Abstract
Infantile neuronal ceroid lipofuscinosis (INCL, also known as Haltia-Santavuori disease) is a lysosomal storage disorder of infants and children characterized by blindness, seizures and a progressive neurodegenerative course. Recent clinical trials have involved neural stem cells and gene therapy directed to the central nervous system; however, enzyme replacement therapy has never been addressed. In the current paper, we describe the production of human recombinant PPT1 (the defective enzyme in INCL) by standard methods in Chinese Hamster Ovary (CHO) cells. The enzyme is largely mannose 6-phosphorylated as assessed by mannose 6-phosphate receptor binding (80% bound) and taken up rapidly by immortalized patient lymphoblasts, where clearance of PPT substrates was demonstrated (EC50 of 0.25 nM after overnight incubation). When injected intravenously into PPT1-deficient mice, the clearance of recombinant human PPT1 from plasma was rapid, with a half-life of 10 min. Most of the injected dose was distributed to the kidney and liver and potentially corrective levels were also observed in heart, lung and spleen. Brain uptake was minimal, as expected based on experience with other intravenously administered lysosomal enzymes. The enzyme may be useful as an adjunct to central nervous system-directed therapies and could be used as a starting point for modifications designed to improve brain delivery.
Keywords: enzyme replacement therapy, lysosomal storage disorder, Batten disease, infantile neuronal ceroid lipofuscinosis
1. INTRODUCTION
Deficiency of the lysosomal enzyme palmitoyl protein thioesterase-1 (PPT1; EC 3.1.2.22) causes a human lysosomal storage disorder, designated ceroid lipofuscinosis, neuronal-1 (CLN1) characterized by progressive blindness, cognitive and motor deterioration, psychiatric disturbances, and seizures, leading to a chronic vegetative state [1]. The onset of the disease varies from infancy to adulthood [2–4]. The pathology is notable for widespread yellow-brown pigment deposition (lipofuscin) with the ultrastructural appearance of granular osmiophilic deposits (GROD) under electron microscopy. Storage material is found throughout most tissues. The disease is not typical of lysosomal storage disorders affecting the brain in that severe neuronal loss and volume contraction (rather than volume expansion due to storage material) is observed [5].
Enzyme replacement therapy has not previously been developed for PPT1 deficiency because peripherally administered enzyme is expected to have only limited access to the brain. However, this has not been tested formally. In the current paper, we confirm that human recombinant PPT1 enzyme produced in a CHO cell line has minimal distribution to the brain but seems to be well tolerated when administered via tail vein to PPT1-deficient mice. Potentially corrective levels of enzyme were achieved in peripheral organs that were tested, including the heart, which may be important clinically [6].
2. MATERIALS AND METHODS
2.1 High-level expression of PPT1 in CHO cells
A fragment corresponding to the entire coding region of the human PPT1 sequence (nucleotides 233 to 1153 of NM_000310 (GenBank)) was generated by polymerase chain reaction amplification from pCMV5-hPPT1 [7] and cloned into XhoI sites in the polylinker region of expression vector pMSXND1 [8]. The nucleotide sequence of the forward primer used was 5′-GCG ATA CTC GAG ATG GCG TCG CCC GGC TGC CTG TGG CTC TTG-3′ and the reverse primer, 5′-GCG ATA CTC GAG TCA TCC AAG GAA TGG TAT GAT GTG GGC-3′. The open reading frame was sequenced to confirm fidelity of the amplification. High-level expression in CHO cells was obtained essentially as described [9] by multiple rounds of selection in methotrexate [10]. Briefly, CHO cells (obtained from the ATCC, #CCL-61™) were maintained in F-12 medium (Invitrogen) supplemented with 10% (v/v) fetal-bovine serum (FBS) and were transfected with pMSXND1-hPPT1 using FuGENE 6 (Roche). Cells were selected with G418 and resistant clones isolated using cloning cylinders. Subclones were analyzed for secretion of PPT1 by measuring enzyme activity in conditioned medium using a fluorescence assay based on hydrolysis of an artificial substrate, 4-methylumbelliferyl-6-thiopalmitoyl-β-D-glucoside (MU-6S-Palm-βGlc) [11]. Highest producing clones were grown in nucleotide-deficient -minimum essential medium (Invitrogen) containing 0.25 μM methotrexate. When resistant colonies appeared, they were expanded, aliquots frozen, and the remaining cells subjected to 2 to 3-fold higher concentration of methotrexate. This process was repeated for a total of 6 cycles, reaching a final maximum methotrexate concentration of 30 μM, after which further increases did not lead to higher enzyme production. The final cell line produced was designated CHO-hPPT1 and has been deposited at the National Cell Culture Center (Minneapolis, MN).
