Abstract
Sepsis is a systemic host response to infection by pathogenic microorganisms. Activation of the coagulation cascade during endotoxemia and sepsis leads to disseminated intravascular coagulation. This review focuses on tissue factor expression by hematopoietic and non-hematopoietic cells and its contribution to the activation of coagulation during endotoxemia and sepsis.
Keywords: Tissue factor, cell type, sepsis, endotoxemia, disseminated intravascular coagulation
Introduction
Tissue factor is the primary initiator of the coagulation cascade[1]. Upon vascular damage or induction of TF expression within vasculature and blood cells, TF comes into the contact with blood. This leads to the formation of the TF:FVIIa complex that activates both FX and FIX, with subsequent thrombin generation, fibrin deposition and activation of platelets[1]. Disseminated intravascular coagulation (DIC) results from a pathological activation of coagulation in response to a variety of diseases, including endotoxemia and sepsis[2]. DIC is associated with a poor prognosis and a high mortality rate. The widespread activation of coagulation leads to the formation of occlusive thrombi within the microvasculature leading to ischemic events and impaired perfusion in multiple organs[2]. Sepsis is also associated with a consumptive coagulopathy and thrombocytopenia resulting in bleeding. In critically ill patients, all of these events may contribute to the development of multisystem organ failure and subsequent mortality[2]. This review focuses on the identification of cellular sources of TF that contribute to the activation of coagulation in endotoxemia and sepsis.
Role of the TF:FVIIa complex in endotoxemia and sepsis
Many studies have demonstrated that inhibition of the TF:FVIIa complex attenuates coagulopathy and reduces morbidity in sepsis. In 1990, Rapaport’s group first showed that treatment with anti-TF antibody reduced disseminated intravascular coagulation in endotoxemic rabbit[3]. Taylor and colleagues then demonstrated that pretreatment with an anti-TF monoclonal antibody not only attenuated the coagulopathy but also reduced mortality in a lethal Escherichia coli sepsis model in baboons[4]. Similar protection was reported after treatment with either an active site-inactivated FVIIa or the natural anticoagulant tissue factor pathway inhibitor (TFPI)[5, 6]. In a baboon model, treatment with an anti-TF monoclonal antibody or an active site-inactivated FVIIa after the establishment of sepsis reduced fibrin deposition and inflammation and attenuated sepsis-induced respiratory and renal failure [7, 8]. Inhibition of the TF:FVIIa complex was also protective in a mouse model of cecal ligation and puncture, an endotoxemic mouse model, and a rabbit peritonitis model[9–11]. Further support for the importance of TF in the pathological activation of coagulation came from studies using genetically modified mice. We demonstrated that mice expressing low levels of TF (~ 1% of wild type) had reduced levels of thrombin-antithrombin (TAT), a marker of coagulation, and reduced mortality compared with littermate controls in a model of endotoxemia[12]. Similar protection was observed in endotoxemic mice genetically modified to express low levels of FVII[13]. These data indicate that the extrinsic coagulation pathway plays a major role in the activation of coagulation in endotoxemia and sepsis. However, despite promising results in animals models, blockade of the TF:FVIIa complex with recombinant TFPI had no significant effect on overall mortality in a randomized phase 3 clinical study[14].
TF expression by hematopoietic cells
In general, circulating blood cells do not express TF in healthy individuals[15, 16], although very low levels of TF antigen have been detected in a small subset of CD14-positive monocytes[17]. However, many studies have demonstrated that bacterial lipopolysaccharide (LPS) stimulation of human monocytes and monocytic cell lines induces TF expression in vitro[17–19]. TF expression was also observed in monocytes from baboons infected with Escherichia coli and from septic patients with a Neisseria meningitidis infection[20, 21]. Furthermore, another study demonstrated that monocytes expressed TF mRNA in a human model of endotoxemia[22]. Our lab and others have shown that either a genetic reduction of TF in hematopoietic cells[12, 23], or selective inhibition of TF expression by these cells reduces activation of coagulation by approximately 50% in endotoxemic mice[24]. In addition, using the Cre-LoxP system, we have found that deletion of TF gene in myeloid cells also reduces LPS-induced coagulation in mice[24]. Other studies have reported TF expression by human neutrophils and eosinophils[25, 26]. However, more recent studies found that neither neutrophils nor eosinophils express TF but can acquire TF by binding monocyte-derived microparticles (MPs)[27–29]. MPs are small membrane vesicles released from activated or apoptotic cells. This concept was also supported by de Vaard and colleagues who showed that TF-positive granulocytes infiltrating organs do not express TF mRNA in a mouse model of endotoxemia[30]. These studies indicate that within the leukocyte population, monocytes are the predominant cell type that expresses TF and are responsible for activation of coagulation during endotoxemia and sepsis.
