Abstract
The nature and frequency of speech production errors in neurodegenerative disease have not previously been precisely quantified. In the present study, 16 patients with a progressive form of nonfluent aphasia (PNFA) were asked to tell a story from a wordless children’s picture book. Errors in production were classified as either phonemic, involving language-based deformations that nevertheless result in possible sequences of English speech segments; or phonetic, involving a motor planning deficit and resulting in non-English speech segments. The distribution of cortical atrophy as revealed by structural MRI scans was examined quantitatively in a subset of PNFA patients (N=7). The few errors made by healthy seniors were only phonemic in type. PNFA patients made more than four times as many errors as controls. This included both phonemic and phonetic errors, with a preponderance of errors (82%) classified as phonemic. The majority of phonemic errors were substitutions that shared most distinctive features with the target phoneme. The systematic nature of these substitutions is not consistent with a motor planning deficit. Cortical atrophy was found in prefrontal regions bilaterally and peri-Sylvian regions of the left hemisphere. We conclude that the speech errors produced by PNFA patients are mainly errors at the phonemic level of language processing and are not caused by a motor planning impairment.
Keywords: Progressive non-fluent aphasia, phonology, speech errors
INTRODUCTION
Errors in the production of the segments comprising speech are made by all speakers of a language. For healthy speakers, this happens infrequently and often to humorous effect, evoking reference to the Reverend William Spooner (1844–1930). A more severe impairment of the production of speech sounds is found in pathological conditions. Much of the literature on speech production errors in subjects with an acquired language impairment has focused on patients who have suffered a cerebral vascular accident (Canter, Trost, & Burns, 1985; Dronkers, 1996; Peach & Tonkovich, 2004; Romani, Olson, Semenza, & Grana, 2002). In the last quarter-century, a language disorder resulting from neurodegenerative disease has been described. This is differentiated from the post-stroke syndrome by its progressive nature and so termed “primary progressive aphasia.” The term “progressive nonfluent aphasia” (PNFA) is now widely applied to a group of those patients whose speech is notably dysfluent (Gorno-Tempini et al., 2004; Grossman & Ash, 2004; Grossman, Mickanin et al., 1996; Hodges & Patterson, 1996; Knibb, Woollams, Hodges, & Patterson, 2009; Snowden, Neary, & Mann, 1996; Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997). This is often a variant of frontotemporal lobar degeneration in which one of the most salient features is effortful speech, with hesitations, retakes, pauses, and errors in the production of words. Patients with this syndrome also characteristically exhibit agrammatism and produce simplified grammatical forms (Ash et al., 2009). They are also impaired in the comprehension of complex syntax (Peelle et al., 2008). Executive resources are impaired as well, but comprehension of single words is relatively spared (Grossman & Ash, 2004; Grossman, Mickanin et al., 1996; Libon, Xie et al., 2007; Snowden et al., 1996).
Little attention has been devoted to the study of speech errors in neurodegenerative disease, even though detailed assessments of speech errors can be quite informative about the nature of the language system and its breakdown in conditions such as PNFA. Discussion of the errors in speech production made by patients with either brain lesions or neurodegenerative disease frequently assumes that the errors are caused by a motor planning impairment (Dronkers, 1996; Duffy, 2006; Gorno-Tempini et al., 2004; Josephs et al., 2006; Peach, 2004) known as apraxia of speech (AOS). The characteristics of AOS that are most often cited are groping towards the articulation of a word, distorted sounds, and dysprosody that often results in the production of a sound that is not part of the speaker’s native language (Croot, 2002; McNeil, Robin, & Schmidt, 1997; Wertz, LaPointe, & Rosenbeck, 1984). While the immediate cause of a speech error must logically be that the speech articulators are not placed in the right place at the right time in the production of a given speech sound or word, the precipitating cause of the production error could occur at a level of implementation upstream from the activation of the articulators (Canter et al., 1985; Romani et al., 2002). A distinction thus is made between AOS and phonemic paraphasias (Canter et al., 1985; Peach & Tonkovich, 2004). Phonemic paraphasic errors are described as phonemes subjected to processes of substitution, deletion, addition (insertion), or transposition (metathesis). These errors are said to involve processes that are more central than activation of the articulators because the speech sounds which are produced are relatively undistorted, actual speech sounds; substitutions and exchanges of speech segments are typically related to the original (intended) speech segments; and misplacement in a word suggests that the error occurs at the level of abstract lexical representation, rather than the surface level of articulation. The present investigation examines the nature of speech errors made by patients with PNFA. We sought to determine whether deficits in speech production in this neurodegenerative disease primarily arise from impairments in the language system or impairments of the motor system.
In the characterization of speech errors it is important to distinguish between two levels: the abstract level of the system of speech sounds in a language and the concrete level of the physical realization of speech sounds. The more abstract level is phonemics, the units of which are phonemes. The phoneme /t/, for example, sounds different depending on its position in a word, as illustrated in Figure 1A; but to the speaker, all the different variants of /t/ are in some sense the same, and the speaker may well not even realize that the /t/s in different positions sound different.
Figure 1.
Terminology
The physical features of the sound that is produced when a person speaks constitute a phone. The different variants of the abstract phoneme, realized as different phones, can be described in terms of their articulatory and acoustic properties, as is illustrated in Figure 1B. Phonetics is the study of speech sounds at this level.
A phonemic error occurs when a person produces a sound that is a well-formed phoneme of the language but not one that was intended by the speaker or anticipated by the listener, as in examples 1a and 1b:
1a) They have a smole ‘smile’ (smole rhymes with mole)
1b) … coming out of the gar ‘jar’ (gar rhymes with bar)
A phonetic error occurs when a speech sound is produced that results in a word which is not a possible sequence of sounds in the speech system of the speaker. This can result from a sound that does not occur in the speech system or from a combination of sounds that does not occur in the language. These productions could be due to an impairment of motor control, or, if produced consistently, they could be caused by an impairment of the mental representation of the phoneme. Examples 2a and 2b show the most frequent type of phonetic error, which results from the lenition of a consonant, that is, the softening of the articulatory force required to produce a well-formed phone. Since these sounds are not part of the inventory of English phonetics, there are no letters of the English alphabet to transcribe them; phonetic symbols borrowed from other alphabets are used. In both examples, the phonetic symbols represent voiced fricatives. The character Đ in (2a) is not a standard phonetic symbol, but it follows the convention of using barred symbols to represent fricatives (Pullum & Ladusaw, 1986). In this case, it transcribes a voiced alveolar slit fricative, in contrast to the voiced grooved fricative [z].
