Abstract
Resistance to antinematodal drugs like levamisole has increased and there is a need to understand what factors affect the responses to these anthelmintics. In our previous study, we examined the role of ryanodine receptors in muscle contraction pathways. Here we have examined interactions of levamisole receptors, ryanodine receptors (RyRs), the excitatory neuropeptide AF2, and coupling to electrophysiological responses. We examined the effects of a brief application of levamisole on Ascaris suum body muscle under current-clamp. The levamisole responses were characterized as an initial primary depolarization, followed by a slow secondary depolarizing response. We examined the effects of AF2 (KHEYLRFamide), 1 μM applied for 2 min. We found that AF2 potentiated the secondary response to levamisole and had no significant effect on the primary depolarization [1]. Further, the reversal potentials observed during the secondary response suggested that more than one ion was involved in producing this potential. AF2 potentiated the secondary response in the presence of 30 μM mecamylamine suggesting the effect was independent of levamisole sensitive acetylcholine receptors. The secondary response, potentiated by AF2, appeared to be dependent on cytoplasmic events triggered by the primary depolarization. Ion-substitution experiments showed that the AF2 potentiated secondary response was dependent on extracellular calcium and chloride suggesting a role for the calcium-activated anion channel. Caffeine mimicked the AF2 secondary response and 0.1 μM ryanodine inhibited it. 1.0 μM ryanodine increased spiking showing that it affected membrane excitability. A model is proposed showing ryanodine receptors mediating effects of AF2 on levamisole responses.
Keywords: Ascaris suum, levamisole, AF2, electrophysiology, ryanodine, caffeine
Introduction
Nematode parasites are a severe burden on the productive lives of humans and animals [2-4]. Treatment of these conditions with anthelimintics is limited to three main classes of drugs, and drug resistance has emerged in humans [2, 3] as well as in animals [4, 5] against each of the three classes of anthelmintic. The appearance of multidrug-resistance in nematode parasites [6] is a worrying development. These concerns emphasize the requirement for understanding the mode of action of these compounds and mechanisms of resistance.
Our laboratory has studied levamisole, pyrantel, oxantel and morantel which belong to an important group of nicotinic anthelmintic drugs [7-12] that are used for treatment of ascariasis, Trichuris sp. and hookworm infections [13]. The target sites of these drugs include the pharmacologically distinctive ion-channels that are nicotinic acetylcholine receptors (nAChRs) found on the body muscles of nematodes [14-16]. These drugs produce spastic paralysis of the parasitic nematode and have an advantage of acting rapidly on the parasite, effecting cures within 4 hours.
We have seen in the previous paper that ryanodine receptors (RyRs) of A. suum modulate the amplitude of the levamisole contraction by affecting gmax but not EC50. The cellular mechanisms that modulate responses to anthelmintics are important to recognize and describe because they may be modified in anthelmintic resistance. In this paper we extend our previous observations on the role of RyRs in muscle contraction in A. suum, using the current-clamp technique. We demonstrate that the RyRs and the entry of calcium play a role in modulating the secondary responses to levamisole and its potentiation by AF2. These observations demonstrate the role of RyRs in modulating the electrophysiological response, and hence, affect the contractile response to levamisole. It is possible that RyRs are modified with the development of resistance to levamisole in parasitic nematodes.
2. Materials and Methods
2.1. Muscle flap preparation for electrophysiology
Adult A. suum were collected weekly from the Tyson's pork packing plant at Storm Lake, Iowa. Worms were maintained in Locke's solution [Composition (mM): NaCl 155, KCl 5, CaCl2 2, NaHCO3 1.5 and glucose 5] at a temperature of 32°C. The Locke's solution was changed daily and each batch of worms was used within 4 days of collection. We prepared 1 cm muscle tissue flaps by dissecting the anterior part of the worm, 2-3 cm caudal to the head. A body muscle flap preparation was then pinned onto a Sylgard™-lined double jacketed bath chamber maintained at 35°C by inner circulation of warm water (Fisher scientific Isotemp 3016H, PA, USA). The intestines were removed to expose the muscle cells [1]. The preparation was continuously perfused, unless otherwise stated, with Ascaris Perienteric Fluid-Ringer (APF-Ringer) composition (mM): NaCl 23, Na-acetate 110, KCl 24, CaCl2 6, MgCl2 5, glucose 11, and HEPES 5; NaOH or acetic acid was used to adjust the pH to 7.6. The incoming perfusate was pre-warmed to 35°C with an inline heating system (SH 27B Warner instruments, CT, USA) before application. The rate of perfusion was 3.5 - 4 ml.min-1 through a 20 gauge needle placed directly above the muscle bag recorded from. The calcium substitution experiments were done using cobalt APF-Ringer, composition (mM): NaCl 23, Na-acetate 110, KCl 24, CoCl2 6, MgCl2 5, glucose 11, and HEPES 5mM; pH 7.6. Chloride was substituted by acetate in chloride free APF-Ringer. The experimental compounds were dissolved in APF-Ringer, cobalt APF-Ringer or chloride free APF-Ringer as described in the results. 1 μM levamisole was applied for a period of 10-20 seconds as described in the results. AF2 (1 μM) was applied for 2 minutes and followed by a minute wash prior to applications of levamisole.
2.2. Electrophysiology
A two-microelectrode current-clamp technique was employed to examine the electrophysiological effects in the bag region of A.suum muscle (Fig.1A). Borosilicate capillary glass (Harvard Apparatus, Holliston, MA, USA, ID-0.86mm, OD- 1.5mm) microelectrodes were pulled on a P-97 Flaming Brown Micropipette puller (Sutter Instrument Co., CA, USA). We used 3M potassium acetate in the micropipettes which had resistances of 20-30 MΩ. The recordings were obtained by impaling the bag region of A. suum muscle with 2 microelectrodes, namely current injecting (I) and voltage recording electrodes (V). All experiments were performed using an Axoclamp 2A amplifier, a 1320A Digidata interface and Clampex 9 software (Molecular Devices, CA, USA). All data were displayed and analyzed on a PC based desktop computer.
Fig. 1.
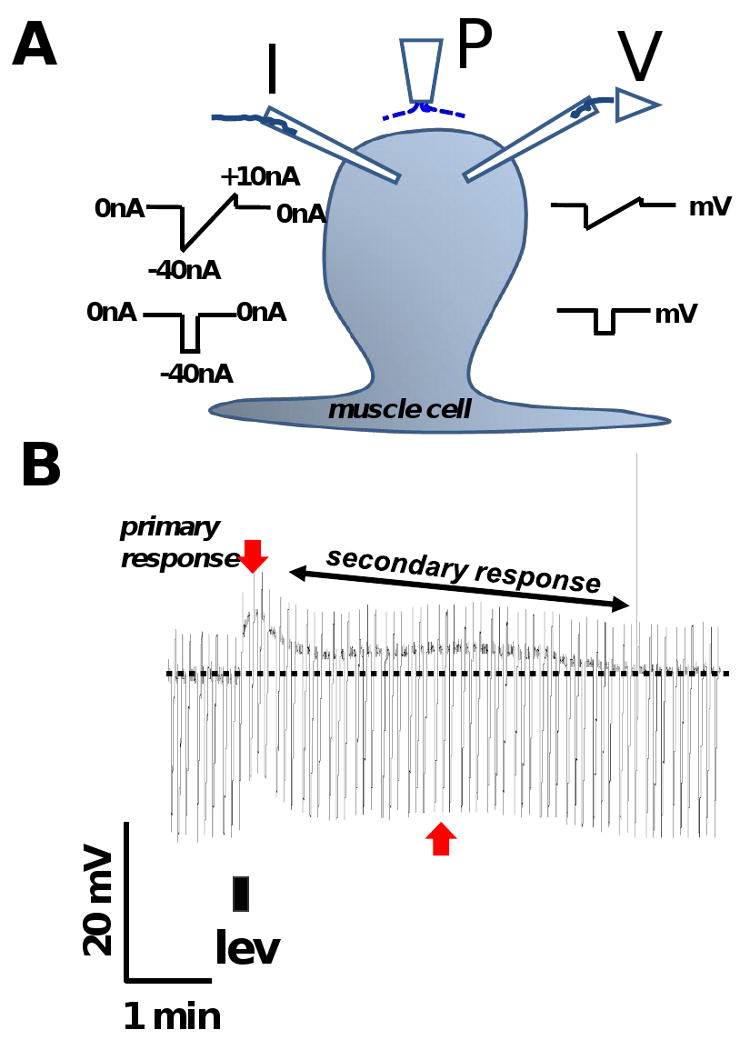
A: Diagram showing the placement of the two micropipettes used for current-clamp and position of the micro perfusion system for continuous perfusion and application of drugs. P: microperfusion pipette. I: current-injecting electrode, injects ramp currents or step currents. V: voltage-recording electrode.
