Abstract
A retrospective study was conducted to determine risk factors for the development of hypertension (HTN) and to describe the prevalence among long-term survivors of pediatric hematopoietic cell transplant (HCT). Records of 689 pediatric patients who survived 5 years or more after HCT from 1969–2004 were reviewed for development of HTN. In children, HTN was defined as either a systolic or diastolic pressure ≥95th percentile according to age, gender, and height. In adults, HTN was defined as systolic pressures ≥140 mmHg and/or diastolic pressures ≥90 mmHg in non-diabetic adults and systolic pressures ≥130 and/or diastolic pressures ≥80 in diabetic adults. Multivariate Cox regression models were used to estimate the hazard ratio (HR) of risk factors associated with HTN. All patients included were off immunosuppressive therapy. Patients had been treated with total body irradiation (TBI) (n=482, 70%) or non-TBI regimens (n=207, 30%) followed by autologous (n=87), related (n=484), or unrelated donor HCT (n=118). Median follow-up was 16 (range, 5–36) years. HTN developed in 120 patients with a 30-year cumulative incidence of 36%. Risk factors associated with HTN were acute kidney injury (doubling of baseline creatinine by day 100 after HCT) (HR=2.5; 95% confidence interval (CI) 1.7–3.7, p <0.0001), TBI in the preparative regimen (HR=2.1; 95% CI 1.3–3.3, p = 0.001), donor type (autologous HR=2.4; 95% CI 1.3–4.4 and unrelated donor HR=1.8; 95% CI 1.0–3.2, p = 0.01), obesity (HR=4.0; 95% CI 2.3–6.8, p <0.0001), diabetes (HR=6.7; 95% CI 3.9–11.0, p <0.0001), and history of growth hormone therapy (HR=1.6; 95% CI 1.0–2.5, p = 0.05). Patients with a positive history of hepatitis C infection were less likely to develop HTN (HR=0.5; 95% CI 0.3–0.9, p = 0.009). Prevalence of HTN was 15% overall and among survivors 11–17 and 18–39 years old, the prevalence was 10% and 14% or triple and double that of the general U.S. population, respectively. Pediatric HCT survivors are more likely to develop HTN than the general population and should be monitored for HTN throughout adulthood.
INTRODUCTION
Survivors of pediatric hematopoietic cell transplant (HCT) are at risk for developing a variety of late complications. As long-term survival increases, assessment of late complications becomes increasingly important. Although hypertension (HTN) following pediatric HCT has been reported to occur in up to 12% of patients, little is known about the prevalence and risk factors for development of HTN in long-term survivors [1–4].
It is estimated that 29% of the adult US population has HTN or are taking anti-hypertensive medication [5]. HTN is more prevalent in African-Americans than whites and more prevalent in men than women [5]. A number of factors are associated with increased blood pressure including older age, obesity, insulin resistance, physical inactivity, excess alcohol consumption, stress, and dietary factors [6]. Other factors include kidney disease, sleep apnea, nicotine, and endocrine disorders [7].
The development of HTN in pediatric HCT patients may be multifactorial. Potential nephrotoxins that can lead to kidney disease include chemotherapy agents such as platinum compounds, carmustine, and ifosfamide, total body irradiation (TBI) and/or abdominal irradiation, antifungal and antiviral agents, aminoglycoside antiobiotics, and immunosuppressive therapy (calcineurin inhibitors) to prevent or treat graft-versus-host disease (GVHD) [8]. Acute kidney injury may occur in up to 53% of HCT patients [3,9–12]. TBI may cause radiation nephropathy that can present more than 6 months after HCT [13]. Pediatric HCT survivors are also at risk for developing the metabolic syndrome, of which HTN is one of the contributing conditions [14–16]. Furthermore, pediatric and adult HCT patients have an increased risk for developing type 2 diabetes mellitus, which may lead to nephropathy and hypertension [17,18].
The individual and cumulative affect of these potential insults on the development of HTN is unknown. The purpose of this study is to identify risk factors associated with the development of HTN and to describe the prevalence of HTN in a large population of long-term pediatric HCT survivors.
PATIENTS AND METHODS
Patient Selection
Between 1969 and January 1, 2004, 2,016 consecutive children less than 18 years of age received a HCT at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA. The records of all 789 patients who survived ≥5 years after transplantation were reviewed. Excluded from the analysis were 91 patients who had relapsed, were receiving immunosuppression for chronic GVHD, or had no follow-up blood pressure readings, 4 patients who did not consent to follow-up, and 5 patients with pre-transplant conditions which may have predisposed them to developing HTN after transplant including polycystic kidney disease (n=2), idiopathic HTN (n=2), and congenital aortic coarctation (n=1). The remaining 689 patients or their responsible guardians consented to follow-up under Protocol 999.02 and the data was reviewed under Protocol 1782 approved by the FHCRC Institutional Review Board.
Transplant preparative regimen, source of stem cells, and supportive care
Treatment prior to referral for HCT varied with the referring institution. Transplant preparative regimens included either chemotherapy or chemoradiotherapy as previously described [19–25]. Most chemotherapy-only regimens utilized cyclophosphamide (CY) 50mg/kg/day for 4 days for aplastic anemia patients or CY 50mg/kg/day for 4 days combined with busulfan 4mg/kg/day for 4 days for patients with a hematologic malignancy and some patients with non-malignant hematologic disorders [19–23]. Most TBI regimens included CY 60 mg/kg/day for 2 days. TBI was delivered from dual opposing cobalt-60 sources at a dose rate in air of 5–8 cGy/min as either 9.2–10.0 Gy single-exposure TBI or fractionated TBI with exposures of 2.0–2.75 Gy for 6–7 consecutive days or hyper-fractionated exposures of 1.2 Gy 2–3 times daily for 4 consecutive days [19,21,24,25]. Non-myeloablative regimens consisted of fludarabine and low-dose (2–6 Gy) TBI [26]. The kidneys were not shielded during TBI.
Autologous transplant recipients received either bone marrow or peripheral blood stem cells. Allogeneic transplant recipients received either bone marrow, peripheral blood, or umbilical cord blood stem cells harvested from related or unrelated donors [27,28]. All allogeneic transplant recipients received prophylaxis for acute GVHD depending on the type of donor and protocol in use at time of HCT and generally included either methotrexate or methotrexate and a calcineurin inhibitor [29–31]. Acute and chronic GVHD were diagnosed, graded, and treated as previously described [32–35]. Hepatitis C virus (HCV) screening began in 1991 as previously described [36].
