Abstract
Background
Kaposi sarcoma (KS) is one of the most common pediatric cancers in sub-Saharan Africa. Few data are available about the clinical presentation or response to treatment of children with epidemic (HIV-associated) KS.
Methods
Medical records of all children with KS and HIV infection referred to the Uganda Cancer Institute in Kampala, Uganda from October 2004 to June 2007 were reviewed. Charts were abstracted for age, sex, location of KS lesions at presentation, biopsy results, CD4 T-cell count and percentage, and KS treatment and outcome.
Results
Seventy-three children with epidemic KS were identified, 37 males and 36 females. The median age was 10.1 years (range 2 - 18). KS presented with lymph node involvement in 60% of cases. The median absolute and percentage CD4 T-cells at presentation were 210 cells/uL and 7.4%, respectively. Those children with lymphadenopathic KS were younger (mean difference 3.7 years; p = 0.01) and had higher CD4 T-cell counts (mean difference 242 cells/uL; p = 0.03) than those without lymph node involvement. Of 32 patients for whom outcome data were available, a complete response to chemotherapy and/or antiretroviral therapy was documented in 20 (62.5%) patients.
Conclusions
In comparison to cutaneous involvement, lymph node involvement of epidemic KS occurs at younger ages and at higher CD4 levels. This clinical presentation may reflect recent infection with human herpesvirus 8 followed by a rapid progression to malignancy. Favorable response to treatment was observed in the majority of cases, but prospective studies are needed to determine optimal management.
Keywords: Epidemic Kaposi sarcoma, human herpesvirus 8, HIV, children, lymphadenopathy
Introduction
Kaposi sarcoma (KS), a vascular tumor caused by infection with human herpesvirus 8 (HHV-8; also called KS-associated herpesvirus), is the most common malignancy among HIV-infected people worldwide [1,2]. Although KS was rare in most parts of the world prior to the HIV/AIDS epidemic[3], an endemic form of KS was described in Uganda and other African countries over 40 years ago [4-8]. Endemic KS occurs more often in adults, but has been reported to be more aggressive in children [6,9]. In Uganda, the incidence of pediatric KS has increased approximately 20-fold during the HIV/AIDS epidemic and is now among the most common childhood cancers [2,8,10].
Few series of epidemic KS have compared the clinical manifestations in children compared to adults [6,9-14]. While cutaneous lesions are observed in >90% of adults with KS in the United States, Europe and Australia, cutaneous KS appears to be less common in children with or without HIV infection [6,10,11,13]. Conversely, KS in young children predominantly involves the lymph nodes (LN), which is an atypical presentation in adults [14-16].
The outcome of epidemic KS in children has not been well characterized. The response of KS to ART alone or with chemotherapy in HIV infected adults is variable, and tends to be less effective in severely immunocompromised patients [17,18]. The optimal management for children with KS have largely been extrapolated from experience in adult patients. Here we describe the clinical characteristics, immune status, and outcomes of large series of HIV-infected children with KS in Uganda.
Methods
Subjects and data collection
Children (≤ 18 years old) with KS and HIV infection who were referred to the Uganda Cancer Institute (UCI) from October 2004 to June 2007 were identified by review of clinical records. HIV infection was confirmed using two rapid tests according to Ugandan national guidelines. For the purposes of this study, children with biopsy results consistent with KS and/or a documented clinical diagnosis of KS by a physician were considered to have KS. Abstraction of medical records was performed using a standardized form to capture the following data: age, sex, location of KS lesions, biopsy results, CD4 T-cell count and percentage, treatment with cancer chemotherapy and antiretroviral therapy, and response to treatment. Patients were categorized as presenting with one or more locations of KS involvement: cutaneous, LN, oral cavity, and visceral. Location of lesions was determined by physician's assessment in the medical record. Response to treatment was determined according to the last available assessment documented in the medical record for each patient, and categorized as: “complete” if there was complete resolution of KS lesions; “partial” if there was any reduction in the size and/or number of lesions; “none” if there was no response or a worsening of any lesions; or “unknown” if this information was not recorded. Institutional Review Board approval was obtained from the University of Washington, Makerere University, Mulago Hospital, and the Ugandan National Council for Science and Technology.