B: Representative trace showing the levamisole response and its 2 components in APF-Ringer namely, a primary depolarization and a secondary depolarizing response (secondary response). The darkest line of the recording is the membrane potential and the downward transients are the responses to injected current. The rapid primary depolarization (downward red arrow) is followed by a slow secondary response (red vertical arrow and oblique black double arrow). 1 μM levamisole was applied for 10 s as indicated by the filled rectangle below the trace. The discontinuous horizontal line indicates the original position of the resting membrane potential. The width of the trace is a reflection of membrane conductance; it gets narrower as membrane-ion channels open. The duration of the secondary response (T80) was measured as the time taken (min) for the peak primary depolarization to decline by 80%.
The current injecting electrode injected hyperpolarizing ramp or step currents, while the voltage recording electrode recorded the change in membrane potential in response to the injected currents. Our ramp current was a hyperpolarizing step of -40 nA changing linearly with time to a depolarizing current of 10 nA over a duration of 3 s at 0.2 Hz. The step current was -40 nA for 500 ms at 0.3 Hz. Each set of experiments were repeated on preparations from separate batches of worms. Cells with constant membrane potentials more negative than -20 mV for 20 minutes and a stable input conductance of < 3.5 μS were selected for the recordings.
2.3. Drugs
AF2 (H - Lys - His - Glu - Tyr - Leu - Arg - Phe - NH2) [Sigma-Genosys, The Woodlands, TX, USA] 1 mM stock solutions were prepared in double distilled water every week and kept in aliquots at -20°C. AF2, stock solutions were thawed just before use. All other chemicals were obtained from Sigma-Aldrich (MO, USA) and Acros-Organics (NJ, USA).
2.4. Analysis
The peak change in membrane potential (δV) and conductance (δG) was determined in response to drug applications. The duration of the secondary depolarizing response (secondary response) to levamisole was measured as the time taken (min) for the peak primary depolarization to decline by 80% (T80). We estimated reversal potentials by extrapolating from the membrane I-V plots using linear regression. We used the intracellular ionic concentration values of Ascaris estimated by Brading and Caldwell [17] to calculate ion-reversal potentials. For a single ion species, the reversal potential was calculated from the Nernst equation using estimates of the intracellular and extracellular concentrations of that ion. When ion-channels selectively permeable to one species of ion open, then the membrane potential will move towards the reversal potential for that ion. We estimated the reversal potential from linear regression of the relationships between injected current and the membrane potential responses (the I-V plots) before and during conductance changes. The potential at which these plots cross, is the reversal potential of the ion-channel that has opened and is determined by ions that flow through the ion-channel. For example, a channel conducting only chloride ions, will have a measured reversal potential that matches the chloride Nernst potential. If the measured reversal potential does not match the Nernst potential of a single ion, it implies that more than one ion is involved in generating the potential.
We defined spikes in A. suum as brief repeating action potentials with amplitude greater than 5 mV appearing as a single spike for a duration up to 500ms. We measured the spike frequency (min-1), amplitude (mV) and spike gradient (mV.s-1). We tested the effects of ryanodine on spike parameters during before levamisole application and or during the rising phase of the levamisole depolarization. The spike gradient was measured on the rising phase and falling phase.
2.5. Statistics
All statistical analysis was done using Graph Pad Prism software (version 4.0/5.0, San Diego, CA, USA). Continuous recordings which had initial control application/s followed by test application/s were compared using paired t-tests. Control and test recordings made from separate preparations were compared using unpaired t-tests. Chi-squared tests were used to evaluate effects on muscle spiking.
3. Results
3.1. The levamisole response has two components: an initial primary depolarization followed by a slower secondary depolarizing response
Fig.1A shows a diagram of the technique used to observe the current-clamp responses of a somatic muscle cell; Fig 1B shows a representative current-clamp recording in APF-Ringer solution. The resting membrane potential of this cell, represented by the dark line in the trace, was -33.2 mV and its input conductance, determined from the amplitude of the downward voltage transients to injected current, was 1.7 μS. A brief application of levamisole (10 s) produced a primary depolarization of 9.3 mV and a change in conductance of 0.06 μS at its peak. After the primary depolarization, a slower secondary depolarizing response (the secondary response) followed which lasted more than 5 min. We quantified the duration of the secondary response as T80 (the time taken for the peak primary depolarization to decay by 80%) which was 4.2 min for the experiment in Fig 1B. During the secondary response, we observed a secondary peak depolarization of 3.8 mV associated with a conductance increase of 0.07 μS. Similar secondary components were observed in more than 20 muscle cell preparations with 1 μM levamisole. The primary response is presumably initiated by the opening of N-, L- and B- subtypes of nAChRs present on the muscle bag membrane [14, 18] during the application of levamisole and supported by voltage-activated channels [19]. However, the cause for the slow secondary response when levamisole is being continuously washed off by APF-Ringer is an interesting, but as yet, unexplained effect of levamisole.
3.2. AF2 potentiates the secondary response to levamisole
Fig 2A shows a representative current-clamp trace where AF2 potentiated the secondary response to levamisole. There are two control levamisole applications (1 μM, 10 s) followed by AF2 treatment (1 μM, 2 min) with a brief wash (1 min) then two test applications of levamisole. The duration of the secondary response, T80, was significantly increased from 4.0 ± 1.4 to 16.1 ± 1.8 min after AF2 treatment (n = 4, p < 0.05 paired t-test, Fig 2B). Before and after the AF2 application the mean primary depolarization responses were 6.9 ± 1.3 and 8.6 ± 2.1 mV respectively (n = 4, p > 0.05 paired t-test).
Fig. 2.
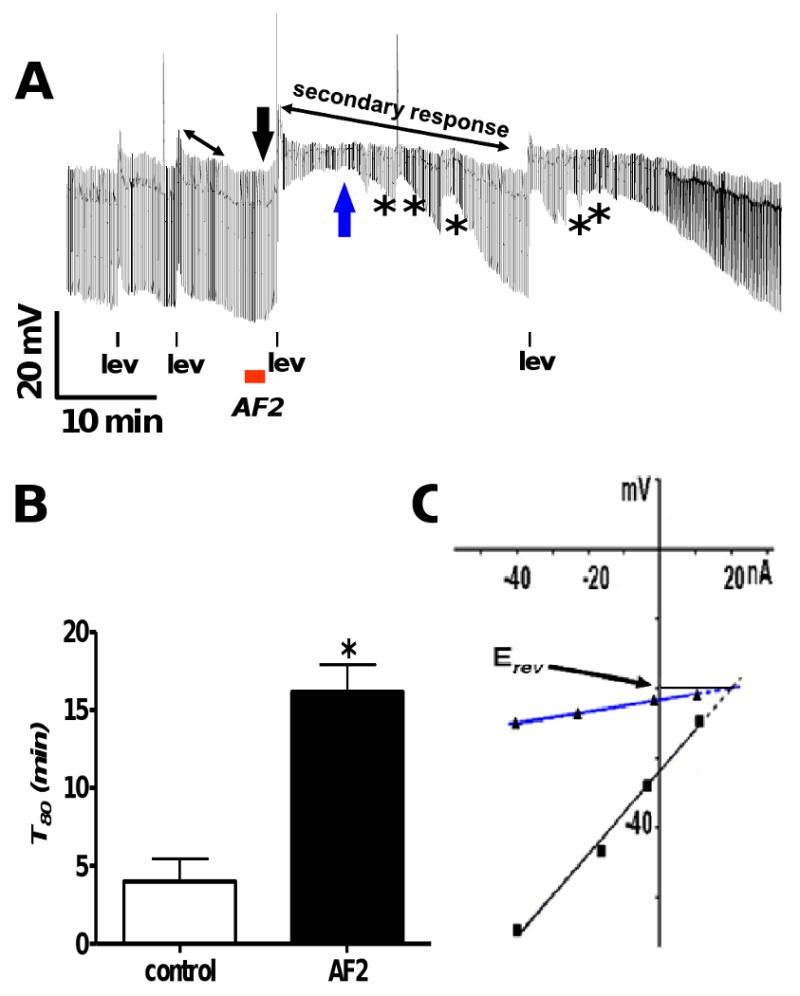
A: Representative current-clamp trace showing AF2 potentiating the secondary response to levamisole. There are two applications of levamisole (1 μM) before and after AF2 treatment. The control levamisole applications are followed by a 2 min application of AF2 (1 μM) with a brief wash (1 min), subsequently; there are two test levamisole applications. The double headed black arrows represent the secondary response before and AF2 treatment. * represents waves of conductance change during the secondary response.
