Abstract
Context
Hyperglycemia resolves quickly after bariatric surgery, but the underlying mechanism and the most effective type of surgery remains unclear.
Objective
To examine glucose metabolism and β-cell function in patients with type 2 diabetes mellitus (T2DM) after two types of bariatric intervention; Roux-en-Y gastric bypass (RYGB) and gastric restrictive (GR) surgery.
Design
Prospective, nonrandomized, repeated-measures, 4-week, longitudinal clinical trial.
Patients
In all, 16 T2DM patients (9 males and 7 females, 52±14 years, 47±9 kg m−2, HbA1c 7.2±1.1%) undergoing either RYGB (N=9) or GR (N=7) surgery.
Outcome measures
Glucose, insulin secretion, insulin sensitivity at baseline, and 1 and 4 weeks post-surgery, using hyperglycemic clamps and C-peptide modeling kinetics; glucose, insulin secretion and gut-peptide responses to mixed meal tolerance test (MMTT) at baseline and 4 weeks post-surgery.
Results
At 1 week post-surgery, both groups experienced a similar weight loss and reduction in fasting glucose (P<0.01). However, insulin sensitivity increased only after RYGB, (P<0.05). At 4 weeks post-surgery, weight loss remained similar for both groups, but fasting glucose was normalized only after RYGB (95±3 mg 100 ml−1). Insulin sensitivity improved after RYGB (P<0.01) and did not change with GR, whereas the disposition index remained unchanged after RYGB and increased 30% after GR (P=0.10). The MMTT elicited a robust increase in insulin secretion, glucagon-like peptide-1 (GLP-1) levels and β-cell sensitivity to glucose only after RYGB (P<0.05).
Conclusion
RYGB provides a more rapid improvement in glucose regulation compared with GR. This improvement is accompanied by enhanced insulin sensitivity and β-cell responsiveness to glucose, in part because of an incretin effect.
Keywords: diabetes remission, insulin secretion, incretin response, hyperglycemic clamp, insulin sensitivity, type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) is a major endocrine disorder that is characterized by progressive β-cell failure and hyperglycemia.1 The development of T2DM is strongly associated with obesity and the accumulation of abdominal and ectopic fat. These fat deposits have been linked to peripheral and hepatic insulin resistance, inflammation and subsequent ‘lipotoxicity’ of β-cells.2,3 Hypocaloric diet, exercise and weight loss improves the pathophysiology of T2DM, preserves β-cell function and represents the first-line treatment for newly diagnosed patients.4 Although the long-term efficacy of these treatments is poor, emerging data suggests that bariatric surgery may provide a more sustained and effective treatment for obesity and related morbidities, including hyperglycemia, hyperlipidemia and T2DM.5,6 Animal studies suggest that the underlying mechanism that is targeted by bariatric surgery includes altered β-cell secretion, and/or improved entero-insulinar responses, specifically the main incretin hormones—glucagon-like peptide-1 (GLP-1) and gastric-inhibitory peptide (GIP).7,8 As different types of surgery may present different stimuli to the pancreas and gut, there is a need to identify the effects of these surgeries on endocrine and gastrointestinal function.
Gastric restrictive surgeries, (laparoscopic adjustable gastric band (LAGB) and laparoscopic sleeve gastrectomy (LSG)) restrict stomach capacityto limit the intake of solid food and calories and as a result facilitate weight loss. Multiple studies,9–11 including a randomized control trial,9 have shown remission of T2DM with LAGB (versus conventional medical therapy) that is primarily mediated by weight loss and improved insulin sensitivity, both of which occur several months following surgery in patients with early stage T2DM. The gut hormones that promote satiety (peptide YY (PYY), GLP-1) do not show the same magnitude of response following gastric restrictive surgery (GR) (LAGB), as has been observed with intestinal bypass surgery.10,11
Roux-en-Y gastric bypass (RYGB) results in the rapid improvement of hyperglycemia within days after surgery and often allows discontinuation of diabetes medications.12,13 These remarkable data suggest that rapid β-cell enhancing effects related to intestinal bypass occur before weight loss. However, the kinetics of in vivo insulin secretion immediately after RYGB has not been characterized in T2DM. Rodent models of diabetes demonstrate immediate anti-diabetic effects of duodenal exclusion of nutrients, independent of gastric restriction,7,14 suggesting that nutrient interaction within the gastro-jejunal intestine has direct anti-diabetic effects. Several studies have demonstrated an improved β-cell response after RYGB and this was related to an incretin effect with increases in GLP-1 and GIP levels.15–17 However, as the deterioration of glucose homeostasis in T2DM can develop in the absence of any impairment in GIP or GLP-1 levels and/or action,18 the improvement of glycemia immediately after RYGB may not be completely attributable to an incretin effect.19
We hypothesized that before marked weight loss; the intestinal bypass component of RYGB has specific effects to enhance β-cell function aside from enforced hypocaloric effects because of gastric restriction. An additional aim of our study was to determine the metabolic effects of intestinal bypass associated with RYGB versus GR procedures alone with respect to fasting glucose, insulin secretion, insulin sensitivity and circulating incretin hormone responses. We examined these effects 1 and 4 weeks post-surgery, which is long before the maximal weight loss that is typically achieved after these surgeries.