Statistical analysis
The t test for unequal variance was used to compare means, Chi square and Fisher's exact test were used to compare proportions, and Spearman's rank correlation or linear regression with robust standard errors was used to test the association between continuous variables. In order to explore the correlates of KS presentation (LN, skin, oral cavity, and visceral involvement), logistic regression with robust standard errors was also used. The independent variables evaluated were age, sex, and CD4 T-cell count. Those variables that were associated with KS presentation with a p value < 0.05 by univariate analysis were included in a multivariate logistic regression analysis. Analyses were performed using Stata 9.2 (College Station, TX).
Results
Characteristics of epidemic pediatric KS
Seventy-three children with KS and HIV infection were identified (Table I), with a median age of 10.1 years (mean = 9.4, standard deviation = 4.4). Twenty-six (36%) patients had biopsy results recorded, all of which were histopathologically-confirmed as KS. Males accounted for 37 (51%) and females for 36 (49%) of the patients. The percentage of children that had documented KS involvement in each of the locations were: LN, 60%; skin, 48%; oral cavity, 21%; and viscera, 12% (Table I).
Table I.
Characteristics of all cases of epidemic pediatric Kaposi sarcoma identified at the Uganda Cancer Institute, Kampala, Uganda over a 32 month period between 2004 and 2007
| Total | Malesa | Females | |
|---|---|---|---|
| KS cases, n (percent) | 73 (100%) | 37 (50.7%) | 36 (49.3%) |
| Age in years, median (range), n=56 | 10.1 (2 – 18) | 9.3 (2 – 16) | 11.0 (2 – 18) |
| CD4 T-cells/uL, median (IQR) n=36 | 210 (21 – 482) | 165 (16 – 538) | 263 (26 – 464) |
| CD4%, median (IQR) n=35 | 7.4 (1.2 – 13.5) | 8.2 (1.9 – 11.1) | 6.7 (1.2 – 14.7) |
| Location of lesions n=42b | |||
| Skin involvement | 20 (47.6%) | 10 (45.5%) | 10 (50.0%) |
| Oral cavity involvement | 9 (21.4%) | 4 (20.0%) | 5 (22.7%) |
| Viscera involvement | 5 (11.9%) | 3 (15.0%) | 2 (9.1%) |
| Lymph node involvement | 25 (59.5%) | 11 (55.0%) | 14 (58.3%) |
No statistically significant differences were observed between males and females for the characteristics listed.
Categories of lesion location not mutually exclusive.
CD4 T-cell values were available for 35 (48%) of the 73 children, and were obtained a median of 2 weeks from the time of presentation (interquartile range (IQR) = 0 - 6 weeks). The median CD4 T-cell count was 210 cells/uL (IQR = 21 - 482), and the median percent CD4 T-cells was 7.4% (IQR = 1.2 - 13.5%). Older children had lower absolute CD4 T-cell counts (absolute CD4 T-cell count decreased by 30.1 cells/uL on average with each additional year over; 95% CI −52.6, −7.6; p = 0.01) and tended to have lower CD4 T-cell percentages (with each additional year of life, the percent CD4 T-cell count decreased by 0.5% on average; 95% CI −1.0, 0.1; p = 0.06). Eight (11%) patients had been started on ART prior to documentation of KS, with a median of 5 months (range 0.5 - 18) on treatment. There was no difference between those patients who were or were not receiving ART at the time of presentation in the mean absolute (p = 0.99) or percent CD4 T-cells (p = 0.40).
Correlates of KS Presentation
KS was more likely to present as involvement of either LN (17/42, 40%) or skin (12/42, 29%), rather than both locations together (8/42, 19%; p = 0.03). Children with LN involvement were also significantly less likely to have oral cavity involvement (2/25 (8%) vs. 7/10 (70%), p = 0.01). There were no correlations observed between cutaneous and oral cavity involvement, nor between visceral involvement and any other KS presentation.
