mRNA and enzyme activity for cathepsin B were also elevated to a similar extent in both MPS VII and MPS I dog aortas in this study, cathepsin B is not stable at the neutral pH expected to be found in the ECM. In addition, mRNA for the aspartic protease cathepsin D was 21- and 10-fold normal in MPS VII and MPS I dog aortas, respectively. Although cathepsin D does not cleave elastin, it can cleave and activate other cathepsins, and may play a role in the marked elevation of cathepsin S activity.
The matrix metalloproteinase (MMP) family has at least 20 members, of which MMP-2, -7, -8, -9, -10, -12, and -14 have elastase activity [52]. MMP-12 could play an important role in elastin fragmentation, as MMP-12 has elastase activity, and its mRNA was 148- and 114-fold normal in MPS VII and MPS I dogs, respectively. However, it was somewhat puzzling that MMP-12 activity was at most marginally increased in MPS VII and MPS I dogs (2-fold or less), which was not significantly different from that in normal dogs. Similarly, in MPS I mice, the increase relative to normal animals in cathepsin S activity was higher than the relative increase in MMP-12 enzyme activity despite the fact that cathepsin S mRNA was less elevated than MMP-12 mRNA [22]. As MMP-12 needs to be activated by proteolysis or other mechanisms, MPS VII and MPS I dog aortas might fail to upregulate activators of MMP-12. Alternatively, there could be a discrepancy between the mRNA and the protein levels due to MMP-12 protein instability, or the fluorogenic substrate that is cleaved by human MMP-12 might not be recognized by canine MMP-12. MMPs also contributed to joint disease in MPS models [47, 53].
Mechanism for upregulation of cathepsin S and MMP-12 mRNA
Since both cathepsin S [54] and MMP-12 [55] are expressed by vascular SMC in vitro, aortic SMC were probably the source of cathepsin S and MMP-12 expression. The JAK-STAT pathway involves binding of a cytokine to a cell surface receptor, which results in phosphorylation and activation of a member of the JAK family of kinases, which in turn can phosphorylate STATs at specific tyrosines and result in translocation to the nucleus and activation of responsive genes [56]. We previously demonstrated that MPS I mouse aortas activated STAT-1 and STAT-3 by phosphorylation, and proposed that this upregulated cathepsin S and MMP-12 mRNA [22]. Indeed, cathepsin S expression can be activated in dendritic cells by IL-6 in a STAT-3-dependent process [57], while the human MMP-12 promoter has a STAT binding sequence [55]. In our study, mRNA for the IL-6-like cytokine OSM was 28-fold normal in MPS VII and MPS I dog aortas, suggesting that this is a cytokine that could contribute to activation of STATs. In addition, there was an increase in TNFα mRNA, which is consistent with the previous studies demonstrating that TNFα mRNA is elevated in joint tissues in MPS [47, 58].
It is important to identify the process that upregulated cytokines in MPS. The TLRs are a family of proteins that bind to various ligands and result in expression of cytokines, at least in part via activation of the JAK-STAT pathway. Indeed, heparan sulfate oligosaccharides from the urine of patients with MPS IIIB induced expression of TNFα and other cytokines in murine microglia cells in vitro, and this depended upon the lipopolysaccharide receptor TLR4 [59], while soluble heparan sulfate stimulated dendritic cell maturation via TLR4 [60]. In addition, Simonaro et al. noted that TLR4 was upregulated in the synovium and cartilage of MPS animals, and proposed that GAGs could activate TLR4 in MPS [47]. The fact that TLR4 was increased to 8-fold normal in the aorta in the canine models of MPS evaluated here is consistent with the hypothesis that TLR4 could play a role in upregulation of cytokines. We, therefore, propose that GAGs activate TLR4 in the aorta, which results in expression of cytokines, activation of STATs, and increased transcription of cathepsin S and MMP-12.