2.2 Purification and characterization of recombinant human PPT1
CHO-hPPT1 cells were maintained in nucleoside-deficient -minimum essential medium (Gibco, catalog number 32561) containing 30 μM methotrexate, and were supplemented with 10% dialyzed FBS (Gemini, catalog number 100–106, lot A12A01X) containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. For production of recombinant protein, confluent adherent CHO-hPPT1 cells were split 1:2 and grown in F-12 medium (Invitrogen) supplemented with 10% FBS to confluence. For recombinant protein production, cells were washed with phosphate-buffered saline (PBS) three times and cultured in serum free medium (Hyclone CDM4CHO, SH30557) for 14 days, with medium collected and replenished with fresh medium every two days. Typically, 30 dishes each containing 5 ml of medium were used. Collected medium was centrifuged at 400 X g, filtered through a 0.2 m filter unit to remove debris and floating cells, then concentrated approximately 20-fold using a stirred cell equipped with a YM-10 membrane (Millipore) at 4 C, and snap-frozen until further use.
Concentrated medium (about 30 ml) was dialyzed overnight using a dialysis cassette (20K molecular weight cut-off, Pierce) against two changes of Mono S equilibration buffer (50 mM Na acetate, 25 mM NaCl, 2 mM EDTA, pH 5.0; 4 liters per change) and centrifuged for 20 min at 8,000 rpm in an SLA-1500 rotor (34,155gmax). The resulting supernatant (40 ml) was loaded on to a Mono S 10/100 GL column (1 × 10 cm, 8 ml bed volume) at a flow rate of 2 ml/min and washed with 40 ml of equilibration buffer. Enzyme activity was eluted with a 160-ml linear gradient of increasing NaCl concentration (25–350 mM) in the same buffer. Fractions (4 ml) containing enzyme activity were pooled, concentrated to 5 mg/ml and exchanged into PBS containing 1 mM EDTA and 1 mM β-glycerol phosphate by repeated rounds of dilution and concentration using a Centricon-30 concentrator.
2.4 Uptake of PPT1 and hydrolysis of cysteinyl lipids by PPT1-deficient lymphoblasts
A PPT1-deficient immortalized lymphoblastoid cell line (UT8-01) was maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 ug/ml). For metabolic labeling experiments, 200 ml of log-phase patient lymphoblastoid cells (0.5-0.75 X106 cells/ml) were pulsed-labeled with [35S] cysteine for 4 h [12], washed, and then incubated overnight in unlabeled cysteine-containing “chase” medium containing various amounts of purified PPT1 in the presence of 5 mM mannose (control) or mannose 6-phosphate (Man 6-P). For time course studies, the labeled cells were incubated for up to 16 h with 0.008 U/ml (0.54 ug/ml) of PPT1 added to the culture medium (1 U= 1 μmol of MU-6S-Palm-βGlc hydrolyzed per min). The cells were harvested into microcentrifuge tubes and washed twice with 0.5 ml of cold PBS and pelleted for 10 seconds at maximum speed in a microcentrifuge at 4°C, followed by chloroform-methanol extraction and analysis by high-performance thin layer chromatography (HP-TLC) as previously described [12].
