Abstract
The present cross-sectional study evaluated whether people who engage in vigorous-intensity exercise are better able to regulate negative affective states, thereby changing core maintenance factors of smoking. Participants were a community sample of adults (n = 270) who completed self-report measures of physical activity, cigarette smoking, anxiety sensitivity, and negative affect. Consistent with hypothesis, vigorous-intensity exercise was related to lower levels of cigarette smoking, accounting for 10% of the variance in smoking. Additionally, negative affect mediated the relationship between vigorous-intensity physical activity and cigarette smoking, accounting for about 12% of this relation. Furthermore, these relationships were stronger for individuals with high anxiety sensitivity than for those with low anxiety sensitivity; including anxiety sensitivity as a moderator of the mediated relationship increased the amount of variance accounted for by negative affect to 17%. The findings are discussed in relation to developing further scientific insight into the mechanisms and pathways relevant to understanding the association among vigorous-intensity exercise, smoking, and emotional vulnerability.
Keywords: Exercise, Physical Activity, Smoking, Negative Affect, Anxiety Sensitivity
1. Introduction
Growing evidence points to the role of negative affect in the maintenance of smoking and smoking cessation relapse. For example, when asked about triggers of smoking cessation relapse smokers consistently point to the experience of stress and negative affect (Brandon & Baker, 1991; Piper et al., 2004). These retrospective reports are complemented by prospective studies that indicate that negative affect is an important precipitating factor in smoking lapses and relapses. Specifically, ecological momentary assessments from 215 smokers collected during the two weeks before and four weeks after initiation of smoking cessation treatment indicate that abrupt increases in negative affect are associated with smoking lapses (Shiffman & Waters, 2004). Similarly, baseline negative affect and increases in negative affect during treatment have been shown to be the most reliable predictors of relapse in clinical trials of smoking cessation treatments (Covey, Glassman, & Stetner, 1990; Hitsman et al., 1999; Kahler et al., 2002; Lerman et al., 2002; Niaura et al., 2001; Zelman, Brandon, Jorenby, & Baker, 1992; Zvolensky et al., 2008). Lastly, reducing negative affect during smoking cessation treatment has been shown to improve abstinence outcomes both with psychological interventions (e.g., Fergusson, Goodwin, & Horwood, 2003; Hall, Muñoz, & Reus, 1994; Haas, Muñoz, Humfleet, Reus, & Hall, 2004) and pharmacological interventions (e.g., Hughes, Stead, & Lancaster, 2007; Prochazka, Kick, Steinbrunn, Miyoshi, & Fryer, 2004; Richmond & Zwar, 2003). Collectively, these findings suggests that addressing negative affect in smokers may be important especially for those who are more prone to experience negative affect (Brown et al., 2001; Haas et al., 2004).
The relatively poor outcomes of standard smoking cessation treatments (Fiore, 2000; Hughes, Keeley, & Naud, 2004; Piasecki, 2006) combined with the observation that targeting negative affect during treatment may be critical to cessation success for many smokers (i.e., those who are prone to experience negative affect) provide justification for investigating the utility of exercise as an intervention for smoking cessation. Indeed, exercise is associated with reduced negative affect (Focht, Knapp, Gavin, Raedeke, & Hickner, 2007; Hassmen, Koivula, & Uutela, 2000; Penedo & Dahn, 2005; Reed & Ones, 2006; Schlicht, 1994) and more importantly, exercise interventions have shown efficacy for the treatment of mood and anxiety problems (Smits et al., 2008; Stathopoulou, Powers, Berry, Smits, & Otto, 2006; Broocks et al., 1998; Martinsen, Hoffart, & Solberg, 1989a, 1989b). Furthermore, cross-sectional surveys have consistently shown a negative relationship between physical activity levels and smoking (e.g. Boutelle, Murray, Jeffery, Hennrikus, & Lando, 2000; Boyle, O’Connor, Pronk, & Tan, 2000; Hu et al., 2002). Likewise, there is initial evidence from randomized controlled trials indicating that exercise interventions can decrease withdrawal symptoms and negative affect in smokers (Bock, Marcus, King, Borrelli, & Roberts, 1999; Schneider, Spring, & Pagoto, 2007; Taylor, Ussher, & Faulkner, 2007) as well as improve smoking cessation outcomes among adults receiving standard cessation treatments (cf. Ussher, Taylor, & Faulkner, 2008; Marcus, Albrecht, Niaura, Abrams, & Thompson 1991; Marcus et al., 1995; Marcus et al., 1999; Martin, Kalfas, & Patten, 1997). For example, Marcus and colleagues (1999) randomized 281 sedentary female smokers to either a 12-week cognitive-behavioral smoking cessation program with vigorous-intensity exercise (three sessions a week of 30 to 40 minutes at 60–85% of heart rate reserve), or a 12-week cognitive-behavioral smoking cessation program with contact control (three 45–60 minute health education sessions a week). All participants initiated the intervention three weeks prior to the quit date of the smoking cessation program. Results revealed that participants receiving the exercise intervention were more likely than participants in the control intervention to be continuously abstinent during the 8, 20, and 60 weeks following the quit date. Unfortunately, neither this study nor other studies in this area have investigated whether the association between exercise and reduced smoking is accounted for by reductions in negative affect. Evidence for this mediational hypothesis would help determine whether exercise is a viable option for smokers for whom negative affect operates prominently in the maintenance of smoking and smoking cessation relapse.