Patients and methods
Patients
In all, 16 consecutive patients with T2DM attending our Bariatric Clinic, who met the NIH criteria for the eligibility for bariatric surgery,20 participated in this study. This cohort included 7 female and 9 male participants, of primarily Caucasian (n=15) descent, with one of African–American ethnicity, a mean age of 52±14 years and a mean body mass index of 47±9 kg m−2. The mean pre-operative fasting plasma glucose was 140±42 mg 100 ml−1 and HbA1c was 7.2±1.1%. The average duration of diabetes based on history was 5±1 years. None of the women were taking hormonal therapy, no subjects used steroid medications and all were non-smokers. Nine subjects underwent RYGB (age: 48±4 years, 5 females and 4 males, body mass index 47±3 kg m−2, HbA1c 7.1±0.4%) and seven underwent GR procedures (age: 59±5 years, 2 females and 5 males, body mass index 48±3 kg m−2, HbA1c 7.2±0.5%). Within the GR group four subjects received LSG and three subjects received LAGB. Diabetes medications for the entire cohort included oral agents for 12 subjects, insulin therapy for 2 subjects and diet control for the remaining 2 subjects. The distribution of diabetes medications for the RYGB group was as follows: 2 out of 9 subjects used sulfonylureas, 4 out of 9 used metformin, 1 out of 9 used TZDs and 1 out of 9 used insulin. The distribution of anti-diabetic medications for GR group was 1 out of 7 subjects used sulfonylureas, 4 out of 7 used metformin, 1 out of 7 used TZDs and 1 out of 7 used insulin. No subjects used incretin mimetics or analogs. After RYGB surgery, 4 out of 9 subjects continued metformin and 1 out of 9 continued TZDs. After GR, 4 out of 7 subjects continued metformin and 1 out of 7 continued TZD. Sulfonylureas and insulin use was discontinued by subjects in both groups. The Cleveland Clinic's Institutional Review Board approved the protocol, and all participants provided written informed consent.
Study design
Patients were studied 1–2 weeks before surgery and again at 1 and 4 weeks after surgery. Insulin secretion and sensitivity were assessed using the hyperglycemic clamp procedure, as previously described,21 and a mixed meal tolerance test (MMTT). The incremental increase in glucose achieved during the hyperglycemic clamp was matched between study visits and between surgery group types, and the clamp was always performed 2–3 days after the MMTT. Pre-operative metabolic testing was carried out when subjects were consuming an 800-calorie liquid diet for 2 weeks before surgery. Oral agents were discontinued 24 h before the procedures. Sulfonylurea medications and insulin were discontinued after surgery; however, metformin and thiazoledindiones were continued as before surgery. No major postoperative complication (infection, bleeding, anastomotic leaks and so on) related to surgery was noted in any of the subjects.
Surgical procedures
All procedures were carried out by the same surgeon (PRS). The techniques we use to perform LSG, LAG banding and laparoscopic RYGB have been previously described13,22,23. An illustration of the relevant procedures is available as a supplementary procedure (available online).
Metabolic studies
Hyperglycemic clamp
A hyperglycemic clamp was performed as previously described.21 A bolus of glucose 0.3 gm kg−1 was given over 2 min. Blood was drawn every 2 min for the first 10 min and every 5 min thereafter for a total of 120 min. A 20% dextrose solution was started at 10 min and adjusted every 5 min as needed to maintain blood glucose levels at a hyperglycemic level of 125 mg 100 ml−1 above the fasting glucose. The magnitude of increase in glucose was matched for subjects before and after surgery and between surgical groups.
MMTT
Ensure plus (4 ounces; calories, 175 kcal; protein, 6.5 g; fat, 5.5 g; carbohydrate, 25 g) was given after a 12–14 h overnight fast. Fasting blood samples were obtained for glucose, c-peptide, insulin, lipids, HbA1c, a complete metabolic panel and thyroid-stimulating hormone. Blood was drawn every 30 min for 120 min during the MMTT for the determination of levels of glucose, insulin and c-peptide levels. Additional blood was drawn at fasting and at 30 and 60 min after ingestion for the determination of GLP-1, GIP and total amylin responses.
Analytical determinations
Blood glucose was measured immediately using the glucose oxidase method (YSI 2300 STAT Plus, Yellow Springs, OH, USA). Plasma insulin was assayed by a double-antibody radioimmunoassay (RIA, Linco Research, St Charles, MO, USA). The intra and inter-assay coefficient of variation was 2.6 and 3.0%, respectively, for level 1, and 1.6 and 4.0% for level 2 respectively. C-peptide was assayed using a chemiluminescence immunoassay (Linco Research, St Charles, MO, USA). The intra and inter-assay coefficient of variation was 3.5 and 7.2%, respectively, for level 1, and 3.2 and 3.0% for level 2. Blood collected for GLP-1 (active), GIP (total), PYY (total) and amylin (total) analysis was treated immediately with a DPP4 and protease cocktail inhibitor (Sigma, St Louis, MO, USA), and assayed with a multiplex ELISA kit (Millipore, Billerica, MA, USA). The intra and inter-assay coefficient of variation was 3.6 and 9.3%, respectively. A high-sensitivity CRP immunoturbidimetric assay was run on a Roche Modular platform (Roche Diagnostics, Indianapolis, IN, USA). This assay involves anti-CRP mouse monoclonal antibodies coupled to latex microparticles that react with the antigen to form an antigen–antibody complex. The analytical sensitivity is >0.425 mg l−1. To correct for inter-assay variability, all pre- and post-measurements for each individual were run on the same plate. Free fatty acid (FFA) levels were determined by standard colorimetric methods (Wako Chemicals, Richmond, VA, USA). The intra and inter-assay coefficient of variation was 3.0 and 4.6% for level 1, respectively, and 5.2 and 5.6% for level 2, respectively.