2.5 Plasma and tissue clearance of PPT1 administered by tail vein injection
Adult PPT1 knockout mice at 8–16 weeks of age of either sex were injected via tail vein with human PPT1 at concentrations of 0 (vehicle), 2.5, 7.5, or 25 mg/kg of human recombinant PPT1 (15 U/mg, in a total volume of 250 μl, four mice per group). Blood (50 μl) was collected at the times indicated from the submandibular vein into Eppendorf tubes containing 1 μl of 0.5 M EDTA at the time indicated, mixed well, centrifuged at 4000 rpm for 10 min in a tabletop microfuge, and the supernatant plasma was snap frozen in liquid nitrogen. At 2, 6, 24, and 72 h after injection, mice were anesthetized with Avertin (250mg/kg) and perfused with 20 ml of heparinized saline through the left ventricle over a period of 20 minutes, and brains, hearts, lungs, livers, kidneys, and spleens were snap frozen in liquid nitrogen. For PPT1 assays, plasma was diluted as appropriate and assayed directly, and tissues were homogenized in 5–10 volumes of 50 mM Tris-HCl, 100 mM NaCl, 2 mM EDTA, pH 7.0, using a Polytron homogenizer, centrifuged for 1 h at 100,000g, and the supernatant assayed according to [11]. All enzyme assays were conducted under conditions where the increase in fluorescent intensity was linear with respect to time and concentration and in comparison with known standards (purified PPT1 and 4-methylumbelliferone). Typically, each assay contained 10–30 μg of protein and incubations were carried out from 15 min to 3 h. Protein content was determined using the Dc protein assay (BioRad).
3. RESULTS
3.1 Production of recombinant human PPT1 in CHO cells
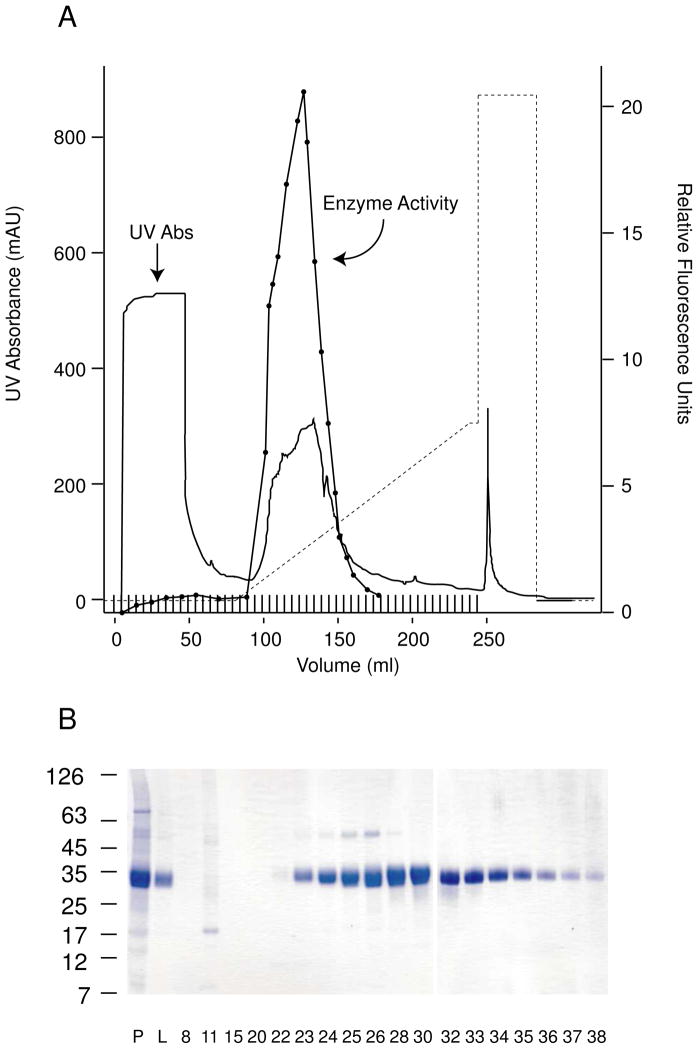
The full-length human PPT1 cDNA was cloned into a plasmid expression vector containing the dihydrofolate reductase resistance marker and used to transfect CHO cells, which were selected by multiple stepwise increases in methotrexate concentration over a period of nine months to achieve a very high and stable level of enzyme expression and secretion. Clones selected following this procedure secreted from 200–250 μg of PPT1 enzyme per ml of culture medium and could be grown in continuous culture in serum-free medium for up to 21 days. The enzyme was stable during concentration and dialysis, and was purified to homogeneity using a Mono-S cation exchange column (Fig. 1). Final enzyme activity was 15 U/mg (1 U= 1 μmol of MU-6S-Palm-βGlc hydrolyzed per min). Approximately 50 mg of purified enzyme was obtained from 600 ml of serum-free medium collected over 14–21 days. We have performed at least three such collections with essentially equivalent results.