This study aimed to provide a preliminary test of the hypothesis that the association between exercise and smoking is, at least in part, accounted for by reduced negative affect. Using cross-sectional data, we examined self-reported negative affect as a mediator of the relationship between self-reported vigorous-intensity exercise levels and smoking. We chose to evaluate the relationship between vigorous-intensity exercise and smoking because there is evidence to suggest that the association between exercise and cigarette smoking is stronger for vigorous-intensity exercise relative to moderate- or low-intensity exercise (cf. Kaczynski, Manske, Mannell, & Grewal, 2008). We also investigated the possibility that the strength of these meditational effects would vary as a function of anxiety sensitivity. Anxiety sensitivity, conceptualized as an emotional vulnerability variable, is a relatively stable trait (Peterson & Plehn, 1999; Weems, Hayward, Killen, & Taylor, 2002) characterized by the fear of both anxiety and related autonomic arousal sensations (e.g., racing heart, sweating, nausea; Reiss, Peterson, Gursky, & McNally, 1986). We selected anxiety sensitivity as a possible moderator of the hypothesized mechanism because of the increasing evidence that individuals with elevated levels of anxiety sensitivity, relative to persons with low levels of anxiety sensitivity, are more likely to smoke in response to negative affect (Brown, Kahler, Zvolensky, Lejuez, & Ramsey, 2001; Brown, Lejuez, Kahler, & Strong, 2002; Novak, Burgess, Clark, Zvolensky, & Brown, 2003; Zvolensky, Bonn-Miller, Bernstein, & Marshall, 2006). Furthermore, smokers with higher levels of anxiety sensitivity are more likely to report negative affect reduction as a smoking outcome expectancy than smokers with lower levels of anxiety sensitivity (Brown et al., 2001; Zvolensky et al., 2007). Accordingly, negative affect reduction as a mechanism underlying the relationship between exercise and smoking may be more salient for individuals with high versus low anxiety sensitivity. We tested the following specific hypotheses: (1) vigorous-intensity exercise engagement would be associated with decreased smoking; (2) the relationship between vigorous-intensity exercise engagement and smoking would be partially mediated by negative affect; and (3) anxiety sensitivity would moderate these mediated relationships such that the mediational role of negative affect would be stronger for individuals with high levels of anxiety sensitivity relative to those with low levels of anxiety sensitivity. Based on the available evidence, we predicted that anxiety sensitivity would moderate the relationship between negative affect and smoking (i.e. the “b” path, see Figure 3) as opposed to the relationship between exercise and negative affect (i.e. the “a” path, see Figure 3).
Figure 3.
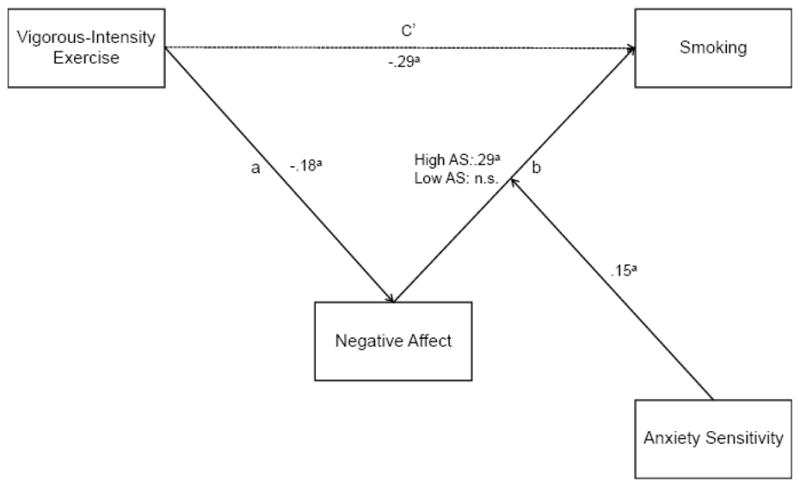
The meditational effects of negative affect as a function of anxiety sensitivity (AS). Note: ap < .01.
