Abstract
Daxx is a multifunctional protein, regulating a wide range of important functions including apoptosis and transcription. However, the way Daxx is regulated is poorly understood. In our previous studies, we have found that Daxx forms a complex with the E3 ubiquitin ligase Mdm2 and the deubiquitinase Hausp. In the present work, we show that Daxx is ubiquitinated by Mdm2 in both in vitro and in vivo systems and Mdm2 reduces Daxx expression upon overe-xpression. We further demonstrate that Hausp critically controls the cellular level of Daxx most likely by inducing Daxx deubiquitination. These results reveal Mdm2 and Hausp as important regulators for Daxx functions by controlling Daxx ubiquitination and stability.
Keywords: Daxx, Mdm2, Hausp, Ubiquitination, Deubiquitination
Introduction
Daxx is a multifunctional protein, regulating a wide range of important cellular functions such as apoptosis, transcription control and embryonic development. Initially identified as a Fas receptor binding protein in a yeast two hybrid screening, Daxx was implicated in Fas-induced apoptosis[1]. Subsequent studies indicate that Daxx is involved in other scenarios of apoptosis. Under stress stimulations, such as TNF-alpha treatment and oxidative stress, Daxx is up-regulated and engaged in JNK mediated apoptosis by activating ASK[2-4]. The Daxx-JNK pathway represents an important anti-tumor program, disruption of which may contribute to tumorigenesis. In contrast to its pro-apoptotic role, it is reported that knockdown endogenous Daxx sensitizes cells to multiple stimuli-induced apoptosis, suggesting Daxx has an anti-apoptotic function in certain cellular context[5]. Daxx is primarily localized in the nucleus[6], however it can translocate into the cytosol upon stress stimulation such as glucose deprivation[7].
Daxx possesses transcriptional regulation activity, which is probably due to its interaction with various transcription factors and epigenetic modifiers, including ATRX, HADCs et al[8-12]. By recruiting ATRX and HADCs to the promoter region through interacting with a specific transcription factor, Daxx suppresses the transcription. One example of Daxx to inhibiting gene expression is Daxx-mediated viral IE gene repression[13; 14]. To counteract Daxx's defense, human cytomegalovirus releases tegument protein pp71 to interact with Daxx by replacing ATRX and inducing Daxx degradation[13-16]. Recent studies suggest that cellular Daxx is also critical for Mdm2 stability and inhibiting the tumor suppressor p53's functions through diverse mechanisms[17; 18]. Daxx knockout in mice results in embryonic lethality suggesting cellular Daxx is required for development[19].
The differential functions of Daxx may be related to its protein level and posttranslational modifications. Peptidyl-prolyl isomerase Pin1 inhibits Daxx-involved apoptosis by inducing Daxx phosphorylation on ser178 which mediated Daxx ubiquitination and degradation[20]. HIPK1 reduces Daxx transcription regulation activity via phosphorylation of Daxx on ser669[21]. Despite that Daxx level is very important in executing its functions; its regulation is poorly understood[20]. Pp71-induced Daxx rapid degradation is ubiquitin independent but proteasome dependant[15]. In contrast, peptidyl-prolyl isomerase Pin1-induced Daxx degradation is ubiquitin dependent, but the responsible E3 is not known[20]. The ubiquitin-proteasome pathway is a major route targeting an ubiquitinated protein for degradation, yet the Daxx level has been found to be controlled by a Cul3-based E3 ubiquitin ligase mediated ubiquitination and degradation process[22]. Concerning the multiple pathways that Daxx is engaged in, it is highly likely that Daxx stability is also regulated by other E3 ligases. De-ubiquitination is a reverse process opposing proteasomal degradation and increasingly recognized in the control of protein stability. However, there is no current report as to whether or not a de-ubiquitinase is involved in Daxx level determination.
In a previous study, we have found that Daxx physically interacts with Mdm2 and the de-ubiquitinase Hausp, and that Daxx facilitates Hausp to de-ubiquitinate Mdm2 whereby stabilizing Mdm2[17]. It has been reported that Mdm2 can induce ubiquitination and degradation of many of its interacting proteins, including p53, Foxo1 and E-cadherin[23; 24]. The interaction between Mdm2 and Daxx suggests that Daxx may be a potential ubiquitination target of Mdm2. In the present study we found that Mdm2 ubiquitinated Daxx in both in vivo and in vitro systems, and that Mdm2 reduced cellular level of Daxx upon overexpression. Furthermore, Hausp de-ubiquitinated Daxx and regulated stability of endogenous Daxx.