Follow-up
All patients had an evaluation for engraftment and GVHD at 80–100 days after transplant. Patients returned to the FHCRC for long-term follow-up at one year and electively thereafter. Follow-up after one year consisted of annual contact with referring physicians from 1969 to 1990. From 1991 to the present, questionnaires were mailed annually to patients and their primary medical provider. Information gathered included new medical conditions, medications, blood pressure, and results of blood testing (complete blood count and chemistries). Study data were collected through April 15, 2009. Of the 689 patients studied, 612 were alive. Follow-up data within the past 4 years was available for 90% of the patients.
Study Data
Data were obtained from the FHCRC clinical information database, pre-transplant medical records, transplant flow-sheets, and long-term follow-up records. Records were reviewed for development of HTN.
In patients <18 years old, HTN was defined as either a systolic or diastolic pressure ≥95th percentile according to age, gender, and height [37]. In patients ≥18 years of age, HTN was defined as systolic pressures ≥140 mmHg and/or diastolic pressures ≥90 mmHg. Patients with diabetes were considered to be hypertensive with systolic pressures ≥130 mmHg and/or diastolic pressures ≥80 mmHg [38].
The onset of HTN was defined as the first of 2 consecutive blood pressure readings showing HTN or the start of drug therapy for HTN. Among patients who died of secondary malignancies, received immunosuppression for non-GVHD conditions (organ transplant, pulmonary disease, or autoimmune disease), or received anti-hypertensive medications for non-hypertensive conditions (cardiac disease, cardiomyopathy, portal hypertension, edema, or headaches), only blood pressure readings prior to these events were considered. However, patients were classified as hypertensive if they developed breakthrough HTN while on anti-hypertensive medications for non-hypertensive conditions.
Body Mass Index (BMI, kg/m2) was calculated based on patient height and weight at diagnosis of HTN or most recent measurements in non-hypertensive patients. Obesity was defined as a BMI ≥95th percentile based on age and gender in patients <18 years old and a BMI ≥30 in patients ≥18 years old [39,40]. Weight loss was defined as a decrease in weight of 5 kg or more in adults or a decrease of 10 or more BMI percentile-points in children. Acute kidney injury was defined as a doubling of baseline serum creatinine within the first 100 days after transplant [11]. Sinusoidal obstruction syndrome (SOS) (previously veno-occlusive disease) was defined according to established criterion [41]. Glomerular filtration rate (GFR) was estimated for children using the equations of Schwartz and the Modification of Diet in Renal Disease equation for adults [42,43]. HCV infection status was based on available medical records.
Statistical methods
The prognostic factors considered for the development of HTN included baseline patient and transplant characteristics: gender, race/ethnicity, family history of HTN (defined as any relative within 2 degrees of separation), single kidney, abdominal irradiation, cranial irradiation, pre-transplant nephrotoxic chemotherapy agents, age at transplantation, diagnosis at transplantation, TBI (yes/no), donor type (autologous, related, unrelated), degree of HLA-antigen mismatching between donor and recipient, acute kidney injury, SOS, cyclosporine/tacrolimus for acute GVHD prophylaxis, acute GVHD grade (0–1 versus 2–4), chronic GVHD (defined as clinical extensive), cyclosporine/tacrolimus, prednisone, or mycophenolate mofetil for chronic GVHD therapy, duration of chronic GVHD therapy, smoking history, and hepatitis C virus infection. Time-dependent risk factors considered includedobesity, diabetes, growth hormone deficiency, and growth hormone therapy. For the purpose of analysis, transplant diagnoses were categorized into 9 groups: acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), myelodysplastic syndrome (MDS), non-Hodgkin lymphoma, neuroblastoma, aplastic anemia, primary immunodeficiencies and red blood cell disorders, and all other diagnoses. Red blood cell disorders included thalassemia, sickle cell anemia, and red cell aplasia. Cox regression analysis was used to evaluate univariate and multivariate associations of pre-transplantation and time-dependent risk factors with the development of HTN. The cumulative incidence of HTN after transplant was estimated by standard methods [44]. Statistical significance was set at p-value < 0.05 and the stability of the estimates reflected by 95% confidence intervals. Statistical data were analyzed using SAS software (SAS Institute, Cary, NC). The point prevalence of HTN among surviving leukemia patients was calculated using the formula [hypertensive patients/total population at risk] as of April 15, 2009 [45]. Descriptive data were analyzed using SPSS software (SPSS Inc., Chicago, IL). Descriptive statistics were described as mean and range.
RESULTS
Patient population
The patient and transplant characteristics of the study population and hypertensive cases are presented in Table 1. Among 689 patients, 120 (17%) developed HTN during a median follow-up period of 16 (5 – 36) years. There were no significant differences between patients with and without HTN with regards to family history of HTN, non-white race, donor type, and acute or chronic GVHD. However, among evaluable patients, acute kidney injury was higher in 47% (51/109) of patients with HTN than 32% without HTN (176/547) (p = 0.003).
Table 1.