Fig. 1.
Purified recombinant PPT1 derived from stable overexpression in CHO cells. A, Mono S chromatography of conditioned medium. Solid line, UV absorption profile; closed circles, PPT1 enzyme activity expressed in arbitrary fluorescence units; dotted line, gradient elution profile (NaCl concentration increased from 25 mM to 350 mM NaCl). B, Fractions from gradient elution. Highly purified PPT1 (migrating as a 34 kDa band) is eluted. P, post-dialysis conditioned medium; L, material loaded onto the column.
3.2 Characterization of recombinant human PPT1
A sample of the purified homogeneous enzyme (5 g) was applied to a 0.5 ml bovine mannose 6-phosphate receptor column (a kind gift of Dr. David Sleat, University of New Jersey School of Medicine and Dentistry) to assess the fraction bound and eluted by mannose 6-phosphate. This assay revealed that 80% of the purified recombinant human CHO-derived enzyme could be bound and eluted in this manner (data not shown).
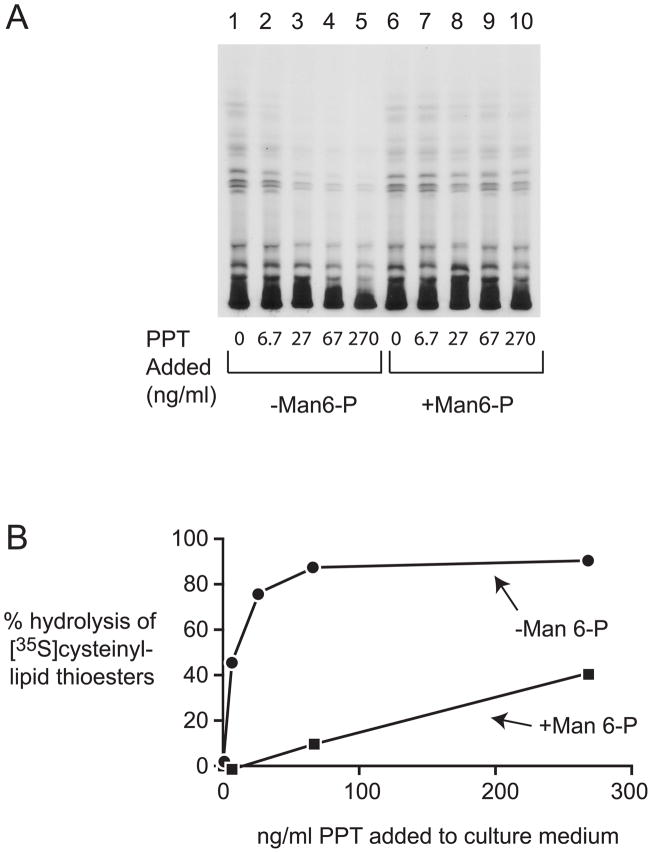
PPT1-deficient immortalized lymphoblasts derived from INCL patients accumulate lipid-soluble metabolites that are substrates for PPT1 in vitro and that can be demonstrated by HP-TLC following [35S]cysteine labeling [12–13]. Purified human recombinant PPT1 was therefore tested for its ability to clear these metabolites from cells (Fig. 2). Cells were labeled with [35S]cysteine for 4 h, washed, and different concentrations of PPT1 added directly to the culture medium for 16 h in the presence and absence of mannose 6-phosphate. As seen in Fig. 2, loss of the labeled compounds in a PPT1 concentration-dependent manner, with an EC50 of 0.25 nM following overnight incubation. Minimal correction was seen in the presence of mannose 6-phosphate (EC50 >20 nM), confirming that the uptake of PPT1 is primarily through the mannose 6-phosphate receptor pathway in this cell type. In parallel with the analysis of [35S] cysteinyl lipids, we also measured PPT1 activity in the washed cell pellet during an experiment similar to that shown in Fig. 2. We found that the EC50 for substrate clearance (0.25 nM in the culture medium) corresponded to an intracellular level of PPT1 that was 0.01 nmoles/min of enzyme activity per mg of total cell protein, which is a level that corresponds to between 0.8 and 1.3% of levels found in wild-type lymphoblasts (data not shown). These data are in agreement with the late-onset disease phenotypes observed in many PPT1 deficient patients, who show residual activity in lymphoblasts of ~2% [2].