2. Material and methods
2.1. Participants
The sample consisted of 270 young adult smokers and non-smokers (see Table 1). Interested persons responded to advertisements for a study on emotional vulnerability within the greater Burlington, Vermont community. Exclusion criteria for the current study included: (1) limited mental competency or the inability to provide informed, written consent; (2) current suicidal or homicidal ideation; (3) current or past history of psychosis; (4) current (past 6-month) Axis I psychopathology (except for substance use disorders); (5) current major medical problems (e.g., heart disease, cancer); (6) current substance dependence (other than nicotine); and (7) self-reported pregnancy.
Table 1.
Demographics of Sample
| Variables | |
|---|---|
| Age | |
| M | 22.4 |
| SD | 9.0 |
| Gender | |
| % Female | 52.6 |
| Race | |
| % White | 90.4 |
| Education | |
| % H.S. Diploma/GED or less | 77.8 |
| % Some college or more | 21.2 |
The racial distribution of the sample generally reflected that of the State of Vermont (State of Vermont Department of Health, 2007; see Table 1). Those that identified themselves as smokers (approximately 50%) averaged 12.99 cigarettes per day (SD = 7.61) with a mean age of onset for daily cigarette use of 16.20 (SD = 3.15) years of age. Mean expired air CO levels among smokers in this sample was 15.3 ppm (2.8%), which is consistent with that of a regular daily smoker (10 ppm cutoff; Cocores, 1993). The mean score on the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) among smokers was 3.53 (SD = 2.05), indicating a relatively low-level of nicotine dependence.
2.2. Measures
2.2.1. Diagnostic screen
The Structured Clinical Interview-Non-Patient Version for DSM-IV (SCID-N/P; First & Gibbon, 2004) screening questions were administered to rule out psychopathology and assess for current suicidal ideation (see exclusionary criteria).
2.2.2. Vigorous-intensity exercise
The Exercise Habits Questionnaire Revised (EHQ-R; Zvolensky, 2008) is a self-report measure used to obtain information about participants’ engagement in physical activity. The EHQ-R asks respondents to indicate for 29 different physical activities (e.g., running, stair stepping, walking/hiking, swimming, hockey, golf, martial arts, rock climbing, yoga) the number of sessions they have completed in the past two weeks as well as the time spent per session (e.g., less than 20 minutes; 20–29 minutes; 30–39 minutes; 40–49 minutes; 50 minutes or more). This information was used in combination with the compendium of physical activities (Ainsworth et al., 2000) to calculate total minutes of weekly vigorous-intensity exercise.1
2.2.3. Smoking
The Smoking History Questionnaire (SHQ; Brown et al., 2002) was used to assess current daily smoking. Our dependent variable was number of cigarettes smoked per day. The SHQ has been successfully used in previous studies as a descriptive measure of smoking history (Brown et al., 2002; Zvolensky, Lejuez, Kahler, & Brown, 2004; Zvolensky et al., 2005).
2.2.4. Nicotine dependence
The Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) was administered to measure tobacco dependence. The FTND has shown high internal consistency, positive relations with key smoking variables (e.g., saliva nicotine; Heatherton et al., 1991), and adequate retest reliability (Pomerleau, Carton, Lutzke, & Flessland, 1994).
2.2.5. Carbon monoxide analysis
Biochemical verification of smoking history was completed by carbon monoxide (CO) analysis of breath samples (10 ppm cutoff; Cocores, 1993). Expired air CO levels were assessed using a CMD/CO Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc.). CO analysis of breath samples were used to verify smoking status (abstinence/smoking).
2.2.6. Negative affect
The Negative Affect subscale of the Positive Affect Negative Affect Schedule (PANAS-NA; Watson, Clark, & Tellegen, 1988) is a 10-item self-report instrument that measures the tendency to experience negative affective symptoms. The PANAS-NA asks respondents to indicate on a 5-point Likert scale (1 = “very slightly” to 5 = “extremely”) the degree to which they typically feel a list of negative affective states (e.g., “irritable,” “upset,” “afraid”) in the past week. Scores can range from 10 (least amount of reported negative affect) to 50 (greatest amount of reported negative affect). The PANAS-NA has demonstrated good internal consistency in both clinical and non-clinical samples (as ranging from .85 to .93), retest reliability (rs ranging from.71 for 2 months to .43 for 72 months), as well as convergent and discriminant validity (Watson, 2000). Internal consistency in the current sample was α = .85.
