Abstract
Aligned nanofibrous scaffolds can recapitulate the structural hierarchy of fiber-reinforced tissues of the musculoskeletal system. While these electrospun fibrous scaffolds provide physical cues that can direct tissue formation when seeded with cells, the ability to chemically guide a population of cells, without disrupting scaffold mechanical properties, would improve the maturation of such constructs and add additional functionality to the system both in vitro and in vivo. In this study, we developed a fabrication technique to entrap drug-delivering microspheres within nanofibrous scaffolds. We hypothesized that entrapping microspheres between fibers would have a less adverse impact on mechanical properties than placing microspheres within the fibers themselves, and that the composite would exhibit sustained release of multiple model compounds. Our results show that microspheres ranging from 10~20 microns in diameter could be electrospun in a dose-dependent manner to form nanofibrous composites. When delivered in a sacrificial PEO fiber population, microspheres remained securely entrapped between slow-degrading PCL fibers after removal of the sacrificial delivery component. Stiffness and modulus of the composite decreased with increasing microsphere density for composites in which microspheres were entrapped within each fiber, while stiffness did not change when microspheres were entrapped between fibers. The release profiles of the composite structures were similar to free microspheres, with an initial burst release followed by a sustained release of the model molecules over 4 weeks. Further, multiple model molecules were released from a single scaffold composite, demonstrating the capacity for multi-factor controlled release ideal for complex growth factor delivery from these structures.
Keywords: Mechanical Properties, Drug Delivery, Microsphere, Nanospun Scaffold
Introduction
Fibrous tissues of the musculoskeletal system are characterized by aligned collagen bundles that impart non-linear and anisotropic mechanical properties, which enable load bearing functionality in demanding mechanical environments over a lifetime of use. Recapitulation of these fundamental structural and mechanical anisotropies is a key determinant in the development of successful engineered analogues for repair or replacement of these tissues. One scaffold fabrication technique, electrospinning, can produce nano- to micron-sized fibers, similar in length scale to native collagen, from a host of natural and synthetic polymers [1–4]. Collection of these nanofibers onto a rotating mandrel [5–7] or implementation of specialized collection surfaces [8, 9] can further refine scaffolds by aligning fibers to create structural and mechanical anisotropy within the forming network. By modifying fiber elements such as composition, diameter, and organization, a wide range of mechanical properties can be achieved, which in turn can be tuned to tissue-specific applications. Indeed, such fibrous scaffolds have been used in a wide range of fibrous tissue engineering applications, including constructs for replacement of the knee meniscus [10], the annulus fibrosus [11, 12], tendons and ligaments [13], blood vessels [14] and articular cartilage [15, 16]. When seeded with cells, nanofibrous scaffolds have demonstrated excellent potential for directing ordered ECM deposition, resulting in improved mechanical properties of the engineered construct with time during in vitro culture [10, 17, 18].
Despite the potential of these aligned micropatterns to guide new tissue formation, further functionalization might be necessary to expand their general utility, both in vitro and with in vivo implantation. One key area for expansion is the ability of nanofibrous scaffolds to release select molecules in a controlled fashion. Previous work in other scaffold formats demonstrates the potential of such an approach, for example by promoting neo-vascularization of porous scaffolds through the dual release of pro-angiogenic factors (VEGF and PDGF) [19]. Recent work by several groups has demonstrated that nanofibrous scaffolds can be modified to achieve a degree of controlled release (as reviewed in [3, 20]). In most cases, molecules or biologic agents (i.e., antibiotic, growth factors) are delivered from the fibers themselves. This is a sensible approach, given the high surface area of fibers relative to the volume of the construct, and the close proximity of the fibers to seeded or infiltrating cells. Specific examples of delivery from nanofibrous scaffolds to date include antibiotics [21–27], anticancer therapeutics [28–31], proteins [32–36], DNA [37–39], and growth factors [40, 41]. These successes have been achieved either through direct blending of the molecule of interest into the polymer solution before electrospinning [23], or via the utilization of coaxial electrospinning, wherein a customized spinneret is employed to trap a secondary fluid layer (containing labile biofactors) within the core of the forming nanofiber [32]. For example, Li and coworkers electrospun silk fibroin fiber scaffolds containing a core of bone morphogenetic protein 2 (BMP-2) and demonstrated increased osteogenic differentiation after one month by seeded human mesenchymal stem cells [42].
Despite this progress, the incorporation of molecules into the electrospun fibers may have adverse consequences. For example, retinoic acid added at low levels increased the mechanical properties of single poly(caprolactone-co-ethyl ethylene phosphate) fibers, while bovine serum albumin incorporated at higher concentrations decreased fiber properties [41]. In another study, Huang and co-workers co-axially electrospun two drugs into PCL fibers and demonstrated that, based on the limited miscibility of the two solvents, mechanical properties were significantly altered [43]. Still other issues may arise when release rates and mechanics are incompatible. For example, Hong and colleagues co-electrospun two populations of fibers, biodegradable poly(ester urethane) urea (PEUU) and poly(lactide-co-glycolide) (PLGA), where the PLGA fibers were loaded directly with the antibiotic tetracycline hydrochloride (PLGA-tet). The PLGA fibers alone had a modulus that was too high and a breaking strain that was too low for the intended application (wound closure in the abdomen). However, addition of a PEUU fiber family decreased the modulus and improved the breaking strain, resulting in more ideal mechanical properties. While promising, this work shows that the remnant fibers contribute to the overall scaffold mechanics, and that drug elution rates are dependent on the fiber properties.