Implications of this study
Our findings suggest that elastin fragmentation and aortic dilatation are ameliorated, but not prevented, by neonatal RV-mediated gene therapy for MPS dogs, which is consistent with data from other investigators that aortic disease is not prevented with HSCT or ERT, as reviewed in the introduction. It is, therefore, likely that some ancillary therapy may be needed to prevent this long-term complication. Since up-regulation of cathepsin S and MMP-12 occurs in MPS I dogs and mice [22], and in MPS VII dogs and mice (JAM and KPP, unpublished data), it is reasonable to propose that upregulation of one or both of these elastases may be pivotal to aortic disease, and could serve as therapeutic targets for inhibitors that are being developed [61–62]. Alternatively, inhibition of signal transduction pathways that were implicated in this study could play a therapeutic role. The existence of canine models of MPS that manifest aortic disease may help to identify therapies that can be effective in patients with MPS.
Supplementary Material
Acknowledgments
We thank Leah Levanduski and Steven Fernadez for assistance in sample collection and animal care, Phil Mason and Monica Bessler for use of a real-time PCR machine, Louis Dehner for help obtaining achieved histopathology samples, and William H. McAlister for help with scanning in a chest radiograph.
Sources of funding
This work was supported by the Ryan Foundation, the MPS Society, and the National Institutes of Health (DK66448, DK54481, HD061879, and RR02512). Histology was supported by P30 DK52574.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: mucopolysaccharidosis (MPS), Online Mendelian Inheritance in Man (OMIM), α-L-iduronidase (IDUA), glycosaminoglycans (GAGs), β-glucuronidase (GUSB), hematopoietic stem cell transplantation (HSCT), mannose 6-phosphate (M6P), enzyme replacement therapy (ERT), intravenous (IV), γ-retroviral vector (RV), extracellular matrix (ECM), elastin binding protein (EBP), matrix metalloproteinase (MMP), smooth muscle cells (SMC), National Institutes of Health (NIH), Verhoeff’s Van Gieson (VVG), reverse transcription (RT), cycle to reach the threshold (CT), dithiothreitol (DTT), benzyloxycarbonyl-L-phenylalanyl-L-arginine-7-amido-4-methylcoumarin (Z-Phe-Arg-AMC), unit (U), ethylenediaminetetraacetic acid (EDTA), standard error of the mean (SEM), tissue inhibitor of metalloproteinase (TIMP), a disintegrin and metalloproteinase with a thrombospondin type 1 motif (ADAMTS), osteopontin (OPN), interleukin (IL), leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin 1 (CTF1), cardiotrophin-like cytokine 1 (CLCF1), glycoprotein 130 (GP130), oncostatin M receptor (OSM-R), IL-11 receptor (IL11-R), the LIF receptor α (LIF-Rα), the ciliary neurotrophic factor (CNTF-R), transforming growth factor β (TGFβ), tumor necrosis factor α (TNFα), interferon γ (IFN-γ), and toll-like receptor (TLR).
References
- 1.Neufeld EF, Muenzer J. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill; 2001. pp. 3421–3452. [Google Scholar]
- 2.Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, Kohlschütter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 3.Boelens JJ, Rocha V, Aldenhoven M, Wynn R, O'Meara A, Michel G, Ionescu I, Parikh S, Prasad VK, Szabolcs P, Escolar M, Gluckman E, Cavazzana-Calvo M, Kurtzberg J. Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with hurler syndrome. Biol Blood Marrow Transplant. 2009;15:618–625. doi: 10.1016/j.bbmt.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Braunlin EA, Stauffer NR, Peters CH, Bass JL, Berry JM, Hopwood JJ, Krivit W. Usefulness of bone marrow transplantation in the Hurler syndrome. Am J Cardiol. 2003;92:882–886. doi: 10.1016/s0002-9149(03)00909-3. [DOI] [PubMed] [Google Scholar]