B: Bar graph comparing T80 (min) before and after AF2 treatment in APF-Ringer. The secondary response to levamisole application was significantly increased after AF2 treatment as indicated by the increase in T80 (Fig 2B, n = 4, p < 0.05, paired t-test).
C: The black line and downward arrow show the control current-voltage plot before the test levamisole application and the blue upward arrow and line show the current-voltage plot during the at the secondary peak from Fig. 2A. Plots were fitted by linear regression. The reversal potential, estimated by extrapolating the two current-voltage plots are shown. The reversal potential, Erev, was -20 mV.
Fig 2A shows effects of AF2 treatment where we observed waves of conductance change during the secondary response. We examined the ionic basis of the secondary response by observing the current-voltage relationships and estimating the reversal potential. Fig 2C shows the I-V plots (fitted by linear regressions) at the two positions in Fig 2 B, after AF2 treatment. These positions were before the test application of levamisole as shown by black arrow and during the secondary peak as shown by the blue arrow. The estimated reversal potential was -20mV; this value did not match that of only one ion, and suggested that more than one ion was involved in its generation. The predicted Nernst potentials for K+, Na+, Cl- and Ca++ were EK = -37.7, ENa = +26.7, ECI = -42.9 and ECa = +45 mV [17, 20].
3.2.1 Potentiation continues after the initial effect of levamisole on nAChRs
We explored the properties of the AF2 potentiated secondary response. Levamisole, a membrane permeable drug [7], adheres to the membrane and may maintain its effect, even after application has stopped. We used a high concentration of mecamylamine (30 μM) during the secondary response to inhibit any residual levamisole from affecting the secondary depolarization, thus isolating the nAChR independent component (Fig 3A). Although, as expected, mecamylamine reduced the duration of secondary response, we observed that AF2 still increased the duration of the secondary response (Fig 3 B). The duration of the secondary response, T80 increased significantly from 0.4 ± 0.1 min (n = 4) to 0.8 ± 0.1 min (n = 5) after AF2 pretreament (p<0.05, unpaired t-test). As an additional test we also measured the area under the response curve (RAUC) from the start of mecamylamine application to the return of the membrane potential to the resting level in control experiments, and in test experiments after AF2. The RAUC increased significantly from 84.6 ± 13.1 mV.s (n = 4) to 157.2 ± 21.5 mV.s (n = 5) after AF2 pretreament (p<0.05, unpaired t-test). Thus, AF2 maintained its potentiating effect on the secondary response after blockade of the levamisole site of action. This indicated the activation of downstream pathways which are initiated during the primary levamisole response and give rise to the secondary response. We proceeded further to identify the ionic basis of the secondary response and its potentiation by AF2.
Fig. 3.
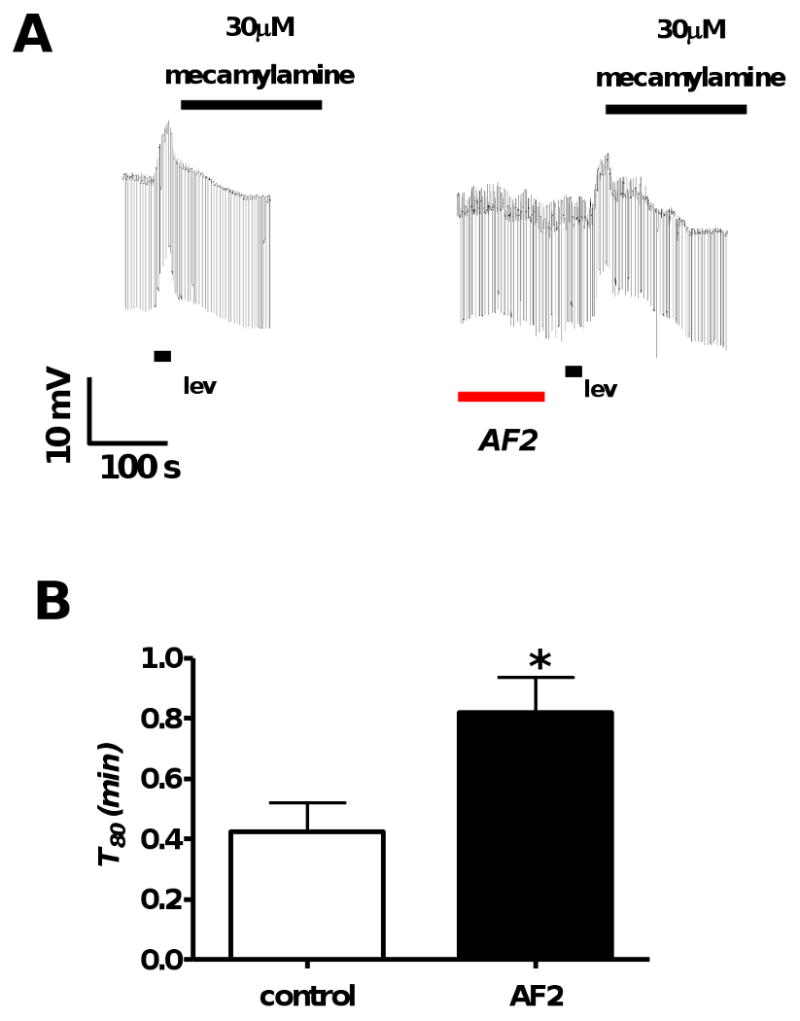
A: Representative current-clamp traces where mecamylamine (30 μM), a nAChR antagonist, was applied immediately after the end of levamisole application in the control (n = 4) and the test (n = 5) recordings. The first trace shows a control and the second trace shows the test response after AF2.
B: Bar graph comparing T80 controls and AF2 test pre-treatments in the presence of mecamylamine. AF2 potentiated the duration of levamisole secondary response T80, in the presence of mecamylamine (p < 0.05, unpaired t-test).
3.2.2 Potentiation requires extracellular calcium
We investigated the ionic basis of the AF2 potentiated levamisole secondary response by substituting calcium with cobalt, a calcium channel blocker. We applied cobalt APF-Ringer (Ca++ free) during the AF2 potentiated secondary response, Fig 4A. Calcium substitution caused a significant reduction in the duration of the secondary response, T80, which was reduced from 16.1 ± 1.8 to 2.6 ± 1.2 min (p < 0.001, n = 4 unpaired t-test Fig 4B). This indicated that external calcium is required for the AF2 potentiation of the secondary response. Calcium entry can initiate calcium induced calcium release mediated by RyRs and other calcium dependent events. Thorn and Martin (1987) demonstrated the presence of a high-conductance calcium-dependent chloride channel in the body muscle membrane of A. suum [21]. This raised the possibility that incoming calcium during the primary response leads to activation of these chloride channels.
Fig. 4.
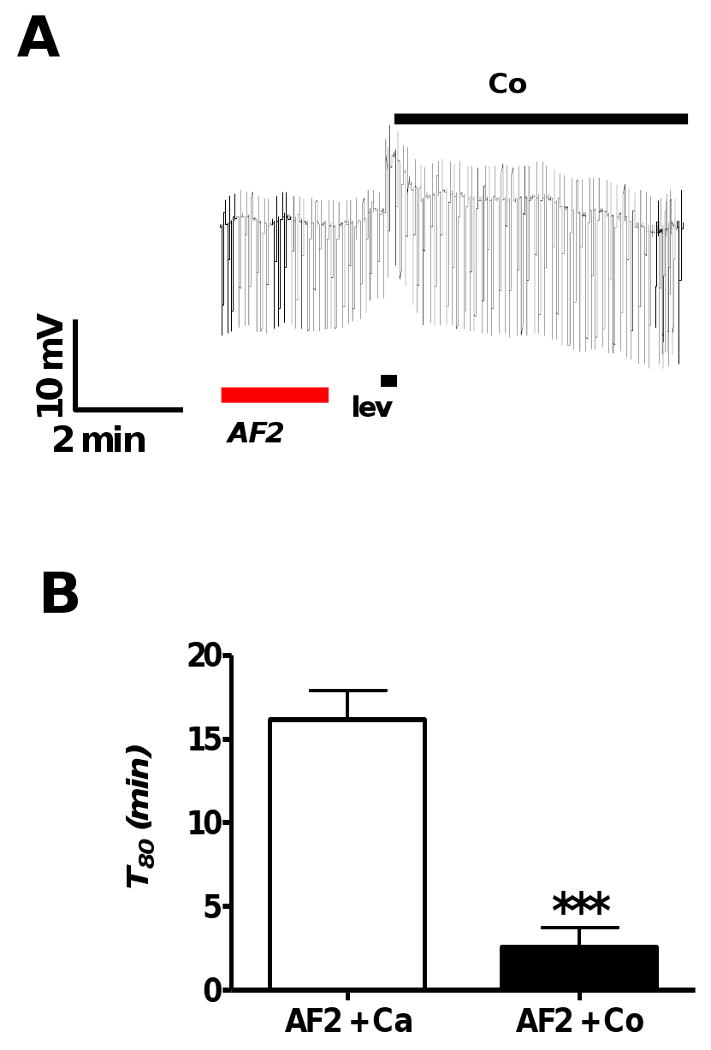
A: Representative current-clamp trace showing the lack of an AF2 potentiated secondary response following replacement of calcium with cobalt APF-Ringer following the end of levamisole application (1 μM).