2a) kiĐ ‘kid’
2b) doγ ‘dog’
It is an empirical question whether the errors of PNFA patients are more often phonemic or phonetic. If the errors are predominantly phonemic, there might be an impairment of language that disturbs the retrieval of the correct phonemes, or there might be an impairment of the executive resources needed to retrieve the appropriate phonemes and assemble them into the correct sequence. If the errors occur at the level of phonetics, this may imply motor difficulty such as an impairment of motor planning. Groping may reflect feedback from an intact phonemic system attempting to guide the motor speech system to produce the correct phonetic segment. If the error occurs consistently, this could implicate either the mental representation of the phoneme itself or its manner of articulation.
In a study of single-word processing in two PNFA patients on tasks of naming, repetition, and reading, Croot et al. found better performance on tasks in which the stimulus provided information about the phonological output: both patients performed best on reading, less well on repetition, and least well on naming (Croot, Patterson, & Hodges, 1998). They propose that these patients have difficulty with phonological encoding, so when the task stimulus provides support for that encoding as—do printed or spoken stimuli—performance is improved. In contrast, a stimulus in the form of a picture of an object provides no clues to the phonological form of the target word, which results in the poorest performance among the tasks. These investigators did not distinguish between phonetic and phonemic errors in transcribing the subjects’ speech, but they address the question of whether the impairment occurs at the level of phonological encoding or at the level of articulation, involving the movements and coordination of the tongue, lips, velum, and vocal folds. They acknowledge that the errors they recorded could have been caused by “phonetic disintegration,” thus leaving open the question of whether the speech errors result from an impairment at the cognitive level or the motor level.
With the goal of illuminating the source of speech errors in PNFA, we undertook to classify speech errors as phonetic or phonemic and to characterize these errors in linguistic terms in order to understand the underlying process of deformation of the speech stream. Further, we examined the linguistic and non-linguistic correlates of speech errors as a means of assessing the neurological underpinnings of speech errors in PNFA.
METHODS
Subjects
We studied 16 mildly to moderately impaired PNFA patients diagnosed by an experienced neurologist (MG) in the cognitive neurology clinic of the Department of Neurology at the University of Pennsylvania according to a revision of published criteria (McKhann et al., 2001; The Lund and Manchester Group, 1994). These patients had the clinical diagnosis of frontotemporal dementia (FTD) (N=14) or corticobasal syndrome (CBS) (N=2) assigned prospectively. Criteria for PNFA included the presence of slowed, effortful speech with agrammatic errors and grammatical simplification in conversation as well as the production of segmental speech errors. These patients also demonstrated some difficulty understanding grammatically complex sentences. This pattern of difficulty was insidiously progressive in nature and was the predominant clinical feature for at least 2 years. We also assessed 10 healthy seniors. Exclusionary criteria included other causes of dementia, such as metabolic, endocrine, vascular, structural, nutritional, and infectious etiologies, and primary psychiatric disorders. The patients were mildly impaired according to the Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975). One-way ANOVAs indicated that the two subject groups were matched for age and education, although the PNFA patients had a significantly lower MMSE than controls. All subjects completed an informed consent procedure in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Pennsylvania. Demographic characteristics are summarized in Table 1.
Table 1.
Mean ± standard deviation demographic and clinical characteristics of PNFA patients and Controls
| PNFA | Controls | |
|---|---|---|
| M/F | 7/9 | 2/8 |
| Age | 69.9 ± 10.9 | 69.1 ± 4.8 |
| Education | 15.1 ± 2.8 | 16.7 ± 2.6 |
| Disease duration (years) | 3.4 ± 2.0 | -- |
| MMSE (max=30) | 24.5** ± 4.4 | 30.0 ± 0.0 |
| Executive functioning: | ||
| Animal fluency | 9.88** ± 5.02 | 25.33 ± 5.43 (6) |
| Reverse digit span | 3.13** ± 1.20 | 5.60 ± 1.34 (5) |
| Trails B time (sec) | 235** ± 82 (9) | 106 ± 44 (5) |
| Semantics: | ||
| Boston Naming Test (% correct) | 81.9** ± 15.7 | 100.0 ± 0.0 (5) |
| Pyramids and Pine Trees (max=52) | 46.8** ± 7.2 (12) | 51.4 ± .9 (5) |
| Comprehension: | ||
| Short sentence comprehension (max=12) | 9.8** ± 1.5 (9) | 12.0 ± 0.0 (5) |
| Complex sentence comprehension (max=48) | 34.2** ± 7.8 (11) | 46.0 ± 1.2 (5) |
p<.01
The patients underwent neuropsychological testing within an average of 115 (± 88) days of the date of recording to determine whether speech errors are related to neuropsychological measures of cognition. We calculated Spearman correlation coefficients of subjects’ phonetic and phonemic error rates with their performance on language measures and a range of neuropsychological tests. The tests evaluated three areas of cognitive functioning. These included, first, executive functioning: animal naming (Lezak, 1983), working memory as demonstrated by reverse digit span (Wechsler, 1987), and mental flexibility as demonstrated by time to complete the Trails B test (Reitan & Wolfson, 1985). We also tested semantic memory by means of the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983) and the Pyramids and Palm Trees test (Howard & Patterson, 1992); and we administered two tests of comprehension of syntax (Grossman, D’Esposito et al., 1996). Performance by PNFA patients and controls on neuropsychological tests is shown in Table 1. PNFA patients are impaired in executive functioning, on semantic measures, and on grammatical comprehension relative to controls.