In 2001, Engelman and colleagues reported that platelets isolated from collagen-stimulated blood contained functional TF[31]. In a subsequent study they found that activation of platelets results in translocation of TF from α-granules to the cell surface[32]. These provocative observations suggested that platelets are a source of intravascular TF. More recently, other groups showed that quiescent and stimulated platelets express variable levels of TF mRNA and protein[33–35].
In collaboration with Dr. Weyrich’s group, we discovered that human platelets contained TF pre-mRNA and that upon activation this is spliced into mRNA and translated into protein[36]. Interestingly, freshly-isolated platelets from septic patients more frequently express mature TF mRNA and have increased levels of TF protein compared to platelets isolated from healthy controls (Rondina, Schwertz, Weyrich – unpublished data). However, it is likely that some of the TF protein associated with platelets in septic patients is due to the binding of leukocyte-derived MPs. Nevertheless, these data suggest that TF expression by platelets may contribute to activation of coagulation during sepsis. It should be noted that other groups have failed to detect any TF protein or TF activity on resting platelets or calcium ionophore-stimulated human platelets[16, 37, 38]. Therefore, TF expression by human platelets remains highly controversial.
Recently, we investigated the role of TF expression by platelets in the activation of coagulation in a mouse model of endotoxemia. Surprisingly, we failed to detect either TF pre-mRNA or mRNA in unstimulated or activated mouse platelets (Pawlinski, Weyrich, Mackman - unpublished data). Moreover, deletion of the TF gene in megakaryocytes, precursors of platelets, had no effect on plasma TAT levels in endotoxemic mice[24]. These results indicate that there are species-specific differences in platelet TF expression between mice and humans, and that TF expression by platelets does not contribute to activation of coagulation in a mouse model of endotoxemia.
TF expression by non-hematopoietic cells
Many cell types surrounding the vasculature constitutively express TF, including pericytes, adventitial fibroblasts and smooth muscles cells[15, 39]. In addition, parenchymal cells in variety of tissues, such as brain, heart, lung, and kidney, express TF[40, 41]. Drake and colleagues proposed that TF expressed by these cells forms a hemostatic envelope that limits bleeding when vascular integrity is compromised[15]. However, during endotoxemia and sepsis, there is an increase in vascular permeability that will expose extravascular TF to blood[42]. Furthermore, in endotoxemic and septic animals, TF expression is increased in many organs, such as the brain, lung, kidney, and spleen[30, 43–45]. Our recent data demonstrate that selective inhibition of TF expressed by non-hematopoietic cells reduces the activation of coagulation in endotoxemic mice by approximately 50%[24]. This means that hematopoietic and non-hematopoietic cells contribute equally to the generation of TAT at 8 hours in the model. At that point, we do not know the cellular source of TF in the non-hematopoietic cell population that contributes to activation of coagulation. We speculate that TF expression in multiple extravascular cell types in multiple organs contributes to activation of coagulation in endotoxemic mice.
In vitro studies demonstrate that activated endothelial cells (ECs) express TF[46–48]. In contrast, only a limited number of studies were able to demonstrate TF expression by ECs in vivo. One study found co-localization of TF and the ECs marker von Willebrand factor within the splenic microvasculature of septic baboons but not in ECs of pulmonary vessels[49]. Another study found TF protein on ECs in endotoxin treated mice and rabbits[44, 50]. More recently, TF protein was observed on ECs at branch points of the aorta of septic baboons[51]. TF protein co-localized with fibrin deposition suggesting that it was functional[51]. However, TF present on ECs was restricted to granular structures some of which were also positive for the leukocyte marker P-selectin glycoprotein ligand-1[51]. This suggests that leukocyte-derived MPs may deliver TF to activated ECs in vivo. In contrast to these studies, we and others did not detect TF expression by ECs in LPS treated mice, rats, and rabbits[43, 45, 52, 53]. These different results may be caused by the relative sensitivity of the various techniques used to detect TF expression.