Associations between each anatomic location of KS and the available clinical characteristics (age, sex, CD4 T-cell values, and having received ART) were evaluated. Location of KS involvement was not associated with the child's sex or receipt of ART. Patients with LN involvement of KS were an average of 3.7 years younger (95% CI 0.9, 6.5 years younger; p = 0.01) than those without LN involvement. In contrast, children with KS skin involvement tended to be older compared with those without cutaneous lesions (11.2 vs. 8.3 years, p = 0.05). The presence of oral cavity or visceral KS involvement did not differ with age. Children with LN involvement had higher absolute CD4 T-cell counts (mean difference 242 cells/uL; 95% CI 51, 434; p = 0.02) and percent CD4 T-cells (mean difference 4.9%; 95% CI 0.5, 9.3; p = 0.03). However, no such trend was observed in CD4 T-cell values between those children with and without other presentations of KS (Figure 1).
Figure 1.
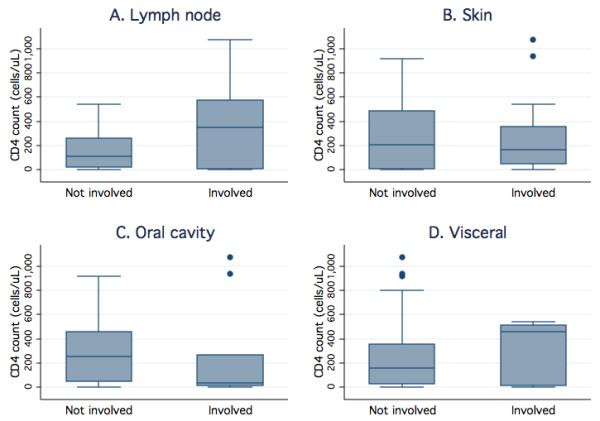
Higher CD4 T-cell counts are associated with KS involvement of lymph nodes, but not other presentations, among HIV-infected children. Box plots display the CD4 T-cell count quartiles (minimum, 25th, 50th, and 75th percentile, and maximum) and outliers (dots) for those patients with and without KS lesions in a given location. CD4 T-cell values are shown for patients with and without KS involvement of the lymph nodes (panel A), skin (panel B), oral cavity (panel C), and viscera (panel D).
In healthy children, CD4 T-cell counts are highest at birth and then decline until adolescence [19]. This decline in CD4 T-cell counts with age is more pronounced among children infected with HIV [19]. To account for potential confounding of age on the observed association between CD4 T-cell count and clinical presentation, therefore, multivariate logistic regression analysis was performed. In the adjusted model, age was no longer significantly associated with lymphadenopathic KS (OR = 0.92; 95% CI 0.75, 1.12; p = 0.39). However, CD4 T-cell count remained predictive of LN involvement of KS, with a 36% increased risk of presenting with lymphadenopathic KS for each increment of 100 cells/uL (OR 1.36, 95% CI 1.01, 1.83; p = 0.05).
Treatment regimens and outcome
Thirty-six (49%) of the children in this cohort were documented to have received cancer chemotherapy (Table II). Thirteen patients received vincristine alone, while an additional 23 received both vincristine and bleomycin. Ten patients received cancer chemotherapy without ART, while 26 received a combination of chemotherapy and ART. Fifteen children received ART alone. Of the ART regimens used, 15 contained a protease inhibitor (all lopinivir/ritonavir) while 26 contained a non-nucleoside reverse transcriptase inhibitor (16 efavirenz and 10 nevirapine). All regimens contained lamivudine, which was combined either with zidovudine (17), stavudine (22), or tenofovir (2).
Table II.
Response of epidemic Kaposi sarcoma in children to chemotherapy and antiretroviral drug combinations
| Responsea, n |
||||
|---|---|---|---|---|
| Therapyb | Complete | Partial | None | Unknown |
| VCR | - | 4 | - | 1 |
| BV | 1 | 1 | - | 3 |
| ART | 2 | - | - | 13 |
| VCR + ART | 4 | 2 | - | 2 |
| BV + ART | 13 | 4 | 1 | - |
| None | - | - | - | 22 |
|
| ||||
| Total | 20 | 11 | 1 | 41 |
Complete response defined as no evidence of residual KS; partial response defined as any appreciable reduction in the size or number of KS lesions; no response defined as the lack of partial response or progression of KS.