Materials
The subjects were shown the wordless children’s picture book, Frog, Where Are You, by Mercer Mayer (1969). The book consists of 24 detailed black-and-white line drawings. All print on the cover and front matter pages was covered with heavy paper stock so that no written words were visible. An outline of the story is given elsewhere (Ash et al., 2006). The elicitation of speech by this means has the advantage of producing a narrative for which the intended content was known, so it was possible to quantify subjects’ performance relative to an externally established standard. The fact that the story was unfamiliar to the subjects prevented the subjects from having preconceived ideas about what was happening or would happen in the course of the story. Extracts from three PNFA subjects’ narratives are given in Appendix 1.
Narrative procedure
The subjects were asked to look through the book to familiarize themselves with the story, then to turn back to the beginning and go through the book again, telling the story as if they were “reading” it to a child. The narrations were recorded digitally and transcribed in detail as described elsewhere (Ash et al., 2006). Seventeen narrations were recorded on a Macintosh Powerbook G3 laptop computer using the Macintosh external microphone (part #590-0670) and the computer program SoundEdit 16, v. 2, with 16-bit recording at a sampling frequency of 44.1 kHz. Five were recorded on a Dell Inspiron 2200 PC using the signal processing software Praat (Boersma & Weenink, 1992–2005) with 16-bit recording at a sampling rate of 22.05 kHz, using a Radio Shack omnidirectional lavaliere electret condenser microphone. Four were recorded on a Marantz PMD 670 digital recorder with 16-bit recording at a sampling frequency of 32 kHz, using a Sennheiser MKE2 omnidirectional lavaliere condenser microphone.
The narratives were analyzed for phonetic and phonemic errors as defined above. All coding was done by a linguist (SA) with expertise in phonetic and grammatical analysis. They were also coded for linguistic features including editing breaks and hesitation markers. Editing breaks are interruptions in the production of a word, repetitions of whole words, or self-corrections when the speaker switches from saying a word or phrase in order to say something else, as in “The dog- the frog climbed out of the jar.” A portion of these editing breaks constitute efforts at producing a word by successive approximations, which might be a feature of AOS. Therefore we tabulated the occurrences of editing breaks that consisted of successive approximations of a word. Hesitation markers include such items as um, er, ah, uh. We coded for additional features of language production as a proportion per utterance: open class words; nouns, total verbs (inflected verbs, participles, and infinitives), complex structures, and percent of well-formed utterances. These terms are defined elsewhere (Ash et al., 2009).
Statistical considerations
Levene’s test of homogeneity of variances revealed that the measures of language production and performance on the neuropsychological tests did not meet the requirement of homogeneity of variances for parametric statistical tests. Therefore nonparametric tests were used to assess the differences between subject groups. Comparisons between subject groups were calculated by the Mann-Whitney U statistic, and correlations were calculated using Spearman’s rho.
Imaging methods
Structural MRI scans were available for a subset of PNFA patients (N=7) and a group of age-, education-, and gender-matched healthy seniors (N=26). MRI images were acquired on a Siemens Trio 3.0T MRI scanner, starting with a rapid sagittal T1-weighted image to determine patient position. Next, high resolution T1-weighted three-dimensional spoiled gradient echo images were acquired with repetition time = 1,620 msec, echo time = 3 msec, slice thickness 1.0 mm, flip angle 15°, matrix = 192 × 256, and in-plane resolution 0.9 × 0.9 mm. To quantify grey matter patterns within participants we used a diffeomorphic registration-based cortical thickness (DiReCT) algorithm reported elsewhere (Das, Avants, Grossman, & Gee, 2009). This approach benefits from the use of a prior constrained estimate of the distance between grey matter and white matter which takes into account the contour of the cortical mantle. This approach also benefits from the use of a diffeomorphic procedure which preserves shape-based constraints. The DiReCT algorithm first involves skull-stripping (Smith, 2002) and segmentation using FMRIB’s Automated Segmentation Tool (FAST) (Zhang, Brady, & Smith, 2001), which labels the brain volumes into grey matter, white matter, CSF, and “other,” with inhomogeneity correction. The grey-white matter interface (GWI) is then calculated, it is defined as voxels in which a grey matter voxel and one neighboring voxel have a grey matter probability greater than p<.05, and a neighborhood-connected white matter voxel has a white matter probability of p<.05. We then calculate from the GWI those grey matter voxels which closely estimate an edge of grey matter cortex and use a novel diffeomorphic mapping between the GWI and an optimized template to determine the resulting measure of cortical thickness. The local template was composed of images from 25 healthy seniors and 25 focal neurodegenerative disease patients, collected using the same imaging sequence and scanner. Lastly, the grey matter cortical thickness images are warped into MNI template space and smoothed with a 6 mm FWHM Gaussian filter. Statistical comparisons of significant reduction in whole-brain grey matter cortical thickness were computed using an independent sample t-test in SPM5. We used an explicit mask of grey matter priors to limit comparisons to include only voxels which contained grey matter values greater than zero, and global calculation was omitted. We set a statistical height threshold of p<.0005 and only accepted clusters with an extent of 100 adjacent voxels because a cluster of this size would also demonstrate a consistent effect in a particular neuroanatomical distribution while minimizing false positives (S. D. Forman et al., 1995). We only report significant reduction in GM thickness for clusters where a peak voxel survived a statistical threshold of p< .001.
RESULTS
Characteristics of the overall speech output of the subjects are shown in Table 2. The PNFA patients took almost twice as long on average to tell the story compared to control subjects (p<.05). In that time, they produced only about two-thirds as many words as controls, so their overall speech rate in words per minute was one-third that of controls (p<.01).
Table 2.