B: Bar graph comparing mean durations of secondary depolarizations, T80, from different preparations recorded after AF2 treatment in the presence and absence of calcium (calcium replaced using cobalt APF-Ringer). Calcium substitution caused a significant reduction in the duration of the secondary response (p < 0.001, n = 4, unpaired t-test).
3.2.3 Potentiation is sensitive to extracellular chloride
We removed chloride replacing it with acetate in our chloride free APF-Ringer solution. AF2 did not potentiate the levamisole secondary response in the absence of extracellular chloride as shown in Fig 5A. There was no significant difference in T80 (p > 0.05, paired t-test) (Fig 5B). The duration of the control secondary response (T80) was 1.6 ± 0.4 min, while after AF2 treatment it was 2.9 ± 1.2 min (n = 5). Fig 5 A also shows that there was no increase in membrane conductance during the secondary responses following AF2; we found in all five experiments in chloride free APF-Ringer, that there was no increase in membrane conductance. These experiments showed that in addition to extracellular calcium, the AF2 potentiation required extracellular chloride.
Fig. 5.
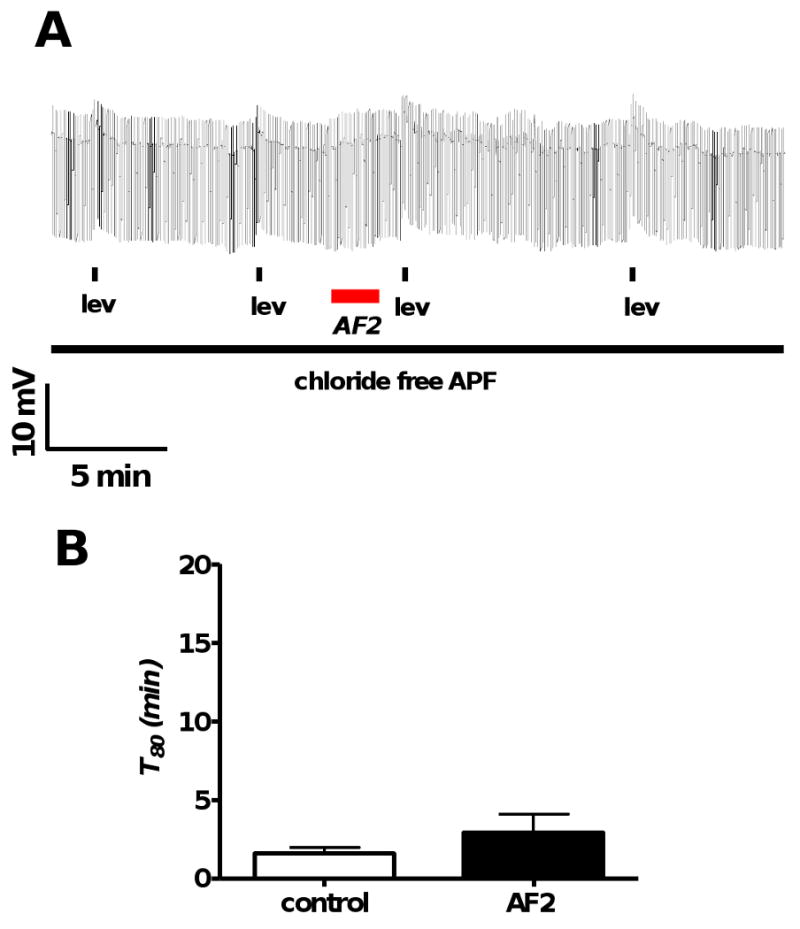
A: Representative current-clamp trace showing levamisole (1 μM) applications before and after AF2 treatment in the chloride free in APF-Ringer.
B: Bar graph comparing T80 before and after AF2 treatment. In the absence of extracellular chloride, T80 in control and test responses were not significantly different (p > 0.05, n = 5, paired t-test). AF2 did not potentiate the levamisole secondary response in the absence of chloride as indicated by T80 measurements.
3.3. Caffeine mimics the AF2 potentiation
Recognizing the importance of extracellular calcium for AF2 potentiation of the levamisole secondary response, we explored the possibility of calcium entry causing calcium induced calcium release via ryanodine receptors (RyRs). The waves of changes in input conductances observed during AF2 potentiation, Fig 2A, suggested that release of intracellular calcium mediated by RyRs was involved. We used caffeine, an agonist of RyRs, to release calcium from the sarcoplasmic stores [22-24]. Fig 6A shows a representative trace of the effects of 30 mM caffeine on the membrane potential and conductance of the muscle cell. Caffeine (30 mM, 4 min) produced a slow depolarization of 3.5 mV associated with an increase in conductance of the cell membrane (0.9 μS). We compared the current-voltage relationship before caffeine application and at the peak of the caffeine response to determine the reversal potential. Similar to the AF2 potentiated secondary response Fig 2C, we observed a reversal potential of -12 mV during caffeine application, Fig 6B. The estimated reversal potential did not match that of one individual ion, suggesting that more that one ion was involved. The slow depolarization with a conductance increase had a similar time course to the AF2 potentiated levamisole secondary response. Based on these observations, we hypothesized that AF2 potentiated the secondary response to levamisole by causing calcium release from the sarcoplasmic stores mediated by RyRs. We tested our hypothesis using ryanodine, a RyR antagonist.
Fig. 6.
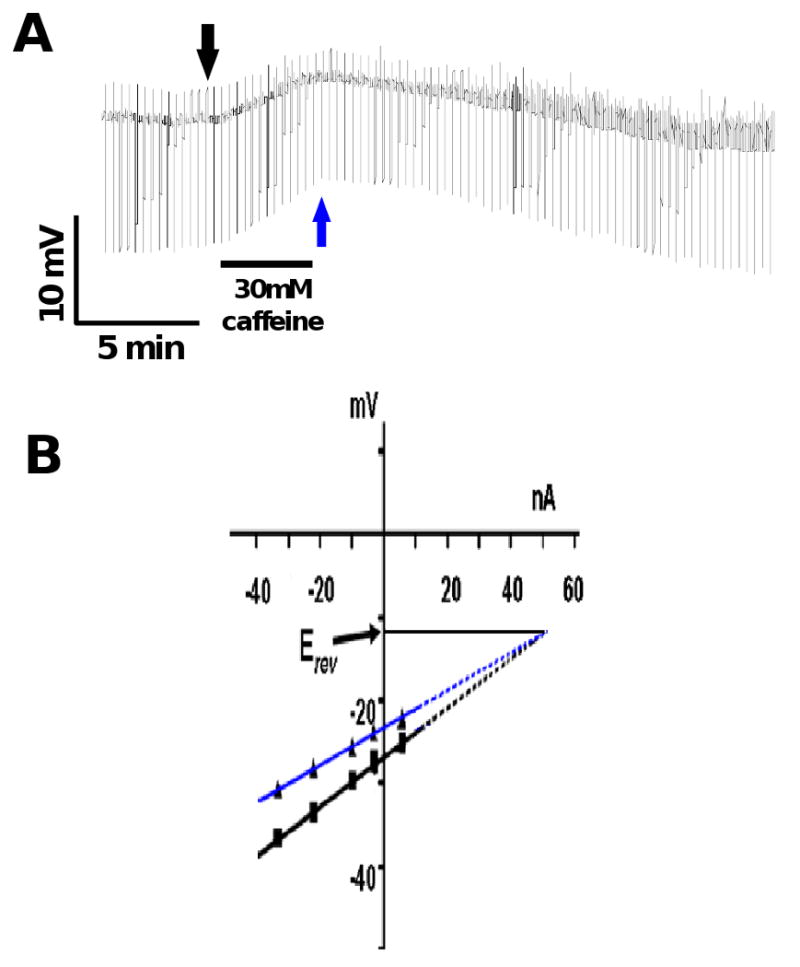
A: Current-clamp trace showing the effect of caffeine (30 mM) on the membrane potential and conductance. Note that the application of caffeine produced a slow depolarization associated with an increase in conductance.