Calculations
The steady-state glucose-infusion rate during the hyperglycemic clamp represents the mean glucose-infusion rate from 80–120 min, corrected for urinary glucose losses. Insulin sensitivity was determined by dividing the average glucose infusion (M value in mg kg−1 min−1) during the last 40 min of the clamp by the average plasma insulin concentration during that same interval (M/I). The first-phase insulin response during the hyperglycemic clamp was determined by the mean insulin concentration during 0–10 min minus the fasting insulin concentration. The second-phase insulin response was determined by the mean insulin concentration during the last 40 min of the clamp minus the fasting insulin concentration. The insulinogenic index during the MMTT was calculated from the difference in insulin (0–30 min) divided by the difference in the corresponding glucose concentration, (ΔI30/ΔG30).
C-peptide modeling kinetics and β-cell function
Insulin-secretion rates were estimated from peripheral plasma C-peptide levels by deconvolution analysis and linear regularization using a two-compartment model with standard parameters for C-peptide kinetics as validated by Van Cauter et al.24 During the hyperglycemic clamp the acute insulin response (AIR) was evaluated as the area under the curve of insulin secretion rate from 0 to 10 min. The disposition index was determined as the AIR relative to the prevailing insulin sensitivity (M/I×1000). During the MMTT, glucose sensitivity of β-cell was calculated by estimating the slope of the dose–response curve of insulin-secretion rate versus glucose concentration at each measured time point.
Statistical analysis
All values represent the mean±s.e.m. Within surgery group differences were determined by the paired two-tailed Student's t-test. Differences between surgery groups were tested by unpaired two-tailed Student's t-test and two-way analysis of variance for repeated measures. Normal distribution was checked before all analyses and the non-parametric estimates were used when appropriate. Comparisons were considered statistically significant if the P value was <0.05.
Results
Clinical and metabolic parameters post-surgery for RYGB versus GR
Before surgery, baseline body mass index, fasting plasma glucose and HbA1c levels were not different between groups (Table 1). However, homeostasis model assessment (HOMA) levels indicated that the RYGB subjects were markedly more insulin resistant (P<0.05). Weight loss was similar at 1 week (∼7–8%) and 4 weeks (∼10%) following both surgery types. Fasting plasma glucose was reduced at 1 week (by ∼20%) in both groups; however, at 4 weeks fasting glucose was further reduced in RYGB, but this was not evident in the GR group (Table 1). Fasting insulin was reduced at 1 and 4 weeks after RYGB, but did not change after GR. C-reactive protein was reduced ∼60% after RYGB and did not change after GR. Liver transaminase levels were within normal limits and did not change significantly 4 weeks following either procedure.
Table 1.
Clinical and metabolic parameters before, 1 week and 1 month after GR and RYGB
| GR | RYGB | |||||
|---|---|---|---|---|---|---|
| Pre-surgery | 1 week | 4 weeks | Pre-surgery | 1 week | 4 weeks | |
| Clinical | ||||||
| Weight (kg) | 147±11 | 137±13 | 133±11† | 139±10 | 127±9‡ | 124±10‡ |
| BMI (kg m−2) | 48±3 | 47±4 | 44±3† | 47±3 | 41±2‡ | 42±3‡ |
| HbA1c (%) | 7.2±0.5 | 6.5±0.4 | 6.5±0.3 | 7.1±0.4 | 6.4±0.2 ‡ | 6.5±0.3‡ |
| Fasting plasma glucose (mg dl−1) | 143±21 | 109±11 | 121±11 | 139±14 | 107±6 | 95±3‡ |
| Fasting insulin (μU ml−1) | 10.4±3 | 13±7 | 9±0.8 | 15±2* | 11±2.4 ‡ | 11±2 ‡ |
| Homeostasis Model Assessment (HOMA-IR) | 3.6±1 | 3.2±1 | 2.7±1.1 | 5.1±1* | 2.7±0.7 ‡ | 2.6±0.9‡ |
| Homeostasis Model Assessment (HOMA-IR) (subgroup) | 3.9±1 | 3.6±1.2 | 2.9±1.2 | 4.2±1 | 2.5±0.5‡ | 2.1±0.3‡ |
| Free fatty acid (mEq l−1) | 0.71±0.05 | 0.69±0.05 | 0.82±0.16 | 0.81±0.07 | ||
| Triglycerides (mg dl−1) | 143±32 | 141±15 | 159±30 | 125±22 | 126±18 | 130 ±13 |
| HDL (mg dl−1) | 41±2.6 | 31±3† | 38±5 | 37±3 | 30±3‡ | 32±2 |
| Alanine transaminase (ALT) (Ul−1) | ||||||
| Aspartate aminotransaminase (AST) (Ul−1) | ||||||
| HS-C reactive protein (mg l−1) | 9.8±2 | 12.5±3 | 11±4 | 4.5±1.4‡ | ||
| Hyperglycemic clamp variables | ||||||
| Fasting ISR (pmol min−1) | 289±34 | 285±51 | 356±75 | 242±35 | 231±27 | 197±33* |
| AIR (pmol 0–10 min) | 6094±2196 | 6848±1150 | 8014±1860 | 5124±935 | 5082±933 | 4718±556* |
| Mean ISR (0–120 min) (pmol min−1) | 451±53 | 473±64 | 517±32 | 509±45 | ||
| M/I (mg kg−1 min−1 per μU mol−1) | 0.16±0.03 | 0.16±0.03 | 0.14±0.03 | 0.06±0.01* | 0.09±0.01 | 0.21±0.05‡ |
| M/I (mg kg−1 min−1 per μU mol−1) (subgroup) | 0.11±0.03 | 0.08±0.03 | 0.08±0.02 | 0.09±0.01* | 0.11±0.01 | 0.26±0.05*‡ |
| MMTT variables | ||||||
| 2 h plasma glucose | 144±14 | 110±11† | 148±14 | 98±5‡ | ||
| Insulinogenic index (ΔI30/ΔG30) | 1.02±0.1 | 0.4±0.05 | 1.09±0.1 | 1.53±0.1‡ | ||
| Slope c peptide vs glucose (ng ml−1×mM−1) | 122±70 | 154±63 | 56±26 | 206±62‡ | ||
Abbreviations: AIR, acute insulin response; BMI, body mass index; GR, gastric restrictive surgery; HDL, high-density lipoprotein; HS, high sensitivity; HOMO-IR, homeostasis model assessment-insulin resistance; ISR, insulin-secretion rate; MMTT, mixed meal tolerance test; RYGB, Roux-en-Y gastric bypass.