Material and Methods
Cells and plasmids
U2OS and 293T cells were grown in Dulbeco's modified Eagles medium (DEME) supplemented with 10% fetus bovine serum and 1% Pen-Strep (Gibco) at 37 °C in a humidified incubator supplied with 5% CO2. For expression in mammalian cells, Daxx, Mdm2 and Hausp were fused an NH2-terminal Flag tag in pRK5 vector. GST-Mdm2 fusion protein was made in pEGX-1ZT and expressed in E. coli.
Purification of recombinant proteins
To purify proteins from mammalian cells, expression plasmids encoding Flag-agged Daxx or -Hausp were transfected into 293T cells, respectively. After 24hrs, the cells were lysed in lysis buffer (20mM Tris-HCl, pH8.0, 150mM NaCl. 0.5% NP-40, 0.5% Triton X-100, 1mM DTT, 1mM EDTA, 10% glycerol) supplemented with 1× complete protease inhibitors. Cell lysates were immunoprecipitated with anti-Flag M2 beads. The non-specific proteins were removed through sequential washes with lysis buffers containing 150mM NaCl, 0.5M NaCl and 1 M NaCl. F-Daxx and F-Hausp were eluted using Flag peptide. For F-Mdm2 purification, the above procedure was followed except that the cells were treated with 20μM MG132 for 4hr before lysis. To purify GST-Mdm2 from E. Coli, the expression plasmid was transformed into strain BL21. Protein expression was induced by 1mM IPTG for 1hr at room temperature. GST-Mdm2 was purified with glutathione-sepharose.
In vitro ubiquitination and deubiquitination assay
For ubiquitination assay, purified F-Daxx was mixed with F-Mdm2 or GST-Mdm2 together with E1, E2, his6-Ub, Mg2-ATP and 2mM DTT as described in [17].The reactions were stopped by adding 2× sample buffers followed by boiling for 5 min. The protein mixture was either directly resolved by SDS-PAGE or diluted 10 times with lysis buffer to reduce the concentration of SDS. F-Daxx was then purified with M2 beads and analyzed by Western blot.
For deubiquitination assay, Daxx was ubiquitinated by F-Mdm2 as above and then diluted 5 times with deubiquitination buffer (50 mM Tris-HCl, pH7.4, 150mM NaCl, 10mM DTT and 5 mM MgCl2). Afterwards F-Hausp was added to the reaction. The samples were incubated at 37C for indicated time.
In vivo ubiquitination assay
U2OS cells were transfected with Ha-Ub and indicated plasmids, 24hrs later treated with MG132 for 4hrs. The cells were then lysed in 1% SDS. After boiling for 5 mins, lysates were diluted 10 times with lysis buffer supplemented with 10 mM N-ethylmaleimide (NEM). F-Daxx was then purified with M2 beads and subject to Western analysis. Ubiquitinated Daxx was detected by an anti-Ha antibody.
siRNA and transfection
Plasmids or si-RNAs were transfected into U2OS cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. The target sequence for the Hausp siRNA is CCCAAATTATTCCGCGGCAAA. Both control siRNA and the Hausp siRNA were purchased from Qiagen.
Cycloheximide treatment
U2OS cells were treated with 50μg/ml cycloheximide for indicated durations and then collected for Western analysis.
Results and Discussion
Daxx is ubiquitinated by Mdm2 in vitro and in vivo
Daxx interacts with E3 ubiquitin ligase Mdm2[17], hinting that Daxx may be subject to Mdm2-induced ubiquitination. To test this possibility, we performed an in vitro ubiquitination assay. Recombinant Mdm2 purified from 293T cells exhibited a strong activity towards Daxx, converting nearly all of the Daxx into high molecular weight species, likely ubiquitinated Daxx (Fig 1A). To confirm this result, we purified the GST-Mdm2 from bacteria and performed the same assay. Compared with the Mdm2 purified from the mammalian expression system, the bacterially purified Mdm2 may exhibit weaker ubiquitination activity, but has the advantage of excluding other E3s which might be co-purified with Mdm2 as in the mammalian system. Like mammalian recombinant Mdm2, bacterial Mdm2 also induced the generation of slow migrating species of Daxx. The high molecular weight species were then determined to be ubiquitinated Daxx (Fig 1B). These results indicated that Daxx can be ubiquitinated by Mdm2 in vitro.
Fig.1.