2.2.7. Anxiety sensitivity
The Anxiety Sensitivity Index (ASI; Reiss et al., 1986) is a 16-item measure, which asks respondents indicate on a five-point Likert-type scale (0 = very little to 4 = very much) the degree to which they are concerned about possible negative consequences of anxiety symptoms (e.g., “When I notice that my heart is beating rapidly, I worry that I might have a heart attack,” ”It scares me when I am nervous”). The ASI is unique from, and demonstrates incremental validity to, trait anxiety (Rapee and Medoro, 1994) and trait-level negative affectivity/neuroticism (Zvolensky et al., 2005). The ASI has sound psychometric properties in both clinical and nonclinical samples including high internal consistency (Peterson & Reiss, 1992; Taylor, Koch, McNally, 1992; Telch, Shermis, & Lucas, 1989), good retest reliability (Peterson & Reiss, 1992; Maller & Reiss, 1992), and good construct validity (McNally & Lorenz, 1987). Internal consistency for this sample was α = .86.
2.3. Procedure
Interested smokers and non-smokers responding to community advertisements contacted the research team via telephone to participate. Potential participants were given a brief detailed description of the study. After providing verbal consent, trained research assistants further screened potential participants by administering the SCID-NP-screener. Those meeting inclusion criteria were scheduled to participate in the study. Upon arrival to the lab, participants (1) provided verbal and written informed consent, (2) provided carbon monoxide analysis of breath samples to biochemically verify their smoking status; and (3) completed the self-report assessments.
3. Results
3.1. Preliminary Analyses
Means, standard deviations, and correlations among the study variables are presented in Table 2. All correlations were significant, except for the relationship between anxiety sensitivity and cigarettes smoked per day (r =.11, p = .08). Additionally, we screened our data for outliers and, due to the large differences in scales standardized all variables, aiding the interpretation of results.
Table 2.
Correlations and Means of Study Variables
| Variable | 1 | 2 | 3 | M (SD) | Range |
|---|---|---|---|---|---|
| 1. Vigorous-intensity exercise | 116.68 (150.64) | 0–933 | |||
| 2. Negative affect | −.18b | 17.85 (5.67) | 10–44 | ||
| 3. Anxiety sensitivity | −.15a | .44b | 16.49 (8.52) | 0–45 | |
| 4. Smoking | −.32b | .27b | .11 | 6.74 (8.46) | 0–40 |
p < .05,
p < .01
Note: Vigorous-intensity exercise reflects minutes per week spent in physical activities (>6 METS; Ainsworth et al., 2000); Negative affect was measured using the PANAS-NA (Watson et al., 1988). Anxiety sensitivity was measured using the ASI (Peterson & Reiss, 1986); Smoking was measured using the SHQ (Zvolensky, 2008).
3.2. The Relationship between Vigorous-intensity Exercise and Smoking
We used linear regression to test the hypothesis that vigorous-intensity exercise would be associated with reduced smoking (i.e., the c path; see Figure 1). Results indicated that higher levels of vigorous-intensity exercise were associated with decreased cigarette smoking (b = −.32, p < .01) with vigorous-intensity exercise accounting for 10% of the variance in smoking (see Figure 1).
Figure 1.
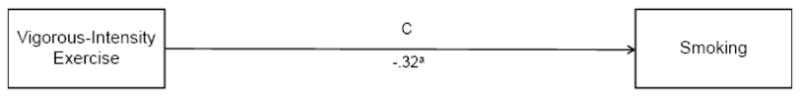
The relationship between vigorous-intensity exercise and smoking. Note: ap < .01
3.3. Negative Affect as a Mediator of the Relationship between Vigorous-intensity Exercise and Smoking
The mediation model is presented in Figure 2. We employed the distribution of products test (MacKinnon, Fairchild, & Fritz, 2007; MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002), which has been shown to have greater power and more accurate Type I error rates (MacKinnon et al., 2007; MacKinnon et al., 2002) than the causal steps approach (e.g., Baron & Kenny, 1986). This test also has virtually the same power as bias-corrected bootstrapping (.72 vs. .73) but with lower Type I error rates (.03 vs. .06; MacKinnon, Lockwood, & Williams, 2004). It also has the advantage of being exactly replicable, which is not the case for bootstrapping (MacKinnon et al., 2004). The distribution of products test calculates the magnitude of the joint mediated pathway (the a–b pathway; see Figure 2) by multiplying the regression coefficients of the two segments of the mediated pathway (i.e., a * b). We then calculated the 95% confidence interval (CI) for this product; CI’s that do not include 0 indicate a significant mediated pathway (MacKinnon et al., 2004). To estimate the effect size of the mediated pathway, we calculated the proportion mediated (PM; Shrout & Bolger, 2002). PM is the proportion of the total effect of the independent variable on the dependent variable (i.e., the c path; see Figure 1) mediated by the mediator and is calculated by the formula (a * b)/c.