VCR = vincristine; BV = bleomycin plus vincristine; ART = antiretroviral therapy, made up of 2 nucleoside reverse transcriptase inhibitors plus either a protease inhibitor (15 patients received ritonavir-boosted lopinavir) or a non-nucleoside reverse transcriptase inhibitor (16 patients received efavirenz and the other 10 received nevirapine).
Thirty-two (46%) children had outcome data available. Compared to those with missing outcome data, children with known outcomes were significantly more likely to have a record of receiving chemotherapy (p = 1.69 × 10−10) or ART (p = 1.35 × 10−4; Table II). However, there was no difference observed with respect to age, sex, CD4 T-cell values, or type of KS presentation between those children with and without available outcome data.
Twenty children (62.5%) of the 32 patients with outcome data available had a complete resolution of KS (Table II). Eleven patients had a partial response, and only one patient had a documented lack of response. No association was apparent between outcome and age, sex or type of KS presentation. Thirty (93.8%) of the 32 patients with outcome data available received cancer chemotherapy (10 with vincristine, 20 with vincristine plus bleomycin). No difference was observed in outcome with respect to whether or not cancer chemotherapy was used, or whether one or two drugs were given. Of those patients with a known outcome, a higher proportion had a complete resolution of KS among those who received any ART regimen compared to those who did not receive ART (19 of 26 vs. 1 of 6; p = 0.02).
Discussion
In this large series of Ugandan children with epidemic KS, LN involvement was observed in the majority of cases. Lymphadenopathic KS appeared to be a discrete clinical presentation, which tended to occur in younger children and at higher CD4 T-cell counts. Of those KS cases with a documented outcome, a large proportion responded to treatment with chemotherapy and/or ART.
In adults with endemic KS, disease is typically limited to the skin, while HIV-associated KS tends to be more aggressive, and oral cavity and visceral lesions are common. Although lymphatic obstruction and lymphedema are frequent features of KS in HIV-infected adults, prominent LN involvement has been reported less often [15,16]. In contrast, we observed LN involvement in the majority of children, which tended to occur more often at younger ages. This association between younger age and KS lymphadenopathy has been reported previously among children both with and without HIV infection [6,9-14].
Lymphadenopathy has been associated with HHV-8 seroconversion in adults [20,21], suggesting that a mononucleosis-like syndrome might occur in some persons with primary HHV-8 infection. An analogous pattern of disease may occur after infection with Epstein-Barr virus (EBV) infection, the most similar human herpesvirus to HHV-8, and the development of EBV-associated Hodgkin lymphoma. Hjalgram et al. [22] found patients with infectious mononucleosis to have a relative risk of EBV-associated Hodgkin disease that peaked in less than 3 years after acquiring EBV infection. Perhaps lymphadenopathic KS in susceptible individuals results from a rapid progression from the onset of HHV-8 infection to the development of malignancy.
Susceptibility to rapid progression to KS would appear to be mediated by factors other than advanced HIV disease, as lymphadenopathic KS was associated with higher CD4 T-cell levels. Furthermore, after adjustment for CD4 T-cell count, age no longer predicted LN involvement of KS. This suggests that, although lymphadenopathic KS is observed more often in children, younger age may not be causal. Rather, the preponderance of lymphadenopathic KS among children may be a function of the early age at which HHV-8 infection is acquired in endemic areas [23-25]. There is evidence that HIV-infected adults who become infected with HHV-8 are more likely to develop KS than those who are infected with HHV-8 prior to HIV [26]. Unlike most adults with KS in Uganda, HIV infection in the majority of these children was likely acquired before HHV-8 infection at birth or through breastfeeding. This temporal difference may contribute to the apparent differences in KS presentation in children compared to those in adults.
