Summary
Over one-quarter of adult Americans are diagnosed with a mental illness like Major Depressive Disorder (MDD), Post-Traumatic Stress Disorder (PTSD), schizophrenia, and Alzheimer’s Disease. In addition to the exceptional personal burden these disorders exert on patients and their families, they also have enormous cost to society. Although existing pharmacological and psychosocial treatments alleviate symptoms in many patients, the comorbidity, severity, and intractable nature of mental disorders strongly underscore the need for novel strategies. As the hippocampus is a site of structural and functional pathology in most mental illnesses, a hippocampal-based treatment approach has been proposed to counteract the cognitive deficits and mood dysregulation that are hallmarks of psychiatric disorders. In particular, preclinical and clinical research suggests that hippocampal neurogenesis, the generation of new neurons in the adult dentate gyrus, may be harnessed to treat mental illness. There are obvious applications and allures of this approach; for example, perhaps stimulating hippocampal neurogenesis would reverse the overt and noncontroversial hippocampal atrophy and functional deficits observed in Alzheimer’s Disease and schizophrenia, or the more controversial hippocampal deficits seen in MDD and PTSD. However, critical examination suggests that neurogenesis may only correlate with mental illness and treatment, suggesting targeting neurogenesis alone is not a sufficient treatment strategy. Here we review the classic and causative links between adult hippocampal neurogenesis and mental disorders, and provide a critical evaluation of how (and if) our basic knowledge of new neurons in the adult hippocampus might eventually help combat or even prevent mental illness.
Keywords: psychiatric illness, mental disorders, dentate gyrus, subgranular zone, neurogenic niche, neural stem cells
Introduction
Each year over 25% of adult Americans carry the diagnosis of at least one mental disorder (Kessler et al., 2005a; Kessler et al., 2005b). By far, the greatest percentage of the adult US population – 18.7% – is diagnosed with an anxiety disorder like Post-Traumatic Stress Disorder (PTSD, 3.5%), but notable percentages of the population are also diagnosed with mood disorders (9.5%) like Major Depressive Disorder (MDD, 6.7%), or with Alzheimer’s Disease (~2%) and schizophrenia (1.1%) (Kessler et al., 2005a; Kessler et al., 2005b). Combined with psychosocial support, pharmacological interventions like anxiolytic, antidepressive, and antipsychotic drugs alleviate many symptoms associated anxiety disorders, MDD, and schizophrenia, respectively. However, the persistence and severity of symptoms of these individual disorders, the high proportion of individuals with comorbid psychiatric disorders, like addiction, or other severe health challenges, like obesity or cardiovascular disease, results in enormous personal and societal cost. Therefore, there is extraordinary interest in identifying and pursuing novel strategies for the treatment and even prevention of mental illness.
While mental disorders are exceptionally diverse and likely have discrete and complex neurobiological underpinnings, one particular brain region has long been studied for its potential involvement in mental illness in general: the hippocampus (Figure 1) (Bloom, 1975; Bloom, 1984; Frith and Done, 1988; Holsboer, 1988; Kling et al., 1987; McEwen et al., 1992; Meaney et al., 1988). Primarily known for its role in learning and memory, the hippocampus also has an important role in general cognition, mood regulation, response to stress, and even in encoding predictions for future events (Bast, 2007; Eichenbaum and Fortin, 2009; Fuchs and Flugge, 1998; Price and Drevets, 2009; Squire, 2004). A large body of literature shows that, in general, mental illness is marked by diminished hippocampal structure and function. For example, MDD, PTSD, schizophrenia, Alzheimer’s disease and even stress – a precipitating factor in many mental disorders – are marked by decreased hippocampal volume, learning and memory deficits, and mood dysregulation (e.g. Bremner, 1999; Campbell and Macqueen, 2004; Geuze et al., 2005; Goldman and Mitchell, 2004; Liberzon and Sripada, 2008; Lupien et al., 2007b; Pfefferbaum and Marsh, 1995; Sala et al., 2004; Sapolsky, 2000b; Savitz and Drevets, 2009; Villarreal and King, 2001). Intriguingly, successful improvement of the behavioral and cognitive symptoms of these disorders is often linked to attenuation or reversal of these changes in hippocampal structure and function. Such work has encouraged consideration of whether hippocampal atrophy is a useful target for the treatment of mental illness (Dhikav and Anand, 2007; Sala et al., 2004; Sapolsky, 2000a).
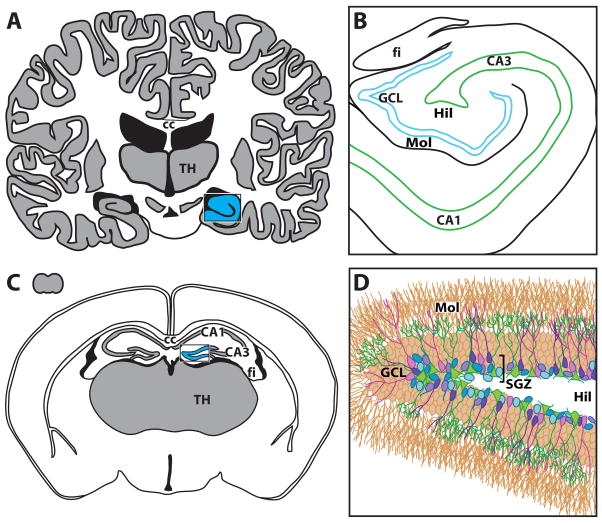
Figure 1. Schematic of hippocamapal dentate gyrus in (A, B) human and (C, D) mouse brains.