P value<0.05 post vs pre GR.
P value<0.05 post vs pre RYGB.
P value<0.05 Pre GR vs RYGB. Mean±s.e.m.
Hyperglycemic clamp after RYGB versus GR
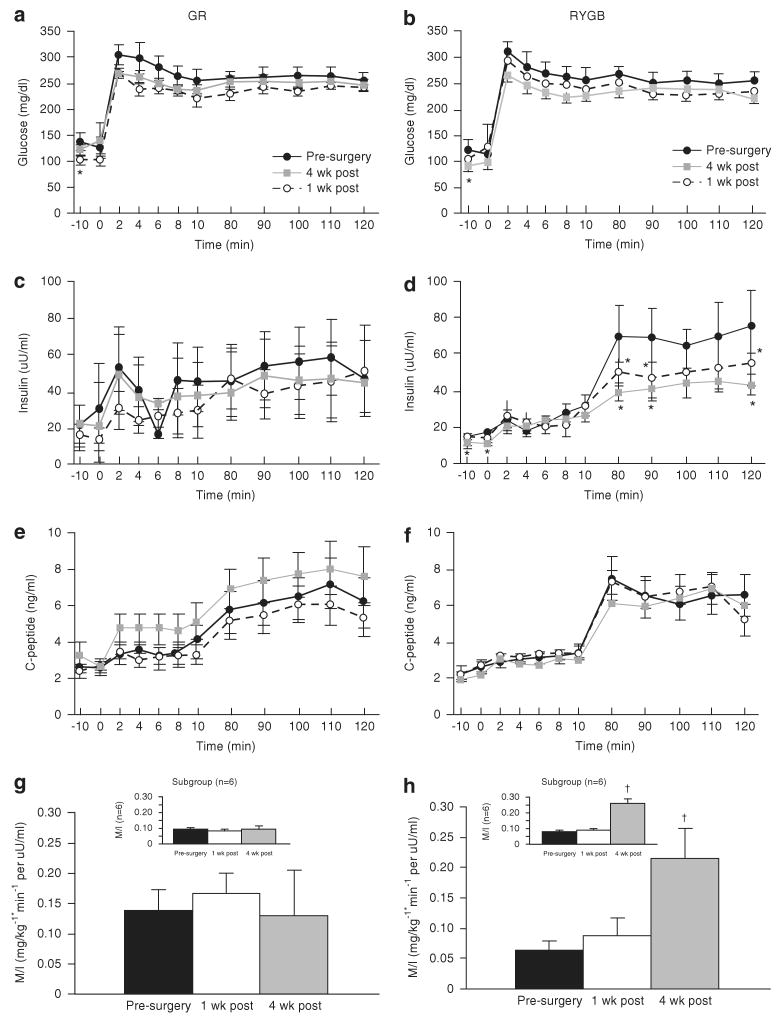
As shown in Figures 1a and b, the increment in plasma glucose above fasting was similar for RYGB (pre: 132±11 versus 1 week: 134±10 versus 4 weeks: 134±5 mg 100 ml−1) and GR surgery (pre: 130±6 versus 1 week: 135±21 versus 4 weeks: 129±19 mg 100 ml−1). Fasting insulin was reduced at 1 week and remained significantly reduced 4 weeks post RYGB surgery, but was not reduced after GR (Table 1). Hence, the HOMA index (Table 1) was reduced twofold at 1 week after RYGB (P<0.01), but was not changed after GR (P=NS). The first-phase insulin concentrations were not changed after either surgery (Figures 1c and d). However, second-phase insulin concentration was reduced by ∼40% at 1 and 4 weeks in the RYGB group, but GR showed no change during second phase (Figures 1c and d). Fasting C-peptide levels were unchanged 1 week post-surgery in both groups, while at 4 weeks C-peptide levels were modestly reduced in RYGB only (Figures 1e and f) (P=NS). There was no change in first or second-phase C-peptide levels at 1 and 4 weeks after RYGB (Figure 1f). However, both first and second-phase C-peptide secretion was increased modestly at 4 weeks after GR (Figure 1e). The M/I ratio, a measure of tissue insulin sensitivity to endogenous insulin, increased slightly at 1 week (0.09±0.01 versus pre: 0.06±0.01, P=NS) and markedly at 4 weeks (0.21±0.05 versus pre: 0.06±0.01, P<0.01) after RYGB only.