B: The membrane potential responses to the injected ramp currents fitted with linear regression before application of caffeine (black arrow Fig. 6 A) and at the peak depolarization (blue arrow Fig 6A) in the IV plot. The reversal potential, Erev, estimated after extrapolating the membrane potential responses was -12 mV.
3.4. Ryanodine antagonizes the AF2 potentiated secondary response
We hypothesized that AF2 potentiation involves calcium induced calcium release mediated by RyRs. Ryanodine is a plant alkaloid that inhibited calcium induced calcium release mediated by RyRs in muscle preparations [25-27]. We tested the sensitivity of AF2 induced potentiation to application of ryanodine (a RyR antagonist). We applied 0.1 μM ryanodine continuously and tested the effects of AF2 on the levamisole responses (Fig 7A). We bathed the preparation for 20 min before control and test levamisole applications (1 μM, 10 s). We observed that AF2 no longer produced significant potentiation of the levamisole secondary response, Fig 7B. The duration of the control levamisole secondary response (T80) was 1.7 ± 0.7 min and after AF2 treatment, it was 3.8 ± 1.6 min (P>0.05, paired t-test, n = 4). This demonstrated the involvement of RyRs during the AF2 potentiation of the levamisole secondary response. Interestingly, we also observed that prolonged ryanodine exposure produced large spikes (Fig 7A, 8A and 8B). We explored some of the properties of these spikes induced by ryanodine in our next experiments.
Fig. 7.
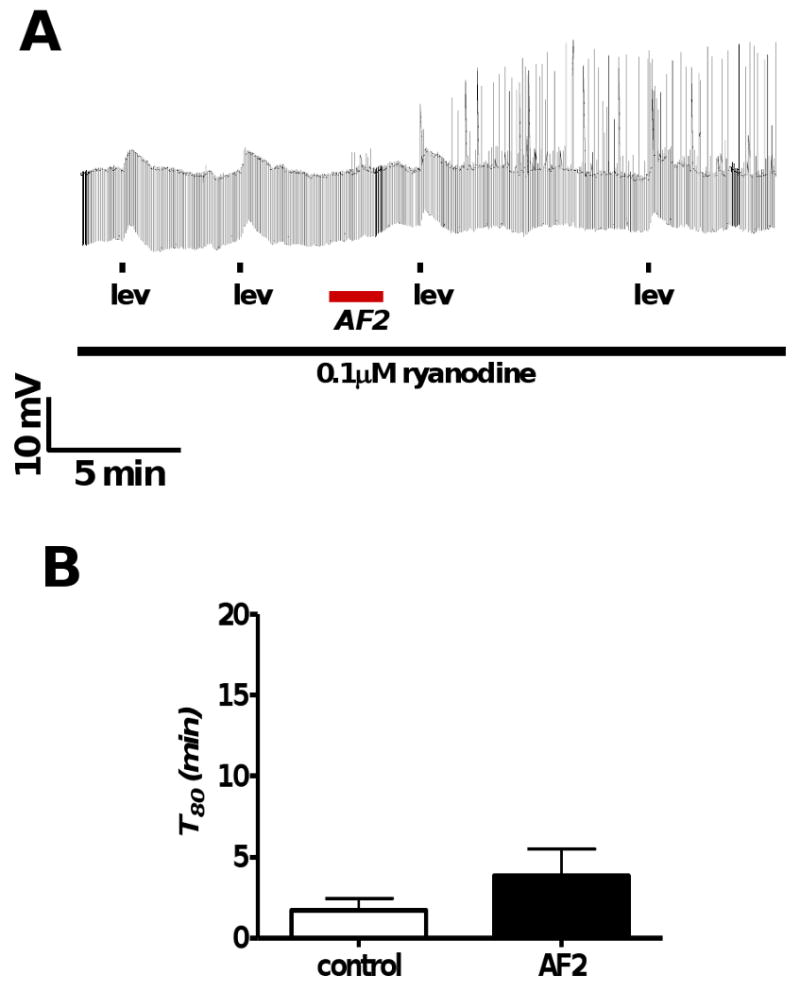
A: Representative current-clamp trace showing levamisole (1 μM) applications before and after AF2 treatment in the presence of 0.1 μM ryanodine.
B: Bar graph depicting T80 (min) in control and test applications. In the presence of 0.1 μM ryanodine, T80 in control and test responses were not significantly different (p > 0.05, n = 4, paired t-test). AF2 did not significantly potentiate the levamisole secondary response in the presence of ryanodine, as indicated by T80 measurements.
Fig. 8.
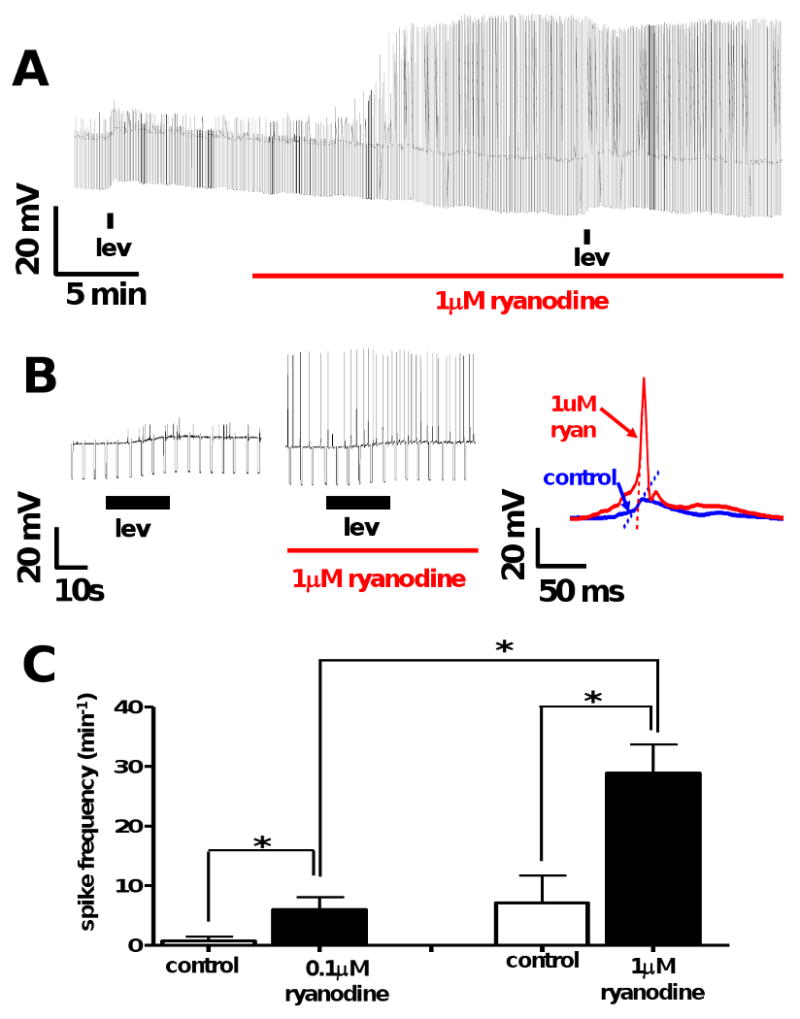
A: Representative current-clamp trace showing levamisole (1 μM) application before and during ryanodine treatment (1 μM).
B: Representative trace of spikes seen at higher time resolution during levamisole application before and in the presence of 1 μM ryanodine. The recordings show an increase the spike amplitudes (left) and an increase in the gradient of the rising phase of the spikes (blue increased in red: right) in the presence of ryanodine.