Fig. 2.
Effect of increasing concentration of PPT1 added to cell culture medium on depletion of [35S]cysteinyl lipid thioesters from cells after overnight incubation. A, Thin layer chromatogram of [35S]cysteinyl lipids extracted from PPT1-deficient lymphoblasts. Cultures were carried out in the presence (closed circles) or absence (closed squares) of 5 mM mannose 6-phosphate. B, Densitometry quantitation of thin layer chromatography bands. Shown is representative data derived from at least three experiments giving similar results.
3.3 Plasma clearance
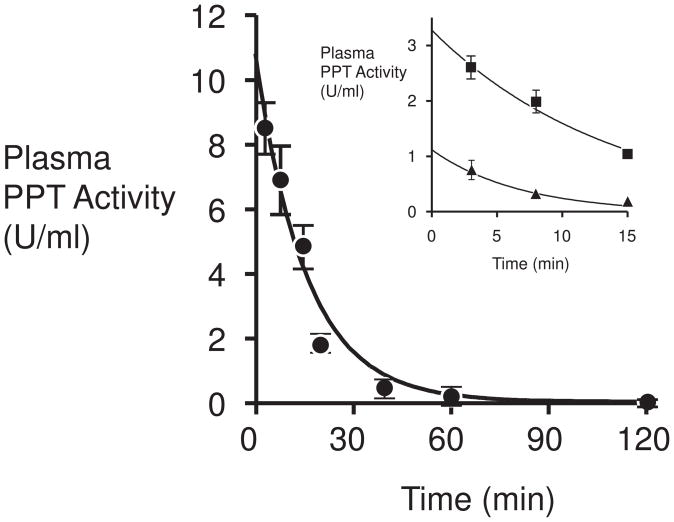
The CHO-derived purified recombinant PPT1 was injected via tail vein into 8-week old PPT1 deficient mice, and blood was collected and plasma assayed for PPT1 activity at varying time points after injection (Fig. 3). Three doses, 2.5, 7.5 and 25 mg/kg of PPT1 were examined. The enzyme was rapidly cleared from the plasma, with a half-life of 10 minutes, consistent with previous measurements of naturally-occurring [14] and recombinant lysosomal enzymes (in the range of 5–10 minutes) [15]. There was no significant effect of the initial concentration on the half-life over the range tested.
Fig. 3.
Time course of clearance of PPT1 activity from mouse plasma after tail vein injection. Closed circles, 25 mg/kg PPT1. Inset, closed squares, 7.5 mg/kg PPT1; closed triangles, 2.5 mg/kg PPT1.
3.4 Time course of tissue distribution following single tail vein injection
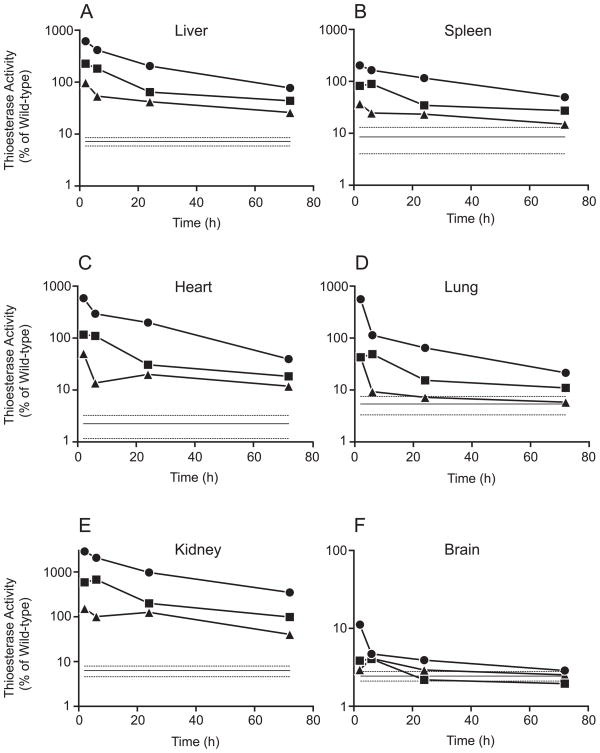
Distribution of the recombinant enzyme to various organs was assessed at 2, 6, 24 and 72 h following injection (Fig. 4). Mice were perfused with heparinized saline and liver, spleen, heart, lung, kidney and brain were harvested, homogenized, and assayed for PPT1 activity (Fig. 4). One mouse was sacrificed at each of the three dose levels and at each time point (total of 12 mice), and tissues from vehicle-injected (n=5) and wild-type (n=4) mice were prepared as controls for comparison. Background (non-PPT1 thioesterase activity) measured in the PPT1 knockout tissues is indicated as a solid line (mean) with stippled bars (±S.D) in each panel, and data is expressed as % of wild-type activity in each organ. As shown in Fig. 4, panels A–F, a dose-dependent increase in enzyme activity was observed in each tissue at all time points examined, with the exception of brain. The highest levels were observed in the kidney, which reached 30.5 times normal at 2 h after injection of 25 mg/kg PPT1 and remained at 3.5 times normal at 72 h (note that Fig. 4 is plotted on a log scale). Even at the lowest dose (2.5 mg/kg) levels in kidney were at potentially corrective levels (minimum, 36%) throughout the 72 h. Despite the high levels in kidney, very little enzyme (maximum, two-fold of wild-type levels) was detected in urine even after injection at the highest dose (data not shown). (Presumably, PPT1 (a 37-kDa protein) is filtered through the glomerulus and taken up and resides in renal tubular cells). In four other tissues examined (liver, spleen, heart, lung), levels of enzyme at 72 h at the 25 mg/kg dose were 75%, 45%, 38% and 17% of wild-type, respectively. These levels may be clinically significant, as adult-onset patient with 7% of residual activity in fibroblasts and blood cells have been described [3], and heterozygous carriers are normal.