If a fibrous scaffold is to serve the dual roles of load bearing and drug delivery simultaneously, then this issue is paramount and must be considered in the fabrication of scaffolds with defined mechanical characteristics. We report herein a new modification of the electrospinning system to allow for the decoupling of scaffold mechanics from biofactor delivery. This fabrication method is based on the well-established ability of microspheres to carry and deliver molecules of therapeutic interest [44]. In this system, drug releasing microspheres are delivered and entrapped within the fibrous network of the scaffold using sacrificial fibers that are removed upon hydration. We have previously demonstrated that removal of these sacrificial fibers, at the proper percentage [45], can act to increase cellular infiltration into these dense fibrous networks. We hypothesize that entrapping microspheres amongst these sacrificial fibers, rather than releasing the drug from the fiber or placing the microspheres inside the fibers, will mitigate any major changes to scaffold properties. Further, this method will decouple the degradation rate of the fibers from microspheres, thus allowing for additional flexibility when designing optimal release profiles. The system is developed so that compatible solvent systems enable polymeric fiber formation from organic solvents, while the solvent for sacrificial fibers (water) can maintain PLGA microsphere in their native form. Finally, by including two populations of microspheres, we show that multiple factors can be released independently from one another, providing further design parameters for tissue-specific applications.
Materials and Methods
Materials
Polystyrene (PS) microspheres (MS) were from either Bangs Laboratories (diameters: 1.94 μm (fluorescent dragon green) and 8.31 μm, Fishers, IN) or Microsphere-Nanosphere (diameter: 15.7 μm, Cold Springs, NY). For nanofiber formation, polyethylene oxide (PEO, 200 kDa) was from Polysciences (Warrington, PA) and poly(ε-caprolactone) (PCL, 80 kDa) was from Sigma-Aldrich (St. Louis MO). Tetrahydrofuran (THF) and N,N-dimethylformamide (DMF), used to dissolve PCL, were from Fisher Chemical (Fairlawn, NJ). Poly lactide co-glycolide 50:50 (PLGA, inherent viscosity: 0.61 dL/g in HFIP) for microsphere fabrication was from DURECT Corp (Pelham, AL). Dichloromethane for microsphere fabrication, bovine serum albumin (BSA, Cohen V fraction), chondroitin 6-sulfate sodium salt (CS), poly vinyl alcohol (PVA, 87–89% hydrolyzed), and fluorescein (free acid) were all from Sigma-Aldrich (Allentown, PA). The bicinchoninic acid (BCA) assay kit was purchased from Pierce Protein Research Products (Thermo Scientific, Rockford, IL). Dulbecco’s phosphate-buffered saline (PBS) was purchased from Gibco (Invitrogen, Grand Island, NY).
Electrospinning Nanofibrous Scaffolds using Pre-Fabricated Microspheres
To electrospin fibers containing pre-fabricated microspheres, a high concentration of PS microspheres (19–109 MS/mL) was dispersed in 10% PEO in 90% ethanol or in 35.7% w/v PCL in a 1:1 mixture of THF and DMF. The suspension was sonicated for 3 minutes to disperse the MS and electrospun as in [10]. Briefly, a 10 mL syringe was filled with the electrospinning solution and fitted with a stainless steel 18G blunt-ended needle that served as a charged spinneret. A flow rate of 2.5 mL/h was maintained with a syringe pump (KDS100, KD Scientific, Holliston, MA). A power supply (ES30N-5W, Gamma High Voltage Research, Inc., Ormond Beach, FL) applied a +15 kV potential difference between the spinneret and the grounded mandrel located at a distance of 12 cm from the spinneret. The mandrel was rotated via a belt mechanism conjoined to an AC motor (Pacesetter 34R, Bodine Electric, Chicago, IL). Additionally, two aluminum shields charged to +10 kV were placed perpendicular to and on either side of the mandrel to better direct the electrospun fibers towards the grounded mandrel.
Fabrication and Electrospinning of PLGA Microsphere-Laden Nanofibrous Scaffolds
Degradable PLGA microspheres were fabricated using a double-emulsion water/oil/water technique based on [44]. Briefly, 0.5 grams of 75:25 PLGA was dissolved in 1 to 4 ml of DCM. The solution was further supplemented with 0.5 ml of 10% BSA and homogenized at Speed 5 for 30 seconds using a Homogenizer 2000 (Omni International, Kennesaw GA). One to 2 mL of 1% PVA was then added and the entire mixture re-emulsified by homogenization for 1 minute at Speed 1. Hardened microspheres were collected after gentle stirring for 3 hours in 100 ml of 0.1% PVA. The collected microsphere solution was then passed through a 70 μm nylon filter (BD Biosciences, Bedford, MA), centrifuged, and washed 3 times in water. Fabricated microspheres were lyophilized and stored at −20°C until use. Light microscope images were taken after fabrication, after filtration, and before lyophilization, and diameters determined using a custom MATLAB program. Microsphere density in formed nanofibers was determined after electrospinning from solutions containing 0.01, 0.03, 0.05, 0.07 and 0.09 g MS/mL PEO solution onto a glass slide for 5 seconds (n=3). For each condition, three light microscope images were obtained with similar fiber density per slide, and microspheres were counted in each image.