C: Bar graphs showing the mean ± S.E. spike frequency during the depolarizing phase of the response to levamisole before and in the presence of 0.1 μM ryanodine and in separate experiments 1 μM ryanodine. Treatment with 0.1 μM ryanodine significantly increased spiking from 0.8 ± 0.8 min-1 to 6 ± 2.1 min-1 (n = 4, p < 0.05, paired t-test). Treatment with 1 μM ryanodine significantly increased spiking from 7.2 ± 4.6 min-1 to 28.9 ± 4.8 min-1 (n = 6, p < 0.05, paired t-test). Spiking frequencies at 1 μM were also significantly greater than at 0.1 μM (p<0.01, unpaired t-test) demonstrating that the effect of ryanodine on spiking was concentration dependent.
3.5. Ryanodine increases the frequency of spikes
Under normal recording conditions (APF-Ringer) spontaneous membrane spikes were rare: 1 out of 10 experiments in this data set (example shown in Fig 8A). Spikes occurred more frequently during the rising phase of the primary levamisole depolarization; 3 out of 10 experiments in this data set. We found that ryanodine (0.1 or 1 μM) treatment increased the likelihood of observing spikes both at rest; 6 out of 10 expts, and during levamisole application; 10 out of 10 expts (Fig 8A & B). In both cases the difference was statistically significant (p < 0.05, Chi-square test, two sided) suggesting that ryanodine increases spiking. Next, we calculated spike frequency (min-1) for recordings before and after ryanodine treatment on the rising phase of the levamisole depolarization. Treatment with 0.1 μM ryanodine significantly increased spiking from 0.8 ± 0.8 min-1 to 6 ± 2.1 min-1 (n = 4, p < 0.05, paired t-test). Treatment with 1 μM ryanodine significantly increased spiking from 7.2 ± 4.6 min-1 to 28.9 ± 4.8 min-1 (n = 6, p < 0.05, paired t-test). Spiking frequencies at 1 μM were also significantly greater than at 0.1 μM (p<0.01, unpaired t-test) demonstrating that the effect of ryanodine on spiking was concentration dependent (Fig 8C). We further characterized spike properties by measuring amplitude, rate of rise and rate of decay. The lack of spikes prior to ryanodine application rendered statistical analysis problematic. However, for the experiment shown in Fig 8A, 1 μM ryanodine caused a significant increase in spike amplitude: at rest the amplitude was 6.5 ± 0.3 mV (n = 9) before and 40.4 ± 3.4 mV (n = 11) after 1 μM ryanodine (p < 0.0001, unpaired t-test). Similarly, 1 μM ryanodine increased both the rate of rise and rate of decay of the spikes. Prior to application of ryanodine, the rise rate was 0.7 ± 0.1 mV.ms-1 and the decay rate was 0.2 ± 0.02 mV.ms-1 (n = 7); in the presence of 1 μM ryanodine, the rate of rise increased to 9.9 ± 0.7 mV.ms-1, and the rate of decay rate to 11.1 ± 0.06 mV.ms-1 (n = 11). The rates of rise before and after ryanodine application were significantly different (p < 0.0001, unpaired t-test), as were the rates of decay (p < 0.0001, unpaired t-test).
Spike action potentials appear to be a combination of voltage activated calcium currents, voltage-activated potassium currents [20] and calcium-activated chloride currents [21] as modeled by Turner [28]. The spike gradient and spike amplitude observations suggest that ryanodine causes an increase in the inward voltage-gated calcium current and the outward voltage-gated potassium current.
4. Discussion
4.1. A model for the electrophysiological effects of AF2 and ryanodine on levamisole responses
Trailovic et al [1] showed that brief application of the neuropeptide, AF2, produces long-lasting potentiation of membrane potential and contraction responses to acetylcholine along with increased action potential generation. The effect on the levamisole response was to extend the duration of the depolarization. To explain these observations Trailovic et al [1], proposed that AF2 increases cytosolic calcium by opening of voltage-gated calcium channels and stimulating release of calcium from sarcoplasmic stores.
Subsequently Verma et al [19], tested the effects of AF2 using voltage-clamp in A. suum muscle and found that AF2 potentiates the calcium currents. In this paper, we have extended these observations and described the more complex response to levamisole. The levamisole response consists of a primary depolarization followed by a slower secondary response and we have observed that AF2 potentiates the secondary response. We found that the AF2 potentiation was inhibited by calcium substitution with cobalt, inhibited by ryanodine, and required the presence of extracellular chloride. Application of caffeine produced a slow depolarization and an increase in membrane conductance like the secondary response. The reversal potentials of the levamisole secondary response when potentiated by AF2 were similar to the potentials produced by caffeine. We also observed that ryanodine alone produced an increase in the frequency of spiking. We tested for the persistence of levamisole following application as an explanation for the secondary response and found that application of a high concentration of mecamylamine did not abolish the secondary response. Importantly, the mecamylamine insensitive component of the secondary response to levamisole was potentiated by AF2.
Fig 9 shows the previously proposed [1] sites of action of AF2 and in addition a currently proposed model that explains observations on the actions of ryanodine and AF2. The model proposes that AF2 acts via one or more G-protein receptors [29] to shift the opening of voltage-sensitive calcium channels to more hyperpolarized potentials so that they open more readily [19]; the model also proposes that there is sensitization of the RyRs by AF2. The model proposes that the primary depolarization is initiated by opening of the nAChRs and the flow of inward current; this depolarization will then secondarily activate voltage-activated channels. Calcium will enter the cytoplasm via the nAChRs and the voltage-activated channels producing an increase in cytoplasmic calcium that activates RyRs and further increase in cytoplasmic calcium. Activation of the calcium-activated anion channels [21] follows the high rise in cytosolic calcium.
Fig. 9.
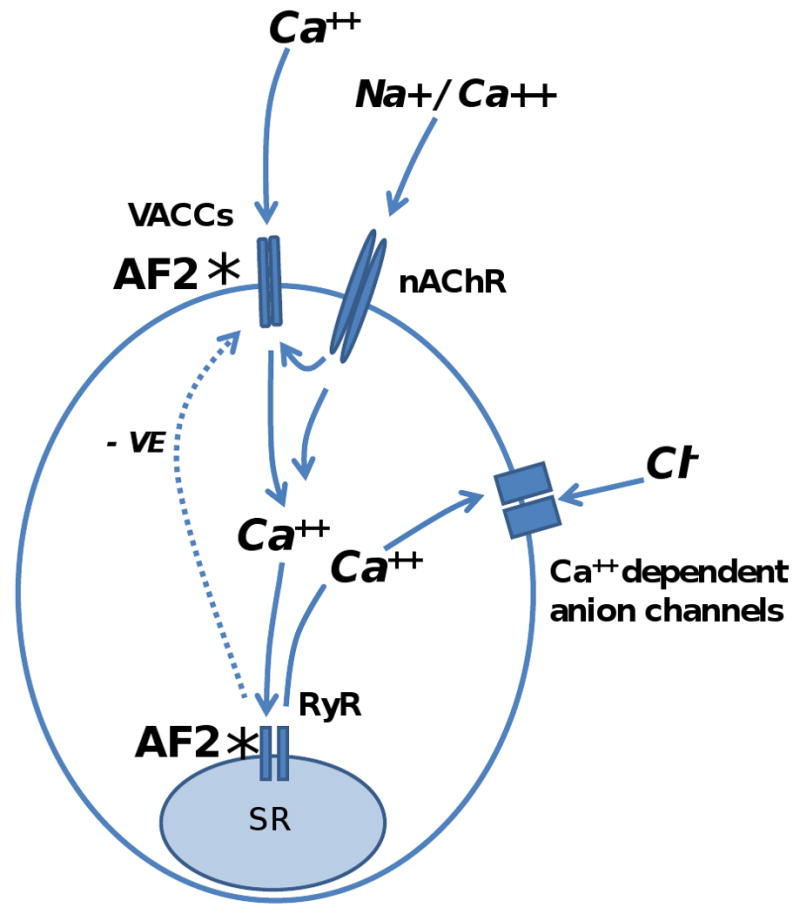
Proposed model and sites of action whereby AF2 modulates the responses to levamisole [1]. The primary depolarization follows levamisole binding to nAChRs and their opening to allow Ca++ and Na+ to enter the cell. The levamisole secondary response is initiated by the primary depolarization and involves activation of voltage-gated calcium channels (VACCs), ryanodine receptors (RyRs) and calcium-activated anion channels. VACCs are activated by the primary depolarization and allow more calcium to enter the cell. Increased intracellular calcium triggers calcium induced calcium release (CICR) from the sarcoplasmic reticulum (SR) and are gated by the ryanodine channels (RyRs). The calcium-activated anion channels are also activated during the cytoplasmic rise in calcium concentration. The calcium induced calcium release can inhibit the voltage-activated calcium channels (VACCs) as a negative feedback (- ve). *: AF2 potentiates the levamisole secondary responses by increasing voltage-activated calcium entry through VACCs [19] and; * by sensitizing the RyRs to release more calcium from the sarcoplasmic reticulum in response to the calcium entry through the nAChRs and VACCs.