Patient and transplant characteristics of study population and hypertensive cases
| Total | Hypertensive | |
|---|---|---|
| Number of patients | 689 | 120 |
| Gender | ||
| Female | 284 (41%) | 45 (37%) |
| Male | 405 (59%) | 75 (63%) |
| Race/Ethnicity | ||
| Non-Hispanic White | 573 (83%) | 102 (85%) |
| Other race/ethnicity | 116 (27%) | 18 (15%) |
| Family history of HTN | ||
| Yes | 96 (14%) | 19 (16%) |
| No | 209 (30%) | 30 (25%) |
| Unknown | 384 (56%) | 71 (59%) |
| Pre-HCT abdominal irradiation (yes) | 22 (3%) | 4 (3%) |
| Pre-HCT single kidney (yes) | 13 (2%) | 4 (3%) |
| Pre-HCT cranial irradiation (yes) | 131 (19%) | 31 (26%) |
| Diagnosis at Transplant | ||
| ALL | 204 (30%) | 45 (38%) |
| AML | 157 (23%) | 22 (18%) |
| Aplastic Anemia | 106 (15%) | 19 (16%) |
| CML | 55 (8%) | 12 (10%) |
| Neuroblastoma | 39 (6%) | 6 (5%) |
| PID/RCD | 33 (5%) | 1 (<1%) |
| MDS | 33 (5%) | 7 (6%) |
| Non-Hodgkin’s Lymphoma | 16 (2%) | 3 (3%) |
| Other* | 46 (7%) | 5 (4%) |
| Age at transplant – years (range) | 9.2 (0.3 – 18.0) | 10.4 (1.0 – 18.0) |
| Donor type at first HCT | ||
| Related | 484 (70%) | 89 (74%) |
| Unrelated | 118 (17%) | 18 (15%) |
| Autologus | 87 (13%) | 13 (11%) |
| Source of stem cells at first HCT | ||
| Bone marrow | 619 (90%) | 111 (93%) |
| Peripheral blood | 62 (9%) | 8 (7%) |
| Cord blood | 8 (1%) | 1 (<1%) |
| Transplant preparative regimen | ||
| Single Fraction 10 Gy TBI | 79 (11%) | 28 (23%) |
| Fractionated 12–15.75 Gy TBI | 356 (52%) | 64 (53%) |
| Low-dose 2–6 Gy TBI | 10 (1%) | -- |
| Non-TBI containing regimens | ||
| Cyclophosphamide | 89 (13%) | 18 (15%) |
| Busulfan and Cyclophosphamide | 80 (12%) | 4 (3%) |
| Other | 29 (4%) | 2 (2%) |
| Two transplants | 46 (7%) | 4 (3%) |
| Acute kidney injury | ||
| Yes | 227 (33%) | 51 (43%) |
| No | 429 (62%) | 58 (48%) |
| Unknown | 33 (5%) | 11 (9%) |
| Acute GVHD grades | ||
| 0–I | 375 (54%) | 69 (58%) |
| II–IV | 314 (46%) | 51 (43%) |
| Chronic GVHD (yes) | 225 (33%) | 44 (37%) |
| Survivors | 612 (89%) | 104 (87%) |
| Years alive post-HCT (range) | 17.4 (5.0 – 36.6) | 18.4 (5.1 – 36.1) |
ALL indicates Acute Lymphoblastic Leukemia; AML, Acute Myelogenous Leukemia; CML, Chronic Myelogenous Leukemia; GVHD, Graft versus Host Disease; HCT, Hematopoietic Cell Transplantation; HTN, Hypertension; MDS, Myelodysplastic Syndrome; PID, Primary Immunodeficiency Disease; RCD, Red Blood Cell Disorder; TBI, Total Body Irradiation.
Other diagnosis include: Fanconi’s anemia (n=12), Juvenile Myelomonocytic Leukemia (n=8), Hodgkin’s disease (n=8), brain tumors (n=5), and other rare diseases (n=13).
Among the 120 patients who developed HTN, 6 (5%) patients had experienced renal complications prior to transplantation (2 acute tubular necrosis, 2 severe tumor lysis syndrome, ≥1 leukemic infiltration of the kidney and transient renal failure, and 1 membraneous glomerulonephropathy). Four of the 6 patients required hemodialysis (data not shown).
Characteristics of patients at onset of HTN are presented in Table 2. At onset of HTN, 17% of patients were obese, 16% had diabetes, 20% were infected with HCV, and 13% were smokers. Chronic kidney disease (GFR <90 ml/min/1.73m2) was present in 23 (32%) of 72 evaluable patients at the time of diagnosis of HTN. Nine (39%) of the 23 patients with chronic kidney disease had a history of acute kidney injury (data not shown). At the onset of HTN, 10 patients had renal imaging performed of whom 8 were normal, one had a solitary kidney with multiple cysts, and one had bilateral increased echogenecity, kidney stones, and hydronephrosis. Three patients had sleep apnea (data not shown). Thirteen children had been on growth hormone therapy for a median of 2.7 (1.7–4.7) years prior to developing HTN.
Table 2.
Characteristics of hypertensive patients at onset of hypertension (n=120)
| Total | Children (age < 18 years) | Adults (age ≥ 18 years) | |
|---|---|---|---|
| Number of patients | 120 | 34 | 86 |
| Age post-HCT at onset - years | 25 (3–46) | 13 (3–17) | 30 (18–46) |
| Time post-HCT at onset - years | 14 (1–32) | 7 (1–14) | 18 (2–32) |
| Hypertension | |||
| Evaluable (unknown) | 105 (15) | 32 (2) | 73 (13) |
| Isolated Systolic | 31 (30%) | 13 (41%) | 18 (25%) |
| Isolated Diastolic | 26 (25%) | 4 (13%) | 22 (30%) |
| Systolic and diastolic | 48 (46%) | 15 (47%) | 33 (45%) |
| Estimated GFR at onset | |||
| Evaluable (unknown) | 72 (48) | 25 (9) | 47 (39) |
| Low (GFR <90) | 23 (32%) | 2 (8%) | 21 (45%) |
| Normal (GFR >= 90) | 49 | 23 | 26 |
| Renal Imaging | |||
| Performed (Abnormal) | 10 | 7 | 3 |
| Abnormal | 2 (20%) | 1 (14%) | 1 (33%) |
| Medications at onset | |||
| Growth Hormone | 13 | 13 | -- |
| Thyroid | 25 | 7 | 18 |
| Sex hormone | 30 | 8 | 22 |
| Anti-depressant | 16 | 3 | 13 |
| Body Mass Index* | |||
| Normal or underweight | 66 | 20 | 46 |
| At-risk or overweight | 34 | 8 | 26 |
| Obese | 20 (17%) | 6 (18%) | 14 (16%) |
| Mean (range) | -- | 64% (1%–98%) | 25.3 (10.4 – 46.3) |
| Diabetes | 19 (16%) | 1 (3%) | 18 (21%) |
| Hepatitis C infection (Yes: No: Unknown) | 24: 71: 25 | 0: 28: 6 | 24: 43: 19 |
| Smoker (Yes: No: Unknown) | 15: 103: 2 | 3: 31: 0 | 12: 72: 2 |
GFR indicates Glomerular Filtration Rate (ml/min/1.73m2); HCT, Hematopoietic Cell Transplantation.
Body mass index (BMI): In children <18 years old, using BMI-for-age-and-gender percentiles, categories defined as normal/underweight <85%, at-risk 85–95%, and obese ≥95%. In adults, using BMI, categories defined as normal/underweight <25, overweight 25–30, and obese ≥30.
When patients were grouped according to the age when they developed HTN, 45% of adult patients had a decreased GFR versus 8% of children, and 21% of adults had diabetes versus 3% of children. Obesity was similar in children and adults (18% versus 16% respectively). Isolated diastolic HTN was found in 30% of adults and only 13% of children, whereas isolated systolic HTN was more common in children (41%) than adults (25%).