Mdm2 induces ubiquitination of Daxx. (A, B) Mdm2 induces ubiquitination of Daxx in vitro. F-Daxx and F-Mdm2 were purified from 293T cells (A). Recombinant GST-Mdm2 was purified from bacteria (B). In vitro ubiquitination assay of Daxx was performed as described in Material and Methods. (C) Mdm2 induces ubiquitination of Daxx in vivo. F-Daxx and Ha-ub were co-transfected with or without Mdm2 into U2OS cells for 24 hrs. F-daxx was then pulled down by M2 beads and subject to Western analysis.
To further confirm these results, we performed an in vivo ubiquitination assay. F-Daxx and Ha-ub were co-transfected with or without Mdm2 into U2OS cells. Overexpressed Mdm2 greatly induced ubiquitination of F-Daxx (Fig 1C), suggesting that Mdm2 has capacity to ubiquitinate Daxx in vivo. A weak ubiquitination signal of F-Daxx is also detected in the absence of overexpressed Mdm2, which is probably induced by endogenous Mdm2 or other E3s.
Daxx is deubiquitinated by Hausp
Besides interacting with Mdm2, Daxx also binds to Hausp[17]. It has been reported that Hausp de-ubiquitinates Mdm2 and p53[25; 26]. The strong interaction between Daxx and Hausp, and that Daxx is ubiquitinated by Mdm2 and other E3s suggest that Hausp might de-ubiquitinate Daxx. To examine this possibility, we first induced Daxx ubiquitination by Mdm2 in vitro, and then added the purified Hausp to assess its ability to deubiquitinate Daxx. Similar to what has been reported with Mdm2 and p53, Hausp strongly de-ubiquitinated Daxx (Fig 2).
Fig. 2.
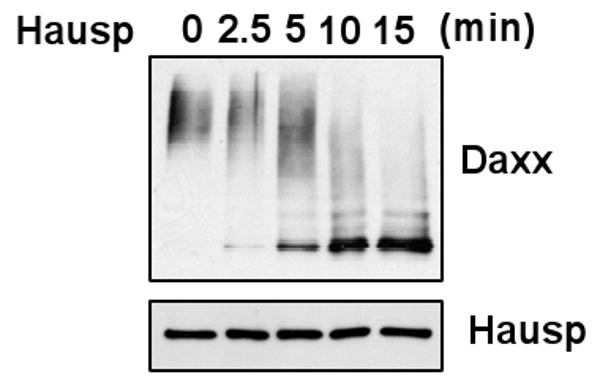
Hausp de-ubiquitinates Daxx in vitro. Ubiquination of Daxx was first induced by F-Mdm2 by an in vitro ubiquitin assay. Hausp was then employed for the indicated periods of time at 37°C.
Mdm2 and Hausp co-regulate the stability of Daxx
One function for protein ubiquitination is to regulate protein stability, and the above results showed that Mdm2 and Hausp reciprocally regulated Daxx ubiquitination. We therefore checked whether the stability of Daxx is regulated by Mdm2 and Hausp. Daxx is a relatively stable protein with a half life longer than 8 hours. However, when endogenous Hausp is knockdown by siRNA, the half-life of Daxx is shortened to less than 8 hours and the steady level of Daxx is obviously decreased (Fig 3B), indicating Hausp plays an important role in stabilizing Daxx. In contrast, when Mdm2 is expressed at a high level, the steady level of Daxx is noticeably reduced (Fig 3C).
Fig. 3.

The stability of Daxx is regulated by Hausp and Mdm2. Knockdown Hausp decreases the steady level (A), and the half-life of Daxx (B). U2OS cells were transfected with con siRNA and Hausp siRNA, and subsequently treated with CHX (10μg/ml) for the indicated time periods. The cell lysates were generated at each time point and analyzed by western blot. Over-expression of Mdm2 reduces the steady level of Daxx (C). U2O2 cells were transfected with increased amounts of F-Mdm2 for 24hrs. Endogenous Daxx, p53 and actin, as well as tranfected F-Mdm2 were analyzed by Western blot.