- 5.Sifuentes M, Doroshow R, Hoft R, Mason G, Walot I, Diament M, Okazaki S, Huff K, Cox GF, Swiedler SJ, Kakkis ED. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab. 2007;90:171–180. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Braunlin EA, Berry JM, Whitley CB. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am J Cardiol. 2006;98:416–418. doi: 10.1016/j.amjcard.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 7.Ponder KP, Haskins ME. Gene therapy for mucopolysaccharidosis. Expert Opin Biol Ther. 2007;7:1333–1345. doi: 10.1517/14712598.7.9.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L L, Mango RL, Sands MS, Haskins ME, Ellinwood NM, Ponder KP. Evaluation of pathological manifestations of disease in mucopolysaccharidosis VII mice after neonatal hepatic gene therapy. Mol Ther. 2002;6:745–758. doi: 10.1006/mthe.2002.0809. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Xu L, Hennig AK, Kovacs A, Fu A, Chung S, Lee D, Wang B, Herati RS, Mosinger OJ, Cai S-R, Ponder KP. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol. Ther. 2005;11:35–47. doi: 10.1016/j.ymthe.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Ma X, Liu Y, Tittiger M, Hennig A, Kovacs A, Popelka S, Wang B, Herati R, Bigg M, Ponder KP. Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation. Mol Ther. 2007;15:889–902. doi: 10.1038/sj.mt.6300112. [DOI] [PubMed] [Google Scholar]
- 11.Herati RS, Ma X, Tittiger M, Ohlemiller KK, Kovacs A, Ponder KP. Improved retroviral vector design results in sustained expression after adult gene therapy in mucopolysaccharidosis I mice. J Gene Med. 2008;10:972–982. doi: 10.1002/jgm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalf JA, Ma X, Linders B, Wu S, Schambach A, Ohlemiller KK, Kovacs A, Bigg M, He L, Tollefsen DM, Ponder KP. A self-inactivating gamma retroviral vector reduces manifestations of mucopolysaccharidosis I in mice. Molecular Therapy. 2009 doi: 10.1038/mt.2009.236. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponder KP, Melniczek JR, Xu L, Weil MA, O'Malley TM, O'Donnell PA, Knox VW, Aguirre GD, Mazrier H, Ellinwood NM, Sleeper M, Maguire AM, Volk SW, Mango RL, Zweigle J, Wolfe JH, Haskins ME. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci USA. 2002;99:13102–13107. doi: 10.1073/pnas.192353499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O'donnell P, Sleeper MM, Vite C, Herati R, Aguirre GD, Haskins M, Ponder KP. Correction of Clinical Manifestations of Canine Mucopolysaccharidosis I with Neonatal Retroviral Vector Gene Therapy. Mol. Ther. 2007;15:1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 15.Xu L L, Haskins ME, Melniczek JR, Gao C C, Weil MA, O'Malley TM, O'Donnell PA, Mazrier H, Ellinwood NM, Zweigle J, Wolfe JH, Ponder KP. Transduction of hepatocytes after neonatal delivery of a Moloney murine leukemia virus based retroviral vector results in long-term expression of beta-glucuronidase in mucopolysaccharidosis VII dogs. Mol Ther. 2002;5:141–153. doi: 10.1006/mthe.2002.0527. [DOI] [PubMed] [Google Scholar]
- 16.Wippermann CF, Beck M, Schranz D, Huth R, Michel-Behnke I, Jüngst BK. Mitral and aortic regurgitation in 84 patients with mucopolysaccharidoses. Eur J Pediatr. 1995;154:98–101. doi: 10.1007/BF01991908. [DOI] [PubMed] [Google Scholar]
- 17.Arn P, Wraith JE, Underhill L. Characterization of Surgical Procedures in Patients with Mucopolysaccharidosis Type I: Findings from the MPS I Registry. J Pediatr. 2009;154:859–864. doi: 10.1016/j.jpeds.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Beaudet AL, Ferrante NM, Ferry GD, Nichols BL, Mullins CE. Variation in the phenotypic expression of beta-glucuronidase deficiency. J Pediatr. 1975;86:388–394. doi: 10.1016/s0022-3476(75)80968-1. [DOI] [PubMed] [Google Scholar]
- 19.Vogler C, Levy B, Kyle JW, Sly WS, Williamson J, Whyte MP. Mucopolysaccharidosis VII: postmortem biochemical and pathological findings in a young adult with beta-glucuronidase deficiency. Mod Pathol. 1994;7:132–137. [PubMed] [Google Scholar]