Mean ± standard deviation of performance on measures of speech production in PNFA patients (N=16) and controls (N=10)1
| PNFA | Controls | |
|---|---|---|
| Speech Output | ||
| Time (sec) | 486 ± 290 | 255 ± 76 |
| Words | 346 ± 218 | 586 ± 110 |
| Words per minute | 45 ± 18 | 143 ± 24 |
| Mean length of utterance (words) | 7.4 ± 2.1 | 10.3 ± 1.8 |
| Editing breaks and hesitation markers (per 100 words) | 12.8 ± 8.2 | 2.0 ± 2.0 |
| Content, structure, grammar | ||
| Open class words per utterance | 2.84 ± .80 | 4.54 ± .71 |
| Nouns per utterance | 1.48 ± .54 | 2.08 ± .41 |
| Total verbs per utterance | 1.12 ± .35 | 1.70 ± .22 |
| Complex structures per utterance | .14 ± .10 | .36 ± .11 |
| Proportion of well-formed utterances | .62 ± .25 | .94 ± .04 |
Note: All between-group comparisons are significant at p<.01 except Time, which is significant at p<.05.
Measures of content, structure, and grammatical competence are also provided in Table 2. PNFA patients exhibited reduced production of open class words, nouns, and total verbs per utterance compared to controls (all contrasts significant at the p<.01 level). The simplification of grammar that is frequently noted in these patients is reflected in their significantly reduced mean length of utterance, reduced rate of complex structures, and reduced frequency of grammatically correct (well-formed) sentences (all contrasts significant at the p<.01 level).
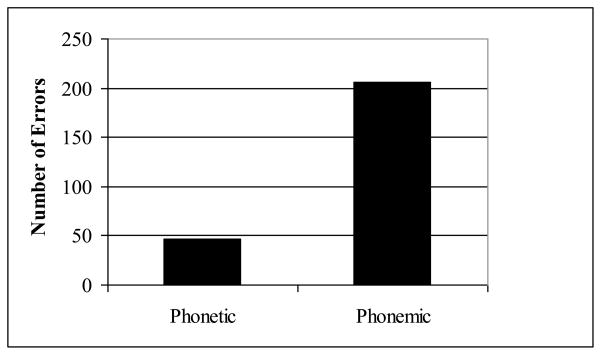
Table 3 presents the frequencies of phonetic and phonemic errors by patients and controls. PNFA patients produced significantly more phonemic errors than controls (p<.01). PNFA patients differed significantly in their production of phonemic vs. phonetic errors (p<.005), producing in total over four times as many phonemic errors as phonetic errors. The preponderance of phonemic errors is illustrated in Figure 2. Only 5 of the 16 patients made phonetic errors, and the majority were produced by one patient (MF) who was extremely impaired. His clinical diagnosis was FTD with onset 4 years prior to testing. His speech consisted mostly of nouns, with little sentence structure. For the other 4 patients who made phonetic errors, the frequency per 100 words was 1.16 ± 1.00, while including the case of MF raised the mean frequency of phonetic errors per 100 words to 7.96 ± 15.23. Thirteen of the 16 patients made phonemic errors. For those patients alone, excluding the three who made no errors, the mean frequency of phonemic errors per 100 words was 6.63 ± 9.99, and excluding MF lowered the rate to 3.97 ± 2.99.
Table 3.
Mean ± standard deviation of speech errors per 100 words by PNFA patients and controls
| PNFA | Controls | |
|---|---|---|
| Phonetic errors | 2.7 ± 9.0 | 0.0 ± 0.0 |
| Phonemic errors | 6.6 ** ± 10.5 | .02 ± .05 |
p<.01
Figure 2.
Phonetic and phonemic errors in PNFA
Most of the patients’ speech was decipherable from either the articulation or the context, but some words were so distorted that it was not possible to tell what the person was trying to say. This was the case for 81 (24%) of the total of 333 errors. Of the 252 interpretable errors, 82% were phonemic: the speaker produced a well-formed speech sound, but it was the wrong sound for that place in that word. In the analyses below, we consider only the errors in words that were interpretable.
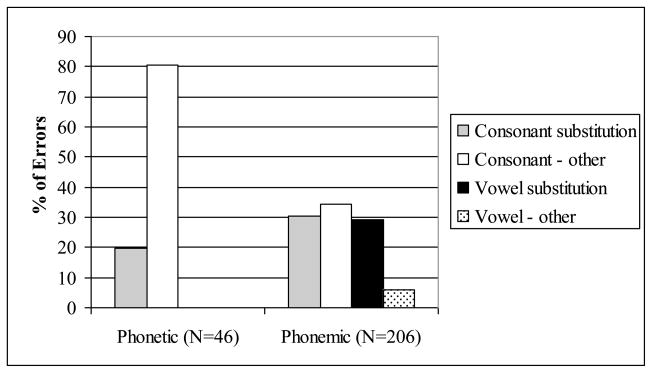
A summary of the distribution of error types is displayed in Figure 3, and some additional detail is provided in Appendix 2. Both consonants and vowels were subject to errors of substitution as well as other processes, including insertion, deletion, lenition (reduction of articulatory force), and metathesis (transposition). The majority of the phonetic errors were the result of non-substitution processes, particularly lenition, as shown in Examples 2a and 2b. In comparison, the majority of the phonemic errors were substitutions. Substitutions in phonemic errors involved the replacement of a segment by one that was a near neighbor in terms of articulation, such as /g/ replacing /k/ or /l/ and /r/ replacing each other. Substitutions were found to be bidirectional. For example, /l/ as a replacement for /r/ occurred, and /r/ as a replacement for /l/ also occurred. The same was found for /m/ and /n/, for nasals and stops, and so on. This implies that these errors are not due to unguided or poorly controlled motor movements, but are made within the phonological system of the speaker. They are not driven by unidirectional processes such as lenition or retraction. Rather, they constitute an incorrect choice of a target phoneme. Most often, the replacement of one segment by another preserves most of the features of articulation of the intended phoneme. Of the 63 consonant substitutions, 41 (65%) involved a substitution of only one feature; of 60 vowel substitutions, 52 (87%) were substitution by a vowel that was adjacent in articulatory space (that is, differing by only one distinctive feature), for an overall rate of substitution by near neighbors of 76%. Substitution by a phoneme that is not a near neighbor of the intended target or that differs by multiple distinctive features could actually be taken as even stronger evidence that the deformation is not caused by motor difficulty. If, for instance, a voiced velar stop /g/ replaces an alveolar affricate /d3/, as in gar for jar in example 1b, it is evident that the speaker had abundant opportunity to produce a segment that was not well formed, with all the articulatory distance intervening between the target and the realization. The production of a well-formed phoneme despite this articulatory distance suggests that the speaker’s conception of the target was incorrect, rather than that the speaker’s motor control of the articulators was faulty.