Fig. 4.
Time course of clearance of PPT1 activity from tissues after tail vein injection. Data from 12 PPT1 knockout mice is shown. Three dose levels were examined: Closed circles, 25 mg/kg PPT1, closed squares, 7.5 mg/kg; closed triangles, 2.5 mg/kg. The solid and dotted lines at the bottom of each panel represent the mean and standard deviation of thioesterase activity measured in tissues of five vehicle-injected PPT1 knockout mice. This activity represents background activity, probably attributable to PPT2 [16]. All values are presented as percent of the mean value of 4 wild-type mice for each tissue.
Only low levels of enzyme were detected in whole brain after PPT1 injection. (Fig. 4, panel F). At the highest dose (25 mg/kg), enzyme activity reached 10% of wild-type levels at 2 h and was barely detectable over background at 24 h. (The background level of non-PPT1 thioesterase activity was 2.4±0.35% of total activity in normal brain, most likely attributable to a known second lysosomal thioesterase, PPT2 [16]. Attempts to diminish this background level, for example, through the use of a more “natural” substrate, such as a palmitoyl-protein (H-Ras) [17] were not successful, data not shown). Whether any enzyme distributed beyond the vasculature and into brain parenchyma is unknown, but initial studies of mouse brain showed no PPT1 immunostaining at 24 h after injection.
4. DISCUSSION
In the current paper, we have demonstrated the feasibility of producing large amounts of human recombinant PPT1 that is competent for uptake by the mannose 6-phosphate receptor using standard technology. The enzyme is cleared rapidly from plasma, and potentially corrective enzyme levels were achieved in major organs except the brain.
Although INCL is usually regarded as a purely neuronal defect, it should be pointed out that the enzyme is widely expressed, and it remains possible that if neuronally-directed therapies (i.e., gene or neural stem cell therapy) turn out to prolong life expectancy in these patients, systemic effects may become manifest. (It is important to note that human neural stem cells for this disorder are being tested in a clinical trial [18] based upon favorable results on neurodegeneration in the mouse model [19]). The human recombinant PPT1 described in the paper may thus become especially pertinent. Of note, gene therapy directed solely at the brains of PPT-deficient mice did not improve lifespan even as it corrected neuropathology [20–21]. The cause of death in the mice was not entirely clear, but they developed cardiac pathology and dysfunction [22], and similar findings has been described in human NCL with GROD as well [6]. Therefore, it may be important that we were able to achieve potentially corrective levels of PPT1 in the heart after intravenous injection.