- 20.Soliman OI, Timmermans RG, Nemes A, Vletter WB, Wilson JH, ten Cate FJ, Geleijnse ML. Cardiac abnormalities in adults with the attenuated form of mucopolysaccharidosis type I. J Inherit Metab Dis. 2007;30:750–757. doi: 10.1007/s10545-007-0586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemes A, Timmermans RG, Wilson JH, Soliman OI, Krenning BJ, ten Cate FJ, Geleijnse ML. The mild form of mucopolysaccharidosis type I (Scheie syndrome) is associated with increased ascending aortic stiffness. Heart Vessels. 2008;23:108–111. doi: 10.1007/s00380-007-1013-x. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Tittiger M, Knutsen RH, Kovacs A, Schaller L, Mecham RP, Ponder KP KP. Upregulation of elastase proteins results in aortic dilatation in mucopolysaccharidosis I mice. Mol Genet Metab. 2008;94:298–304. doi: 10.1016/j.ymgme.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woloszynek JC, Kovacs A, Ohlemiller KK, Roberts M, Sands MS. Metabolic adaptations to interrupted glycosaminoglycan recycling. J Biol Chem. 2009 Aug 21; doi: 10.1074/jbc.M109.020818. [Epub ahead of print]PMID: 19700765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sleeper MM, Kusiak CM, Shofer FS, O'Donnell P, Bryan C, Ponder KP, Haskins ME. Clinical characterization of cardiovascular abnormalities associated with feline mucopolysaccharidosis I and VI. J Inherit Metab Dis. 2008;31:424–431. doi: 10.1007/s10545-008-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gompf RE, Shull RM, Breider MA, Scott JA, Constantopoulos GC. Cardiovascular changes after bone marrow transplantation in dogs with mucopolysaccharidosis I. Am J Vet Res. 1990;51:2054–2060. [PubMed] [Google Scholar]
- 26.Sammarco C, Weil M, Just C, Weimelt S, Hasson C, O'Malley T, Evans SM, Wang P, Casal ML, Wolfe J, Haskins M. Effects of bone marrow transplantation on the cardiovascular abnormalities in canine mucopolysaccharidosis VII. Bone Marrow Transplant. 2000;25:1289–1297. doi: 10.1038/sj.bmt.1702448. [DOI] [PubMed] [Google Scholar]
- 27.Sleeper MM, Fornasari B, Ellinwood NM, Weil MA, Melniczek J, O'Malley TM, Sammarco CD, Xu L, Ponder KP, Haskins ME. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation. 2004;110:815–820. doi: 10.1161/01.CIR.0000138747.82487.4B. [DOI] [PubMed] [Google Scholar]
- 28.Sleeper MM, Haskins ME, Ponder KP. Gene therapy for cardiovascular manifestations of lysosomal storage diseases. Heart and Metabolism. 2009;41:21–24. [PMC free article] [PubMed] [Google Scholar]
- 29.Krettek A, Sukhova GK, Libby P P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler Thromb Vasc Biol. 2003;123:582–587. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- 30.Cattell MA, Anderson JC, Hasleton PS. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta. 1996;245:73–84. doi: 10.1016/0009-8981(95)06174-6. [DOI] [PubMed] [Google Scholar]
- 31.Cantini C, Kieffer P, Corman B, Limiñana P, Atkinson J. Lartaud-Idjouadiene I. Aminoguanidine and aortic wall mechanics, structure, and composition in aged rats. Hypertension. 2001;38:943–948. doi: 10.1161/hy1001.096211. [DOI] [PubMed] [Google Scholar]
- 32.Mithieux SM, Weiss AS. Elastin. Adv. Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- 33.Jordan MD, Zheng Y, Ryazantsev S, Rozengurt N, Roos KP, Neufeld EF. Cardiac manifestations in the mouse model of mucopolysaccharidosis I. Mol. Genet. Metab. 2005;86:233–243. doi: 10.1016/j.ymgme.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renteria VG, Ferrans VJ, Roberts WC. The heart in the hurler syndrome: Gross, histologic and ultrastructural observations in five necropsy cases. Am. J. Cardiol. 1976;38:487–501. doi: 10.1016/0002-9149(76)90468-9. [DOI] [PubMed] [Google Scholar]
- 35.Braunlin E, Mackey-Bojack S, Panoskaltsis-Mortari A, Berry JM, McElmurry RT, Riddle M, Sun LY, Clarke LA, Tolar J, Blazar BR. Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I. Pediatr Res. 2006;59:27–32. doi: 10.1203/01.pdr.0000190579.24054.39. [DOI] [PubMed] [Google Scholar]