(A) Schematic of human brain, cut through the frontal/coronal plane at the level of the thalamus (TH) and corpus callosum (cc). The hippocampus is a bilateral structure nestled within the temporal lobe; the right hippocampus is shaded in blue. (B) Human hippocampus, enlarged from blue region in (A). Adult-generate neurons in the human dentate gyrus reside in the granule cell layer (GCL; blue) and the nearby hilus (Hil). For context, other human hippocampal regions are also depicted, such as the molecular layer of the dentate gyrus (Mol), regions of Ammon’s Horn (CA1, CA3), and the nearby white matter structure the fimbria (fi). (C) Small grey form in upper-left represents the mouse brain in size relation to the human brain in (A). Larger image is schematic of the adult mouse brain in the coronal plane, with the majority of the hippocampal dentate gyrus highlighted in blue. For context, regions of Ammon’s horn (CA1, CA3) and nearby gray matter (TH) and white matter structures (cc, fi) are provided. (D) Mouse dentate gyrus, enlarged from blue region in (C). Detail is shown in (D) to highlight the current view on the “stages” or phases of neurogenesis and the cellular diversity that thus exists in the neurogenic region of the subgranular zone (SGZ). Note many aspects of the neurogenic niche are not depicted, including vasculature, inhibitory interneurons, and astrocytes. Based on current understanding, the putative stem cell (green) gives rise to progenitor cells (blue), some of which survive and mature into immature neurons (purple), which eventually mature into granule cell neurons (brown) and incorporate into hippocampal circuitry by projecting to CA3 via the mossy fiber pathway. In the rodent, projections may enter the mossy fiber pathway in less than 7 days and adult-generated neurons can present indices of morphological and phenotypic maturity from 2 weeks-2 months later. In the human, the timing of adult hippocampal neurogenesis is unknown. In addition, the anatomic and morphologic features of the human hippocampal stem cell remains unknown. CA1, CA3, Cornu Ammon subregions 1, 3; cc, corpus callosum; fi, fimbria; GCL, granule cell layer; Hil, hilus; Mol, molecular layer; SGZ, subgranular zone; TH, thalamus.
The hippocampus is one of most “responsive” brain structures in that it demonstrates rapid plasticity at the molecular, cellular, structural, and functional levels after specific stimuli. Thus, it has been challenging for scientists to narrow which aspect of hippocampal plasticity might be best targeted to counteract the symptoms of such diverse disorders. One particular aspect of hippocampal plasticity that has received significant attention is adult hippocampal neurogenesis, or the ability of the hippocampus to generate new neurons throughout life. First discovered by Joseph Altman more than forty-five years ago (Altman, 1963), it is now accepted that stem-like and progenitor cells residing in the aptly-named subgranular zone (SGZ; Figure 1) give rise to dentate gyrus granule neurons that integrate into circuitry and contribute to discrete aspects of hippocampal functions (Balu and Lucki, 2009; Pathania et al., in press). As reviewed in detail elsewhere (Abrous et al., 2005; Kempermann et al., 2008) and briefly here (Table 1), there is an enormous amount of correlative evidence linking hippocampal neurogenesis with mental disorders. More recent work has provided striking causative links as well (e.g. Li et al., 2008b; Revest et al., 2009; Santarelli et al., 2003). The surge of primary and review papers on this topic urge revisiting the question, “Is manipulation of hippocampal neurogenesis a promising target for the treatment of mental disorders?”
Table 1. Summary of data from rodent models regarding links between adult hippocampal neurogenesis and mental illness.
Schematics in top row depict the phenotypic and morphological criteria delineate the putative stages of neurogenesis with cartoon representions of various cell types in vivo.
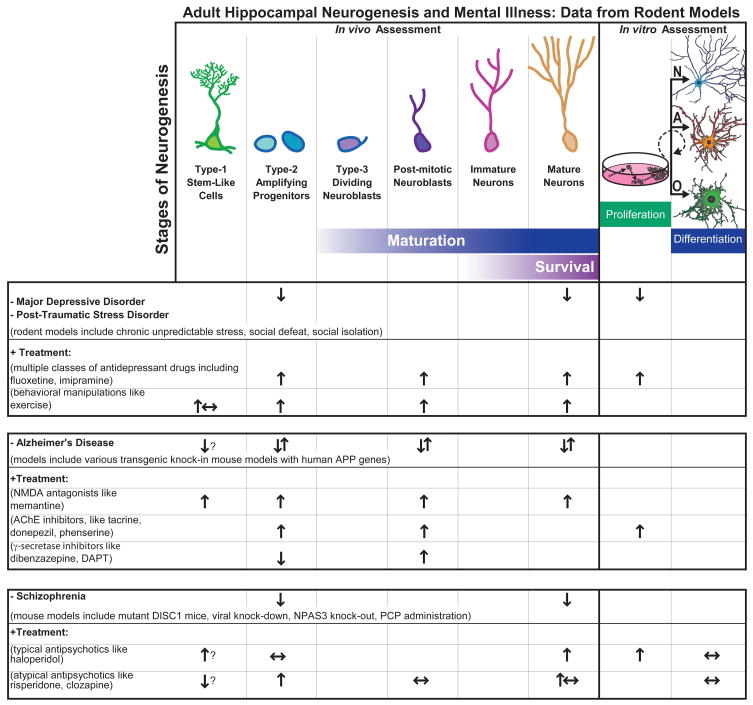 |
As indicated on the far right side of the top tow, in vitro assays are also used to assess proliferation of progenitor cells, as well as potential of progenitors to differentiate into three lineages: neurons (N), astrocytes (A), oligodendrocytes (O). Table below summarizes data that has emerged from animal models of mental diseases (demarcated with “−“) and clinically-relevant treatments (“+”) which have provided critical information regarding how adult neurogenesis is altered in rodents. ? Inconclusive; ↑ Increase; ↓ Decrease; ↔ No change; ↑↓ Contradictory literature showing increase and decrease in different models.
A number of excellent reviews have recently tackled questions including “What is neurogenesis good for?” and “Is targeting hippocampal atrophy useful for mental illness?” (e.g. Aimone et al., 2006; Becker and Wojtowicz, 2007; Bruel-Jungerman et al., 2007; Drew and Hen, 2007; Eisch et al., 2008; Elder et al., 2006; Gould et al., 1999; Kempermann et al., 2008; Kempermann and Kronenberg, 2003; Ming and Song, 2005; Morgan, 2007; Perera et al., 2008; Sahay and Hen, 2007; Thomas and Peterson, 2008; Thompson et al., 2008; Vaidya et al., 2007). Therefore, the goal for this brief review is to critically evaluate hippocampal neurogenesis as a viable treatment aim and to provide an “update” to previous reviews that have been more narrowly focused on individual psychiatric disorders, such as depression (e.g. Drew and Hen, 2007, Feldmann, 2007 #10060) or schizophrenia/DISC1 (e.g. Dranovsky and Hen, 2007). While the preponderance of literature to date has provided insight into psychiatric illnesses through rodent models, there is surprisingly little known about normal and pathological neurogenesis in humans. Much recent work in human neurogenesis focuses on seizure activity and epilepsy, known to robustly increase neurogenesis in both rodents and humans. However, the lack of data on other human psychiatric illnesses has not been emphasized elsewhere, and another stated purpose of the current review is to highlight how little is known regarding the human diseases. We critically evaluate the hopes in targeting adult hippocampal neurogenesis for treating mental disorders, then highlight major obstacles to overcome before translational applications of adult hippocampal neurogenesis can be realized. We particularly hope this review will engage those readers outside the fields of adult neurogenesis and mental illness research, because stimulating interdisciplinary research is likely critical to future integration of our basic knowledge of new cells in the adult brain with clinical need.