Fabrication of PCL/MS Composite Nanofibrous Scaffolds
Composite nanofibrous scaffolds (PCL/PCL and PCL/PEO) containing PS microspheres (15.7 micron diameter) were formed by dual-electrospinning from two opposing spinnerets onto a common rotating mandrel as in [45]. In one configuration, a PCL jet (2.5 mL, +15 kV, 12 cm) and a PCL jet with microspheres (2.5 mL/hr, +11 to +16 kV, 6 cm) were electrospun together. In the second configuration, a PCL jet and a PEO jet with microspheres (2 mL/hr, +16 kV, 6 cm) were electrospun together. Microsphere densities in the spinning solutions were 0, 0.05, 0.1 and 0.2 g PS microspheres/mL electrospinning solution. After fabrication, scaffold samples containing PEO were taken along the length of the scaffold, weighed, hydrated in 50% ethanol for 10 minutes to remove PEO, lyophilized and reweighed to determine PEO content as a function of position. Scaffolds were imaged via SEM (Philips XL20 by FEI, Hillsboro, Oregon) before and after PEO elution to visualize MS inclusions.
Mechanical Properties of PCL/MS Composite Nanofibrous Scaffolds
For mechanical testing, 30 × 5 mm strips of scaffold were excised with their long axes oriented in the fiber direction (along the circumference of the collecting mandrel). For PCL/PEO-MS scaffolds, strips containing ~15% PEO were utilized. Prior to mechanical testing, all samples were soaked in 50% ethanol for 10 minutes, and then stored in PBS until testing. The cross-sectional area of each sample was measured using an OptoNCDT laser measuring device (Micro-Epsilon, Raleigh, NC) combined with a custom Matlab program [46]. Samples were loaded into an Instron 5848 Microtester equipped with serrated vise grips and a 50 N load cell (Instron, Canton, MA). Strips were pre-loaded for 2 minutes to 0.5N, after which the gauge length was noted. Samples were then preconditioned with extension to 0.5% of the gauge length at a frequency of 0.1 Hz for 10 cycles. Finally, samples were extended to failure at a rate of 0.1% of the gauge length per second. Stiffness was determined from the linear portion of the force-elongation curve, and modulus calculated by considering sample cross-sectional area and gauge length.
Dual Release from Composite Nanofibrous Scaffolds
PLGA microspheres were formed containing two representative molecules, bovine serum albumin (BSA) and chondroitin sulfate (CS). BSA-containing microspheres were prepared as above with a 10% mass/volume BSA solution encapsulated in 50:50 PLGA. CS-containing microspheres were prepared from a 20% mass/volume CS solution that was mixed with 100 μl of 1% PVA with encapsulation in 50:50 PLGA. The initial encapsulation efficiency of BSA was determined by dissolving 50 mg of fresh MS in 0.1N NaOH containing 5% SDS with vigorous agitation for 16 hours. The supernatant was assessed via the BCA assay, with standards containing 0.1N NaOH with 5% SDS. To determine CS encapsulation efficiency, 50 mg of MS were dissolved in 8 mL of a 1:1 solution of DCM and H20 with vigorous agitation for 4 hours. After overnight phase separation, the aqueous phase was removed and CS content determined using the DMMB assay [47].
Long term release of CS or BSA from PLGA microspheres was evaluated via incubation in PBS (30 mg MS per 1 mL PBS) at 37°C on a 3-D mini-rocker (Denville Scientific, South Plainfield, NJ). At defined intervals over 5 weeks, microspheres were pelleted by centrifugation and the supernatant tested for CS content (via the DMMB assay) or BSA content (via the BCA assay) as above. At each sampling, fresh PBS was added and MS re-dispersed by gentle vortexing. Next, composites were formed to evaluate release from MS when entrapped in a PCL network. In preliminary studies, to image the composite, PCL was doped with fluorescein and PLGA microspheres were fabricated with rhodamine B. Fluorescent and light micrographs were overlaid to identify each component within the composite system. Subsequently, three microsphere-laden nanofibrous composites were constructed: one with CS-containing microspheres, one with BSA-containing microspheres, and one with a 1:1 mixture of CS- and BSA-containing microspheres. For these studies, rectangles of scaffold (80 mg) were cut across the length of the mandrel to ensure sample uniformity. Scaffolds were soaked in 5 ml of 50% ethanol for 10 minutes and washed in PBS to remove PEO. Scaffolds were then transferred to PBS (1 mL) and incubated as above for the MS release study. At set intervals, the supernatant was removed and CS and BSA quantified as above.
Statistical Analyses
One-way analysis of variance (ANOVA) was carried out using GraphPad Prism software (Graphpad Software, La Jolla, CA) with Bonferonni’s post-hoc tests (n=3 for characterization of MS density, n=5 for mechanical testing, n=5 for evalution of release kinetics), with significance set at p < 0.05.
Results
Formation of Nanofibers with Microspheres
Electrospinning from a solution of PEO and pre-fabricated fluorescent polystyrene microspheres resulted in the formation of fibers with embedded microspheres (Figure 1A). Similar findings were noted when PS microspheres were electrospun from a PCL solution, with thickened regions of PCL visible around the microsphere via SEM (Figure 1B).
Figure 1. Fabrication of microsphere-laden nanofibrous scaffolds.