HTN treatment, complications, and survival
Among the 120 patients who developed HTN, 78 were started on anti-hypertensive medications, 38 did not receive medications, and 4 developed breakthrough HTN while on anti-hypertensive medications for other reasons. Among the 78 patients started on anti-hypertensive medications, 41 started therapy within 1 year of developing HTN, 32 started therapy a median of 2.2 (1.0–9.9) years later, and the start date was unknown for 5 patients. Of the 82 patients treated with anti-hypertensive medications (78 for HTN and 4 for breakthrough HTN), 74 patients continued therapy, 4 discontinued therapy and remained hypertensive, and 4 discontinued therapy and were normotensive (2 had achieved weight loss). Among the 74 patients remaining on therapy, 43 had achieved adequate blood pressure control of <140/90 mm Hg in non-diabetic patients and <130/80 mm Hg in diabetic patients, 17 were not adequately controlled, and 14 were not evaluable. Among the 38 untreated patients, 27 continued to be hypertensive, 8 became normotensive (5 had achieved weight loss), and 3 were not evaluable. Renal imaging was performed in 14 patients at a median of 4.3 (1.7 – 11.2) years after the onset of HTN, of whom 8 had normal renal ultrasounds and 6 had abnormalities, including renal artery stenosis (n=2), renal cysts (n=2), small kidneys (n=1), and nephrolithiasis (n=1). Among the 120 patients, three developed end-stage renal disease (two received kidney transplants and one was on dialysis). Four patients developed congestive heart failure and four had strokes. Sixteen patients died, but only one death was related to HTN after renal transplantation.
Risk factors for HTN
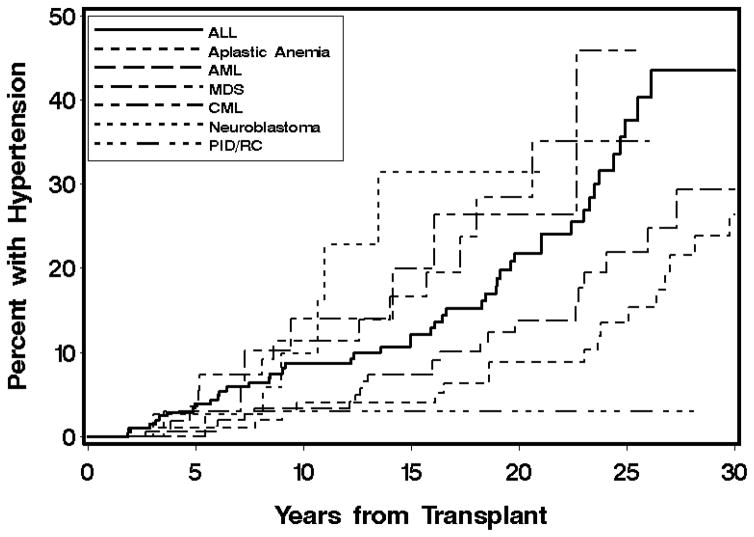
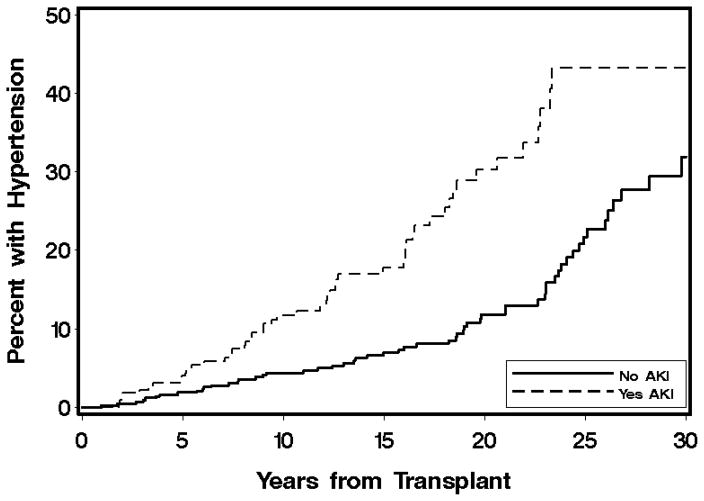
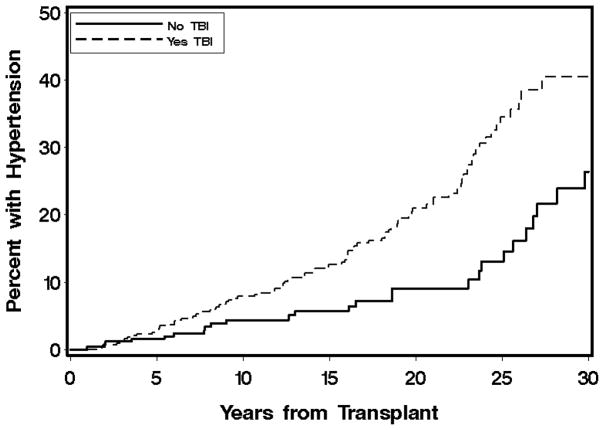
Variables associated with the development of HTN in multivariate analyses are shown in Table 3. In multivariate analysis, the strongest baseline predictors of HTN incidence were acute kidney injury and TBI. TBI fractionation was considered, but did not improve the model. HCV infection was associated with a decreased risk of HTN. Autologous and unrelated donors were more likely to develop HTN. Among time-dependent factors, obesity, diabetes, and growth hormone therapy were associated with an increased risk of HTN. The cumulative incidences of HTN by transplant diagnosis, acute kidney injury, and TBI are shown in Figures 1, 2, and 3. The 20-year cumulative incidence of HTN was ALL 22%, AML 14%, CML 28%, MDS 26%, aplastic anemia 9%, neuroblastoma 31%, and primary immunodeficiencies and red blood cell disorders 3%. The 30-year cumulative incidence of HTN was ALL 44%, AML 29%, and aplastic anemia 26%.
Table 3.
Multivariate analysis of risk factors for the development of hypertension
| Risk factor | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Acute kidney injury | <0.0001 | ||
| Yes | 2.53 | (1.7 – 3.7) | |
| No | 1.0 | --- | |
| Total body irradiation* | 0.001 | ||
| Yes | 2.06 | (1.3 – 3.3) | |
| No | 1.0 | --- | |
| Hepatitis C virus infection | 0.009 | ||
| Yes | 0.52 | (0.3 – 0.9) | |
| Unknown | 0.55 | (0.3 – 0.9) | |
| No | 1.0 | --- | |
| Donor type | 0.01 | ||
| Autologous | 2.39 | (1.3 – 4.4) | |
| Unrelated | 1.79 | (1.0 – 3.2) | |
| Related | 1.0 | --- | |
| Obesity | 3.98 | (2.3 – 6.8) | <0.0001 |
| Diabetes | 6.59 | (3.9 – 11.0) | <0.0001 |
| Growth hormone therapy | 1.58 | (1.0 – 2.5) | 0.05 |
Total body irradiation (TBI) included single fraction (10 Gy) and fractionated (12–15.75 Gy). Low-dose TBI (2–6 Gy) was included in the No-TBI category.