In a previous study, we have demonstrated that Daxx-Mdm2-Hausp forms a ternary complex, in which Daxx facilitates Hausp-Mdm2 interaction resulting in Mdm2 deubiquitination and stabilization[17]. In the present study, we uncovered another layer of regulation that Mdm2 and Hausp co-regulate Daxx. Besides forming a ternary complex with Mdm2 and Hausp, Daxx can bind these two proteins respectively. Although we observed that Mdm2 stimulates Daxx ubiquitination, knockdown endogenous Mdm2 did not change Daxx levels (data not shown). We speculate that Mdm2-mediated Daxx downregulation could only exist in certain Mdm2 over-expressing cancer cells, which needs validation in further studies. In cells with normal Mdm2 level, Daxx tethers Hausp to Mdm2 and removes ubquitin adducts from both Mdm2 and Daxx, nullifying Mdm2's effect on Daxx. Mdm2 is an important negative regulator of p53, whose amplification is found in 7% human tumors[27]. Mdm2 over-expression correlates with poor prognosis, which can not be explained simply by inhibiting p53 functions[28; 29]. Increasing evidence suggests that up-regulation of Mdm2 induces tumor formation though multiple mechanisms, many of which are p53-independent, such as inducing Rb degradation[30; 31]. Our results suggest that Mdm2 may inhibit Daxx-mediated apoptosis programs by inducing Daxx degradation. This effect on Daxx may also contribute to Mdm2-mediated tumorigenesis.
The strong effect of Hausp knockdown on Daxx stability suggests that Hausp plays an essential role in regulating the cellular level of Daxx. Besides Mdm2, a Cul3-based E3 and CHIP also induce Daxx ubiquitination[22; 32]. Hausp likely stabilizes Daxx by reversing these E3 induced ubiquitination.
In conclusion, we have provided evidence that Mdm2 and Hausp regulate the stability of Daxx by inducing its ubiquitination and deubquitination, suggesting that Mdm2 and Hausp could be involved in the regulation of Daxx-mediated biological processes. Based on these results and our previous finding that Daxx-Hausp regulates the stability of Mdm2, we propose that in normal cells Mdm2 is regulated by Daxx and Hausp, however, under pathological conditions when Mdm2 is up-regulated, Mdm2 could inhibit Daxx-mediated apoptosis leading to tumor progression.
Acknowledgments
We thank Dr. Shijun Zheng for assistance and valuable discussions. This work was supported by the National Natural Sciences Foundation of China (30971487) and National Institute of Health (USA CA088868).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuyo Y, Kitamura T, Inoue M, Horikoshi NT, Higashikubo R, Hunt CR, Usheva A, Horikoshi N. Phosphorylation-dependent Lys63-linked polyubiquitination of Daxx is essential for sustained TNF-{alpha}-induced ASK1 activation. Cancer Res. 2009;69:7512–7517. doi: 10.1158/0008-5472.CAN-09-2148. [DOI] [PubMed] [Google Scholar]
- 3.Khelifi AF, D'Alcontres MS, Salomoni P. Daxx is required for stress-induced cell death and JNK activation. Cell Death Differ. 2005;12:724–733. doi: 10.1038/sj.cdd.4401559. [DOI] [PubMed] [Google Scholar]
- 4.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 5.Chen LY, Chen JD. Daxx silencing sensitizes cells to multiple apoptotic pathways. Mol Cell Biol. 2003;23:7108–7121. doi: 10.1128/MCB.23.20.7108-7121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torii S, Egan DA, Evans RA, Reed JC. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs) EMBO J. 1999;18:6037–6049. doi: 10.1093/emboj/18.21.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JJ, Lee YJ. Tryptophan 621 and serine 667 residues of Daxx regulate its nuclear export during glucose deprivation. J Biol Chem. 2004;279:30573–30578. doi: 10.1074/jbc.M404512200. [DOI] [PubMed] [Google Scholar]
- 8.Wethkamp N, Klempnauer KH. Daxx is a transcriptional repressor of CCAAT/enhancer-binding protein beta. J Biol Chem. 2009;284:28783–28794. doi: 10.1074/jbc.M109.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue Y, Gibbons R, Yan Z, Yang D, McDowell TL, Sechi S, Qin J, Zhou S, Higgs D, Wang W. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc Natl Acad Sci U S A. 2003;100:10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R, Grosveld G. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein. Dek J Cell Sci. 2002;115:3319–3330. doi: 10.1242/jcs.115.16.3319. [DOI] [PubMed] [Google Scholar]
- 11.Li R, Pei H, Watson DK, Papas TS. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene. 2000;19:745–753. doi: 10.1038/sj.onc.1203385. [DOI] [PubMed] [Google Scholar]