Figure 3.
Error types in PNFA
The tabulation of editing breaks involved in successive approximations of a word showed that groping for a word accounted for a low proportion of these disruptions in the flow of speech. For 15 of the 16 patients, the rate of editing breaks that constituted groping for a word was 17%, with an average frequency of 4.1 occurrences out of an average of 23.5 editing breaks per patient. Of these instances of groping for a word, the majority (69%) involved phonemic errors. Phonetic errors accounted for only 11%, and 16% were simple false starts. The 16th patient was an exception, for whom 76 of 147 (52%) editing breaks consisted of groping for words. For this patient too, far more of the segmental speech errors in groping for words were phonemic (22%) than phonetic (3%), but most were false starts (75%). Thus most editing breaks by PNFA patients were the same kinds of false starts and changes of intent that are exhibited by healthy speakers.
Correlations of phonetic and phonemic speech errors with fluency, structural, and content measures of language use are given in Table 4. Phonemic errors occur more when editing breaks and hesitation markers interrupt the flow of speech, although there is no association with speech rate. Both phonetic and phonemic errors occur less in utterances that are grammatically well-formed, but phonetic errors do not correlate significantly with any other aspects of speech production. We also considered a neuropsychological account of speech errors. The correlation analysis revealed no significant correlation of phonetic or phonemic errors with executive functioning, semantic memory, or comprehension of syntax.
Table 4.
Correlations of speech errors with fluency, language structure, and content in PNFA (N=16)
| Phonetic errors | Phonemic errors | |
|---|---|---|
| Words per minute | −.33 | −.27 |
| Editing breaks & hesitation markers (per 100 words) | .46 | .74** |
| Open class words per utterance | −.01 | −.19 |
| Nouns per utterance | −.04 | −.29 |
| Total verbs per utterance | −.24 | −.18 |
| Complex structures | .01 | −.01 |
| % Well-formed utterances | −.54* | −.72** |
p<.05
p<.01
Figure 4A displays the cortical atrophy image for the 7 PNFA patients for whom structural MRI scans were available; the Talairach coordinates of the areas of significant atrophy are given in Table 5. The image shows cortical atrophy for PNFA patients bilaterally in frontal regions and in the left peri-Sylvian region. Atrophy in parietal regions may reflect underlying corticobasal syndrome in some of the patients. The area of atrophy is diminished when patients with a diagnosis of CBD are removed from the analysis. Figure 4B shows a sagittal section through the left insula illustrating significant atrophy in a prefrontal region but no significant atrophy in the insula.
Figure 4. Significant atrophy in PNFA: Cortical thickness (N=7).
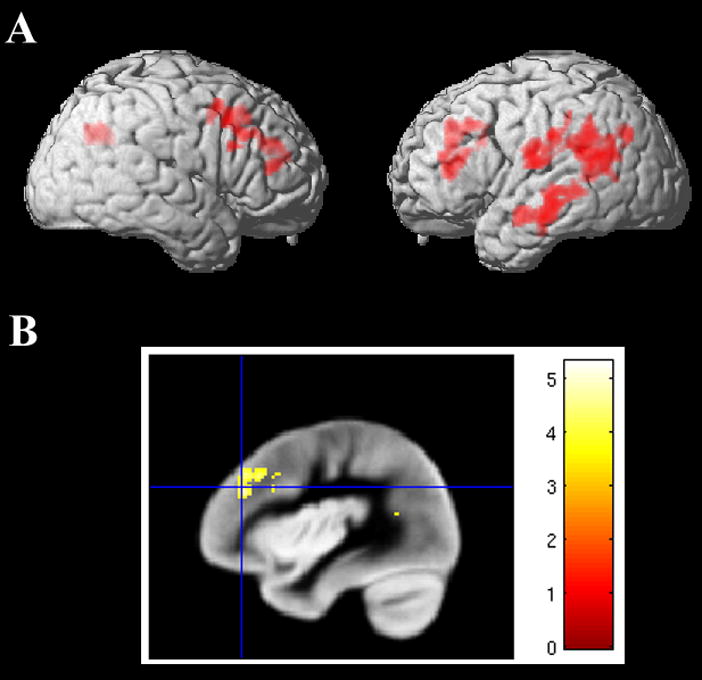
A. Lateral views
B. Sagittal section (x = −38) showing significant atrophy in left prefrontal cortex but not the insula.
Table 5.
Regional distribution of significant anatomic atrophy in PNFA (N=7)
| Anatomic locus (Brodmann area) | Coordinates | Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left inferior prefrontal (46) | −44 | 35 | 7 | 4.20 | 338 |
| Left postcentral (43) | −65 | −13 | 17 | 4.38 | 270 |
| Left middle temporal (21) | −53 | −16 | −11 | 4.23 | 552 |
| Left inferior parietal (40) | −55 | −49 | 23 | 4.15 | 950 |
| Right dorsolateral prefrontal (9) | 44 | 18 | 40 | 4.45 | 541 |
| Right temporal-parietal (19/39) | 34 | −62 | 36 | 4.15 | 248 |
DISCUSSION
We studied the production of speech errors in semi-structured speech samples of a cohort of PNFA patients in order to describe the characteristics of these errors and to determine their source. We found that speech errors in PNFA can be phonetic or phonemic, with phonemic errors accounting for more than 80% of errors. These errors are most often substitutions, and they are most often close approximations of the target phoneme, affecting both vowels and consonants. They do not appear to be subject to a general process such as simplification, since substitutions occur bidirectionally; that is, if x is replaced in some cases by y, then it is also found that y is replaced by x in other cases. The errors are variants of the intended targets, but they are constrained by the system of phonemes of the language, with its particular set of distinctive features and inventory of salient contrasts. These findings imply that phonemic errors occur within the language faculty more than within the motor-speech planning apparatus.