Adult hippocampal neurogenesis in a nutshell
The hippocampus is one of two well-accepted regions of the adult brain in which new neurons are added through mammalian life. While the human and rodent hippocampi maintain the same basic anatomic regions (Figure 1A–C), the process of neurogenesis has been best characterized within the rodent dentate gyrus and has been the subject of many extensive reviews. However, we would be remiss if we did not provide essential information regarding basic progression of neurogenic stages. The reader is strongly encouraged to see the supplemental material for a more detailed (albeit still abbreviated) discussion of the hallmark stages of proliferation, maturation, and survival of hippocampal progenitors (see “Adult hippocampal neurogenesis: where, how, and for what purpose?” in Supplemental Information).
In brief, neurogenesis proceeds in the adult hippocampus but is restricted to the subgranular zone (SGZ) of the dentate gyrus (Figure 1D). Along the border of the granule cell layer and the hilus, stem-like cells (referred to as Type-1 cells, Type B cells, or quiescent neural precursors) putatively divide asymmetrically to produce daughter precursor cells (called Type-2 cells, Type D cells, or transiently-amplifying progenitor cells). Type-2 and their slightly more mature counterparts (called Type-3 cells or neuroblasts) divide frequently and constitute the majority of dividing cells within the SGZ. In a process that remains poorly understood, proliferative neuroblasts become post-mitotic and differentiate into immature neurons. During this process, immature neurons mature and extend dendritic branches into the molecular layer to receive synaptic input from the perforant pathway and also extend a nascent axon through the hilus to the CA3 region of the hippocampus via the mossy fiber pathway. Over the course of several weeks to months, adult-born neurons survive and incorporate into the hippocampal circuitry. Cell-intrinsic and -extrinsic factors governing neurogenesis, as well as the role of neurotransmitters, are reviewed elsewhere within this issue (Pathania et al., in press).
Several facets of neurogenesis remain elusive. In particular, hippocampal stem-like cells have been difficult to study in part because they divide infrequently and the putative stem cells express markers of immature astrocytes and maintain unique morphology with an irregularly-shaped soma and an elaborate series of processes in the inner molecular layer. Also, it remains unclear at which point progenitor cells become fated to become neurons. Under culture conditions, hippocampally-derived progenitors can differentiate into three hallmark lineages, including neurons, astrocytes, and oligodendrocytes (Table 1, far right column). However, under normal conditions in vivo, the vast majority (70–90%) of surviving adult-born cells become neurons. In addition, the behavioral and functional significance of adult neurogenesis remain poorly understood, though recent studies emphasize that new granule neurons may play a role in discrete hippocampal memory tasks and in aspects of mood regulation.
Hippocampal dysfunction in mental illness: the case for targeting neurogenesis
Adult hippocampal neurogenesis has been suggested as a target for the amelioration or prevention of mental illness (Balu and Lucki, 2009; Kaneko and Sawamoto, 2009). Each of the psychiatric disorders discussed here – MDD, PTSD, Alzheimer’s Disease, and schizophrenia – is linked to decreased hippocampal volume and function. As summarized in Table 1, an enormous amount of evidence links animal models of mental illness to altered hippocampal neurogenesis. Here we provide a selective review of this preclinical work alongside translational work in human and non-human primates for MDD, PTSD, schizophrenia and Alzheimer’s Disease to highlight why researchers are so excited about the prospect of reversing deficits in hippocampal neurogenesis to treat these disorders.
MDD, PTSD, and Stress
In humans, stress often precipitates or exacerbates mental illness. Thus, animal models of MDD and PTSD generally use stress to elicit behavioral phenotypes approximating clinical symptoms (Bremner, 2006; Greenwood and Fleshner, 2008; Krishnan and Nestler, 2008; Stam, 2007; Takemura and Kato, 2008). Animal models of MDD and PTSD almost ubiquitously result in acute suppression of in vivo and in vitro proliferation and in vivo survival of hippocampal neurons, and treatments like antidepressant drugs and exercise can reverse these changes (Banasr and Duman, 2007; Feldmann et al., 2007; Perera et al., 2008; Sahay and Hen, 2007; Sahay and Hen, 2008; Schloesser et al., 2009; Thomas and Peterson, 2008; Vollmayr et al., 2007; Wang et al., 2008). However, while suppression of hippocampal neurogenesis alone does not result in a depressive phenotype (e.g. Airan et al., 2007; David et al., 2009), adult-generated hippocampal neurons appear to play a role in some aspects of mood regulation. Interestingly, loss of adult-born neurons may interfere with the efficacy of antidepressant drugs (Santarelli et al., 2003). However, since loss of antidepressant efficacy is likely drug-, behavior- and species-specific and has not been demonstrated in all models of diminished neurogenesis or models of depression (e.g. David et al., 2009; Holick et al., 2008; Singer et al., 2009; Surget et al., 2008), far more work is needed on this topic.
While preclinical models of MDD and PTSD generally show decreased hippocampal proliferation and survival immediately after the stressful experience, the clinical effects are still unclear. Initial work on post-mortem tissue found no change in proliferation in MDD patients (Reif et al., 2006). Another recent report has replicated that there is no decrease in proliferating Ki-67 cells between MDD and control samples (Boldrini et al., 2009), and recent data indicate that antidepressant treatments enhance discrete facets of neurogenesis in human and non-human primate models (Boldrini et al., 2009; Perera et al., 2007). Unfortunately, there are many aspects of these and other postmortem experiments that urge caution in their interpretation. These include low sample size, lack of information on agonal index or serum toxicology at death, and variations in the region of the hippocampus chosen for analysis. Thus, firm conclusion on the relationship between depression and neurogenesis awaits assessment in other studies. It is reasonable however at this juncture to indicate the following striking links: clinical and preclinical data support that the depressed disease state does not correlate with diminished neurogenesis, whereas treatment of the disease state may result in normalization or enhancement of neurogenesis.