Figure 1.
Cumulative incidence of hypertension by selected diagnoses at transplant.
Figure 2.
Cumulative incidence of hypertension by development of acute kidney injury (defined as a doubling of baseline serum creatinine by day 100 after transplantation), p-value <0.0001.
Figure 3.
Cumulative incidence of hypertension by total body irradiation, p-value 0.0003.
Prevalence of HTN
At the time of analysis, 612 of the 689 patients in the study were alive including 104 of the 120 patients who developed HTN. Eleven of the 104 patients who developed HTN became normotensive and were not included in the prevalence calculation. Thus, the overall prevalence of HTN among surviving patients was 15% (93/612). The prevalence increased with age from 7% among <11 year olds, 10% for 11–17 year olds, 13% for 18–39 year olds, and 31% for 40–59 (mean, 44) year olds. The prevalence increased with time after transplantation from 7% at 5–9 years post-HCT, 12% at 10–19 years, 17% at 20–29 years, and 36% at ≥30 years. The prevalence of HTN was higher in obese (33%) versus non-obese (5%) children and higher in obese (26%) versus non-obese (15%) adults. HTN was also more prevalent in patients with diabetes than without (52% versus 11%).
Table 4 presents the prevalence of HTN by selected diagnosis at transplant. Among other diagnosis not shown in Table 4, the prevalence of HTN was 20% (3/15) non-Hodgkin lymphoma, 20% (1/5) brain tumors, 17% (1/6) juvenile myelomonocytic leukemia, 13% (5/39) neuroblastoma, 13% (1/8) Hodgkin disease, 10% (1/10) Fanconi’s anemia, and 6% (1/18) primary immunodeficiencies. There were no cases of HTN among 25 patients diagnosed with rare diseases.
Table 4.
Prevalence of hypertension by selected diagnosis and risk factors
| ALL | AML | CML | MDS | AA | |
|---|---|---|---|---|---|
| % (Cases/Survivors) | % (Cases/Survivors) | % (Cases/Survivors) | % (Cases/Survivors) | % (Cases/Survivors) | |
| Total patients | 20% (32/162) | 12% (17/145) | 17% (9/52) | 20% 6/30) | 17% (16/97) |
| Preparative regimen | |||||
| Total Body Irradiation based | 20% (32/161) | 15% (14/94) | 20% (9/46) | 26% (5/19) | 8% (1/12) |
| CY | --- | --- | --- | --- | 21% (15/71) |
| BUCY | --- | 6% (2/36) | 0% (0/3) | 11% (1/9) | |
| Two transplants | 0% (0/1) | 7% (1/15) | 0% (0/3) | 0% (0/2) | 0% (0/11) |
| Survival period | |||||
| 30 years or more | 70% (7/10) | 38% (3/8) | --- | --- | 24% (9/37) |
| 20–30 years | 18% (11/61) | 15% (10/67) | 32% (6/19) | 40% (2/5) | 9% (3/32) |
| 10–20 years | 18% (12/67) | 4% (2/56) | 11% (3/27) | 19% (4/21) | 18% (4/22) |
| 5–10 years | 8% (2/24) | 14% (2/14) | 0% (0/6) | 0% (0/4) | 0% (0/6) |
| Mean (range) years | 19.0 (5.5 – 34.5) | 20.3 (5.6 – 37.0) | 18.0 (6.1 – 28.0) | 16.7 (6.2 – 27.3) | 25.0 (5.5 – 37.0) |
| Age at analysis | |||||
| 40–59 yrs. | 19% (4/21) | 36% (9/25) | 0% (0/2) | 100% (1/1) | 31% (11/35) |
| 18–39 yrs. | 22% (24/111) | 7% (8/110) | 18% (9/49) | 17% (4/23) | 9% (5/59) |
| 11–17 yrs. | 13% (3/24) | 0% (0/7) | 0% (0/1) | 20% (1/5) | 0% (0/2) |
| <11 yrs | 17% (1/6) | 0% (0/3) | --- | 0% (0/1) | 0% (0/1) |
| Diabetes | |||||
| Yes | 64% (14/22) | 36% (9/25) | 57% (4/7) | 100% (2/2) | 71% (5/7) |
| No | 13% (18/140) | 7% (8/120) | 11% (5/45) | 14% (4/28) | 12% (11/90) |
| Body Mass Index* | |||||
| Child – Not obese (<95th) | 8% (2/24) | 0% (0/9) | 0% (0/1) | 17% (1/6) | 0% (0/1) |
| Child - Obese (≥95th) | 40% (2/5) | 0% (0/1) | --- | --- | 0% (0/2) |
| Adult – Not obese (<30) | 21% (26/123) | 11% (14/123) | 17% (8/48) | 24% (5/21) | 13% (9/70) |
| Adult – Obese (≥30) | 20% (2/10) | 25% (3/12) | 33% (1/3) | 0% (0/3) | 29% (7/24) |
AA indicates Aplastic Anemia; ALL, Acute Lymphoblastic Leukemia; AML, Acute Myelogenous Leukemia; BU, Busulfan; Chronic Myelogenous Leukemia; CY, Cyclophosphamide; HCT, Hematopoietic Cell Transplantation; MDS, Myelodysplastic syndrome.
Body Mass Index (BMI, kg/m2). Obesity was defined as a BMI ≥95th percentile based on age and gender in patients <18 years old and a BMI ≥30 in patients ≥18 years old
DISCUSSION
This is the first study to report the prevalence of hypertension in a large population of pediatric HCT survivors. HTN developed in 17% of 689 long-term survivors with up to 36 years of follow-up. These patients developed HTN at an average age of 25 years old, earlier than the general population which typically develops HTN after 40 years of age [5]. The early onset of HTN appears to be multi-factorial resulting from both multiple risk factors at the time of transplantation and the development of diabetes and obesity after transplantation.