Several limitations of this study should be noted. Histopathology reports were documented for only 26 patients. Although biopsy is routinely performed for confirmation of KS diagnosis among patients at the UCI, records were unavailable for some patients. Therefore, some lymphadenopathy may have been due to causes other than KS, including HIV itself [14]. The treatment response rate observed was almost certainly biased by the large number of children lost to follow-up, among whom we expect disproportionately poor outcomes. Furthermore, interpretation of these outcome data is complicated by the lack of information about co-morbidities, length of treatment, and duration of response.
The number of favorable outcomes recorded for children in this series is encouraging. However, prospective studies in children, including controlled trials of antiretroviral and cancer chemotherapy drugs are needed. Children with KS may respond differently to chemotherapy than adults, requiring alternate regimens. Current state of the art regimens used for adult KS patients in resource-rich countries, such as liposomal doxorubicin, have not been evaluated in children and may not be appropriate for use in resource-poor settings [27]. Future studies to elucidate the viral and host factors that lead to KS may result in additional treatment options. Ideally, such knowledge would also inform rational interventions to prevent HHV-8 infection and/or its progression to malignancy.
Acknowledgements
The authors thank Drs. Vincent Tukei and Adeodata Kekitiinwa for their help with patient referrals and identification; Huong Nguyen and Evelyn Babirye for technical assistance; Annet Nakaganda, Erica Sessle and Kirsten Hauge for administrative support. Financial support was provided by the National Institutes of Health (1 KL2 RR 025015-01 (SG), K23 AI 054162-05S1 (CC), K24 AI-071113 (AW), and 5 P01 AI030731-17 (LC)) and the Doris Duke Charitable Foundation (Clinical Scientist Development Award (CC)).
Abbreviations
- KS
Kaposi sarcoma
- LN
lymph node
- HHV-8
human herpesvirus 8
- EBV
Epstein-Barr virus
- ART
antiretroviral therapy
- UCI
Uganda Cancer Institute
Footnotes
Conflict of Interest Statement: none of the authors has affiliations with any organization that has a direct interest in the subject matter discussed.
References
- 1.Cancer incidence in five continents IARC scientific publications. 2002;VIII(155):1–781. [PubMed] [Google Scholar]
- 2.Parkin DM, Wabinga H, Nambooze S, et al. AIDS-related cancers in Africa: maturation of the epidemic in Uganda. Aids. 1999;13(18):2563–2570. doi: 10.1097/00002030-199912240-00010. [DOI] [PubMed] [Google Scholar]
- 3.Beral V, Peterman TA, Berkelman RL, et al. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335(8682):123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 4.Davies JN, Wilson BA, Knowelden J. Cancer incidence of the African population of Kyadondo (Uganda) Lancet. 1962;2:328–330. doi: 10.1016/s0140-6736(62)90111-3. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JF, Smith PG, Bull D, et al. Kaposi's sarcoma in Uganda: geographic and ethnic distribution. Br J Cancer. 1972;26(6):483–497. doi: 10.1038/bjc.1972.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olweny CL, Kaddumukasa A, Atine I, et al. Childhood Kaposi's sarcoma: clinical features and therapy. Br J Cancer. 1976;33(5):555–560. doi: 10.1038/bjc.1976.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wabinga HR, Parkin DM, Wabwire-Mangen F, et al. Cancer in Kampala, Uganda, in 1989-91: changes in incidence in the era of AIDS. Int J Cancer. 1993;54(1):26–36. doi: 10.1002/ijc.2910540106. [DOI] [PubMed] [Google Scholar]
- 8.Wabinga HR, Parkin DM, Wabwire-Mangen F, et al. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. Br J Cancer. 2000;82(9):1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutz W, Stout AP. Kaposi's sarcoma in infants and children. Cancer. 1960;13:684–694. doi: 10.1002/1097-0142(196007/08)13:4<684::aid-cncr2820130408>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler JL, Katongole-Mbidde E. Kaposi's sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer. 1996;65(2):200–203. doi: 10.1002/(SICI)1097-0215(19960117)65:2<200::AID-IJC12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 11.Davies JN, Lothe F. Kaposi's sarcoma in African children. Acta - Unio Internationalis Contra Cancrum. 1962;18:394–399. [PubMed] [Google Scholar]