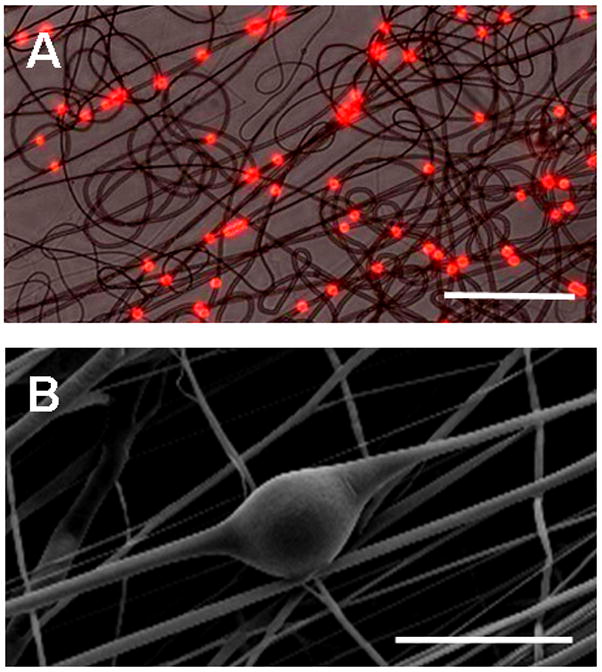
(A) Composite light and fluorescent micrograph showing electrospun PEO fibers with embedded PS microspheres (diameter 2 microns) distributed along the fiber length (Scale bar = 50 μm). (B) SEM micrograph demonstating alterations in PCL fiber morphology local to the inclusion of an 15.7 micron diameter PS microsphere (Scale bar = 25 μm).
PLGA microspheres were fabricated via the water/oil/water double emulsion process (Figure 2A). Microsphere diameters were on the order of 10–20 microns (Figure 2B), with little change through the washing process (data not shown). Increasing the density of PLGA microspheres in the PEO electrospinning solution increased the density of microspheres in the resulting fibers (Figure 2C, D). Microsphere numerical density within the fibrous scaffold was higher for solutions starting with microspheres at 0.07 and 0.09 g/mL compared to those starting with lower microsphere concentrations (Figure 2C, p<0.05).
Figure 2. Dose-dependent inclusion of PLGA microspheres in nanofibrous mats.
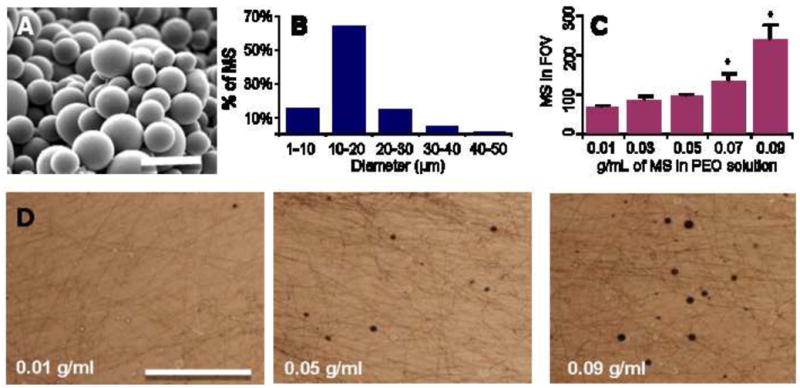
(A) SEM micrograph showing PLGA microspheres fabricated by the double emulsion technique (Scale bar = 50 μm). (B) Histogram of microsphere diameter. (C) PLGA microsphere density with a field of view (FOV) of a PEO fiber mat increases with increasing microsphere density in the electrospinning solution. *indicates significant difference compared with lower values, p<0.05. (D) Bright-field images of PEO fiber mats formed from solutions of increasing PLGA MS density (Scale bar = 500 μm).
Fabrication and Electrospinning of Microsphere-Laden Nanofibrous Scaffolds
As described above, and shown schematically in Figure 3, a fabrication system was developed to entrap microspheres within a fibrous scaffold. In this technique, the sacrificial PEO fiber population containing microspheres is co-electrospun with PCL fibers onto a common rotating mandrel. Fluorescent labeling of PCL fibers (green) and microspheres (blue), while the PEO component remained unlabelled, identifies the blend of the three components (Figure 4A). Upon hydration, the sacrificial PEO fibers dissolve away, resulting in a structure in which microspheres are entrapped between aligned PCL fibers. SEM images of composites before (Figure 4B) and after (Figure 4C, D) PEO removal shows that microspheres remain entrapped between the aligned fibers throughout the fabrication process. Notably, this dispersion is seen through the thickness of the composite when cross sections are viewed end on (Figure 4D).
Figure 3. An approach for decoupling drug delivery from scaffold mechanics.
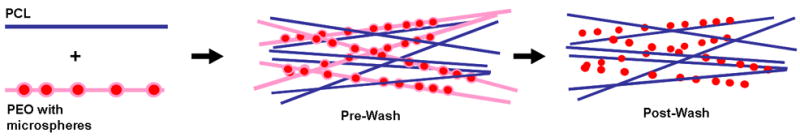
Composite scaffolds are formed from microspheres delivered through a sacrificial PEO fiber fraction coupled with a stable PCL fiber fraction (Pre-Wash). With dissolution of the PEO (After-Wash), MS remain entrapped within the slow degrading and surrounding fibrous PCL fibrous network.
Figure 4. Realization of composite MS-laden scaffolds with sacrificial content.
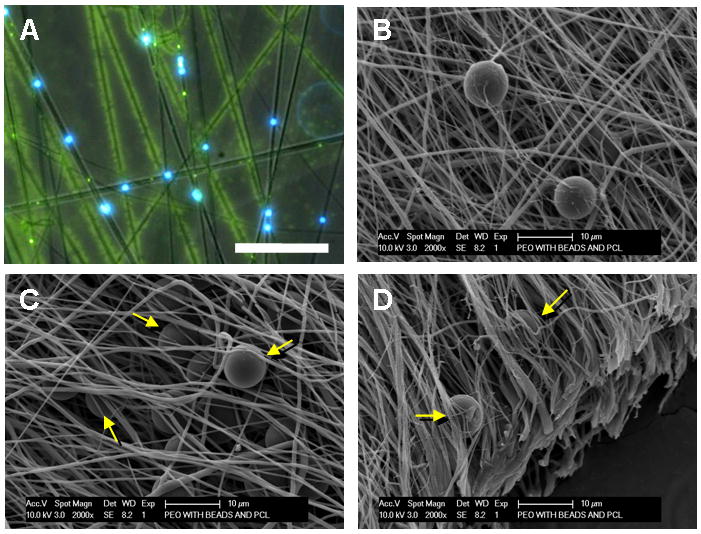
Bright-field with overlaid fluorescent image (A, 4X, Scale bar = 50 μm) and SEM (B, Scale bar = 20 μm) of PEO/PCL/MS composite. In (A), blue shows MS, green shows PCL fibers, and black shows sacrificial PEO fibers within the composite structure. After PEO removal, microspheres remain entrapped and distributed between the remaining PCL fibers (C and D, arrows, Scale bar = 10 μm).