It has been pointed out that motor difficulty can result in production of a segment that sounds like a well-formed phoneme. For example, a timing error in the onset of voicing, as a consequence of the motor programming of vocal fold vibration, could produce a well-formed segment differing from the target only in voicing. We acknowledge that such an error could occur. If errors of motor programming were the main source of speech errors, however, then we would expect to hear many more phonetic errors than phonemic errors. It would be unlikely that most of the errors of articulation would produce well-formed segments; poorly controlled articulatory gestures would be expected to produce segments with random phonetic errors most of the time. In fact, we find that the large majority (more than 80%) of errors produce well-formed phonemes and fewer than 20% produce sounds that are not compatible with American English phonology. Furthermore, these phonetic errors were produced by only 5 of the 16 patients, and 32 of the 46 phonetic errors (70%) were produced by one subject. His speech was extremely impaired in other respects: in almost 8 minutes (477 seconds), he produced only 91 interpretable words, 66 of which were nouns; he used necessary determiners in only 4 cases out of 60 (7%); and in his 48 utterances he produced only 6 verbs. Most often, such patients would be regarded as untestable. In a sense, it may not be surprising that a patient whose speech has deteriorated to this extent would suffer in part from an impairment of motor planning or programming. However, there is another possibility. In light of the evidence of the earlier stages in the progression of this syndrome as represented by the other patients, it appears that this patient’s ability to select the features that compose phonemes has deteriorated, in conjunction with the profound breakdown in his ability to represent, identify, and/or select the correct phoneme. Further study is needed to explore this possibility.
We have also found that speech errors do not correlate with features of language structure such as speech rate, frequency of content words, or complexity of grammatical structure. This implies that the phonemic system is dissociable from other components of the language system. The production of speech sounds and the errors produced in PNFA do not appear to be governed by resources related to organization, attention, and other such capacities that are routinely considered in neuropsychological evaluation. Furthermore, the evidence does not point to a motor planning impairment as the cause of the errors. We conclude that phonemic errors in PNFA appear to come from within the neural network for language that supports the system of speech sounds.
Several previous studies have examined speech errors in patients with an aphasia consequent to stroke (Ackermann & Riecker, 2004; Dronkers, 1996; Mumby, Bowen, & Hesketh, 2007; Peach & Tonkovich, 2004; Riecker et al., 2004). Apraxia of speech has been well documented in stroke patients (Dronkers, 1996), and other authors have explicitly compared errors of speech production in patients with fluent vs. nonfluent aphasia due to stroke (Canter et al., 1985; Romani et al., 2002). These studies have generally associated AOS with a nonfluent aphasia, also known as Broca’s aphasia. Canter et al. (1985) suggest that phonemic paraphasias in conduction and Wernicke’s aphasics result from a breakdown of the retrieval of phonological word patterns, while the AOS of Broca’s aphasics follows from an impairment of the encoding of phonological patterns into speech movements.
It has perhaps seemed reasonable to characterize speech errors in PNFA in the same way as in Broca’s aphasia and therefore to attribute them to the same source, i.e., a motor planning impairment. Although PNFA is a non-fluent form of progressive aphasia that partly resembles Broca’s aphasia, there are also important differences between PNFA and Broca’s aphasia following a stroke (Patterson, Graham, Lambon Ralph, & Hodges, 2006). For example, Broca’s aphasia typically is associated with frank motor weakness in the face and right upper extremity, implicating direct involvement of motor regions of the brain, while PNFA is rarely associated with weakness. Limb apraxia and oral apraxia also may be seen in Broca’s aphasia but appear to be much less common in PNFA (Libon, Massimo et al., 2007). We also find significant executive resource limitations in PNFA that may not be present in Broca’s aphasia, and this may have an impact on the nature of speech and language processing in PNFA (Grossman et al., 2008; Libon et al., 2009; Libon, Xie et al., 2007). Thus, the presence of AOS in Broca’s aphasia does not necessarily mean that AOS should also be present in PNFA. One PNFA patient in our series nevertheless had prominent AOS without the lateralized motor features of a Broca’s aphasia. We suspect that other PNFA patients may also have prominent AOS, but it is uncommon in our series. Our study thus adds weight to the view that PNFA does not replicate the deficit seen in Broca’s aphasia following stroke.
There have also been reports on the occurrence of phonemic paraphasias in primary progressive aphasia as contrasted with stroke (Patterson et al., 2006) or other syndromes (Weintraub, Rubin, & Mesulam, 1990). Patterson et al. (2006) concluded that PNFA patients are superior to non-fluent aphasic stroke patients in phonological processing but are more impaired at producing self-generated speech, consistent with our observation of a high rate of phonemic paraphasias seen in PNFA patients’ self-generated speech. Weintraub (1990) compared primary progressive aphasia (PPA) to probable Alzheimer’s disease (PRAD) in a longitudinal study of four patients and found that the high of rate of phonemic paraphasias in PPA was one of the features that most clearly distinguished between the two conditions. Semantic substitutions and circumlocutions were found to be prominent in PRAD but not in PPA. Another study examined errors in spontaneous speech production in patients with the neurodegenerative condition of semantic dementia (SD), characterized by a selective deterioration of semantic knowledge (Meteyard & Patterson, 2009). These authors found that SD patients produced grammatical abnormalities as well as semantic errors, but phonological errors were extremely rare.