HTN developed in 17% of our patients, which is higher than 0–12% incidence previously reported in pediatric HCT studies [1–4], which may be due to differences in TBI dose, donor types, and length of follow-up. The majority (87%) of patients in the present study received allogeneic transplants and the median length of follow-up was 16 years compared to 5–10 years in the prior studies. Kist-van Holthe et al. [4] observed no HTN among patients treated with TBI given as 4–8 Gy in one fraction or 12 Gy in two fractions, whereas in our study TBI was generally given at 10 Gy in a single fraction or 12–15.75 Gy in 6–7 fractions.
The prevalence of HTN was higher in our patient population than in the general U.S. population [5,46]. In the U.S., the prevalence of HTN among children ages 11–17 years of age is estimated to be 3.2% [46]. In the present study, the prevalence of HTN among patients 11–17 years old was 10% or 3 times higher than expected. In the U.S., the prevalence of HTN based on the National Health and Nutrition Examination Survey 2003–2004 survey was estimated to be 7.3% among 18–39 year olds and 32.6% among 40–59 year olds [5]. Among survivors 18–39 years of age, the prevalence of HTN was 13% or 2 times higher than expected, but no different among survivors 40–59 years old (31%). One possible explanation for this finding is due to the fact that survivors were relatively younger at a mean age of 44 years, compared to an expected mean age of 50 years among 40–59 year olds in the general population. In calculating prevalence, we exclude patients who developed HTN, but had become normotensive by non-pharmacological means, such as weight loss. Given the retrospective design of this study, it is possible that HTN may be either underdiagnosed or underreported. Hence, the present study confirms that HTN is a prevalent late-effect of pediatric HCT and the true prevalence may be higher than presently reported.
In multivariate analysis, the most significant baseline risk factor identified for the development of HTN was acute kidney injury, suggesting that renal damage plays a role in the pathophysiology of HTN. Identified risk factors for acute kidney injury include SOS, amphotericin use, younger age (<5 years) and older age (>25 years), jaundice, weight gain, donor type, and a high pre-HCT serum creatinine [3,9–12,47]. TBI has not been associated with acute kidney injury, except in a study by Frisk et al. [3]. In the present study, acute kidney injury occurred in 34% of patients, which is identical to the 34% incidence in pediatric patients reported by Kist-van Holthe et al. [10] and lower than the 36–53% incidence reported in two prior reviews of Seattle patients (adult and pediatric) undergoing allogeneic transplantation in 1986 and 1997–2000 [9,11]. Our incidence may also be lower due to the inclusion of autologous recipients which have a lower reported incidence of acute kidney injury ranging from 2.5–6.5% [3,12].
We hypothesize that acute kidney injury may lead to chronic kidney disease and HTN in a subset of patients. In two previous Seattle studies, acute kidney injury has been shown to be a risk factor for chronic kidney disease in myeloablative and non-myeloablative transplants [48,49]. In the present study, 32% of evaluable patients had evidence of chronic kidney disease (GFR <90 ml/min/1.73m2) at the onset of HTN, of whom 39% had developed acute kidney injury during HCT. Although HTN is known to be both a cause and a complication of chronic kidney disease, the chronic kidney disease in our patients preceded the development of HTN, thus supporting the hypothesis that acute kidney injury had progressed to chronic kidney disease and HTN in one-third of our patients. Furthermore, the associated of chronic kidney disease and HTN has also been observed in a study of adult HCT patients [50].
The second most significant risk factor for the development of HTN was TBI. TBI is a recognized risk factor for radiation nephropathy, chronic kidney disease, and may increase the risk of diabetes. Radiation nephropathy, also known as “bone marrow transplantation nephropathy,” may occur several months to years after irradiation and typically presents with azotemia, HTN, and anemia [13]. Renal shielding may reduce the incidence of radiation nephropathy, but none of our patients had renal shielding [51]. TBI has been associated with chronic kidney disease in several studies, but not all [3,4,52,53]. TBI has been associated with an increased risk of diabetes [18]. The present study is the first to find a direct association between TBI and HTN. In a previous study by Baker et al. [18], TBI was not associated with HTN, but the patient population included both pediatric and adult HCT survivors and the mean follow-up period was shorter (9 versus 17 years).
The third most significant risk factor for HTN was HCV infection status. Patients with HCV infection had a lower risk of developing HTN than uninfected patients. The finding that subjects with HCV infection are less likely to be hypertensive was also observed in a large study of 171,665 veterans, although the reasons for this association are not fully understood [54].
The fourth most significant risk factor for the development of HTN was donor type. Autologous and unrelated donor recipients were found to be at higher risk of developing HTN than related donor recipients. The higher risk of developing HTN among autologous recipients contrasts with two previous studies that found autologous transplantation carries a lower risk of HTN [3,18]. One possible explanation for this finding is that 40% of autologous recipients in the present study were transplanted for neuroblastoma, which had the highest incidence of HTN as shown in Figure 1. Treatment of neuroblastoma patients may include nephrectomy, abdominal radiation, and nephrotoxic platinum compounds, which might predispose them to developing renal problems. In one study, 72% (8/11) of neuroblastoma patients receiving HCT developed late renal problems associated with radiation [55]. Thus, the inclusion of neuroblastoma patients may explain the higher risk of autologous patients developing HTN than related donor recipients. However, it is unclear why unrelated donor recipients would have a higher risk of developing HTN than related donors. Although unrelated and related donor recipients differed in both the composition of diseases leading to HCT and the incidence of developing chronic GVHD, neither diagnosis at transplant, chronic GVHD, type of immunosuppression, nor duration of chronic GVHD therapy were associated with HTN in multivariate analysis. Thus, the lack of an association between HTN and chronic GVHD or immunosuppressive therapies suggests that some other characteristic of unrelated donor transplants predisposes patients to HTN.
Other significant risk factors for the development of HTN in multivariate analysis were time-dependent variables, including obesity, diabetes, and growth hormone usage. Obesity and diabetes are well established risk factors for HTN. In the present study, 17% of patients were obese and 16% were diabetic when they developed HTN. We have previously reported an increased risk of type 2 diabetes in our patient population [17]. Taken together, HTN, diabetes, and obesity suggest the possibility that the etiology of HTN in some of our patients may be a result of having developed the metabolic syndrome, defined as the clustering of adverse metabolic conditions (hypertension, insulin resistance, dyslipidemia, and obesity.) Pediatric HCT survivors appear to be at risk for developing the metabolic syndrome [14–16]. Additionally, diabetes and obesity can worsen underlying chronic kidney disease, thereby accelerating the development of HTN. Lastly, growth hormone therapy was marginally associated with an increased risk of HTN (p = 0.05). These patients had received growth hormone therapy for a median of 2.7 (1.7–4.7) years prior to developing HTN. We are not aware of an increased risk of HTN associated with growth hormone therapy. However, growth hormone deficiency has been associated with a higher risk of developing of developing features of the metabolic syndrome in adult survivors of childhood leukemia [56].