Mechanical Properties of Composite Scaffolds as a Function of Microsphere Inclusion To better understand the mechanical consequences of microsphere inclusion, networks were formed in which a graded concentration of polystyrene microspheres was entrapped either within or between the nanofibers of the scaffold. PS microspheres (15.7 μm diameter) were used here because PLGA microspheres would dissolve when mixed into the solvents employed for electrospinning PCL. Scaffolds were fabricated as depicted in Figure 5A and 5D, with one jet used to produce a PCL fiber population, and a second jet used to produce a microsphere-containing fiber population of either PCL or PEO. Tensile testing showed that when microspheres were included within the PCL fiber population, both the stiffness and modulus decreased with each step of increasing microsphere density (Figure 5B and C). Conversely, in composites where the microspheres were entrapped between fibers after sacrificial fiber removal, no change in stiffness was observed at any microsphere density (Figure 5E). Likewise, modulus in these composites did not differ from control values at Low microsphere densities.
Figure 5. Construction and mechanical analysis of composite MS-laden scaffolds.
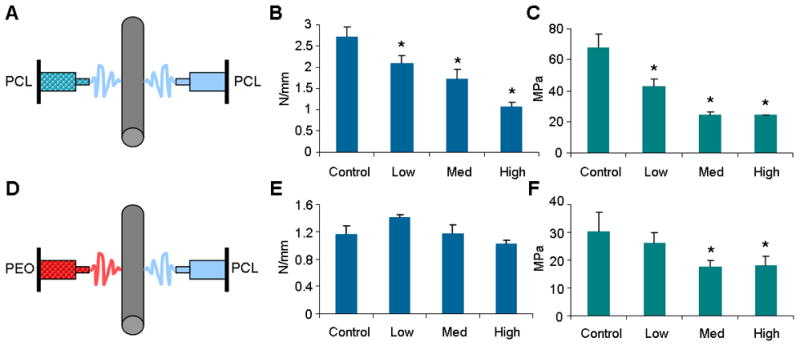
(A) Schematic of electrospinning PCL/PCL-MS scaffold. (B) Stiffness of scaffold decreases with increasing MS density (Control = 0, Low = 0.05, Med = 0.1, High = 0.2 g MS/mL electrospinning solution). (C) Modulus decreases with increasing MS density. (D) Schematic of electrospinning PCL/PEO-MS scaffold. (E) Stiffness does not change with increasing MS density. (F) Modulus decreases at medium and high density MS inclusion, but not at low inclusion density. *indicates p < 0.05 from control.
Controlled Release from Microsphere-Laden Nanofibrous Composites
To determine if molecules could be released from the composite in a controlled fashion, BSA- and CS-containing PLGA microspheres were fabricated and release rates determined for both free microspheres and microspheres entrapped within the composite structures. The encapsulation rate for each molecule was 13% and 11%, respectively, with a burst release occurring over the first day for free microspheres, followed by a sustained release over 27 days (Figure 6B). The initial burst release was larger from the CS-containing microspheres compared to BSA-containing microspheres. By day 27, free microspheres had degraded to the point where clumping of the polymer was apparent (data not shown). When either BSA- or CS-containing microspheres were electrospun into the composite, a slightly-more gradual release profile was observed over the first 5 days, with sustained released occurring thereafter (Figure 6C) perhaps due to washing steps that occurred during PEO removal. Contrary to free microspheres, microspheres entrapped in nanofibrous scaffolds remained distinct, most likely due to physical protection and isolation when media was changed (data not shown). When the microsphere populations were mixed 1:1 and electrospun into a single nanofibrous composite (Figure 6D, black = CS, red = BSA), a similar graded release profile for each molecule was observed over 35 days.
Figure 6. Controlled release from composite MS-laden scaffolds.
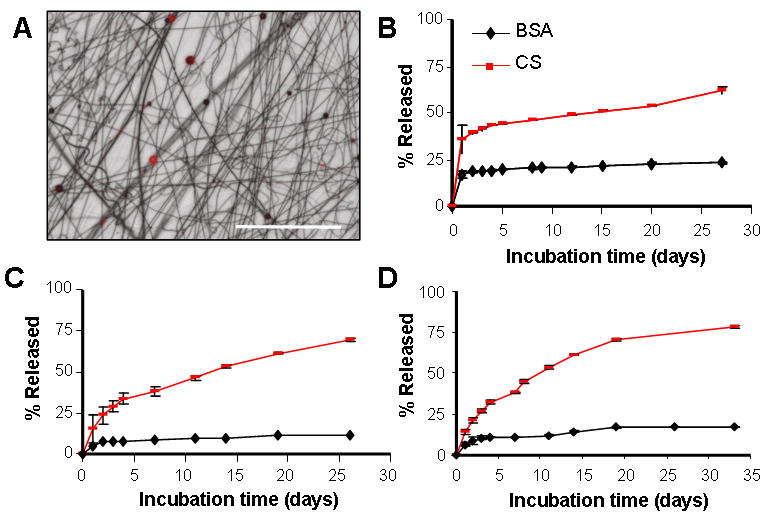
(A) Overlay of light and fluorescent micrographs showing mixed MS population (BSA MS = red, CS MS = black, scale bar = 250 μm). (B) Sustained release of bovine serum albumin (BSA) or chondroitin sulfate (CS) from PLGA microspheres with time in physiologic conditions. (C) Sustained release of BSA and CS from composite PCL/PEO-MS scaffold containing either BSA or CS microspheres. (D) Sustained release of both BSA and CS from a single composite system containing both BSA and CS microspheres at a 1:1 ratio.