Clearly, more studies in clinical and preclinical paradigms are required. In addition to the call raised above for more detailed human post-mortem experiments, additional basic and clinical research is required to identify how neurogenesis is altered basally in MDD and PTSD, and how effective pharmacobehavioral therapy might differentially alter neurogenesis. In addition, the hypothesis that adult-generated neurons are required for antidepressant efficacy (Santarelli et al., 2003) still needs to be sufficiently addressed through humans, primate, and rodent studies, particularly in light of more recent work from the same group that may draw the generality of this hypothesis into question (David et al., 2009; Holick et al., 2007; Surget et al., 2008). However, the enormity of the correlative and the emerging causative links between adult hippocampal neurogenesis and MDD, PTSD and stress provides a remarkable amount of fuel for this ongoing research effort.
Alzheimer’s Disease
It has long been known that the hippocampus is target for neurodegeneration in Alzheimer’s Disease, and imaging studies now support that the hippocampus is among the first brain areas influenced during disease progression. While links between Alzheimer’s Disease and neurogenesis have also long been proposed (German and Eisch, 2004), the direction of the relationship remains highly controversial due to the opposing results that have emerged from animal models of the disorder (Table 1) and work with tissue from Alzheimer’s Disease patients.
For example, discrete stages of neurogenesis are decreased in certain mouse models (Donovan et al., 2006; Zhang et al., 2007), but increased in other mouse models (Jin et al., 2004a; Yu et al., 2009). The attention to effect of neurogenic stages has particularly paid off here, as some studies have shown that proliferation is decreased but survival of ectopic granule cell neurons is actually enhanced (Donovan et al., 2006), while other have shown that proliferation is increased although the cells have delayed maturation (Li et al., 2008a). The human work has been similarly challenging to interpret, as some aspects of neurogenesis like maturation are increased in Alzheimer’s Disease brains (Jin et al., 2004b) while other facets including proliferation and self-renewal are decreased in cultured cells derived from patients with Alzheimer’s disease in vitro (He and Shen, 2009).
Although the direction of the links between Alzheimer’s Disease and hippocampal neurogenesis await further validation, there is an active literature on strategies to promote new neurons with direct relevance for Alzheimer’s Disease (Schaeffer et al., 2009). Given the complexity of this disorder, future clinical studies likely will have to take a multipronged approach, such as assessing links between sleep, Alzheimer’s Disease, and neurogenesis (Meerlo et al., 2009).
Schizophrenia
Schizophrenia has among the most robust clinical evidence for a deficit in hippocampal volume (e.g. MacDonald and Schulz, 2009). In addition, there is an intriguing story developing on Disrupted-in-Schizophrenia-1 (DISC1), a schizophrenia susceptibility gene that also enhances susceptibility to other mental illnesses including MDD (Dranovsky and Hen, 2007; Welberg, 2009). DISC1 appears to be critical in neurogenesis (Enomoto et al., 2009; Kim et al., 2009; Maekawa et al., 2009b; Mao et al., 2009; Ming and Song, 2009), and has recently been found to be expressed in higher levels specifically in the hippocampi of patients diagnosed with schizophrenia (Nakata et al., 2009). Another gene linked to susceptibility of schizophrenia in humans is the bHLH transcription factor NPAS3 (Kamnasaran et al., 2003; Pickard et al., 2009). While it is intriguing that mice lacking NPAS3 in all cells from birth show deficits in hippocampal neurogenesis (Pieper et al., 2005), further assessment of links between NPAS3 and neurogenesis will benefit from cell-targeting strategies.
In addition to the requirement of DISC1 and NPAS3 for normal neurogenesis, there is ample preclinical evidence that schizophrenia is linked to decreased in vivo and in vitro neurogenesis (Table 1) (Arango et al., 2001; Kempermann et al., 2008; Kobayashi, 2009; Reif et al., 2007). For example, mice that constitutively lack NPAS3, that have a mutant form of DISC1, or that were administered phencyclidine display decreased in vivo proliferation and survival (Duan et al., 2007; Liu et al., 2006; Maeda et al., 2007; Pieper et al., 2005). In contrast, drugs that are used to counteract schizophrenic symptoms in people, like antipsychotics (clozapine), NMDA antagonists (memantine), acetylcholinesterase inhibitors (tacrine, donepezil, phenserin), and -secretase inhibitors (dibenzazepine, DAPT) in general enhance cells in discrete stages of neurogenesis in vivo and in vitro (Table 1) (Breunig et al., 2007; Jin et al., 2006; Kaneko et al., 2006; Kotani et al., 2008; Maeda et al., 2007; Maekawa et al., 2009a; Marutle et al., 2007; Namba et al., 2009; Wang et al., 2009). It is perhaps not surprising that a drug like DAPT actually enhances proliferation given that it also prevents cleavage of stem-cell promoting proteins like Notch1 (Breunig et al., 2007; Favaro et al., 2009).
Human work supports these links between schizophrenia and decreased hippocampal neurogenesis, as tissue from human schizophrenics has fewer proliferating Ki-67 cells than tissue from control subjects (Reif et al., 2006). However, caution is advised in the interpretation of Reif et al., because a decrease in Ki-67-expressing cells is not an index of overall neurogenesis, as not all dividing cells become neurons (Eisch and Mandyam, 2007). Even so, the links between hippocampal neurogenesis and schizophrenia are promising enough to inspire the proposition that an immature dentate gyrus, as might result from decreased neurogenesis, is a novel endophenotype of psychiatric disorders like schizophrenia (Yamasaki et al., 2008).