Figure 2.
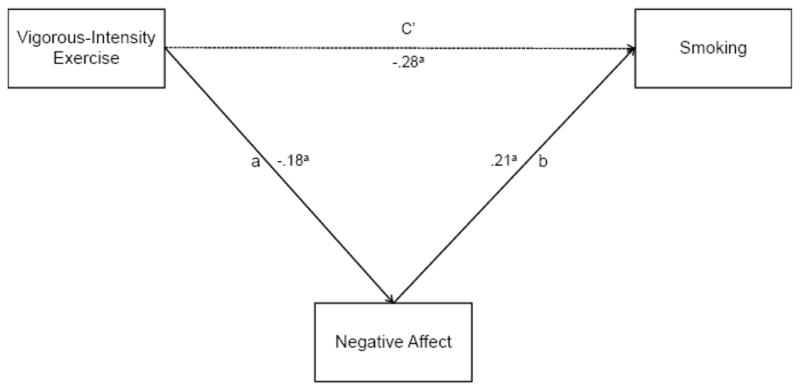
Negative affect as a mediator of the relationship between vigorous-intensity exercise and smoking. Note: ap < .01.
Consistent with prediction, regression analyses revealed that vigorous-intensity exercise was inversely associated with negative affect (a path coefficient; b = −.18, p < .01) and negative affect was positively associated with smoking (b path coefficient; b = .21, p < .01). As indicated by the 95% CI of the a * b product (−.08 to −.01), the mediated pathway was significant. The proportion of the relationship between vigorous-intensity exercise and cigarette smoking mediated by negative affect (PM) was 11.9%.
3.4 Anxiety Sensitivity as a Moderator of the Mediated Effect
In order to test the hypothesis that anxiety sensitivity would moderate the mediated pathway (i.e., the negative affect to smoking path [b]), we repeated the regression analyses described above, but entered anxiety sensitivity and the anxiety sensitivity by negative affect interaction term as additional predictor variables in the analysis calculating the b path (see Figure 3). To interpret the meaning of the interaction, we followed the procedures suggested by Aiken and West (1991). Specifically, we first centered the moderator (anxiety sensitivity) at 1 SD above and below the mean and then reran the mediation model separately for those with anxiety sensitivity centered at high anxiety sensitivity and those centered at low anxiety sensitivity (Tein, Sandler, MacKinnon, & Wolchik, 2004; Edwards & Lambert, 2007). These procedures yielded b path coefficients for those high in anxiety sensitivity and for those low in anxiety sensitivity. We used these path coefficients to ascertain the relations among the variables and to determine the significance and effect size (PM) of the mediation model separately for those high and low in anxiety sensitivity (as per Tien et al., 2004; Edwards & Lambert, 2007).
The regression analyses revealed that the interaction between anxiety sensitivity and negative affect was associated with cigarette smoking (b = .15, p < .01). Examining the relation between negative affect and smoking for those high and low in anxiety sensitivity, we found that negative affect was associated with increased smoking for those with high anxiety sensitivity (b = .29, p < .01; see Figure 3), but not for individuals with low levels of anxiety sensitivity (b = .00, n.s.). The joint mediated pathway among individuals with elevated anxiety sensitivity was significant (i.e., the 95% CI of the a * b product, −.11 to −.02). Further, the proportion of the relationship between vigorous-intensity exercise and smoking mediated by negative affect was 16.8%. These results suggest that, consistent with hypothesis, negative affect as a mechanism underlying the relationship between vigorous-intensity exercise and smoking is more salient among persons with high anxiety sensitivity relative to those with low anxiety sensitivity.