- 36.Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol. 2000;156:925–938. doi: 10.1016/S0002-9440(10)64961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, Baxter BT. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005;137:457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Lesauskaite V, Epistolato MC, Castagnini M, Urbonavicius S, Tanganelli P. Expression of matrix metalloproteinases, their tissue inhibitors, and osteopontin in the wall of thoracic and abdominal aortas with dilatative pathology. Hum Pathol. 2006;37:1076–1084. doi: 10.1016/j.humpath.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Sukhova GK, Zhang Y-O, Pan J-H, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi G-P. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birkenmeier EH, Barker JE, Vogler CA, Kyle JW, Sly WS, Gwynn B, Levy B, Pegors C. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood. 1991;78:3081–3092. [PubMed] [Google Scholar]
- 42.Simonaro CM, Haskins ME, Kunieda T, Evans SM, Visser JW, Schuchman EH. Bone marrow transplantation in newborn rats with mucopolysaccharidosis type VI: biochemical, pathological, and clinical findings. Transplantation. 1997;63:1386–1393. doi: 10.1097/00007890-199705270-00003. [DOI] [PubMed] [Google Scholar]
- 43.Ellinwood NM, Colle MA, Weil MA, Casal ML, Vite CH, Wiemelt S, Hasson CW, O'Malley TM, He X, Prociuk U, Verot L, Melniczek JR, Lannon A, Aguirre GD, Knox VW, Evans SM, Vanier MT, Schuchman EH, Walkley SU, Haskins ME. Bone marrow transplantation for feline mucopolysaccharidosis I. Mol Genet Metab. 2007;91:239–250. doi: 10.1016/j.ymgme.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auclair D, Hopwood JJ, Brooks DA, Lemontt JF, Crawley AC. Replacement therapy in Mucopolysaccharidosis type VI: advantages of early onset of therapy. Mol Genet Metab. 2003;78:163–174. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 45.Werle B, Staib A, Julke B, Ebert W, Zladoidsky P, Sekirnik A, Kos J, Spiess E. Fluorometric microassays for the determination of cathepsin L and cathepsin S activities in tissue extracts. Biol. Chem. 1990;380:1109–1116. doi: 10.1515/BC.1999.138. [DOI] [PubMed] [Google Scholar]
- 46.Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005;68:155–166. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 47.Simonaro CM CM, D'Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am J Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bromme D, Bonneau PR, Lachance P, Wiederanders B, Kirschke H, Peters C, Thomas DY, Storer AC, Vernet R. Functional expression of human cathepsin S in Saccharomyces cerevisiae. J. Biol. Chem. 1993;268:4832–4838. [PubMed] [Google Scholar]
- 49.Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 50.Stoka V, Turk B, Turk V. Lysosomal cysteine proteases: structural features and their role in apoptosis. IUBMB Life. 2005;57:347–353. doi: 10.1080/15216540500154920. [DOI] [PubMed] [Google Scholar]
- 51.Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fingleton B. MMPs as therapeutic targets--still a viable option? Semin Cell Dev Biol. 2008;19:61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simonaro CM, D'Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr. Res. 2005;57:701–707. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 54.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L, Tanimoto A, Murata Y, Sasaguri T, Fan J, Sasaguri Y, Watanabe T. Matrix metalloproteinase-12 gene expression in human vascular smooth muscle cells. Genes Cells. 2003;8:225–234. doi: 10.1046/j.1365-2443.2003.00628.x. [DOI] [PubMed] [Google Scholar]
- 56.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Simonaro CM, Haskins ME, Schuchman EH. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab Invest. 2001;81:1319–1328. doi: 10.1038/labinvest.3780345. [DOI] [PubMed] [Google Scholar]
- 59.Ausseil J, Desmaris N, Bigou S, Attali R, Corbineau S, Vitry S, Parent M, Cheillan D, Fuller M, Maire I, Vanier MT, Heard JM. Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice. PLoS One. 2008;3:1–11. doi: 10.1371/journal.pone.0002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 61.Link JO, Zipfel S. Advances in cathepsin S inhibitor design. Curr. Opin. Drug Discov. Devel. 2006;9:471–482. [PubMed] [Google Scholar]
- 62.Devel L, Rogakos V, David A, Makaritis A, Beau F, Cuniasse P, Yiotakis A, Dive V. Development of selective inhibitors and substrate of matrix metalloproteinase-12. J. Biol. Chem. 2006;281:11152–11160. doi: 10.1074/jbc.M600222200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.