The AF2 potentiated secondary response to levamisole, in APF-Ringer, was sometimes associated with a large conductance change which had an oscillating pattern suggestive of intracellular calcium waves. The reversal potential, ∼ -20 mV, was more depolarized than the predicted chloride reversal potential of -43 mV. We found that replacement of the chloride in the APF-Ringer with acetate inhibited the increase in conductance of the secondary depolarization, indicating the supporting role of the ca-activated anion channel [21]. The actual reversal potential of the calcium-activated anion channel is likely to be more positive than the chloride reversal potential because of the presence of significant amounts of intracellular carboxylic acids from anaerobic respiration [30] that permeates this anion channel [31]. A rise in cytosolic calcium will, in addition, initiate homeostatic mechanisms that then lead to reduction of the cytosolic calcium, returning it to control levels; these mechanisms are expected to include the high capacity, low affinity Na-Ca exchanger and the low capacity high affinity calcium-ATPase systems that remove cytosolic calcium [32].
4.2 Ryanodine receptors and effects of AF2
In the following discussion, we suggest that AF2 increases cAMP in muscle cytoplasm [33] and that the raised camp leads to sensitization of RyRs and to the increases in cytoplasmic calcium. Nematode RyRs, like those of other eukaryotes are homo-tetramer calcium release channels found in the membrane of sarcoplasmic reticulum and that bind the alkaloid ryanodine [34]. In C. elegans, a single gene, unc-68, encodes for RyRs.C. elegans RyRs are located near the muscle surface membrane in vesicles that resemble junctional sarcoplasmic reticulum of the vertebrate striated muscle [27] and the RyRs of C. elegans show 42% homology to mammalian RyRs [26]. RyR channels in C. elegans have two conductance states, a 215 pS state and a 78 pS state; ryanodine at a concentration of 4 μM locks the RyR channel in the 78 pS (subconductance state) preventing transition between the states [25]. The locking of the channel in the subconductance state can lead to emptying of the calcium stores in the SR so that the effect of application of ryanodine on C. elegans is to produce an incomplete hypercontraction and overall paralysis [25]. In our muscle strips of A. suum however, ryanodine did not produce hypercontraction but reduced the maximum force of levamisole-induced contractions. The lack of contraction may be explained if there is slow emptying of the SR in A. suum which could be accommodated by homeostatic mechanisms like the sodium-calcium exchanger [Robertson et al, this issue].
RyRs release calcium from sarcoplasmic reticulum in vertebrate smooth muscle. Triggers for this release include an increase in cytosolic calcium and other ligands like caffeine [35, 36]. In regular vertebrate skeletal muscle, RyRs release calcium in response to each action potential as a result of being coupled directly to the T-tubule system [37, 38]. In Ascaris suum however, we know from the work of Weisblat et al., [39] that contraction of body muscle is not coupled to each spike, rather contraction is coupled to slower depolarizations referred to as modulation waves. In C. elegans RyRs play an important, but non-essential role in excitation-contraction coupling: calcium entry through plasma membrane voltage-activated channels can initiate contraction in the absence of RyRs [27, 35]. Thus RyRs enhance contraction in nematode body muscle by amplifying the calcium signal initiated by opening of nAChRs and voltage-activated calcium channels in the plasma membrane [27]. AF2 has been shown to produce a long-lasting increase in muscle cytoplasmic cAMP in A. suum [33]. Activation of cAMP-dependent protein kinase-A can phosphorylate RyRs and reduce the effects of the RyR inhibitor proteins (FKBPs) [40]. By this mechanism, AF2 could increase the probability that RyRs open in response to cytoplasmic calcium [40] and in turn increase contraction in response to a nicotinic anthelmintic like levamisole.
4.3 Ryanodine effects on spiking
We have observed that ryanodine increased the frequency of spiking and in two preparations, where spikes were present before the application of ryanodine; ryanodine increased the gradient of the rising phase of the spikes. Spikes in A. suum are produced by inward calcium currents and not sodium currents [19, 39]. Ryanodine increased the gradient of the rising phase of the spike (dV/dt) which is taken to be proportional to the inward calcium current [20, 28] indicating that ryanodine increased the inward calcium current. It is of interest to consider what the mechanism for the spiking and increased calcium current could be. Voltage-activated calcium channels are subject to calcium-induced inactivation mediated by calmodulin [41, 42]. Since ryanodine inhibits the calcium-induced calcium release (we believe by emptying the calcium SR store), ryanodine could lead to the removal of inhibition of calcium channels by calmodulin. Other less likely mechanisms include ryanodine modulation of presynaptic transmitter release or inhibition of potassium channels in muscle. Modulation of presynaptic transmitter release does not seem likely because ryanodine (100 μM) has been shown to reduce excitatory neurotransmitter release in C. elegans [46]; this would be seen as an inhibitory effect not an excitatory effect. With regard to an effect on potassium channels, we found that ryanodine did not affect the resting membrane potential of A. suum muscle nor was it observed to have an effect on the duration of the A. suum spikes. A closing of potassium channels would be expected to depolarize the cell membrane, to decrease the rate of spike decay and increase spike duration; it did not. To our knowledge, there are no reports of direct effects of ryanodine on C. elegans potassium channels. However, we cannot rule out an indirect effect via changes in cytosolic calcium having effects on calcium-activated potassium channels [47]. Taken together, these observations suggest that ryanodine affects spiking via an action on calcium channels. Despite this increase in spiking, we have observed that ryanodine does not increase the force of muscle contraction [Robertson et al, this issue] demonstrating the physiological separation of spikes and contraction in A. suum muscle.
4.5 Nematode ryanodine receptors and levamisole resistance
We have seen that the force of levamisole induced contractions in A. suum is sensitive to ryanodine as is the secondary response of levamisole following AF2 treatment. The genetic basis of resistance to levamisole has been studied in C. elegans where it has been found that null-mutants of components of the levamisole-signaling excitation-contraction cascade cause resistance [43]. Components of the levamisole-signaling pathway include the subunits of the levamisole receptor: UNC-38, UNC-63, UNC-29, LEV-1 and LEV-8. They also include the RyRs encoded by the unc-68 gene. unc-68 C. elegans null-mutants show reduced sensitivity to levamisole [44]. It is likely that null-mutants of unc-68 of parasitic nematodes would also show reduced response to levamisole because the reduction in gmax [Robertson et al., this issue]. Mutations of RyRs in parasitic nematodes are predicted to reduce the response to treatment with nicotinic anthelmintics like levamisole and pyrantel and to be associated with resistance.