Figure 1.
Glucose (a, b), insulin (c, d) and C-peptide (e, f) responses during the hyperglycemic clamp (Δ 125 mg 100 ml−1 glucose) performed before surgery (-●-), 1 week (-○-) and 4 weeks ( ) after surgery. Insulin sensitivity was estimated from the M/I ratio by dividing the average glucose infusion (M value in mg kg−1 min−1) during the last 40 min of the clamp by the average plasma insulin concentration during that same interval (M/I) (g, h). Subgroup analysis of subjects with matched insulin sensitivity values are shown in panels for g, h. Values represent mean±s.e.m. *Significantly lower than the pre-surgery response, P<0.05. †Significantly greater than the pre-surgery response P<0.05.
) after surgery. Insulin sensitivity was estimated from the M/I ratio by dividing the average glucose infusion (M value in mg kg−1 min−1) during the last 40 min of the clamp by the average plasma insulin concentration during that same interval (M/I) (g, h). Subgroup analysis of subjects with matched insulin sensitivity values are shown in panels for g, h. Values represent mean±s.e.m. *Significantly lower than the pre-surgery response, P<0.05. †Significantly greater than the pre-surgery response P<0.05.
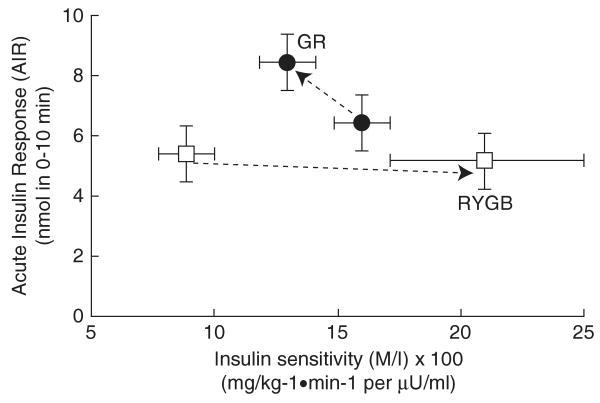
At 4 weeks, fasting insulin-secretion rate was modestly reduced after RYGB and was increased after GR (Table 1). In addition, the AIR was reduced after RYGB and increased after GR (both P=NS). The disposition index was calculated from glucose infused during the clamp, was unchanged after RYGB, but was increased by 30% following GR (P=0.1) (Figure 2).
Figure 2.
Data are shown for the disposition index determined at pre-surgery and 4 weeks after Roux-en-Y gastric bypass (RYGB) (□) and gastric restrictive surgery (GR) (●) groups respectively. The disposition index is determined as the acute insulin response (AIR) during 0–10 min relative to insulin sensitivity (M/I) multiplied by 100 during the hyperglycemic clamp. The AIR was evaluated as the area under the curve of insulin secretion rate from 0 to 10 min. Values represent mean±s.e.m.
Subgroup analysis of RYGB and GR subjects with matched insulin sensitivity
Pre-surgery insulin sensitivity values determined by the M/I ratio and HOMA index indicated that the RYGB subjects were markedly more insulin resistant than the GR subjects. A subgroup analysis was carried out in insulin sensitivity matched subjects. Six RYGB and six GR subjects with similar pre-surgery M/I ratio (RYGB: 0.09±0.01 versus GR: 0.11±0.01, P=NS) were compared. Insulin sensitivity rose threefold 4 weeks after RYGB (0.26±0.03 versus 0.09±0.01, P<0.01) and did not change after GR (Table 1; Figures 1g and h). The increase in insulin sensitivity noted 4 weeks after RYGB in the subgroup is similar to that seen in the original cohort. Similarly, homeostasis model assessment-insulin resistance (HOMA-IR) values reduced markedly after RYGB at 1 and 4 weeks and did not change after GR (Table 1).