4. Discussion
The present study provides preliminary evidence for the hypothesis that negative affect partially mediates the positive effects of exercise on smoking behavior. First, we observed a medium effect size for the relationship between vigorous-intensity exercise and cigarette smoking (i.e., r = −.32). This adds to the growing literature indicating a meaningful relationship between exercise and smoking (Kaczynski et al., 2008). Second, our findings suggest that negative affect accounts for significant variance (PM = 11.9%) in the relationship between vigorous-intensity exercise and smoking. Lastly, consistent with our prediction, these mediated effects varied as a function of anxiety sensitivity. Here, the hypothesized mechanism was evident and clinically meaningful (i.e., PM =16.8%; translating into a 40% increase in effect size) among persons with high levels of anxiety sensitivity, but not significant among persons with low levels of anxiety sensitivity. These results are consistent with extant work suggesting that smokers who are high in anxiety sensitivity are less tolerant of negative affect and more likely to regulate negative affect by smoking (Brown et al., 2001; Novak et al., 2003; Zvolensky et al., 2006). Collectively, the present results offer an emerging, albeit initial cross-sectional perspective on the relationship among vigorous-intensity exercise, smoking, and emotional vulnerability. Theoretically, the present results are important as they begin to elucidate the pathways and subgroups related to the interconnection between vigorous exercise and smoking. As in much past work (Ziedonis et al., 2008), the current data are consistent with the perspective that negative mood vulnerability plays a key role in determining for whom vigorous-intensity exercise influences smoking behavior. Clinically, the current data may point to the possible therapeutic option of using exercise among emotionally vulnerable subgroups of daily smokers in the context of cessation. Although the current sample was not treatment-seeking, nor selected on the basis of anxiety/mood disorders, and in that sense should be considered preliminary, the data nonetheless provide support for considering vigorous-intensity exercise in the treatment of smoking cessation.
A number of limitations of the present investigation and points for future direction should be considered. First, the present study included daily, but not necessarily heavy, smokers as indexed by the rate of smoking per day and level of nicotine dependence. One next step for future work would be to study participants who are heavier smokers and manifest greater levels of nicotine dependence to aid in understanding the generalizability of the observed effect. Second, the present sample is limited in that it is comprised of a relatively homogenous (e.g., primarily young, and Caucasian) and active group. Of particular note, participants’ mean time spent in weekly vigorous-intensity exercise was 117 minutes, suggesting that participants, on average, met the recommended public health dose for physical activity (Department of Health and Human Services, 2008). Accordingly, our work can be extended by examining the observed relationships in samples that are less active and more representative of the U.S. adult population. Third, given that self-report measures were employed as the assessment methodology, shared method variance may have contributed to the observed results. Hence, future work in this area should include objective assessment of physical activity (e.g., motion sensors, physiological monitoring). The inclusion of physiological measures may also offer insight into potential alternative or complementary biological mechanisms underlying the vigorous-intensity exercise-negative affect-smoking relationship. Finally, the present study utilized a cross-sectional design. This methodological design cannot elucidate processes over time or isolate causal relations between variables. Thus, the study results are best construed as a “snapshot” of mediational/moderational relations. Experimental work is needed to evaluate the temporal relations between smoking, exercise, and emotional vulnerability over time.
Overall, the present study offers novel empirical insight into the nature of the associations among smoking, exercise, and emotional vulnerability. Results indicate that negative affect accounts for significant variance in the relationship between vigorous-intensity exercise and smoking. Moreover, such mediated effects are particularly evident among persons with high anxiety sensitivity. The potential importance of these findings is amplified when considering that approximately one out five adults who is a daily smoker has a mood or anxiety disorder (Grant, Hassin, Chou, Stinson, & Dawson, 2004) and that quit rates with traditional treatments are significantly lower for these individuals relative to those without mood or anxiety disorders (Lasser et al., 2000).
Footnotes
For minutes spent per session, we used the midpoint of the range (e.g. 1 = < 20 minutes equaled 10 minutes; 2 = 20–29 minutes equaled 24.5 minutes) and for “50 minutes or more,” we used 50 minutes. We classified activities associated with metabolic equivalent (METS) values greater than 6 as vigorous (Ainsworth et al., 2000).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET Intensities. Medicine & Science of Sports & Exercise. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Murray DM, Jeffery RW, Hennrikus DJ, Lando HA. Associations between exercise and health behaviors in a community sample of working adults. Preventive Medicine. 2000;30:217–224. doi: 10.1006/pmed.1999.0618. [DOI] [PubMed] [Google Scholar]
- Boyle RG, O’Connor P, Pronk N, Tan A. Health behaviors of smokers, ex-smokers, and never smokers in an HMO. Preventive Medicine. 2000;31:177–182. doi: 10.1006/pmed.2000.0699. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Baker TB. The Smoking Consequences Questionnaire: The subjective expected utility of smoking in college students. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:484–491. [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, et al. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. American Journal of Psychiatry. 1998;155:603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180. [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE. Anxiety sensitivity: Relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addictive Behaviors. 2001;26:887–899. doi: 10.1016/s0306-4603(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed November 24, 2009.];2005 http://apps.nccd.cdc.gov/brfss/index.asp.