Acknowledgments
The project was supported by Grant Number R 01 AI 047194 from the national Institute of Allergy and Infectious Diseases to RJM and by an Iowa Center for Advanced Neurotoxicology grant to APR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trailovic SM, et al. Brief application of AF2 produces long lasting potentiation of nAChR responses in Ascaris suum. Mol Biochem Parasitol. 2005;139:51–64. doi: 10.1016/j.molbiopara.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–10. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Albonico M, et al. Development of the egg hatch assay for detection of anthelmintic resistance in human hookworms. Int J Parasitol. 2005;35:803–11. doi: 10.1016/j.ijpara.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Jackson F. Anthelmintic resistance-the state of play. Br Vet J. 1993;149:123–38. doi: 10.1016/S0007-1935(05)80083-1. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–81. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Jones PM, George AM. Multidrug resistance in parasites: ABC transporters, P-glycoproteins and molecular modelling. Int J Parasitol. 2005;35:555–66. doi: 10.1016/j.ijpara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Robertson SJ, Martin RJ. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br J Pharmacol. 1993;108:170–8. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale VM, Martin RJ. Oxantel-activated single channel currents in the muscle membrane of Ascaris suum. Parasitology. 1995;110:437–48. doi: 10.1017/s0031182000064775. [DOI] [PubMed] [Google Scholar]
- 9.Evans AM, Martin RJ. Activation and cooperative multi-ion block of single nicotinic-acetylcholine channel currents of Ascaris muscle by the tetrahydropyrimidine anthelmintic, morantel. Br J Pharmacol. 1996;118:1127–40. doi: 10.1111/j.1476-5381.1996.tb15515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson AP, Bjorn HE, Martin RJ. Pyrantel resistance alters nematode nicotinic acetylcholine receptor single-channel properties. Eur J Pharmacol. 2000;394:1–8. doi: 10.1016/s0014-2999(00)00135-7. [DOI] [PubMed] [Google Scholar]
- 11.Levandoski MM, et al. Single-channel properties of N- and L-subtypes of acetylcholine receptor in Ascaris suum. Int J Parasitol. 2005;35:925–34. doi: 10.1016/j.ijpara.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Martin RJ, et al. Oxantel is an N-type (methyridine and nicotine) agonist not an L-type (levamisole and pyrantel) agonist: classification of cholinergic anthelmintics in Ascaris. Int J Parasitol. 2004;34:1083–90. doi: 10.1016/j.ijpara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Hotez PJ, et al. Hookworm: “the great infection of mankind”. PLoS Med. 2005;2:e67. doi: 10.1371/journal.pmed.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006;20:2606–8. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- 15.Robertson AP, Bjorn HE, Martin RJ. Resistance to levamisole resolved at the single-channel level. FASEB J. 1999;13:749–60. doi: 10.1096/fasebj.13.6.749. [DOI] [PubMed] [Google Scholar]
- 16.Robertson SJ, et al. The action of pyrantel as an agonist and an open channel blocker at acetylcholine receptors in isolated Ascaris suum muscle vesicles. Eur J Pharmacol. 1994;271:273–82. doi: 10.1016/0014-2999(94)90784-6. [DOI] [PubMed] [Google Scholar]
- 17.Brading AF, Caldwell PC. The resting membrane potential of the somatic muscle cells of Ascaris lumbricoides. J Physiol. 1971;217:605–24. doi: 10.1113/jphysiol.1971.sp009588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin RJ, et al. Drug resistance and neurotransmitter receptors of nematodes: recent studies on the mode of action of levamisole. Parasitology. 2005;131:S71–84. doi: 10.1017/S0031182005008668. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Robertson AP, Martin RJ. The nematode neuropeptide, AF2 (KHEYLRF-NH2), increases voltage-activated calcium currents in Ascaris suum muscle. Br J Pharmacol. 2007;151:888–99. doi: 10.1038/sj.bjp.0707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin RJ, et al. Voltage-activated currents in somatic muscle of the nematode parasite Ascaris suum. J Exp Biol. 1992;173:75–90. doi: 10.1242/jeb.173.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Thorn P, Martin RJ. A high-conductance calcium-dependent chloride channel in Ascaris suum muscle. Q J Exp Physiol. 1987;72:31–49. doi: 10.1113/expphysiol.1987.sp003053. [DOI] [PubMed] [Google Scholar]
- 22.Aoki S, Ito K. Time- and use-dependent inhibition by ryanodine of caffeine-induced contraction of guinea-pig aortic smooth muscle. Biochem Biophys Res Commun. 1988;154:219–26. doi: 10.1016/0006-291x(88)90673-0. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, et al. Effects of ryanodine and 9,21-didehydroryanodine on caffeine-induced contraction of rat and guinea pig aortae. Jpn J Pharmacol. 1989;51:531–8. doi: 10.1254/jjp.51.531. [DOI] [PubMed] [Google Scholar]
- 24.Sitsapesan R, McGarry SJ, Williams AJ. Cyclic ADP-ribose, the ryanodine receptor and Ca2+ release. Trends Pharmacol Sci. 1995;16:386–91. doi: 10.1016/s0165-6147(00)89080-x. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, et al. High molecular weight proteins in the nematode C. elegans bind [3H]ryanodine and form a large conductance channel. Biophys J1992. 63:1379–84. doi: 10.1016/S0006-3495(92)81702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakube Y, Ando H, Kagawa H. Cloning and mapping of a ryanodine receptor homolog gene of Caenorhabditis elegans. Ann N Y Acad Sci. 1993;707:540–5. doi: 10.1111/j.1749-6632.1993.tb38121.x. [DOI] [PubMed] [Google Scholar]
- 27.Maryon EB, Coronado R, Anderson P. unc-68 encodes a ryanodine receptor involved in regulating C. elegans body-wall muscle contraction J Cell Biol. 1996;134:885–93. doi: 10.1083/jcb.134.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner RE. A model for an Ascaris muscle cell. Exp Physiol. 2001;86:551–9. doi: 10.1113/eph8602161. [DOI] [PubMed] [Google Scholar]
- 29.Kubiak TM, et al. AF2 interaction with Ascaris suum body wall muscle membranes involves G-protein activation. Biochem Biophys Res Commun. 2003;301:456–9. doi: 10.1016/s0006-291x(02)03054-1. [DOI] [PubMed] [Google Scholar]
- 30.Komuniecki R, Campbell T, Rubin N. Anaerobic metabolism in Ascaris suum: acyl CoA intermediates in isolated mitochondria synthesizing 2-methyl branched-chain fatty acids. Mol Biochem Parasitol. 1987;24:147–54. doi: 10.1016/0166-6851(87)90101-0. [DOI] [PubMed] [Google Scholar]
- 31.Valkanov MA, Martin RJ. A Cl channel in Ascaris suum selectivity conducts dicarboxylic anion product of glucose fermentation and suggests a role in removal of waste organic anions. J Membr Biol. 1995;148:41–9. doi: 10.1007/BF00234154. [DOI] [PubMed] [Google Scholar]
- 32.DiPolo R, Beauge L. The squid preparation as a general model for ionic and metabolic Na+/Ca2+ exchange interactions: physiopathological implications. Ann N Y Acad Sci. 2007;1099:135–51. doi: 10.1196/annals.1387.049. [DOI] [PubMed] [Google Scholar]
- 33.Reinitz CA, et al. Changes in locomotory behavior and cAMP produced in Ascaris suum by neuropeptides from Ascaris suum or Caenorhabditis elegans. Mol Biochem Parasitol. 2000;111(1):185–97. doi: 10.1016/s0166-6851(00)00317-0. [DOI] [PubMed] [Google Scholar]
- 34.Bennett DL, et al. Expression and function of ryanodine receptors in nonexcitable cells. J Biol Chem. 1996;271:6356–62. doi: 10.1074/jbc.271.11.6356. [DOI] [PubMed] [Google Scholar]
- 35.Kimball BC, Yule DI, Mulholland MW. Caffeine- and ryanodine-sensitive Ca2+ stores in cultured guinea pig myenteric neurons. Am J Physiol. 1996;270:594–603. doi: 10.1152/ajpgi.1996.270.4.G594. [DOI] [PubMed] [Google Scholar]
- 36.Fabiato A, Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res. 1977;40:119–29. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- 37.Rios E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991;71:849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- 38.Yano M, el-Hayek R, Ikemoto N. Conformational changes in the junctional foot protein/Ca2+ release channel mediate depolarization-induced Ca2+ release from sarcoplasmic reticulum. J Biol Chem. 1995;270:3017–21. doi: 10.1074/jbc.270.7.3017. [DOI] [PubMed] [Google Scholar]
- 39.Weisblat DA, Byerly L, Russell RL. Ionic mechanisms of electrical activity in somatic muscle of the nematode Ascaris lumbricoides. J Comp Physiol. 1976;111:93–113. [Google Scholar]
- 40.Petrovic MM, et al. Ryanodine receptors, voltage-gated calcium channels and their relationship with protein kinase A in the myocardium. Physiol Res. 2008;57:141–9. doi: 10.33549/physiolres.931171. [DOI] [PubMed] [Google Scholar]
- 41.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 42.Dunlap K. Calcium channels are models of self-control. J Gen Physiol. 2007;129:379–383. doi: 10.1085/jgp.200709786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dick IE, et al. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–4. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robertson AP, Martin RJ. Ion-channels on parasite muscle: pharmacology and physiology. Invert Neurosci. 2007;7:209–17. doi: 10.1007/s10158-007-0059-x. [DOI] [PubMed] [Google Scholar]
- 45.Sakube Y, Ando H, Kagawa H. An abnormal ketamine response in mutants defective in the ryanodine receptor gene ryr-1 (unc-68) of Caenorhabditis elegans. J Mol Biol. 1997;267:849–64. doi: 10.1006/jmbi.1997.0910. [DOI] [PubMed] [Google Scholar]
- 46.Liu Q, Chen B, Yankova M, Morest KD, Maryon E, Hand AR. Nonet ML, Wang ZW. Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabidits elegans neuromuscular junction. J Neurosci. 2005;25:6745–54. doi: 10.1523/JNEUROSCI.1730-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salkoff L, et al. Potassium channels in C. elegans. WormBook. 2005:1–15. doi: 10.1895/wormbook.1.42.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