MMTT following RYGB versus GR
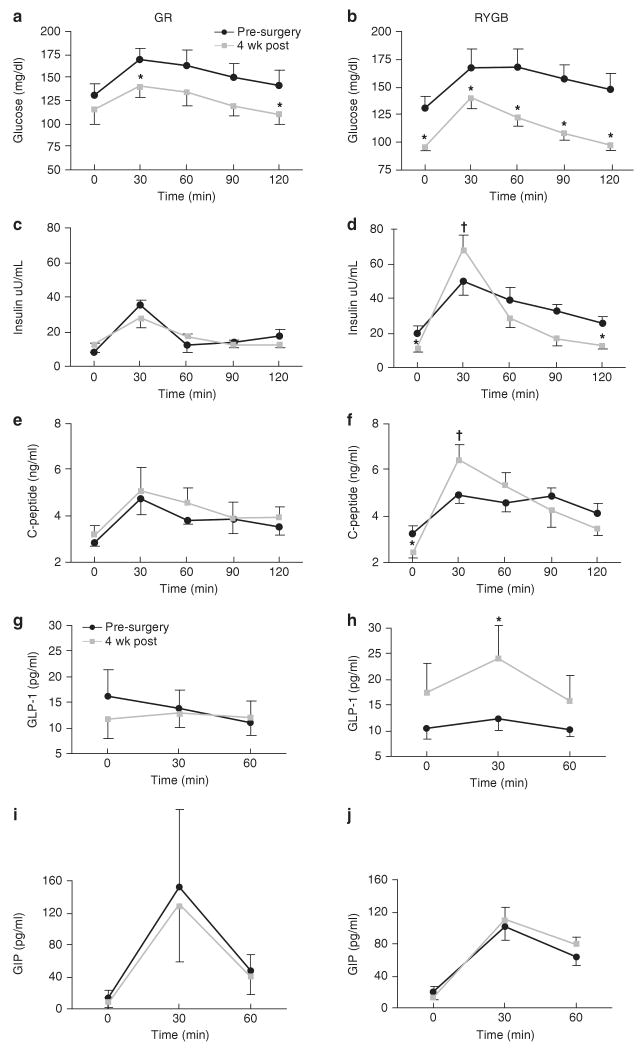
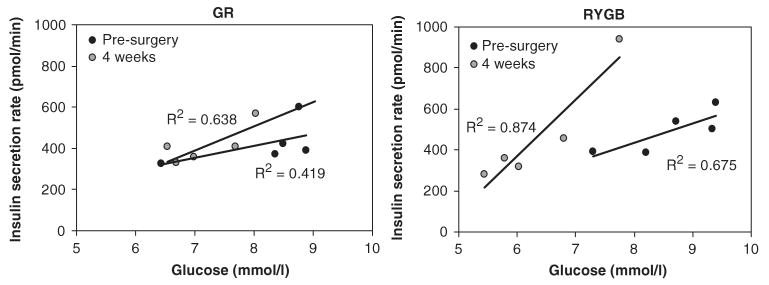
Fasting glucose, insulin and C-peptide levels were significantly reduced (P<0.05) in the RYGB group 4 weeks post-surgery, whereas fasting insulin and C-peptide levels rose slightly after GR, with only a trend for reduction in fasting glucose (Figure 3). Ingestion of the mixed meal resulted in an increase in the insulinogenic index (ΔI30/ΔG30) after RYGB (Table 1). In contrast, the insulinogenic index trended towards a decrease after GR (P=0.1). C-peptide AUC showed a 3.4-fold increase in RYGB group 4 weeks after surgery (pre: 84±7 versus 4 weeks: 284±16, P<0.05). The twofold increase in C-peptide AUC following GR was not statistically significant (pre: 103±27 versus 4 weeks: 208±89). Insulin sensitivity determined by Matsuda index was twofold higher (4 weeks: 6.5±0.07 versus pre: 3.3±0.06, P<0.05) 4 weeks after RYGB and did not change after GR surgery (4 wk: 6.3±1.6 vs pre: 7.8±2.8, P=NS). Insulin secretion rates, calculated by C-peptide deconvolution and kinetic modeling, during the MMTT revealed a significant fivefold improvement in β-cell sensitivity after 4 weeks in the RYBG group, but did not change markedly after GR (Figure 4). Fasting free fatty acid concentrations (Table 1) and meal-induced suppression of free fatty acids at 120 min post-ingestion was similar following both RYGB (120 min post: 0.68±0.09 versus 120 min pre: 0.55±0.1 mEq l−1) and GR surgery (120 min post: 0.75±0.1 versus 120 min pre: 0.65±0.05 mEq l−1).
Figure 3.
Glucose (a, b), insulin (c, d), c-peptide (e, f), glucagon-like peptide-1 (GLP-1) (g, h), and gastric-inhibitory peptide (GIP) (i, j) responses during the mixed meal tolerance test (MMTT) carried out before surgery and 4 weeks after surgery. Mixed meal consisted of Ensure 4oz with 30 min interval blood sampling for glucose, insulin and C-peptide values. GLP and GIP levels were obtained at fasting, 30 and 60 min during MMTT. Values represent means±s.e.m. *Significantly reduced compared with the corresponding pre-surgery response, P<0.05. †Significantly increased compared with the corresponding pre-surgery response, P<0.05.
Figure 4.
Beta (β)-cell function in response to the prevailing plasma glucose observed during the mixed meal tolerance test (MMTT) at fasting, 30, 60, 90 and 120 min timepoints. Data represent the average insulin-secretion rate for each timepoint during the MMTT carried out at pre-surgery (●) and 4 weeks after gastric restrictive surgery (GR) and Roux-en-Y gastric bypass (RYGB) ( ). The values of the slopes are indicated in Table 1. Comparison between slopes for RYGB (pre-surgery versus 4 weeks) was statistically different P<0.05. Comparison between pre-surgery slopes for RYGB versus GR was also statistically different P<0.05.
). The values of the slopes are indicated in Table 1. Comparison between slopes for RYGB (pre-surgery versus 4 weeks) was statistically different P<0.05. Comparison between pre-surgery slopes for RYGB versus GR was also statistically different P<0.05.
Fasting and postprandial gut-hormone responses
Fasting GLP-1, GIP and amylin were not altered by surgery in either group. However, the postprandial GLP-1 response was increased (P<0.05) in the RYGB group (Figure 3h). There was no change in postprandial GIP or amylin (data not shown) responses after RYGB (Figure 3j) and there was no change in any of the gut-hormone responses after GR.
Discussion
This is the first study to rigorously evaluate the effects of RYGB on glucose regulation and β-cell function during the first weeks after surgery when weight loss is relatively modest. It is also the first study to compare the acute anti-diabetic effects of RYGB against GR surgery in obese patients with type 2 diabetes. RYGB was associated with an immediate and sustained reduction of fasting hyperglycemia, improved β-cell function and insulin sensitivity. These outcomes were linked to an enhanced incretin response during an MMTT, which indicates that one of the potential mechanisms that may be facilitating these improvements in glucoregulation lies along the gastroenteroinsular axis. Further, the data show that in the first weeks after surgery, RYGB is effective in reversing hyperglycemia and improving β-cell function.