- Cocores J. Nicotine dependence: Diagnosis and treatment. Psychiatric Clinics of North America. 1993;16:49–60. [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S: 2008. [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990;31:350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Lambert LS. Methods for integrating moderation and mediation: A general analytical framework using moderated path analysis. Psychological Methods. 2007;12:1–22. doi: 10.1037/1082-989X.12.1.1. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: Results of a 21-year longitudinal study. Psychological Medicine. 2003;33:1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Fiore MC. A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. JAMA. 2000;283:3244–3254. [PubMed] [Google Scholar]
- First MB, Gibbon M. Comprehensive handbook of psychological assessment, Vol. 2: Personality assessment. Hoboken, NJ US: John Wiley & Sons Inc; 2004. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) pp. 134–143. [Google Scholar]
- Focht BC, Knapp DJ, Gavin TP, Raedeke TD, Hickner RC. Affective and self-efficacy responses to acute aerobic exercise in sedentary older and younger adults. Journal of Aging and Physical Activity. 2007;15:123–138. doi: 10.1123/japa.15.2.123. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Haas AL, Muñoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:563–570. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. Journal of Consulting and Clinical Psychology. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hassmen P, Koivula N, Uutela A. Physical exercise and psychological well-being: A population study in Finland. Preventive Medicine. 2000;30:17–25. doi: 10.1006/pmed.1999.0597. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerström K. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Pingitore R, Spring B, Mahableshwarkar A, Mizes JS, Segraves KA, et al. Antidepressant pharmacotherapy helps some cigarette smokers more than others. Journal of Consulting and Clinical Psychology. 1999;67:547–554. doi: 10.1037//0022-006x.67.4.547. [DOI] [PubMed] [Google Scholar]
- Hu G, Pekkarinen H, Hanninen O, Yu Z, Guo Z, Tian H. Commuting, Leisure-time physical activity, and cardiovascular risk factors in China. Medicine & Science in Sports & Exercise. 2002;34:234–238. doi: 10.1097/00005768-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keeley J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database System. 2007;4:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- Kaczynski AT, Manske SR, Mannell RC, Grewal K. Smoking and physical activity: A systematic review. American Journal of Health Behaviors. 2008;32:93–110. doi: 10.5555/ajhb.2008.32.1.93. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology. 2002;111:670–675. doi: 10.1037//0021-843x.111.4.670. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Reviews in Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller RG, Reiss S. Anxiety sensitivity in 1984 and panic attacks in 1987. Journal of Anxiety Disorders. 1992;6:241–247. [Google Scholar]
- Marcus B, Albrecht A, Niaura R, Abrams D, Thompson P. Usefulness of physical exercise for maintaining smoking cessation in women. American Journal of Cardiology. 1991;68:406–407. doi: 10.1016/0002-9149(91)90843-a. [DOI] [PubMed] [Google Scholar]
- Marcus B, Albrecht A, King T, Parisi A, Pinto B, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Archives of Internal Medicine. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus B, Albrecht A, Niaura R, Taylor E, Simkin LR, Feder S, et al. Exercise enhances the maintenance of smoking cessation in women. Addictive Behaviors. 1995;20:87–92. doi: 10.1016/0306-4603(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Martin J, Kalfas K, Patten C. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: One-year results of project SCRAP-Tobacco. Journal of Consulting and Clinical Psychology. 1997;65:190–194. doi: 10.1037//0022-006x.65.1.190. [DOI] [PubMed] [Google Scholar]
- Martinsen E, Hoffart A, Solberg O. Aerobic and non-aerobic forms of exercise in the treatment of anxiety disorders. Stress and Medicine. 1989a;5:115–120. [Google Scholar]
- Martinsen E, Hoffart A, Solberg O. Comparing aerobic with nonaerobic forms of exercise in the treatment of clinical depression: A randomized trial. Comprehensive Psychiatry. 1989b;30:324–331. doi: 10.1016/0010-440x(89)90057-6. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Lorenz M. Anxiety sensitivity in agoraphobics. Journal of Behavior Therapy and Experimental Psychiatry. 1987;18:3–11. doi: 10.1016/0005-7916(87)90065-6. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Novak A, Burgess E, Clark M, Zvolensky M, Brown R. Anxiety sensitivity, self-reported motives for alcohol and nicotine use, and level of consumption. Journal of Anxiety Disorders. 2003;17:165–180. doi: 10.1016/s0887-6185(02)00175-5. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Plehn K. Measuring anxiety sensitivity. In: Peterson RA, Plehn K, Taylor S, editors. Anxiety sensitivity: Theory, research, and treatment of the fear of anxiety. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1999. pp. 61–81. [Google Scholar]