In contrast to these studies, other authors do not ascribe the speech errors in PNFA to phonemic paraphasia. Josephs, Duffy, et al. (2006) claim that phonemic paraphasias do not occur in PNFA but state that the term is “probably used to refer to phonetic (i.e. motor) rather than phonemic (i.e. linguistic) distortions” (p. 1386). Gorno-Tempini et al. (2004) suggest that “labored speech,” agrammatism, and impaired production and comprehension of syntax are the defining characteristics of PNFA. However, the definition of AOS is not clearly specified in these studies, and the authors did not distinguish between AOS and phonemic paraphasia. Phonetic errors in our patients were relatively uncommon. This raises the question of an ascertainment bias in the differences between our series and patients reported from other centers, where PNFA patients may have had more pronounced motor impairments associated with movement disorders such as CBS or PSP. Also, these patients may have been evaluated later in the course of their disease (Josephs et al., 2006). Longitudinal studies of patients with well-characterized motor and non-motor disorders would be useful in the future to help resolve these differences.
Another potential source of discrepancy between our reports and those of other authors may be an inconsistency in terminology. Most authors appear to follow the descriptions which define AOS as an impairment of speech production characterized by effortful groping for articulatory gestures, difficulty with the initiation of utterances, distortions of phonemes, impaired transitions between segments, and abnormalities of speech rate, stress assignment, and dysprosody unrelieved by extended stretches of normal rhythm, stress, and prosodic contours (Duffy, 1995; McNeil, Doyle, & Wambaugh, 2000; McNeil et al., 1997; Ogar, Dronkers, Brambati, Miller, & Gorno-Tempini, 2007; Wertz et al., 1984). These features are attributed to a motor impairment. The criterion of “distortions” of speech segments appears to apply to the phonetic errors we observe in PNFA, and thus these errors may also be due to a motor impairment. Likewise, some of the editing breaks we observed appear to correspond to the “groping” characteristic of AOS. Certainly these types of speech errors occur in PNFA, and we have documented this in one patient in particular. However, phonetic errors are far less frequent than phonemic errors in our series, and a detailed analysis of groping indicates that phonetic errors occur no more commonly in groping sequences than in the remainder of the speech sample. Characteristics of AOS thus appear to occur in PNFA speech, but they are not common.
We found little association of speech errors with measures of fluency, content, and syntax in PNFA (Table 4). The correlation of phonemic errors with editing breaks and hesitation markers is unsurprising, as both features stem from difficulty in producing smoothly flowing speech. The correlation of phonetic and phonemic errors with the production of well-formed utterances, among higher-level language measures, seems to reflect the same effect of fluency as editing breaks and hesitation markers: the frequency of these errors of dysfluency is closely correlated with the frequency of well-formed utterances (rho=.78, p<.001). We found no relationship between the features of segmental speech production and measures of executive functioning, semantics, and grammatical processing, suggesting that errors in the production of speech segments come from within another component of the language system. Canter, et al. proposes that phonemic paraphasias result from a deficit in retrieval of the phonological pattern. This is an inviting avenue for further investigation in PNFA.
Our imaging findings showed that these PNFA patients have significant atrophy affecting the frontal lobe bilaterally. This is expected in PNFA, based on prior work assessing the distribution of cortical atrophy in these patients (Gorno-Tempini et al., 2004; Grossman & Ash, 2004). The PNFA patients also have some atrophy in a left peri-Sylvian distribution. This may reflect corticobasal syndrome seen in some of our PNFA patients (Gorno-Tempini et al., 2004; Murray, Koenig, Antani, McCawley, & Grossman, 2007).
Some authors have associated left insula disease with AOS in stroke or PNFA. However, reports of insular atrophy in stroke (Dronkers, 1996) may have limited bearing on studies of PNFA, as suggested by Hillis, et al. (2004). An imaging study of PNFA (Nestor et al., 2003) found limited left peri-Sylvian atrophy on MRI but significant hypoperfusion of the left insula on PET. These findings are inconsistent with a number of studies that have demonstrated frontal atrophy in PNFA (Gorno-Tempini et al., 2004; Grossman & Ash, 2004; Grossman et al., 2008). CBS is also associated with AOS, albeit less consistently. Additional work is needed to determine whether clinical conditions such as these have an impact on the frequency and nature of speech errors and the anatomic distribution of disease associated with these errors. Further, it appears that up to 30% of PNFA patients have Alzheimer’s disease (AD) as the underlying pathology (Hodges, Davies, Xuereb, Kril, & Halliday, 2003; Knibb, Xuereb, Patterson, & Hodges, 2006). This is also true to some extent in our autopsy series (M. S. Forman et al., 2006; Grossman et al., 2008). AD is associated with peri-Sylvian disease that may have a more posterior anatomic distribution. Additional work is needed with imaging studies in a larger group of patients so that regression analyses can be used to relate speech errors directly to cortical atrophy.
We conclude that errors of word formation at the phonemic level are frequent, and they are a prominent feature of PNFA. We find that speech errors in PNFA are partially systematic in that they most often involve replacement of one phoneme by a phoneme that is similar in articulation. This and other characteristics suggest that speech errors are guided in large part by the linguistic properties of the phonologic system. In contrast to phonemic errors, phonetic errors in PNFA are relatively infrequent. We hypothesize that these errors are more likely to be caused by a motor planning impairment such as AOS.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (AG17586, AG15116, NS44266, and NS53488).