One limitation of the above studies was that they all investigated TF expression by ECs but did not analyze the functional contribution of this expression to the activation of coagulation. Recently, we addressed this question using a combination of the Cre-LoxP system and bone marrow transplantation. We found that deletion of the TF gene in ECs had no significant effect on activation of coagulation at 8 hours [24]. One possible explanation for our result is that only small subsets of ECs express TF and/or the expression levels are very low.
Summary
In summary, TF expression by both hematopoietic and non-hematopoietic cells plays a significant role in the activation of the coagulation cascade during endotoxemia and sepsis. General inhibition of the TF:FVIIa complex reduces coagulation but can also compromise hemostasis, as was observed with the clinical trial using TFPI[14]. We believe that future studies should focus on strategies that selectively inhibit inducible TF expression, for example on monocytes, without affecting TF activity on extravascular cells. This should decrease pathological activation of coagulation and minimize bleeding complications.
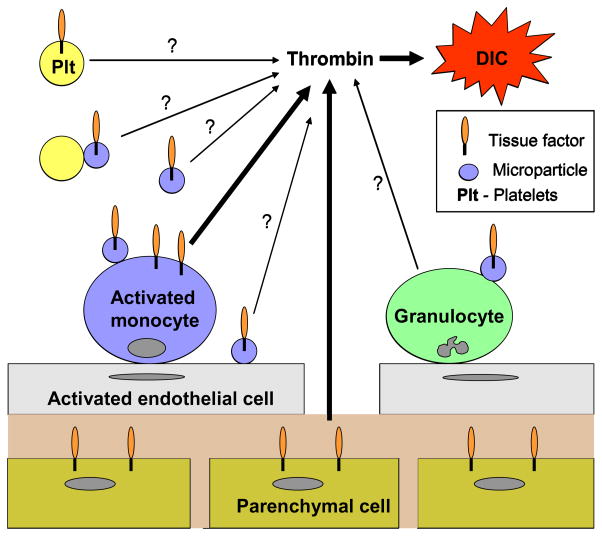
Figure 1. Cellular sources of TF.
Activated monocytes and parenchymal cells are two major sources of TF that contribute to activation of coagulation and DIC during sepsis and endotoxemia. In addition, activated monocytes are the main source of TF-positive microparticles which bind to endothelial cells, platelets and granulocytes. The contribution of TF-positive microparticles to the development of DIC has not been determined.
Acknowledgments
This work was support by National Institutes of Health and American Heart Association grants. We thank Jeremiah Boles for comments on the manuscript.
Abbreviation
- TF
tissue factor
- FVII
factor VII
- DIC
disseminated intravascular coagulation
- LPS
lipopolysaccharide
- TAT
thrombin-antithrombin
- TFPI
tissue factor pathway inhibitor
- ECs
endothelial cells
Footnotes
Conflict of interest
The authors state that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackman N. The many faces of tissue factor. J Thromb Haemost. 2009;7 (Suppl 1):136–9. doi: 10.1111/j.1538-7836.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi M. Disseminated intravascular coagulation: What’s new? Crit Care Clin. 2005;21:449–67. doi: 10.1016/j.ccc.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Warr TA, Rao LV, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75:1481–9. [PubMed] [Google Scholar]
- 4.Taylor FB, Jr, Chang A, Ruf W, Morrissey JH, Hinshaw L, Catlett R, Blick K, Edgington TS. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–34. [PubMed] [Google Scholar]
- 5.Taylor FB, Chang AC, Peer G, Li A, Ezban M, Hedner U. Active site inhibited factor VIIa (DEGR VIIa) attenuates the coagulant and interleukin-6 and -8, but not tumor necrosis factor, responses of the baboon to LD100 Escherichia coli. Blood. 1998;91:1609–15. [PubMed] [Google Scholar]
- 6.Creasey AA, Chang AC, Feigen L, Wun TC, Taylor FB, Jr, Hinshaw LB. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest. 1993;91:2850–60. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welty-Wolf KE, Carraway MS, Ortel TL, Ghio AJ, Idell S, Egan J, Zhu X, Jiao JA, Wong HC, Piantadosi CA. Blockade of tissue factor-factor X binding attenuates sepsis-induced respiratory and renal failure. Am J Physiol Lung Cell Mol Physiol. 2006;290:L21–31. doi: 10.1152/ajplung.00155.2005. [DOI] [PubMed] [Google Scholar]
- 8.Carraway MS, Welty-Wolf KE, Miller DL, Ortel TL, Idell S, Ghio AJ, Petersen LC, Piantadosi CA. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med. 2003;167:1200–9. doi: 10.1164/rccm.200204-287OC. [DOI] [PubMed] [Google Scholar]
- 9.Dackiw AP, McGilvray ID, Woodside M, Nathens AB, Marshall JC, Rotstein OD. Prevention of endotoxin-induced mortality by antitissue factor immunization. Arch Surg. 1996;131:1273–8. doi: 10.1001/archsurg.1996.01430240027003. [DOI] [PubMed] [Google Scholar]