Hippocampal dysfunction in mental illness: the case against targeting neurogenesis
While Table 1 and the review above support the breadth and depth of neurogenesis as target for the treatment of mental illness, there are problems with hippocampal neurogenesis as a clinically-viable treatment. First, none of these psychiatric disorders is marked by overt loss of dentate gyrus granule neurons as a primary pathology. While both Alzheimer’s Disease and schizophrenia are incontrovertibly accompanied by hippocampal atrophy, decreased hippocampal volume may be correlative, and this atrophy is not regionally restricted to the dentate gyrus granule cell layer where adult neurogenesis occurs. Further, it remains unclear if a smaller hippocampus predisposes a person to these disorders. Second, the majority of these disorders and their pathophysiology are not, in fact, rooted in the hippocampus. As an example, symptomology of PTSD is coincident with structural and functional changes in the amygdala and cortex, which may play a more prominent role than the hippocampus (e.g. Lupien et al., 2007a). Third, increased numbers of adult-born neurons might not always better (Scharfman and Hen, 2007), and in fact stimulation of aberrant neurogenesis as in the epileptic brain may do more harm than good (Parent et al., 2007; Parent and Murphy, 2008).
Another major concern regards the potential for adult neurogenesis in the dentate gyrus to overcome generalized hippocampal atrophy. In some rodent experiments, antidepressant treatment reversed stress-induced decreases in hippocampal volume (e.g. tianeptine in Czeh et al., 2001); the behavioral effects of antidepressant treatment have been explored elsewhere. In rodent models, adult neurogenesis contributes approximately 5% of the total cells in the dentate gyrus (Lagace et al., 2007b); in humans, however, estimates of total contribution of neurogenesis remain unclear but are generally very low (e.g. Kornack and Rakic, 1999). Therefore, it seems unlikely that the relatively modest neurogenesis in the human could reverse the estimated 10–15% loss of total hippocampal volume observed in MDD clinically (Czeh and Lucassen, 2007). Therefore, while targeting atrophy seems a realistic treatment goal, we do not believe that normalization of neurogenesis alone would be sufficient to overcome atrophy.
This does not mean the concept of targeting adult hippocampal neurogenesis for the treatment of mental illnesses should be abandoned. Rather, the concept should be re-articulated in a more practical way: understanding hippocampal neurogenesis will give us a better understanding of brain plasticity in general, which then might be harnessed to tackle and perhaps prevent a wide variety of disorders, including psychiatric disorders. The next section details obstacles that need to be overcome in order for the translational application of hippocampal neurogenesis to become a reality.
Harnessing hippocampal neurogenesis: obstacles to overcome
Many obstacles stand in the way of fully harnessing hippocampal neurogenesis for the treatment of mental illness and other brain disorders. One of the most glaring knowledge gaps is the lack of understanding about differences between rodent and human neurogenesis. More specifically, almost nothing is known about human neurogenesis except that it persists into adulthood (Eriksson et al., 1998; Manganas et al., 2007). More information is needed on the extent of neurogenesis, the function of adult-generated neurons, the location and function of the adult stem cell, and even whether the stages of neurogenesis that are so well-characterized in the rodent are recapitulated in the human. We need to understand these fundamental concepts before we can fully appreciate how these dependent measures are changed in mental illness. For example, the recent pioneering study in human MDD patients examined the number of dividing Ki-67- and nestin-expressing cells (Boldrini et al., 2009). However, it remains to be proven in the human and even in the rodent brain whether nestin expression is a sufficient requirement to label the stem cells. We also need better understanding of how new neurons integrate into hippocampal circuitry. To this end, more functional approaches combined with computational modeling may provide critical insight (Gradin and Pomi, 2008). A more thorough examination of human post-mortem samples is also desperately needed to better understand human neurogenesis and how it is similar to, and distinct from, rodent neurogenesis.
On a related note, more work is needed on precisely what other neurogenic niches exist in the basal (Cameron and Dayer, 2008; Gould, 2007) or injured brain (Magavi and Macklis, 2002). Such information in the rat or mouse brain but especially in the human brain would be immensely useful for understanding the etiology of mental illnesses as well as delineating treatment options and limitations. The importance of the niche reminds us that while in vitro work will remain invaluable for the mechanistic insight it can provide to us about adult hippocampal neurogenesis, it is necessary to pursue as much work in vivo or in situ as possible. In fact, there is growing appreciation that while “stem cell genes” have been identified, the concept of “stemness” in fact may rely solely on the niche in which the cell develops (Lander, 2009). Powerful new tools for imaging new neurons in the living brain are likely to help bridge this knowledge gap (Manganas et al., 2007; Pereira et al., 2007), and such data would bring us closer to being able to direct new neurons to places of primary pathology in each disease, and to design approaches to specifically target select stages of neurogenesis.
It is also critical to improve our fundamental understanding of mental illnesses and their pharmacological treatments (Dhikav and Anand, 2007), and to parlay key aspects of the disorders into common use in basic animal models. For example, one key assessment lacking in the MDD and PTSD literature is whether there is a heterogeneous response of neurogenesis to stress, though a heterogeneous behavioral response has been described (Krishnan et al., 2007). Given the differences in allostatic load according to experience (McEwen, 2007), examination of whether neurogenesis is correlated with – or perhaps causative to – the development of individual susceptibility or resilience to stress is a high priority for the field.
A more complete understanding of disease pathophysiology and neuromechanisms of effective treatments will also require additional and more complete model systems. For example, the prairie vole has a well-delineated social structure and can provide an excellent model for depression, anxiety, social isolation, and perhaps even PTSD (e.g. Grippo et al., 2008). Also, recently-developed mouse models to track and inducibly manipulate stem cells and their progeny need to be incorporated with existing disease models to greatly advance our understanding of the complex interplay between disease models and neurogenesis (Lagace et al., 2007b; Mori et al., 2006). Even more commonly-used approaches to suppress hippocampal neurogenesis, like cranial irradiation, are proving very useful in uncovering novel roles for adult neurogenesis in psychiatric disorders like addiction (Noonan et al., in press). Perhaps using such approaches more widely and rigorously will allow us to learn more about how we might encourage migration of SGZ-generated neurons into nearby regions, as has been shown in other brain regions (Yamashita et al., 2006). This would potentially allow directed migration of adult-generated neurons to the site of pathology in each brain disorder, vastly enhancing the utility of this approach for translational use.