- 12.Ziegler JL, Templeton AC, Vogel CL. Kaposi's sarcoma: a comparison of classical, endemic, and epidemic forms. Seminars in oncology. 1984;11(1):47–52. [PubMed] [Google Scholar]
- 13.Patil PS, Elem B, Gwavava NJ, et al. The pattern of paediatric malignancy in Zambia (1980-1989): a hospital-based histopathological study. The Journal of tropical medicine and hygiene. 1992;95(2):124–127. [PubMed] [Google Scholar]
- 14.Arkin LM, Cox CM, Kovarik CL. Kaposi's sarcoma in the pediatric population: the critical need for a tissue diagnosis. The Pediatric infectious disease journal. 2009;28(5):426–428. doi: 10.1097/INF.0b013e318193ee21. [DOI] [PubMed] [Google Scholar]
- 15.Friedman-Kien AE, Laubenstein LJ, Rubinstein P, et al. Disseminated Kaposi's sarcoma in homosexual men. Annals of internal medicine. 1982;96(6 Pt 1):693–700. doi: 10.7326/0003-4819-96-6-693. [DOI] [PubMed] [Google Scholar]
- 16.Duprez R, Lacoste V, Briere J, et al. Evidence for a multiclonal origin of multicentric advanced lesions of Kaposi sarcoma. J Natl Cancer Inst. 2007;99(14):1086–1094. doi: 10.1093/jnci/djm045. [DOI] [PubMed] [Google Scholar]
- 17.Stebbing J, Sanitt A, Nelson M, et al. A prognostic index for AIDS-associated Kaposi's sarcoma in the era of highly active antiretroviral therapy. Lancet. 2006;367(9521):1495–1502. doi: 10.1016/S0140-6736(06)68649-2. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen HQ, Magaret AS, Kitahata MM, et al. Persistent Kaposi sarcoma in the era of highly active antiretroviral therapy: characterizing the predictors of clinical response. Aids. 2008;22(8):937–945. doi: 10.1097/QAD.0b013e3282ff6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Embree J, Bwayo J, Nagelkerke N, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. The Pediatric infectious disease journal. 2001;20(4):397–403. doi: 10.1097/00006454-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Wang QJ, Jenkins FJ, Jacobson LP, et al. Primary human herpesvirus 8 infection generates a broadly specific CD8(+) T-cell response to viral lytic cycle proteins. Blood. 2001;97(8):2366–2373. doi: 10.1182/blood.v97.8.2366. [DOI] [PubMed] [Google Scholar]
- 21.Casper C, Wald A, Pauk J, et al. Correlates of prevalent and incident Kaposi's sarcoma-associated herpesvirus infection in men who have sex with men. J Infect Dis. 2002;185(7):990–993. doi: 10.1086/339605. [DOI] [PubMed] [Google Scholar]
- 22.Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349(14):1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 23.Olsen SJ, Chang Y, Moore PS, et al. Increasing Kaposi's sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi's sarcoma endemic region, Zambia in 1985. Aids. 1998;12(14):1921–1925. doi: 10.1097/00002030-199814000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Mayama S, Cuevas LE, Sheldon J, et al. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77(6):817–820. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Mbulaiteye SM, Pfeiffer RM, Whitby D, et al. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187(11):1780–1785. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 26.Rezza G, Dorrucci M, Serraino D, et al. Incidence of Kaposi's sarcoma and HHV-8 seroprevalence among homosexual men with known dates of HIV seroconversion. Italian Seroconversion Study. Aids. 2000;14(11):1647–1653. doi: 10.1097/00002030-200007280-00021. [DOI] [PubMed] [Google Scholar]
- 27.Dedicoat M, Vaithilingum M, Newton R. Treatment of Kaposi's sarcoma in HIV-1 infected individuals with emphasis on resource poor settings. Cochrane database of systematic reviews (Online) 2003;(3):CD003256. doi: 10.1002/14651858.CD003256. [DOI] [PubMed] [Google Scholar]