- 10.Opal SM, Palardy JE, Parejo NA, Creasey AA. The activity of tissue factor pathway inhibitor in experimental models of superantigen-induced shock and polymicrobial intra-abdominal sepsis. Crit Care Med. 2001;29:13–7. doi: 10.1097/00003246-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Camerota AJ, Creasey AA, Patla V, Larkin VA, Fink MP. Delayed treatment with recombinant human tissue factor pathway inhibitor improves survival in rabbits with gram-negative peritonitis. J Infect Dis. 1998;177:668–76. doi: 10.1086/514246. [DOI] [PubMed] [Google Scholar]
- 12.Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, Andrade-Gordon P, Frank RD, Mackman N. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103:1342–7. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Ploplis VA, Castellino FJ. A coagulation factor VII deficiency protects against acute inflammatory responses in mice. J Pathol. 2006;210:488–96. doi: 10.1002/path.2073. [DOI] [PubMed] [Google Scholar]
- 14.Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettila V, Artigas A, Percell SR, Shu V, Zwingelstein C, Tobias J, Poole L, Stolzenbach JC, Creasey AA. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–47. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 15.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 16.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–70. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 17.Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–8. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989;9:2752–5. doi: 10.1128/mcb.9.6.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand K, Fowler BJ, Edgington TS, Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Mol Cell Biol. 1991;11:4732–8. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrissey JH, Drake TA, Schlag G, Redl H. Procoagulant response of the endothelium and monocytes. Berlin, New York: Springer-Verlag; 1993. [Google Scholar]
- 21.Osterud B, Flaegstad T. Increased tissue thromboplastin activity in monocytes of patients with meningococcal infection: related to an unfavourable prognosis. Thromb Haemost. 1983;49:5–7. [PubMed] [Google Scholar]
- 22.Franco RF, de Jonge E, Dekkers PE, Timmerman JJ, Spek CA, van Deventer SJ, van Deursen P, van Kerkhoff L, van Gemen B, ten Cate H, van der Poll T, Reitsma PH. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96:554–9. [PubMed] [Google Scholar]
- 23.Schoenmakers SH, Groot AP, Florquin S, Reitsma PH, Spek CA. Blood cell-derived tissue factor influences host response during murine endotoxemia. Blood Cells Mol Dis. 2004;32:325–33. doi: 10.1016/j.bcmd.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Pawlinski R, Mackman N. Cellular sources of tissue factor that contribute to activation of coagulation in endotoxemic mice. J Thromb Haemost. 2009;7:AS-MO-044. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, de Gaetano G, Cerletti C. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 2006;4:1323–30. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 26.Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, Lohse P, Patel KD, Engelmann B. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995–1002. doi: 10.1182/blood-2006-02-004945. [DOI] [PubMed] [Google Scholar]
- 27.Osterud B, Rao LV, Olsen JO. Induction of tissue factor expression in whole blood: lack of evidence for the presence of tissue factor expression in granulocytes. Thromb Haemost. 2000;83:861–7. [PubMed] [Google Scholar]
- 28.Sovershaev MA, Lind KF, Devold H, Jorgensen TO, Hansen JB, Osterud B, Egorina EM. No evidence for the presence of tissue factor in high-purity preparations of immunologically isolated eosinophils. J Thromb Haemost. 2008;6:1742–9. doi: 10.1111/j.1538-7836.2008.03105.x. [DOI] [PubMed] [Google Scholar]
- 29.Egorina EM, Sovershaev MA, Olsen JO, Osterud B. Granulocytes do not express but acquire monocyte-derived tissue factor in whole blood: evidence for a direct transfer. Blood. 2008;111:1208–16. doi: 10.1182/blood-2007-08-107698. [DOI] [PubMed] [Google Scholar]
- 30.de Waard V, Hansen HR, Spronk HH, Timmerman JJ, Pannekoek H, Florquin S, Reitsma PH, ten Cate H. Differential expression of tissue factor mRNA and protein expression in murine sepsis. The role of the granulocyte revisited. Thromb Haemost. 2006;95:348–53. doi: 10.1160/TH05-07-0512. [DOI] [PubMed] [Google Scholar]