Another critical obstacle to address is the differences in incidence and treatment of mental disorders by sex. It is clear that sex steroids exert potent influences on the dentate gyrus physiology and in stress responses (Goel and Bale, 2009; Hajszan et al., 2007). For example, sex appears to play a critical role in mental disorders and in regulation of neurogenesis in the rat and human (Boldrini et al., 2009; Galea, 2008). As the mouse is a major model species in neuroscience, it is notable that evidence for sex differences in the mouse remains inconclusive (Lagace et al., 2007a; Sakata et al., 2009; Silasi et al., 2004). Given the demonstrated implications that phytoestrogens have on laboratory animal and human cognition and behavior (e.g. Lephart et al., 2002; Patisaul and Polston, 2008), it will be critical for future preclinical studies to account for phytoestrogens in laboratory animal chow as this will make translational application of the data more feasible.
While many of the obstacles noted above relate to human neurogenesis and translational efforts, one final obstacle falls squarely in the realm of the basic researchers. We also need a more complete understanding the function of adult-generated neurons, and the dentate gyrus as a whole. This likely will emerge as researchers employ better models of inducible and reversible ablation of neurogenesis (e.g. Dupret et al., 2008; Saxe et al., 2006; Singer et al., 2009), and as cleaner and more sophisticated ways to assess hippocampal function in behavioral tasks are developed.
Present and future approaches to the promote hippocampal neurogenesis
Even if adult hippocampal neurogenesis is not an immediate target for the amelioration of the symptoms and trajectory of mental disorders, it does makes sense both to study it and harness it where feasible and practical, and to in general promote hippocampal health. Indeed, driving hippocampal neurogenesis is tightly correlated with other things presumed to be good for the hippocampus, such as angiogenesis, blood flow, and growth factor production and cytokine reduction (e.g. Pereira et al., 2007). We offer a few words on this topic in order to highlight some commonly-used approaches as well as developing techniques to enhance neurogenesis.
One of the most clear-cut ways to both enhance adult hippocampal neurogenesis and thus perhaps hippocampal plasticity and health is via exercise (Fabel and Kempermann, 2008; Pietropaolo et al., 2008; van Praag, 2008). For example, exercise is an effective antidepressant, and it enhances neurogenesis even under conditions of forced exercise. Environmental enrichment, also referred to as environmental complexity, is another robust way of promoting neurogenesis and hippocampal plasticity. Interestingly, environmental enrichment and voluntary exercise promote hippocampal neurogenesis via dissociable pathways (Olson et al., 2006), indicating there are likely multiple pathways to enhanced neurogenesis that remain to be understood. These strong links between exercise and enhanced neurogenesis are quite clinically compelling since exercise is an effective treatment for many mental disorders, including MDD and Alzheimer’s Disease (Andrade and Radhakrishnan, 2009; Mead et al., 2009).
On a molecular basis, it will be increasingly important to identify cell-intrinsic and niche factors important to discrete stages of neurogenesis, such as the recently identified Cdk5, NeuroD, Sox2, Notch, and CREB (Favaro et al., 2009; Gao et al., 2009; Jagasia et al., 2009; Lagace et al., 2008). CREB is particularly intriguing in this regard because it drives brain derived neurotrophic factor (BDNF), a factor strongly correlated with neurogenesis in animal models and decreased in post-mortem tissue from MDD patients (Brunoni et al., 2008; Duman, 2004). A final approach that may be utilized to drive adult neurogenesis is the application of stem cell technology to enhance neurogenesis, either via viral-mediated gene transfer or nanoparticles as has been done for other stem cell populations (e.g. Brown and Naldini, 2009; Yang et al., 2009).
Conclusion
Because the primary pathology in MDD, PTSD, schizophrenia and Alzheimer’s Disease is not an early and overt loss of dentate gyrus granule cells, it is obvious that dentate gyrus granule cell replacement strategies via stimulation of adult hippocampal neurogenesis alone will not be sufficient for treatment of these disorders. However, the general consensus among researchers and physicians is that activities that promote overall health and wellness, such as physical and mental exercises, are beneficial to hippocampal health and can help prevent or combat mental illness (e.g. Valenzuela et al., 2008). As these activities enhance neurogenesis in animal models, they may very well enhance neurogenesis in humans too. Thus, the advancement of our understanding of adult hippocampal neurogenesis has and will likely continue to reveal neuromechanisms of plasticity that can then be co-opted for translational application to these and other disorders, such as addiction and epilepsy, to help restore or enhance hippocampal plasticity.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85(2):523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–7. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317(5839):819–23. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–91. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- Andrade C, Radhakrishnan R. The prevention and treatment of cognitive decline and dementia: An overview of recent research on experimental treatments. Indian J Psychiatry. 2009;51(1):12–25. doi: 10.4103/0019-5545.44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango C, Kirkpatrick B, Koenig J. At issue: stress, hippocampal neuronal turnover, and neuropsychiatric disorders. Schizophr Bull. 2001;27(3):477–80. doi: 10.1093/oxfordjournals.schbul.a006888. [DOI] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33(3):232–52. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6(5):311–20. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18(3–4):253–81. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11(2):70–6. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Bloom FE. Modern concepts in electrophysiology for psychiatry. Psychopharmacol Commun. 1975;1(6):579–85. [PubMed] [Google Scholar]
- Bloom FE. Endorphins and psychiatry: pre-clinical perspectives. Psychiatr Dev. 1984;2(1):1–21. [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–89. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Alterations in brain structure and function associated with post-traumatic stress disorder. Semin Clin Neuropsychiatry. 1999;4(4):249–55. doi: 10.153/SCNP00400249. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445–61. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(51):20558–63. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet. 2009;10(8):578–85. doi: 10.1038/nrg2628. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18(2):93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11(8):1169–80. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Dayer AG. New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry. 2008;63(7):650–5. doi: 10.1016/j.biopsych.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29(6):417–26. [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):250–60. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98(22):12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhikav V, Anand KS. Is hippocampal atrophy a future drug target? Med Hypotheses. 2007;68(6):1300–6. doi: 10.1016/j.mehy.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. DISC1 puts the brakes on neurogenesis. Cell. 2007;130(6):981–3. doi: 10.1016/j.cell.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 2007;6(3):205–18. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin NJ. The neurobiology of memory based predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1183–91. doi: 10.1098/rstb.2008.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28(46):11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Mandyam CD. Adult neurogenesis: can analysis of cell cycle proteins move us “Beyond BrdU”? Curr Pharm Biotechnol. 2007;8(3):147–65. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- Elder GA, De Gasperi R, Gama Sosa MA. Research update: neurogenesis in adult brain and neuropsychiatric disorders. Mt Sinai J Med. 2006;73(7):931–40. [PubMed] [Google Scholar]