Footnotes
Portions of this report were presented at the American Academy of Neurology, Seattle, 2009
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89(2):320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, et al. Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics. 2009;22:370–383. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat. 4.3.27. Institute of Phonetic Sciences, University of Amsterdam; 1992–2005. [Google Scholar]
- Canter GJ, Trost JE, Burns MS. Contrasting speech patterns in apraxia of speech and phonemic paraphasia. Brain Lang. 1985;24(2):204–222. doi: 10.1016/0093-934x(85)90131-2. [DOI] [PubMed] [Google Scholar]
- Croot K. Diagnosis of AOS: definition and criteria. Semin Speech Lang. 2002;23(4):267–280. doi: 10.1055/s-2002-35800. [DOI] [PubMed] [Google Scholar]
- Croot K, Patterson K, Hodges JR. Single word production in Nonfluent Progressive Aphasia. Brain and Language. 1998;61:226–273. doi: 10.1006/brln.1997.1852. [DOI] [PubMed] [Google Scholar]
- Das SR, Avants BB, Grossman M, Gee JC. Registration based cortical thickness measurement. Neuroimage. 2009;45(3):867–879. doi: 10.1016/j.neuroimage.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. St. Louis: Mosby; 1995. [Google Scholar]
- Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20(6):511–527. [Google Scholar]
- Folstein MF, Folstein SF, McHugh PR. “Mini Mental State.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: Clinicopathological correlations. Annals of Neurology. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary progressive aphasia: A review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grossman M, D’Esposito M, Hughes E, Onishi K, Biassou N, White-Devine T, et al. Language comprehension difficulty in Alzheimer’s disease, vascular dementia, and fronto-temporal degeneration. Neurology. 1996;47:183–189. doi: 10.1212/wnl.47.1.183. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding XS, et al. Progressive nonfluent aphasia: Language, cognitive and PET measures contrasted with probable Alzheimer’s disease. Journal of Cognitive Neuroscience. 1996;8(2):135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Grossman M, Xie SX, Libon DJ, Wang X, Massimo L, Moore P, et al. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology. 2008;70(22):2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies R, Xuereb J, Kril JJ, Halliday GM. Survival in frontotemporal dementia. Neurology. 2003;61:349–354. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: A comparative neuropsychological study. Journal of the International Neuropsychological Society. 1996;2:511–524. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm Trees: A Test of Semantic Access from Pictures and Words. Nature Publishing Group; 1992. [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Knibb JA, Woollams AM, Hodges JR, Patterson K. Making sense of progressive non-fluent aphasia: an analysis of conversational speech. Brain. 2009 doi: 10.1093/brain/awp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59(1):156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. Oxford: Oxford University Press; 1983. [Google Scholar]
- Libon DJ, Massimo L, Moore P, Coslett HB, Chatterjee A, Aguirre GK, et al. Screening for frontotemporal dementias and Alzheimer’s disease with the Philadelphia Brief Assessment of Cognition: a preliminary analysis. Dementia & Geriatric Cognitive Disorders. 2007;24(6):441–447. doi: 10.1159/000110577. [DOI] [PubMed] [Google Scholar]
- Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, et al. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology. 2009;73(7):535–542. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Mayer M. Frog, Where Are You? New York: Penguin Books; 1969. [Google Scholar]
- McKhann G, Trojanowski JQ, Grossman M, Miller BL, Dickson D, Albert M. Clinical and pathological diagnosis of frontotemporal dementia: Report of a work group on frontotemporal dementia and Pick’s disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Doyle PJ, Wambaugh J. Apraxia of speech: A treatable disorder of motor planning and programming. In: Nadeau SE, Gonazales Rothi LJ, Crosson B, editors. Aphasia and language: Theory to practice. New York: Guilford Press; 2000. pp. 221–266. [Google Scholar]
- McNeil MR, Robin DA, Schmidt RA. Apraxia of speech: Definition, differentiation, and treatment. In: McNeil MR, editor. Clinical management of sensorimotor speech disorders. New York: Thieme; 1997. pp. 311–344. [Google Scholar]
- Meteyard L, Patterson K. The relation between content and structure in language production: an analysis of speech errors in semantic dementia. Brain Lang. 2009;110(3):121–134. doi: 10.1016/j.bandl.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Mumby K, Bowen A, Hesketh A. Apraxia of speech: how reliable are speech and language therapists’ diagnoses? Clin Rehabil. 2007;21(8):760–767. doi: 10.1177/0269215507077285. [DOI] [PubMed] [Google Scholar]
- Murray R, Koenig P, Antani S, McCawley G, Grossman M. Lexical acquisition in progressive aphasia and frontotemporal dementia. Cogn Neuropsychol. 2007;24(1):48–69. doi: 10.1080/02643290600890657. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21(4):S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Patterson K, Graham NL, Lambon Ralph MA, Hodges JR. Progressive non-fluent aphasia is not a progressive form of non-fluent (post-stroke) aphasia. Aphasiology. 2006;20(9):1018–1034. [Google Scholar]
- Peach RK. Acquired apraxia of speech: features, accounts, and treatment. Top Stroke Rehabil. 2004;11(1):49–58. doi: 10.1310/ATNK-DBE8-EHUQ-AA64. [DOI] [PubMed] [Google Scholar]
- Peach RK, Tonkovich JD. Phonemic characteristics of apraxia of speech resulting from subcortical hemorrhage. J Commun Disord. 2004;37(1):77–90. doi: 10.1016/j.jcomdis.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L, et al. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics. 2008;21(5):418–432. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullum GK, Ladusaw WA. Phonetic Symbol Guide. Chicago: University of Chicago Press; 1986. [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Riecker A, Gerloff C, Wildgruber D, Nagele T, Grodd W, Dichgans J, et al. Transient crossed aphasia during focal right-hemisphere seizure. Neurology. 2004;63(10):1932. doi: 10.1212/01.wnl.0000140690.72955.1b. [DOI] [PubMed] [Google Scholar]
- Romani C, Olson A, Semenza C, Grana A. Patterns of phonological errors as a function of a phonological versus an articulatory locus of impairment. Cortex. 2002;38(4):541–567. doi: 10.1016/s0010-9452(08)70022-4. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Neary D, Mann DM. Fronto-temporal Lobar Degeneration: Fronto-temporal Dementia, Progressive Aphasia, Semantic Dementia. New York: Churchill Livingstone; 1996. [Google Scholar]
- The Lund and Manchester Group, A. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–331. [Google Scholar]
- Wechsler D. Wechsler memory scale - revised. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia: Longitudinal course, neuropsychological profile, and language features. Archives of Neurology. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbeck JC. Apraxia of speech: The disorders and its management. New York: Grune and Stratton; 1984. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.