- 31.Zillmann A, Luther T, Muller I, Kotzsch M, Spannagl M, Kauke T, Oelschlagel U, Zahler S, Engelmann B. Platelet-associated tissue factor contributes to the collagen-triggered activation of blood coagulation. Biochem Biophys Res Commun. 2001;281:603–9. doi: 10.1006/bbrc.2001.4399. [DOI] [PubMed] [Google Scholar]
- 32.Muller I, Klocke A, Alex M, Kotzsch M, Luther T, Morgenstern E, Zieseniss S, Zahler S, Preissner K, Engelmann B. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17:476–8. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 33.Camera M, Frigerio M, Toschi V, Brambilla M, Rossi F, Cottell DC, Maderna P, Parolari A, Bonzi R, De Vincenti O, Tremoli E. Platelet activation induces cell-surface immunoreactive tissue factor expression, which is modulated differently by antiplatelet drugs. Arterioscler Thromb Vasc Biol. 2003;23:1690–6. doi: 10.1161/01.ATV.0000085629.23209.AA. [DOI] [PubMed] [Google Scholar]
- 34.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–50. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 35.Mobarrez F, Antovic J, Egberg N, Hansson M, Jorneskog G, Hultenby K, Wallen H. A multicolor flow cytometric assay for measurement of platelet-derived microparticles. Thromb Res. 2009:S0049–3848. doi: 10.1016/j.thromres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–40. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- 38.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 39.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–37. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 40.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 41.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, Marynen P, Mackman N. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Zhang D, Scafidi J, Wu X, Cramer CC, Davis AE., 3rd C1 inhibitor prevents Gram-negative bacterial lipopolysaccharide-induced vascular permeability. Blood. 2005;105:2350–5. doi: 10.1182/blood-2004-05-1963. [DOI] [PubMed] [Google Scholar]
- 43.Pawlinski R, Pedersen B, Kehrle B, Aird WC, Frank RD, Guha M, Mackman N. Regulation of tissue factor and inflammatory mediators by Egr-1 in a mouse endotoxemia model. Blood. 2003;101:3940–7. doi: 10.1182/blood-2002-07-2303. [DOI] [PubMed] [Google Scholar]
- 44.Song D, Ye X, Xu H, Liu SF. Activation of endothelial intrinsic NF-{kappa}B pathway impairs protein C anticoagulation mechanism and promotes coagulation in endotoxemic mice. Blood. 2009;114:2521–9. doi: 10.1182/blood-2009-02-205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erlich J, Fearns C, Mathison J, Ulevitch RJ, Mackman N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect Immun. 1999;67:2540–6. doi: 10.1128/iai.67.5.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colucci M, Balconi G, Lorenzet R, Pietra A, Locati D, Donati MB, Semeraro N. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983;71:1893–6. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986;83:4533–7. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:612–21. doi: 10.1161/01.atv.15.5.612. [DOI] [PubMed] [Google Scholar]
- 49.Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142:1458–70. [PMC free article] [PubMed] [Google Scholar]
- 50.Semeraro N, Triggiani R, Montemurro P, Cavallo LG, Colucci M. Enhanced endothelial tissue factor but normal thrombomodulin in endotoxin-treated rabbits. Thromb Res. 1993;71:479–86. doi: 10.1016/0049-3848(93)90121-4. [DOI] [PubMed] [Google Scholar]
- 51.Lupu C, Westmuckett AD, Peer G, Ivanciu L, Zhu H, Taylor FB, Jr, Lupu F. Tissue factor-dependent coagulation is preferentially up-regulated within arterial branching areas in a baboon model of Escherichia coli sepsis. Am J Pathol. 2005;167:1161–72. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hara S, Asada Y, Hatakeyama K, Marutsuka K, Sato Y, Kisanuki A, Sumiyoshi A. Expression of tissue factor and tissue factor pathway inhibitor in rats lungs with lipopolysaccharide-induced disseminated intravascular coagulation. Lab Invest. 1997;77:581–9. [PubMed] [Google Scholar]
- 53.Mackman N, Sawdey MS, Keeton MR, Loskutoff DJ. Murine tissue factor gene expression in vivo. Tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol. 1993;143:76–84. [PMC free article] [PubMed] [Google Scholar]