- Enomoto A, Asai N, Namba T, Wang Y, Kato T, Tanaka M, Tatsumi H, Taya S, Tsuboi D, Kuroda K, et al. Roles of disrupted-in-schizophrenia 1-interacting protein girdin in postnatal development of the dentate gyrus. Neuron. 2009;63(6):774–87. doi: 10.1016/j.neuron.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10(2):59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009 doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Feldmann RE, Jr, Sawa A, Seidler GH. Causality of stem cell based neurogenesis and depression--to be or not to be, is that the question? J Psychiatr Res. 2007;41(9):713–23. doi: 10.1016/j.jpsychires.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Frith CD, Done DJ. Towards a neuropsychology of schizophrenia. Br J Psychiatry. 1988;153:437–43. doi: 10.1192/bjp.153.4.437. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 1998;23(2):295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57(2):332–41. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12(9):1090–2. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Eisch AJ. Mouse models of Alzheimer’s disease: insight into treatment. Rev Neurosci. 2004;15(5):353–69. doi: 10.1515/revneuro.2004.15.5.353. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10(2):160–84. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21(4):415–20. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Mitchell CP. What is the functional significance of hippocampal pathology in schizophrenia? Schizophr Bull. 2004;30(2):367–92. doi: 10.1093/oxfordjournals.schbul.a007086. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8(6):481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3(5):186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Pomi A. The role of hippocampal atrophy in depression: a neurocomputational approach. J Biol Phys. 2008;34(1–2):107–20. doi: 10.1007/s10867-008-9099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, Learned Helplessness, and the Stress-Resistant Brain. Neuromolecular Med. 2008 doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25(6):E17–26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog Brain Res. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J Neurosci. 2009;29(20):6545–57. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral Effects of Chronic Fluoxetine in BALB/cJ Mice Do Not Require Adult Hippocampal Neurogenesis or the Serotonin 1A Receptor. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Implications of altered limbic-hypothalamic-pituitary-adrenocortical (LHPA)-function for neurobiology of depression. Acta Psychiatr Scand Suppl. 1988;341:72–111. doi: 10.1111/j.1600-0447.1988.tb08556.x. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29(25):7966–77. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc Natl Acad Sci U S A. 2004a;101(36):13363–7. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004b;101(1):343–7. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Xie L, Mao XO, Greenberg DA. Alzheimer’s disease drugs promote neurogenesis. Brain Res. 2006;1085(1):183–8. doi: 10.1016/j.brainres.2006.02.081. [DOI] [PubMed] [Google Scholar]
- Kamnasaran D, Muir WJ, Ferguson-Smith MA, Cox DW. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J Med Genet. 2003;40(5):325–32. doi: 10.1136/jmg.40.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11(10):1145–59. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63(3):155–64. doi: 10.1016/j.neures.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21(3):290–5. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54(5):499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005a;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005b;352(24):2515–23. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63(6):761–73. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling MA, Kellner CH, Post RM, Cowdry RW, Gardner DL, Coppola R, Putnam FW, Gold PW. Neuroendocrine effects of limbic activation by electrical, spontaneous, and pharmacological modes: relevance to the pathophysiology of affective dysregulation in psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11(4):459–81. doi: 10.1016/0278-5846(87)90016-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39(1):24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768–73. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact. 2008;175(1–3):227–30. doi: 10.1016/j.cbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105(47):18567–71. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007a;17(3):175–80. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007b;27(46):12623–9. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD. The ‘stem cell’ concept: is it holding us back? J Biol. 2009;8(8):70. doi: 10.1186/jbiol177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24(1):5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, Iqbal K, Grundke-Iqbal I. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008a;67(1):78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008b;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–69. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Suzuki T, Seki T, Namba T, Tanimura A, Arai H. Effects of repeated phencyclidine administration on adult hippocampal neurogenesis in the rat. Synapse. 2006;60(1):56–68. doi: 10.1002/syn.20275. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007a;34(2):479–85. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007b;65(3):209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Schulz SC. What we know: findings that every theory of schizophrenia should explain. Schizophr Bull. 2009;35(3):493–508. doi: 10.1093/schbul/sbp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Sugino H, Hirose T, Kitagawa H, Nagai T, Mizoguchi H, Takuma K, Yamada K. Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J Pharmacol Sci. 2007;103(3):299–308. doi: 10.1254/jphs.fp0061424. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Namba T, Suzuki E, Yuasa S, Kohsaka S, Uchino S. NMDA receptor antagonist memantine promotes cell proliferation and production of mature granule neurons in the adult hippocampus. Neurosci Res. 2009a;63(4):259–66. doi: 10.1016/j.neures.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y, Kiso Y, Yoshikawa T, et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One. 2009b;4(4):e5085. doi: 10.1371/journal.pone.0005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magavi SS, Macklis JD. Induction of neuronal type-specific neurogenesis in the cerebral cortex of adult mice: manipulation of neural precursors in situ. Brain Res Dev Brain Res. 2002;134(1–2):57–76. doi: 10.1016/s0165-3806(01)00316-9. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318(5852):980–5. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–31. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc Natl Acad Sci U S A. 2007;104(30):12506–11. doi: 10.1073/pnas.0705346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gould EA, Sakai RR. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry Suppl. 1992;15:18–23. [PubMed] [Google Scholar]