A source of recombinant PPT1 will also be useful for exploring alternative delivery methods, such as injection into brain ventricles [23–26], chemical modifications [15] or chronic high-dose therapy [27–28], which have shown efficacy in mouse models of other lysosomal storage disorders and which are being explored for human use.
Acknowledgments
The authors wish to thank Dr. Peter Lobel and Dr. David Sleat for many helpful suggestions and Dr. Sleat for the mannose 6-phosphate receptor affinity column. This work was funded by the Batten Disease Support and Research Association and the National Institute for Neurological Disorders and Stroke (NS036867 to S.L.H).
ABBREVIATIONS
- INCL
infantile neuronal ceroid lipofuscinosis
- PPT1
palmitoyl-protein thioesterase-1
- CHO
Chinese Hamster Ovary
- CLN1
ceroid lipofuscinosis, neuronal-1
- GROD
granular osmiophilic deposits
- FBS
fetal bovine serum
- MU-6S-Palm-βGlc
4-methylumbelliferyl-6-thiopalmitoyl-β-D-glucoside
- PBS
phosphate-buffered saline
- HP-TLC
high performance-thin layer chromatography
- Man 6-P
mannose 6-phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 2.Das AK, Becerra CHR, Yi W, Lu JY, Siakotos AN, Wisniewski KE, Hofmann SL. Molecular genetics of palmitoyl-protein thioesterase deficiency in the U. S. J Clin Invest. 1998;102:361–370. doi: 10.1172/JCI3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Diggelen OP, Thobois S, Tilikete C, Zabot MT, Keulemans JL, van Bunderen PA, Taschner PE, Losekoot M, Voznyi YV. Adult neuronal ceroid lipofuscinosis with palmitoyl-protein thioesterase deficiency: first adult-onset patients of a childhood disease. Ann Neurol. 2001;50:269–272. doi: 10.1002/ana.1103. [DOI] [PubMed] [Google Scholar]
- 4.Ramadan H, Al-Din AS, Ismail A, Balen F, Varma A, Twomey A, Watts R, Jackson M, Anderson G, Green E, Mole SE. Adult neuronal ceroid lipofuscinosis caused by deficiency in palmitoyl protein thioesterase 1. Neurology. 2007;68:387–388. doi: 10.1212/01.wnl.0000252825.85947.2f. [DOI] [PubMed] [Google Scholar]
- 5.Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6:107–126. doi: 10.1007/s10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- 6.Hofman IL, van der Wal AC, Dingemans KP, Becker AE. Cardiac pathology in neuronal ceroid lipofuscinoses--a clinicopathologic correlation in three patients. Eur J Paediatr Neurol. 2001;5(Suppl A):213–217. doi: 10.1053/ejpn.2000.0465. [DOI] [PubMed] [Google Scholar]
- 7.Camp LA, Verkruyse LA, Afendis SJ, Slaughter CA, Hofmann SL. Molecular cloning and expression of palmitoyl-protein thioesterase. J Biol Chem. 1994;269:23212–23219. [PubMed] [Google Scholar]
- 8.Lee SJ, Nathans D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988;263:3521–3527. [PubMed] [Google Scholar]
- 9.Lin L, Lobel P. Production and characterization of recombinant human CLN2 protein for enzyme-replacement therapy in late infantile neuronal ceroid lipofuscinosis. Biochem J. 2001;357:49–55. doi: 10.1042/0264-6021:3570049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman RJ, Sharp PA. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982;159:601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- 11.van Diggelen OP, Keulemans JL, Winchester B, Hofman IL, Vanhanen SL, Santavuori P, Voznyi YV. A rapid fluorogenic palmitoyl-protein thioesterase assay: pre- and postnatal diagnosis of INCL. Mol Genet Metab. 1999;66:240–244. doi: 10.1006/mgme.1999.2809. [DOI] [PubMed] [Google Scholar]
- 12.Lu JY, Verkruyse LA, Hofmann SL. Lipid thioesters derived from acylated proteins accumulate in infantile neuronal ceroid lipofuscinosis: correction of the defect in lymphoblasts by recombinant palmitoyl-protein thioesterase. Proc Natl Acad Sci U S A. 1996;93:10046–10050. doi: 10.1073/pnas.93.19.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu JY, Verkruyse LA, Hofmann SL. The effects of lysosomotropic agents on normal and INCL cells provide further evidence for the lysosomal nature of palmitoyl-protein thioesterase function. Biochim Biophys Acta. 2002;1583:35–44. doi: 10.1016/s1388-1981(02)00158-0. [DOI] [PubMed] [Google Scholar]