Discussion
Electrospun nanofibrous scaffolds are a promising tool for fibrous tissue engineering as they provide excellent structural cues and can foster development of anisotropic mechanical properties similar to native tissues [10]. Indeed, we have grown constructs in vitro, under chemically defined conditions and with the addition of matrix-promoting growth factors that reach 50–100% of the tensile properties of native meniscus and annulus fibrosus [3, 12]. Simply providing a guided micropattern for tissue formation may not be enough, however, as both tissue development and regeneration occur in the context of a host of biologic factors whose timing and doses vary considerably. Moreover, upon implantation of a scaffold, our ability to control the chemical environment (i.e., the provision of pro-matrix forming growth factors in culture medium) is lost. Further functionalization of these scaffolds to enable delivery of drugs, growth factors or other chemicals would further our ability to both guide construct maturation and dictate cell behavior in vivo and in vitro.
Several recent reports have shown that micro-and nano-particles can be incorporated into electrospun nanofibers. In one report, Lim and colleagues demonstrated that silica particles ranging in size from 100–1000 nanometers could be electrospun from a solution of polyacrylimide to create a ‘bead on a string’ fiber morphology [48]. Also, Dong et al. incorporated two distinct populations of nanospheres into electrospun polyurethane fibers, suggesting the ability to multiplex delivered factors, but did not evaluate release [49]. Towards drug delivery, Melaiye et al. incorporated silver(I)-imidazole cyclophane gem-diol complexes into tecophilic polymer electrospun fibers, and demonstrated that release of this molecule from particles within the fibers could prevent microbial growth [50]. Finally, Qi et al. fabricated BSA-loaded Ca-alginate microspheres and emulsion electrospun the spheres within PLLA fibers. In this context, BSA released at a slower rate and with a lower initial burst than from free Ca-alginate microspheres [51]. While these previous studies represent early efforts to protect a molecule during fabrication and release it from a particle in a fiber, they did not address the mechanical characteristics of the system or how to decouple the fiber function, degradation, and microsphere release kinetics.
Given the mechanical roles these scaffolds must play upon in vivo placement (where the tensile moduli of fiber reinforced tissues are on the order of 100 MPa [52]), we endeavored to create a system where microspheres could be delivered without significantly disrupting the overall scaffold mechanics. The inclusion of particles within fibers disrupts individual fiber architecture (Figure 1B) and creates local stress concentrations, thereby modifying the overall mechanical properties of the scaffold. When the microspheres were included within the load-bearing PCL fibers, scaffold stiffness decreased even at low microsphere concentrations (Figure 5B). Conversely, in our composite system that contains particles between the fibrous network but not the fibers themselves, stiffness remained unchanged (Figure 5E) at all microsphere densities explored. Of note, while stiffness did not change in our composite, the modulus did decrease at the medium and high microsphere concentrations. This result was due to a slight increase in scaffold cross sectional area with microsphere inclusion caused by decreased fiber packing, and may be considered a limitation of our design.
Spatial and temporal control of growth factor presentation is an important consideration in directing cell behavior during development and repair. Delivery of particles within a fiber may complicate release by coupling molecular diffusion within a fiber and/or fiber degradation with the release kinetics of the factor from the particle itself. Our approach delivers particles via a sacrificial fiber population that is removed immediately upon hydration. When particles are of sufficient size (20 microns, in this case), they remain entrapped within the fibrous network but are exposed directly to the aqueous environment. This approach decouples the release kinetics of the microspheres from the degradation kinetics of the fibers themselves. Furthermore, using PEO allows for a compatible solvent system (water) for sacrificial fiber production, such that the PLGA microsphere structure is not disrupted with exposure to organic solvents (i.e., the DMF/THF solution used to dissolve PCL). When two model agents, BSA and CS, were included in microspheres in the composite, release kinetics were independent from one another and comparable to free microspheres, suggesting that release is indeed independent of the surrounding fiber population (Figure 6).
The potential applications of a composite nanofibrous system that can deliver multiple factors in a controlled fashion while maintaining mechanical functionality are enumerable. For example, a cascade of growth factors (i.e., PDGF followed by VEGF) might be delivered to promote vascularization of the implanted construct [19]. This would be particularly suited for the knee meniscus, whose dense structure and limited vascularity does not allow for endogenous repair. Alternatively, one might engineer the system to provide for instantaneous release of mitogenic (i.e., FGF) or migratory factors, followed by a delayed release of a pro-matrix forming compound (i.e., TGF-β). This construction would promote cell infiltration from surrounding tissue and division during an initial period of repair, followed by transition towards a matrix deposition phase of development. Delivered factors also need not be solely anabolic/growth promoting. For example, microparticles might be designed to deliver proteases locally to engender local matrix disruption to enhance bridging of new matrix between the host tissue and the implanted material. Similarly, the distribution of particles need not be homogenous, with gradients of local release established both through the depth and along the fiber plane.