- Peterson RA, Reiss S. Anxiety Sensitivity Index Manual. 2. Worthington: 1992. [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addictive Behaviors. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Prochazka AV, Kick S, Steinbrunn C, Miyoshi T, Fryer GE. A randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation. Archives of Internal Medicine. 2004;164:2229–2233. doi: 10.1001/archinte.164.20.2229. [DOI] [PubMed] [Google Scholar]
- Rapee R, Medoro L. Fear of physical sensations and trait anxiety as mediators of the response to hyperventilation in nonclinical subjects. Journal of Abnormal Psychology. 1994;103:693–699. doi: 10.1037//0021-843x.103.4.693. [DOI] [PubMed] [Google Scholar]
- Reed J, Ones DS. The effect of acute aerobic exercise on positive activated affect: A meta-analysis. Psychology of Sport and Exercise. 2006;7:477–514. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug and Alcohol Review. 2003;22:203–220. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- Schlicht W. Does physical exercise reduce anxious emotions? A meta-analysis. Anxiety, Stress, & Coping: An International Journal. 1994;6:275–288. [Google Scholar]
- Schneider K, Spring B, Pagoto S. Affective benefits of exercise while quitting smoking: Influence of smoking-specific weight concern. Psychology of Addictive Behaviors. 2007;21:255–260. doi: 10.1037/0893-164X.21.2.255. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and non-experimental studies: New procedures and recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Smits J, Berry A, Rosenfield D, Powers M, Behar E, Otto M. Reducing anxiety sensitivity with exercise. Depression and Anxiety. 2008;25:689–699. doi: 10.1002/da.20411. [DOI] [PubMed] [Google Scholar]
- State of Vermont Department of Health. 2007 Vermont population estimates: Overview of race and ethnicity data. 2007 Retrieved April 28, 2009, from http://healthvermont.gov/research/2007pop/documents/RACENOTE07.PDF.
- Stathopoulou G, Powers M, Berry A, Smits J, Otto M. Exercise interventions for mental health: a quantitative and qualitative review. Clinical Psychology: Science and Practice. 2006;13:179–193. [Google Scholar]
- Taylor S, Koch WJ, McNally RJ. How does anxiety sensitivity vary across the anxiety disorders? Journal of Anxiety Disorders. 1992;6:249–259. [Google Scholar]
- Taylor S, Ussher M, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behavior: A systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Tein JY, Sandler IN, MacKinnon DP, Wolchik SA. How did it work? Who did it work for? Mediation in the context of a moderated prevention effect for children of divorce. Journal of Consulting and Clinical Psychology. 2004;72:617–624. doi: 10.1037/0022-006X.72.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch MJ, Shermis MD, Lucas JA. Anxiety sensitivity: Unitary personality trait or domain-specific appraisals? Journal of Anxiety Disorders. 1989;3:25–32. [Google Scholar]
- Ussher M, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2008;4:CD002295. doi: 10.1002/14651858.CD002295.pub3. [DOI] [PubMed] [Google Scholar]
- Watson D. Mood and temperament. New York: Guilford Press; 2000. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weems C, Hayward C, Killen J, Taylor C. A longitudinal investigation of anxiety sensitivity in adolescence. Journal of Abnormal Psychology. 2002;111:471–477. [PubMed] [Google Scholar]
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. Journal of Consulting and Clinical Psychology. 1992;60:943–952. doi: 10.1037//0022-006x.60.6.943. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hittsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine and Tobacco Research. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ. The Exercise Habits Questionnaire Revised. 2008. Unpublished manuscript. [Google Scholar]
- Zvolensky MJ, Bonn-Miller M, Bernstein A, Marshall E. Anxiety sensitivity and abstinence duration to smoking. Journal of Mental Health. 2006;15:659–670. [Google Scholar]
- Zvolensky M, Gibson L, Vujanovic A, Gregor K, Bernstein A, Kahler C, et al. Impact of Posttraumatic Stress Disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research. 2008;10:1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA. Panic attack history and smoking cessation: An initial examination. Addictive Behaviors. 2004;29:825–830. doi: 10.1016/j.addbeh.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, Antony MM, McCabe RE, Forsyth JP, Feldner MT, et al. Evaluating the role of panic disorder in emotional sensitivity processes involved with smoking. Journal of Anxiety Disorders. 2005;19:673–686. doi: 10.1016/j.janxdis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Bonn-Miller MO, Bernstein A, Yartz AR, Gregor KL, et al. Incremental validity of anxiety sensitivity in terms of motivation to quit, reasons for quitting, and barriers to quitting among community-recruited daily smokers. Nicotine and Tobacco Research. 2007;9:965–975. doi: 10.1080/14622200701540812. [DOI] [PubMed] [Google Scholar]