HTN represents a significant health risk for pediatric HCT survivors. HTN is an important risk factor for a variety of serious diseases and conditions, including heart disease (ischemic heart disease and heart failure), stroke, chronic kidney disease, peripheral artery disease, retinopathy, and dementia [38]. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of Hypertension [38], defined control of adult blood pressure as a systolic pressures < 140 mm Hg and diastolic pressures <90 mm Hg and <130/80 mm Hg in diabetic patients. In the present study, 72% of treated patients achieved adequate blood pressure control, but 27 hypertensive patients were untreated. Unfortunately, three patients developed end stage renal disease, four developed congestive heart failure, four had strokes, and one patient died of HTN related complications.
Screening for HTN is recommended for long-term survivors of HCT. Current guidelines suggest that blood pressure should be checked at least every 2 years [8]. Although determining the optimal screening interval was beyond the scope of the present study, the high prevalence of HTN in the present study suggests the need for more frequent screening. In screening patients, it is noteworthy to mention that at onset of HTN, 41% of children and 25% of adults had isolated systolic HTN (ISH), whereas in the general population, ISH occurs in roughly 18% of untreated hypertensive adults less than 40 years of age [57]. Current guidelines also recommend a trial of non-pharmacologic treatments for mild hypertension and include moderate dietary sodium restriction, weight reduction in the obese, avoidance of excess alcohol intake, and regular aerobic exercise [8]. Twelve patients successfully became normotensive with non-pharmacologic treatments. However, given the association between HTN and chronic kidney disease, early treatment and referral to a nephrologist may be indicated.
The major limitation of our retrospective study was that the identification of hypertensive cases was dependent on the availability of follow-up records and patient access to medical care, therefore the prevalence of HTN may be under-estimated. We were also limited in our ability to assess certain potential risk factors including excess alcohol consumption, diet, physical inactivity, and cumulative steroid exposure which has been significantly associated with hypertension in non-HCT acute lymphoblastic leukemia patients [58]. Lastly, assessing associations between HTN and hypercholesterolemia and the metabolic syndrome were beyond the scope of the present study.
CONCLUSION
Survivors of pediatric HCT appear more likely to develop HTN than the general population. HTN typically occurred in non-obese, adolescent and young adult HCT survivors. Risk factors for the development of HTN included acute kidney injury, TBI, negative history of HCV infection, autologous (primarily neuroblastoma patients) and unrelated donor types, obesity, and diabetes. At-risk patients should be educated and monitored for the development of HTN. Medical providers should screen patients and refer cases to a nephrologist for specialized care. Research to further understand the etiology and pathogenesis of HTN in transplant patients is needed.
Acknowledgments
Supported in part by the National Cancer Institute (CA 018029, HL 36444, CA 15704, and CA 78902).
We are thankful to the FHCRC Long-term Follow-up staff and to our patients who participate in our long-term follow-up program.
Footnotes
Financial Disclosure: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kumar M, Kedar A, Neiberger RE. Kidney function in long-term pediatric survivors of acute lymphoblastic leukemia following allogeneic bone marrow transplantation. Ped Hematol Oncol. 1996;13:375–379. doi: 10.3109/08880019609030844. [DOI] [PubMed] [Google Scholar]
- 2.Leahey AM, Teunissen H, Friedman DL, Moshang T, Lange BJ, Meadows AT. Late effects of chemotherapy compared to bone marrow transplantation in the treatment of pediatric acute myeloid leukemia and myelodysplasia. Med Pediatr Oncol. 1999;32:163–169. doi: 10.1002/(sici)1096-911x(199903)32:3<163::aid-mpo1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Frisk P, Bratteby LE, Carlson K, Lonnerholm G. Renal function after autologous bone marrow transplantation in children: a long-term prospective study. Bone Marrow Transplant. 2002;29:129–136. doi: 10.1038/sj.bmt.1703312. [DOI] [PubMed] [Google Scholar]
- 4.Kist-van Holthe JE, Bresters D, Ahmed-Ousenkova YM, et al. Long-term renal function after hemopoietic stem cell transplantation in children. Bone Marrow Transplant. 2005;36:605–610. doi: 10.1038/sj.bmt.1705110. [DOI] [PubMed] [Google Scholar]
- 5.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 6.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology (Review) Circulation. 2000;101:329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 7.Onusko E. Diagnosing secondary hypertension (Review) American Family Physician. 2003;67:67–74. [PubMed] [Google Scholar]
- 8.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Zager RA, O’Quigley J, Zager BK, et al. Acute renal failure following bone marrow transplantation: A retrospective study of 272 patients. Am J Kidney Dis. 1989;13:210–216. doi: 10.1016/s0272-6386(89)80054-x. [DOI] [PubMed] [Google Scholar]
- 10.Kist-van Holthe JE, van Zwet JM, Brand R, van Weel MH, Vossen JM, van der Heijden AJ. Bone marrow transplantation in children: consequences for renal function shortly after and 1 year post-BMT. Bone Marrow Transplant. 1998;22:559–564. doi: 10.1038/sj.bmt.1701388. [DOI] [PubMed] [Google Scholar]
- 11.Hingorani SR, Guthrie K, Batchelder A, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int. 2005;67:272–277. doi: 10.1111/j.1523-1755.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 12.Gruss E, Bernis C, Tomas JF, et al. Acute renal failure in patients following bone marrow transplantation: prevalence, risk factors and outcome. Am J Nephrol. 1995;15:473–479. doi: 10.1159/000168889. [DOI] [PubMed] [Google Scholar]
- 13.Cohen EP, Robbins ME. Radiation nephropathy (Review) Semin Nephrol. 2003;23:486–499. doi: 10.1016/s0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 14.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 15.Lorini R, Cortona L, Scaramuzza A, et al. Hyperinsulinemia in children and adolescents after bone marrow transplantation. Bone Marrow Transplant. 1995;15:873–877. [PubMed] [Google Scholar]