- 14.Stahl P, Six H, Rodman JS, Schlesinger P, Tulsiani DR, Touster O. Evidence for specific recognition sites mediating clearance of lysosomal enzymes in vivo. Proc Natl Acad Sci U S A. 1976;73:4045–4049. doi: 10.1073/pnas.73.11.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grubb JH, Vogler C, Levy B, Galvin N, Tan Y, Sly WS. Chemically modified beta-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci U S A. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calero G, Gupta P, Nonato MC, Tandel S, Biehl ER, Hofmann SL, Clardy J. The crystal structure of palmitoyl protein thioesterase-2 (PPT2) reveals the basis for divergent substrate specificities of the two lysosomal thioesterases, PPT1 and PPT2. J Biol Chem. 2003;278:37957–37964. doi: 10.1074/jbc.M301225200. [DOI] [PubMed] [Google Scholar]
- 17.Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993;268:22566–22574. [PubMed] [Google Scholar]
- 18.Taupin P. HuCNS-SC (StemCells) Curr Opin Mol Ther. 2006;8:156–163. [PubMed] [Google Scholar]
- 19.Tamaki SJ, Jacobs Y, Dohse M, Capela A, Cooper JD, Reitsma M, He D, Tushinski R, Belichenko PV, Salehi A, Mobley W, Gage FH, Huhn S, Tsukamoto AS, Weissman IL, Uchida N. Neuroprotection of host cells by human central nervous system stem cells in a mouse model of infantile neuronal ceroid lipofuscinosis. Cell Stem Cell. 2009;5:310–319. doi: 10.1016/j.stem.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Griffey M, Bible E, Vogler C, Levy B, Gupta P, Cooper J, Sands MS. Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16:360–369. doi: 10.1016/j.nbd.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Griffey MA, Wozniak D, Wong M, Bible E, Johnson K, Rothman SM, Wentz AE, Cooper JD, Sands MS. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13:538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Galvin N, Vogler C, Levy B, Kovacs A, Griffey M, Sands MS. A murine model of infantile neuronal ceroid lipofuscinosis-ultrastructural evaluation of storage in the central nervous system and viscera. Pediatr Dev Pathol. 2008;11:185–192. doi: 10.2350/07-03-0242.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang M, Cooper JD, Sleat DE, Cheng SH, Dodge JC, Passini MA, Lobel P, Davidson BL. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- 24.Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, Hanson S, Passage M, Kakkis E. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol Genet Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemsley KM, King B, Hopwood JJ. Injection of recombinant human sulfamidase into the CSF via the cerebellomedullary cistern in MPS IIIA mice. Mol Genet Metab. 2007;90:313–328. doi: 10.1016/j.ymgme.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Hemsley KM, Luck AJ, Crawley AC, Hassiotis S, Beard H, King B, Rozek T, Rozaklis T, Fuller M, Hopwood JJ. Examination of intravenous and intra-CSF protein delivery for treatment of neurological disease. Eur J Neurosci. 2009;29:1197–1214. doi: 10.1111/j.1460-9568.2009.06666.x. [DOI] [PubMed] [Google Scholar]
- 27.Vogler C, Levy B, Grubb JH, Galvin N, Tan Y, Kakkis E, Pavloff N, Sly WS. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci U S A. 2005;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanz J, Stroobants S, Lullmann-Rauch R, Morelle W, Ludemann M, D’Hooge R, Reuterwall H, Michalski JC, Fogh J, Andersson C, Saftig P. Reversal of peripheral and central neural storage and ataxia after recombinant enzyme replacement therapy in alpha-mannosidosis mice. Hum Mol Genet. 2008;17:3437–3445. doi: 10.1093/hmg/ddn237. [DOI] [PubMed] [Google Scholar]