- 12.Tang J, Wu S, Liu H, Stratt R, Barak OG, Shiekhattar R, Picketts DJ, Yang X. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein 3. J Biol Chem. 2004;279:20369–20377. doi: 10.1074/jbc.M401321200. [DOI] [PubMed] [Google Scholar]
- 13.Saffert RT, Kalejta RF. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J Virol. 2006;80:3863–3871. doi: 10.1128/JVI.80.8.3863-3871.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantrell SR, Bresnahan WA. Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication 1. J Virol. 2006;80:6188–6191. doi: 10.1128/JVI.02676-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang J, Kalejta RF. Proteasome-dependent, ubiquitin-independent degradation of Daxx by the viral pp71 protein in human cytomegalovirus-infected cells. Virology. 2007;367:334–338. doi: 10.1016/j.virol.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Lukashchuk V, McFarlane S, Everett RD, Preston CM. Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection 1. J Virol. 2008;82:12543–12554. doi: 10.1128/JVI.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang J, Qu LK, Zhang J, Wang W, Michaelson JS, Degenhardt YY, El-Deiry WS, Yang X. Critical role for Daxx in regulating Mdm2. Nat Cell Biol. 2006;8:855–862. doi: 10.1038/ncb1442. [DOI] [PubMed] [Google Scholar]
- 18.Zhao LY, Liu J, Sidhu GS, Niu Y, Liu Y, Wang R, Liao D. Negative regulation of p53 functions by Daxx and the involvement of MDM2. J Biol Chem. 2004;279:50566–50579. doi: 10.1074/jbc.M406743200. [DOI] [PubMed] [Google Scholar]
- 19.Michaelson JS, Bader D, Kuo F, Kozak C, Leder P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 1999;13:1918–1923. doi: 10.1101/gad.13.15.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryo A, Hirai A, Nishi M, Liou YC, Perrem K, Lin SC, Hirano H, Lee SW, Aoki I. A suppressive role of the prolyl isomerase Pin1 in cellular apoptosis mediated by the death-associated protein Daxx. J Biol Chem. 2007;282:36671–36681. doi: 10.1074/jbc.M704145200. [DOI] [PubMed] [Google Scholar]
- 21.Ecsedy JA, Michaelson JS, Leder P. Homeodomain-interacting protein kinase 1 modulates Daxx localization, phosphorylation, and transcriptional activity. Mol Cell Biol. 2003;23:950–960. doi: 10.1128/MCB.23.3.950-960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH, Baek SH, Chiba T, Tanaka K, Bang OS, Joe CO, Chung CH. BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. J Biol Chem. 2006;281:12664–12672. doi: 10.1074/jbc.M600204200. [DOI] [PubMed] [Google Scholar]
- 23.Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, Nawaz Z, Shimojima T, Wang H, Yang Y, Shen Z, Zhang Y, Zhang X, Nicosia SV, Zhang Y, Pledger JW, Chen J, Bai W. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation 1. J Biol Chem. 2009;284:13987–14000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang JY, Zong CS, Xia W, Wei Y, li-Seyed M, Li Z, Broglio K, Berry DA, Hung MC. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation 8. Mol Cell Biol. 2006;26:7269–7282. doi: 10.1128/MCB.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway 24. Mol Cell. 2004;13:879–886. doi: 10.1016/s1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization 25. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 27.Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database 41 Nucleic. Acids Res. 1998;26:3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Oliver P, Zhang Z, Agrawal S, Zhang R. Chemosensitization and radiosensitization of human cancer by antisense anti-MDM2 oligonucleotides: in vitro and in vivo activities and mechanisms 11. Ann N Y Acad Sci. 2003;1002:217–235. doi: 10.1196/annals.1281.025. [DOI] [PubMed] [Google Scholar]
- 29.Moller MB, Nielsen O, Pedersen NT. Oncoprotein MDM2 overexpression is associated with poor prognosis in distinct non-Hodgkin's lymphoma entities 24. Mod Pathol. 1999;12:1010–1016. [PubMed] [Google Scholar]
- 30.Bouska A, Eischen CM. Mdm2 affects genome stability independent of p53 8. Cancer Res. 2009;69:1697–1701. doi: 10.1158/0008-5472.CAN-08-3732. [DOI] [PubMed] [Google Scholar]
- 31.Miwa S, Uchida C, Kitagawa K, Hattori T, Oda T, Sugimura H, Yasuda H, Nakamura H, Chida K, Kitagawa M. Mdm2-mediated pRB downregulation is involved in carcinogenesis in a p53-independent manner 24. Biochem Biophys Res Commun. 2006;340:54–61. doi: 10.1016/j.bbrc.2005.11.148. [DOI] [PubMed] [Google Scholar]
- 32.McDonough H, Charles PC, Hilliard EG, Qian SB, Min JN, Portbury A, Cyr DM, Patterson C. Stress-dependent Daxx-CHIP interaction suppresses the p53 apoptotic program. J Biol Chem. 2009;284:20649–20659. doi: 10.1074/jbc.M109.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]