- 16.Shalitin S, Phillip M, Stein J, Goshen Y, Carmi D, Yaniv I. Endocrine dysfunction and parameters of the metabolic syndrome after bone marrow transplantation during childhood and adolescence. Bone Marrow Transplant. 2006;37:1109–1117. doi: 10.1038/sj.bmt.1705374. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmeister PA, Storer BE, Sanders JE. Diabetes mellitus in long-term survivors of pediatric hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2004;26:81–90. doi: 10.1097/00043426-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:832–843. 895–902. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 20.Sanders JE, Storb R, Anasetti C, et al. Marrow transplant experience for children with severe aplastic anemia. Am J Ped Hematol Oncol. 1994;16:43–49. [PubMed] [Google Scholar]
- 21.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84:2036–2043. [PubMed] [Google Scholar]
- 22.Nemecek ER, Gooley TA, Woolfrey AE, Carpenter PA, Matthews DC, Sanders JE. Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transplant. 2004;34:799–806. doi: 10.1038/sj.bmt.1704689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeg HJ, Storer B, Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–1207. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 24.Thomas ED, Sanders JE, Flournoy N, et al. Marrow transplantation for patients with acute lymphoblastic leukemia in remission. Blood. 1979;54:468–476. [PubMed] [Google Scholar]
- 25.Balduzzi A, Gooley T, Anasetti C, et al. Unrelated donor marrow transplantation in children. Blood. 1995;86:3247–3256. [PubMed] [Google Scholar]
- 26.Carella AM, Champlin R, Slavin S, McSweeney P, Storb R. Mini-allografts: ongoing trials in humans (Editorial) Bone Marrow Transplant. 2000;25:345–350. doi: 10.1038/sj.bmt.1702204. [DOI] [PubMed] [Google Scholar]
- 27.Hansen JA, Mickelson EM, Choo SY, et al. Clinical bone marrow transplantation: Donor selection and recipient monitoring. In: Rose NR, De Macario EC, Fahey JL, Friedman H, Penn GM, editors. Manual of Clinical Laboratory Immunology. Washington: American Society for Microbiology; 1992. pp. 850–866. [Google Scholar]
- 28.Dupont B, Yang SY. Histocompatibility. In: Forman SJ, Blume KG, Thomas ED, editors. Bone Marrow Transplantation. Boston: Blackwell Scientific Publications; 1994. pp. 22–40. [Google Scholar]
- 29.Storb R, Deeg HJ, Pepe M, et al. Graft-versus-host disease prevention by methotrexate combined with cyclosporin compared to methotrexate alone in patients given marrow grafts for severe aplastic anaemia: Long-term follow-up of a controlled trial. Br J Haematol. 1989;72:567–572. doi: 10.1111/j.1365-2141.1989.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 30.Deeg HJ, Lin D, Leisenring W, et al. Cyclosporine or cyclosporine plus methylprednisolone for prophylaxis of graft-versus-host disease: a prospective, randomized trial. Blood. 1997;89:3880–3887. [PubMed] [Google Scholar]
- 31.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 32.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 33.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 34.Martin PJ, Carpenter PA, Sanders JE, Flowers MED. Diagnosis and clinical management of chronic graft-versus-host disease. Int J Hematol. 2004;79:221–228. doi: 10.1532/ijh97.03176. [DOI] [PubMed] [Google Scholar]
- 35.Sala-Torra O, Martin PJ, Storer B, et al. Serious acute or chronic graft-versus-host disease after hematopoietic cell transplantation: a comparison of myeloablative and non-myeloablative conditioning regimens. Bone Marrow Transplant. 2008;41:887–893. doi: 10.1038/sj.bmt.1705987. [DOI] [PubMed] [Google Scholar]
- 36.Shuhart MC, Myerson D, Childs BH, et al. Marrow transplantation from hepatitis C virus seropositive donors: Transmission rate and clinical course. Blood. 1994;84:3229–3235. [PubMed] [Google Scholar]
- 37.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114 (Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 38.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [erratum appears in JAMA. 2003 Jul 9;290(2):197] JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 39.National Institutes of Health, National Heart, Lung and Blood Institute; 1998. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults--executive summary. www.nhlbi.nih.gov. [Google Scholar]
- 40.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and Health Statistics National Center for Health Statistics. 2002;Series 11:1–190. [PubMed] [Google Scholar]
- 41.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 43.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate [erratum appears in Ann Intern Med. 2008 Oct 7;149(7):519] Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 44.Andersen PK, Borgan O, Gill RD, Keiding N. Statistical Models Based on Counting Processes. New York: Springer-Verlag; 1993. [Google Scholar]
- 45.Hennekens CH, Buring JE. Measures of disease frequency and association. In: Mayrent SL, editor. Epidemiology in Medicine. Boston: Little, Brown and Company; 1987. pp. 54–98. [Google Scholar]
- 46.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 644(150):640–644. doi: 10.1016/j.jpeds.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 47.Esiashvili N, Chiang KY, Hasselle MD, Bryant C, Riffenburgh RH, Paulino AC. Renal toxicity in children undergoing total body irradiation for bone marrow transplant. Radiother Oncol. 2009;90:242–246. doi: 10.1016/j.radonc.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Weiss AS, Sandmaier BM, Storer B, Storb R, McSweeney PA, Parikh CR. Chronic kidney disease following nonmyeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6:89–94. doi: 10.1111/j.1600-6143.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 49.Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39:223–229. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 50.Kersting S, Verdonck LF. Chronic kidney disease after nonmyeloablative stem cell transplantation in adults. Biol Blood Marrow Transplant. 2008;14:403–408. doi: 10.1016/j.bbmt.2007.12.495. [DOI] [PubMed] [Google Scholar]
- 51.Lawton CA, Cohen EP, Murray KJ, et al. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant. 1997;20:1069–1074. doi: 10.1038/sj.bmt.1701022. [DOI] [PubMed] [Google Scholar]
- 52.Gronroos MH, Bolme P, Winiarski J, Berg UB. Long-term renal function following bone marrow transplantation. Bone Marrow Transplant. 2007;39:717–723. doi: 10.1038/sj.bmt.1705662. [DOI] [PubMed] [Google Scholar]
- 53.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplant: epidemiology, pathogenesis and treatment. Journal of the American Society of Nephrology. 2006;17:1995–2005. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 54.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarbell NJ, Guinan EC, Chin L, Mauch P, Weinstein HJ. Renal insufficiency after total body irradiation for pediatric bone marrow transplantation. Radiotherapy & Oncology. 1990;18 (Suppl 1):139–142. doi: 10.1016/0167-8140(90)90195-3. [DOI] [PubMed] [Google Scholar]
- 56.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 57.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 58.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]