Although both types of gastrointestinal surgery significantly reduced fasting hyperglycemia within the first week, only RYGB sustained this lower glycemic level by the fourth week. The rapid glycemic improvement following both surgery procedures may be explained, in part, by the known beneficial effects of food restriction and weight loss on glycemic control.1,4 This is in sharp contrast to the relapse of glucose control frequently encountered with the acute inflammation associated with non-bariatric surgical procedures (for example, coronary artery bypass) and noted to some extent after GR surgery in our cohort. Inflammation reflected by CRP levels reduced dramatically 4 weeks after RYGB. In addition to glucose, the RYGB group also experienced a reduction in fasting insulin levels and improved HOMA-IR, suggestive of improved sensitivity. Similar findings of acute normalization of fasting hyperglycemia and insulin levels were demonstrated by Rubino et al.7 after gastro-jejunal anastomosis in rodent models of diabetes and in T2DM patients 4 weeks after bilopancreatic diversion surgery.25 Some indication of how these responses are achieved may be gleaned from the work of Troy et al.,26 who observed that direct gastro-jejunal contact of nutrients enhanced intestinal gluconeogenesis, thus resulting in the suppression of hepatic glucose production by a hepatoportal sensor pathway.
A new finding from this study was the rapid restoration of β-cell function in the RYGB group. By 4 weeks, there was a slight reduction in the fasting insulin secretion rate and AIR that is likely adaptive to an increase in insulin sensitivity (increased M/I); this resulted in an unchanged disposition index during the clamp. These data suggest a ‘resting’ of β-cells that is characteristically observed with diet-induced weight loss, exercise interventions and insulin sensitizing drugs, and was recently noted acutely after bilopancreatic diversion in patients with T2DM.4,27–29 These effects are in contrast to the modest increase in insulin secretion (particularly AIR) after GR surgery in the setting of unchanged insulin sensitivity, which resulted in a 30% increase in the disposition index. Although impaired post-hepatic insulin clearance is documented in obese T2DM,30 and mediated in part by insulin resistance,21 it is unclear if the rapid lowering of second-phase insulin concentrations after RYGB was determined by weight loss, improved insulin sensitivity or altered gut anatomy. However, a similar weight loss after GR did not result in a similar outcome. Reduction of the AIR after bilopancreatic diversion has been demonstrated in T2DM patients31 and suggests that the effects we observed are mediated by rapid improvement in insulin sensitivity related to bypassing the small intestine and are independent of weight loss.
Despite comparable weight loss by the two groups, only RYGB was associated with marked improvement in insulin sensitivity 4 weeks after surgery, as reflected in the HOMA-IR, Matsuda Index (during MMTT) and M/I levels during the clamp studies. This enhanced insulin sensitivity after RYGB, which is known to be a partially malabsorptive procedure with respect to fat,32 was not associated with reduced free fatty acid levels in response to insulin or reduced triglyceride levels after RYGB. There are limited data on lipids and lipolysis in the period immediately after bariatric surgery. Recently, Johansson et al.33 reported an increase in FFA in patients 1 month after RYGB, despite improvements in insulin sensitivity. By 6 and 12 months after surgery FFA levels were either reduced, or like the data in this study, were similar to pre-surgery values.34–36 These later data are consistent with improved control of lipolysis arising from enhanced adipocyte insulin sensitivity. More detailed mechanistic studies are warranted to resolve this particular issue.
The lack of change in insulin secretion in response to intravenous infusion of glucose after RYGB is in sharp contrast to the robust increase in β-cell sensitivity to glucose during the MMTT and suggests that this phenomenon may not merely be consistent with an incretin effect as widely reported by others.16,17,19 The fivefold increase in the response of β-cells to glucose as indicated by the slope of insulin-secretion rate during the meal after RYGB, suggests a positive β-cell adaptation that is stimulated by both altered nutrient intestinal interactions and robust improvements in insulin sensitivity; this response was not seen in the GR group. The lack of a change in β-cell responsiveness after GR suggests that weight loss alone induced by enforced caloric restriction may be insufficient to reverse β-cell failure in T2DM. Although robust increases in both GLP-1 and GIP levels have been noted previously after RYGB,9,15,17 our study only observed a marked rise in GLP-1, with no change in the GIP response to meal ingestion. Although fatty acids are reported to regulate intestinal GLP-1 secretion,37 no changes in fasting or prandial levels during the mixed meal were observed after RYGB.
A limitation in this study design was that subjects were not randomized to the surgical procedures. Thus, subjects were not exactly matched for baseline clinical measures. RYGB group was more insulin resistant and hyperinsulinemic at pre-surgery than GR, hence the robust improvement in insulin sensitivity after surgery may be confounded by this. However, key measures such as body mass index, HbA1c and fasting plasma glucose were very similar for both groups. In addition, we employed a repeated measures design so that the subjects were their own controls, and thus comparisons and changes within the groups have strong statistical validity. The subgroup analysis of 12 subjects with matched baseline insulin sensitivity demonstrated significant improvement in insulin sensitivity after RYGB adds strength to our results. Second, although LAGB and LSG procedures are similar with respect to induction of reduced caloric intake and subsequent weight loss, we recognize that they are different anatomically and may consequently differ with respect to nutrient transit and gut-hormone effects. The sleeve procedure involves removing the majority of the gastric fundus and some studies have documented greater weight loss immediately after this procedure.23 Furthermore, there is some evidence of a decreased ghrelin response to meals and an increased postprandial PYY response from distal L cells, thus suggesting a rapid transit of nutrients to the distal ileum. It remains unclear whether the sleeve procedure alters incretin hormones; however, our subjects undergoing this procedure (n=3) did not experience a rise in GLP-1 or GIP levels after surgery. Hence, further study is necessary to distinguish the metabolic effects specific to LSG from LAGB and RYGB.