While the results of this study are promising, and the system meets our stated design criteria, some issues remain to be optimized. First, it is not clear how microsphere size influences mechanical properties; in this work, microspheres were on the order of 20–30 microns. Larger microsphere sizes might further disrupt mechanical properties, while smaller particles could be lost from the scaffold through the porous structure. Additional studies are required to examine this variable. Another point of optimization involves the steric and biologic influence of the particles themselves. We have previously demonstrated that both meniscus fibrochondrocytes and mesenchymal stem cells attach to and infiltrate electrospun PCL scaffolds [10–12, 45]. While the microspheres in this formulation are composed of a biocompatible material (PLGA), local pH changes with PLGA degradation might influence cellular activity. Further, sacrificial fibers were used here to deliver microspheres. We previously utilized these sacrificial fibers (at a level of ~40–60% of the composite) to increase scaffold porosity and enhance cell infiltration into the depth of the aligned nanofibrous structure [45]. For microsphere inclusion, our highest PEO content was on the order of 15%. It remains to be determined how this low level of sacrificial fibers (and the potential decrease in fiber packing due to the microspheres themselves) influences cell infiltration. Future iterations may utilize a multiple spinneret system comprised of one source jet delivering PCL or another slow-degrading structural fiber population, one source jet delivering PEO fibers, and the final jet delivering microspheres through additional sacrificial PEO fibers. Such a multi-jet system would also allow for the provision of additional mechanical functionality via variation in the mechanical properties of the PCL or slow eroding component [53]. A final point of optimization is the microspheres themselves. We used a traditional fabrication technique (water/oil/water emulsions) to entrap model compounds in order to demonstrate multi-factor release. While sufficient for proof of principle, we did observe the commonly seen burst release with each compound. Others have shown that microsphere fabrication methods can be tuned to enable release with a multitude of profiles, including constant, early burst, and late burst [54]; such methods would be useful in further tuning towards the intended biologic applications described above.
Conclusions
Overall, this study describes a approach for the creation of drug-delivering anisotropic nanofibrous scaffolds for fibrous tissue engineering. In this fabrication method the inclusion of microspheres does not significantly modify the mechanical properties of the scaffold or the release properties of the microspheres entrapped within the composite. Importantly, multiple populations of microspheres releasing unique factors can be incorporated, allowing for the complex control of cellular behavior through spatially and temporally-tuned release. Vascular recruitment, cellular phenotype and matrix elaboration may all be dictated via the proper release of single or multiple factors from these composites. Rather than creating a simple template for new ECM deposition, this advanced composite provides higher order functionality for mechanical and biologic guidance of tissue regeneration.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AR056624 and T32 AR007132), the Penn Center for Musculoskeletal Disorders, the University Of Pennsylvania Department Of Orthopaedic Surgery, and the Department of Veterans Affairs (VA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li WJ, Mauck RL, Tuan RS. Electrospun nanofibrous scaffolds: production, characterization, and applications for tissue engineering and drug delivery. J Biomed Nanotechnol. 2005;1(3):259–275. [Google Scholar]
- 2.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007 Dec 10;59(14):1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Baker BM, Handorf AM, Ionescu LC, Li WJ, Mauck RL. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev Med Devices. 2009;6(5):515–532. doi: 10.1586/erd.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauck RL, Baker BM, Nerurkar NL, Burdick JA, Li WJ, Tuan RS, et al. Engineering on the straight and narrow: The mechanics of nanofibrous assemblies for fiber-reinforced tissue regeneration. Tissue Eng Part B Rev. 2009;15(2):171–193. doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2006;40(8):1686–1693. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27(19):3631–3638. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Ayres CE, Bowlin GL, Pizinger R, Taylor LT, Keen CA, Simpson DG. Incremental changes in anisotropy induce incremental changes in the material properties of electrospun scaffolds. Acta Biomater. 2007;3(5):651–661. doi: 10.1016/j.actbio.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Ouyang G, McCann JT, Xia Y. Collecting electrospun nanofibers with patterned electrodes. Nano Letters. 2005;5(5):913–916. doi: 10.1021/nl0504235. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Wang Y, Xia Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv Mater. 2004;16(4):361–366. [Google Scholar]
- 10.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007 Apr;28(11):1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 12.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8(12):986–992. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffat KL, Wang IN, Rodeo SA, Lu HH. Orthopedic interface tissue engineering for the biological fixation of soft tissue grafts. Clin Sports Med. 2009;28(1):157–176. doi: 10.1016/j.csm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49(23):2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12(7):579–587. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 16.Li WJ, Jiang YJ, Tuan RS. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue Eng Part A. 2008 May;14(5):639–648. doi: 10.1089/tea.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker BM, Nathan AS, Huffman GR, Mauck RL. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthr Cartilage. 2009;17(3):336–345. doi: 10.1016/j.joca.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nerurkar NL, Mauck RL, Elliott DM. ISSLS prize winner: integrating theoretical and experimental methods for functional tissue engineering of the annulus fibrosus. Spine. 2008;33(25):2691–2701. doi: 10.1097/BRS.0b013e31818e61f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 20.Hadjiargyrou M, Chiu JB. Enhanced composite electrospun nanofiber scaffolds for use in drug delivery. Expert Opin Drug Deliv. 2008;5(10):1093–1106. doi: 10.1517/17425247.5.10.1093. [DOI] [PubMed] [Google Scholar]