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev. 2009;(3):CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239(4841 Pt 1):766–8. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13(3):187–94. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. DISC1 partners with GSK3beta in neurogenesis. Cell. 2009;136(6):990–2. doi: 10.1016/j.cell.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. Amyloid, memory and neurogenesis. Exp Neurol. 2007;205(2):330–5. doi: 10.1016/j.expneurol.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia. 2006;54(1):21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- Nakata K, Lipska BK, Hyde TM, Ye T, Newburn EN, Morita Y, Vakkalanka R, Barenboim M, Sei Y, Weinberger DR, et al. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc Natl Acad Sci U S A. 2009;106(37):15873–8. doi: 10.1073/pnas.0903413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T, Maekawa M, Yuasa S, Kohsaka S, Uchino S. The Alzheimer’s disease drug memantine increases the number of radial glia-like progenitor cells in adult hippocampus. Glia. 2009;57(10):1082–90. doi: 10.1002/glia.20831. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of Adult Hippocampal Neurogenesis Confers Vulnerability in an Animal Model of Cocaine Addiction. J Neurosci. doi: 10.1523/JNEUROSCI.4256-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Parent JM, Jessberger S, Gage FH, Gong C. Is neurogenesis reparative after status epilepticus? Epilepsia 48 Suppl. 2007;8:69–71. doi: 10.1111/j.1528-1167.2007.01355.x. [DOI] [PubMed] [Google Scholar]
- Parent JM, Murphy GG. Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia 49 Suppl. 2008;5:19–25. doi: 10.1111/j.1528-1167.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- Pathania M, Yan LD, Bordey A. A symphony of signals directs early and late stages of adult neurogenesis. Neuropharmacology. doi: 10.1016/j.neuropharm.2010.01.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Polston EK. Influence of endocrine active compounds on the developing rodent brain. Brain Res Rev. 2008;57(2):352–62. doi: 10.1016/j.brainresrev.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27(18):4894–901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Park S, Nemirovskaya Y. Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist. 2008;14(4):326–38. doi: 10.1177/1073858408317242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Marsh L. Structural brain imaging in schizophrenia. Clin Neurosci. 1995;3(2):105–11. [PubMed] [Google Scholar]
- Pickard BS, Christoforou A, Thomson PA, Fawkes A, Evans KL, Morris SW, Porteous DJ, Blackwood DH, Muir WJ. Interacting haplotypes at the NPAS3 locus alter risk of schizophrenia and bipolar disorder. Mol Psychiatry. 2009;14(9):874–84. doi: 10.1038/mp.2008.24. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, et al. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci U S A. 2005;102(39):14052–7. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192(1):42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of Mood Disorders. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11(5):514–22. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Reif A, Schmitt A, Fritzen S, Lesch KP. Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):290–9. doi: 10.1007/s00406-007-0733-3. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–5. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Hippocampal neurogenesis and depression. Novartis Found Symp. 2008;289:152–60. doi: 10.1002/9780470751251.ch12. discussion 160–4, 193–5. [DOI] [PubMed] [Google Scholar]
- Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Fujita T, Iwai M, Ito M, Horiuchi M. Sex -different effect of angiotensin II type 2 receptor on ischemic brain injury and cognitive function. Brain Res. 2009 doi: 10.1016/j.brainres.2009.08.068. [DOI] [PubMed] [Google Scholar]
- Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, Brambilla P. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14(5):393–405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000a;57(10):925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000b;48(8):755–65. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EL, Novaes BA, da Silva ER, Skaf HD, Mendes-Neto AG. Strategies to promote differentiation of newborn neurons into mature functional cells in Alzheimer brain. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(7):1087–102. doi: 10.1016/j.pnpbp.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315(5810):336–8. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20(6):553–7. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silasi G, Diaz-Heijtz R, Besplug J, Rodriguez-Juarez R, Titov V, Kolb B, Kovalchuk O. Selective brain responses to acute and chronic low-dose X-ray irradiation in males and females. Biochem Biophys Res Commun. 2004;325(4):1223–35. doi: 10.1016/j.bbrc.2004.10.166. [DOI] [PubMed] [Google Scholar]
- Singer BH, Jutkiewicz EM, Fuller CL, Lichtenwalner RJ, Zhang H, Velander AJ, Li X, Gnegy ME, Burant CF, Parent JM. Conditional ablation and recovery of forebrain neurogenesis in the mouse. J Comp Neurol. 2009;514(6):567–82. doi: 10.1002/cne.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–7. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: a tale of brain and body Part 2: animal models. Neurosci Biobehav Rev. 2007;31(4):558–84. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Takemura NU, Kato N. Adult neurogenesis and systemic adaptation: animal experiments and clinical perspectives for PTSD. Prog Brain Res. 2008;167:99–109. doi: 10.1016/S0079-6123(07)67007-1. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Peterson DA. Even neural stem cells get the blues: evidence for a molecular link between modulation of adult neurogenesis and depression. Gene Expr. 2008;14(3):183–93. [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Boekhoorn K, Van Dam AM, Lucassen PJ. Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes Brain Behav 7 Suppl. 2008;1:28–42. doi: 10.1111/j.1601-183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Fernandes K, Jha S. Regulation of adult hippocampal neurogenesis: relevance to depression. Expert Rev Neurother. 2007;7(7):853–64. doi: 10.1586/14737175.7.7.853. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P, Wen W, Chen X, Brodaty H. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi: 10.1371/journal.pone.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and Exercise: Past and Future Directions. Neuromolecular Med. 2008 doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- Villarreal G, King CY. Brain imaging in posttraumatic stress disorder. Semin Clin Neuropsychiatry. 2001;6(2):131–45. doi: 10.1053/scnp.2001.21840. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Mahlstedt MM, Henn FA. Neurogenesis and depression: what animal models tell us about the link. Eur Arch Psychiatry Clin Neurosci. 2007;257(5):300–3. doi: 10.1007/s00406-007-0734-2. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–84. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Zhang RL, Zhang L, Letourneau Y, Feng YF, Jiang A, Morris DC, Zhang ZG. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience. 2009;158(4):1356–63. doi: 10.1016/j.neuroscience.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L. Assisted birth with DISC1. Nat Rev Neurosci. 2009;10(5):314. doi: 10.1038/nrn2640. [DOI] [PubMed] [Google Scholar]
- Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, Soma M, Takao K, Tanda K, Ohira K, Toyama K, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1(1):6. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26(24):6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, et al. Regenerative Medicine Special Feature: Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, He J, Zhang Y, Luo H, Zhu S, Yang Y, Zhao T, Wu J, Huang Y, Kong J, et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus. 2009 doi: 10.1002/hipo.20587. [DOI] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007;204(1):77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.