In conclusion, our data provide new insights into the acute metabolic effects of the intestinal bypass component of gastrointestinal bariatric surgery on hyperglycemia and β-cell function. The reversal of hyperglycemia and enhanced β-cell sensitivity after RYGB was more marked than after GR. Uniquely, these data show that the metabolic adaptations are independent of prolonged enforced caloric restriction and were achieved with equivalent weight-loss effects after both surgery types. The rapid improvement in β-cell function after RYGB has significant clinical implications for the further development and application of surgical approaches for the treatment of obesity and T2DM.
Supplementary Material
Acknowledgments
We are grateful to the nurses and technicians in the Cleveland Clinic, Clinical Research Unit for providing the skilled assistance that enabled the successful implementation of this study. This work was supported in part by National Institutes of Health, National Center for Research Resources (NCRR), Multidisciplinary Clinical Research Career Development Programs Grant 5K12RR023264 (SRK), National Institutes of Aging Award RO1 AG12834 (JPK), National Center for Research Resources, CTSA 1UL1RR024989 and by Ethicon Endo-Surgery (PRS, SRK). Grant support: NIH, NCRR, Multidisciplinary Clinical Research Career Development Programs Grant 5K12RR023264, National Center for Research Resources, CTSA 1UL1RR024989 and Ethicon Endo-Surgery. Disclosure statement: Dr Kashyap discloses relationships with Ethicon endo-surgery (research grants); Dr Schauer discloses relationships with Ethicon endo-surgery (research grants).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
References
- 1.Defronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 3.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Defronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47:31–39. doi: 10.1007/s00125-003-1263-9. [DOI] [PubMed] [Google Scholar]
- 4.Polonsky KS, Gumbiner B, Ostrega D, Griver K, Tager H, Henry RR. Alterations in immunoreactive proinsulin and insulin clearance induced by weight loss in NIDDM. Diabetes. 1994;43:871–877. doi: 10.2337/diab.43.7.871. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 7.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 10.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 11.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y bypass. Int J Obes. 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 16.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 19.Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94:884–891. doi: 10.1210/jc.2008-1620. [DOI] [PubMed] [Google Scholar]
- 20.Burguera B, Agusti A, Arner P, Baltasar A, Barbe F, Barcelo A, et al. Critical assessment of the current guidelines for the management and treatment of morbidly obese patients. J Endocrinol Invest. 2007;30:844–852. doi: 10.1007/BF03349226. [DOI] [PubMed] [Google Scholar]
- 21.Defronzo RA, Tobin J, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and insulin resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien PE, Dixon JB, Laurie C, Anderson M. A prospective randomized trial of placement of the laparoscopic adjustable gastric band: comparison of the perigastric and pars flaccida pathways. Obes Surg. 2005;15:820–826. doi: 10.1381/0960892054222858. [DOI] [PubMed] [Google Scholar]
- 23.Roa PE, Kaidar-Person O, Pinto D, Cho M, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes Surg. 2006;16:1323–1326. doi: 10.1381/096089206778663869. [DOI] [PubMed] [Google Scholar]
- 24.Van CE, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 25.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 26.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 28.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol. 1993;48:M84–M90. doi: 10.1093/geronj/48.3.m84. [DOI] [PubMed] [Google Scholar]
- 30.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 32.Guijarro A, Suzuki S, Chen C, Kirchner H, Middleton FA, Nadtochiv S, et al. Characterization of weight loss and weight regain mechanisms following Roux-en-Y gastric bypass in rats. Am J Physiol Regul Intreg Comp Physiol. 2007;293:1474–1489. doi: 10.1152/ajpregu.00171.2007. [DOI] [PubMed] [Google Scholar]
- 33.Johansson L, Roos M, Kullberg J, Weis J, Ahlstrom H, Sundbom M, et al. Lipid mobilization following Roux-en-Y gastric bypass examined by magnetic resonance imaging and spectroscopy. Obes Surg. 2008;18:1297–1304. doi: 10.1007/s11695-008-9484-0. [DOI] [PubMed] [Google Scholar]
- 34.Bikman BT, Zheng D, Pories WJ, Chapman W, Pender JR, Bowden RC, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93:4656–4663. doi: 10.1210/jc.2008-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingrone G, DeGaetano A, Greco AV, Capristo E, Benedetti G, Castagneto M, et al. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia. 1997;40:599–605. doi: 10.1007/s001250050721. [DOI] [PubMed] [Google Scholar]
- 36.Phillips ML, Lewis MC, Chew V, Kow L, Slavotinek JP, Daniels L, et al. The early effects of weight loss surgery on regional adiposity. Obes Surg. 2005;15:1449–1455. doi: 10.1381/096089205774859353. [DOI] [PubMed] [Google Scholar]
- 37.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.