- 21.He CL, Huang ZM, Han XJ, Liu L, Zhang HS, Chen LS. Coaxial electrospun poly(lactic acid) ultrafine fibers for sustained drug delivery. J Macromol Sci Phys. 2006;45:515–524. [Google Scholar]
- 22.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater. 2004;70(2):286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 23.Kenawy ER, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, et al. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release. 2002;17(81):57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 24.Zong X, Kim K, Fang D, Ran SH, Hsiao BS, Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43(16):4403–4412. [Google Scholar]
- 25.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98(1):47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, et al. Biodegradable electrospun fibers for drug delivery. J Control Release. 2003;92(3):227–231. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 27.Hong Y, Fujimoto K, Hashizume R, Guan J, Stankus JJ, Tobita K, et al. Generating elastic, biodegradable polyurethane/poly(lactide-co-glycolide) fibrous sheets with controlled antibiotic release via two-stream electrospinning. Biomacromolecules. 2008;9(4):1200–1207. doi: 10.1021/bm701201w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff H, Greiner A. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules. 2005;6(3):1484–1488. doi: 10.1021/bm0492576. [DOI] [PubMed] [Google Scholar]
- 29.Zeng J, Yang L, Liang Q, Zhang X, Guan H, Xu X, et al. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J Control Release. 2005;105(1–2):43–51. doi: 10.1016/j.jconrel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Wang CH. Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm Res. 2006;23(8):1817–1826. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Yang L, Xu X, Wang X, Chen X, Liang Q, et al. Ultrafine medicated fibers electrospun from W/O emulsions. J Control Release. 2005;108(1):33–42. doi: 10.1016/j.jconrel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Hu Y, Li Y, Zhao P, Zhu K, Chen W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J Control Release. 2005;108(2–3):237–243. doi: 10.1016/j.jconrel.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Hu Y, Zhao P, Li Y, Zhu K. Modulation of protein release from biodegradable core-shell structured fibers prepared by coaxial electrospinning. J Biomed Mater Res B Appl Biomater. 2006;79B(1):50–57. doi: 10.1002/jbm.b.30510. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YZ, Wang X, Feng Y, Li J, Lim CT, Ramakrishna S. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(e-caprolactone) nanofibers for sustained release. Biomacromolecules. 2006;7(4):1049–1057. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- 35.Maretschek S, Greiner A, Kissel T. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. J Control Release. 2008;127(2):180–187. doi: 10.1016/j.jconrel.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Li X, Qi M, Zhou S, Weng J. Release pattern and structural integrity of lysozyme encapsulated in core-sheath structured poly(dl-lactide) ultrafine fibers prepared by emulsion electrospinning. Eur J Pharm Biopharm. 2008;69(1):106–116. doi: 10.1016/j.ejpb.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J Control Release. 2003;89(2):341–353. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 38.Nie H, Wang CH. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J Control Release. 2007;120(1–2):111–121. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Liang D, Luu YK, Kim K, Hsiao BS, Hadjiargyrou M, Chu B. In vitro non-viral gene delivery with nanofibrous scaffolds. Nucleic Acids Res. 2005;33(19):170. doi: 10.1093/nar/gni171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chew SY, Wen J, Yim EKF, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6(4):2017–2024. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- 41.Liao IC, Chew SY, Leong KW. Aligned core-shell nanofibers delivering bioactive proteins. Nanomedicine. 2006;1(4):465–471. doi: 10.2217/17435889.1.4.465. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Huang ZM, He CL, Yang A, Zhang Y, Han XJ, Yin J, et al. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J Biomed Mater Res A. 2006;77A(1):169–179. doi: 10.1002/jbm.a.30564. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 45.Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29(15):2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27(3):416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 48.Lim JM, Moon JH, Yi GR, Heo CJ, Yang SM. Fabrication of one-dimensional colloidal assemblies from electrospun nanofibers. Langmuir. 2006;22(8):3445–3449. doi: 10.1021/la053057d. [DOI] [PubMed] [Google Scholar]
- 49.Dong B, Smith ME, Wnek GE. Encapsulation of multiple biological compounds within a single electrospun fiber. Small. 2009;5(13):1508–1512. doi: 10.1002/smll.200801750. [DOI] [PubMed] [Google Scholar]
- 50.Melaiye A, Sun Z, Hindi K, Milsted A, Ely D, Reneker DH, et al. Silver imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: formation of nanosilver particles and antimicrobial activity. J Am Chem Soc. 2005;127(7):2285–2291. doi: 10.1021/ja040226s. [DOI] [PubMed] [Google Scholar]
- 51.Qi H, Hu P, Xu J, Wang A. Encapsulation of drug reservoirs in fibers by emulsion electrospinning: morphology characterization and preliminary release assessment. Biomacromolecules. 2006;7(8):2327–2330. doi: 10.1021/bm060264z. [DOI] [PubMed] [Google Scholar]
- 52.Bursac P, York A, Kuznia P, Brown LM, Arnoczky SP. Influence of donor age on the biomechanical and biochemical properties of human meniscal allografts. Am J Sports Med. 2009;37(5):884–889. doi: 10.1177/0363546508330140. [DOI] [PubMed] [Google Scholar]
- 53.Tan AR, Ifkovits JL, Baker BM, Brey DM, Mauck RL, Burdick JA. Electrospinning of photocrosslinked and degradable fibrous scaffolds. J Biomed Mater Res A. 2008;15(87):1034–1043. doi: 10.1002/jbm.a.31853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee W, Wiseman ME, Cho NJ, Glenn JS, Frank CW. The reliable targeting of specific drug release profiles by integrating arrays of different albumin-encapsulated microsphere types. Biomaterials. 2009;30(34):6648–6654. doi: 10.1016/j.biomaterials.2009.08.035. [DOI] [PubMed] [Google Scholar]


