Abstract
Cell exposed tissue factor (TF) is generally in a low procoagulant (“cryptic”) state, and requires an activation step (decryption) to exhibit its full procoagulant potential. Recent data suggest that TF decryption may be regulated by the redox environment through the oxidoreductase activity of protein disulfide isomerase (PDI). In this article we review PDI contribution to different models of TF decryption, namely the disulfide switch model and the phosphatidylserine dynamics, and hypothesize on PDI contribution to TF self-association and association with lipid domains. Experimental evidence debate the disulfide switch model of TF decryption and its regulation by PDI. More recently we showed that PDI oxidoreductase activity regulates the phosphatidylserine equilibrium at the plasma membrane. Interestingly, PDI reductase activity could maintain TF in the reduced monomeric form, while also maintaining low exposure of PS, both states correlated with low procoagulant function. In contrast, PDI inhibition or oxidants may promote the adverse effects with a net increase in coagulation. The relative contribution of disulfide isomerization and PS exposure needs to be further analyzed to understand the redox control of TF procoagulant function. For the moment however TF regulation remains cryptic.
Keywords: tissue factor, decryption, protein disulfide isomerase, phosphatidylserine
Introduction
Tissue factor (TF) is the main initiator of coagulation in both physiologic and pathologic conditions, functioning as the receptor and allosteric cofactor for plasma protease factor VIIa (FVIIa). The extrinsic tenase complex (TF-FVIIa) initiates the coagulation cascade through limited proteolysis of factor X (FX), autoactivation of FVII and activation of the intrinsic tenase protease factor IX (FIX). As such, TF has a pivotal role in the explosive process that ultimately leads to thrombin generation and fibrin formation.
Under physiologic conditions, TF is expressed by cells that are not exposed to the circulating coagulation factors[1]. Combined with the low concentration and procoagulant activity of plasma TF[2, 3], this distribution prevents the initiation of coagulation in the absence of vascular damage. In pathologic conditions TF may become exposed by endothelial cells[4, 5] and monocytes[6], initiating thrombotic events associated with sepsis[4], cancer[7] or atherosclerosis[8]. In vitro studies have indicated that most of TF exposed in different cellular environments is in a low procoagulant, or “cryptic” state, and that an activation step, named decryption, is required for the maximal expression of TF procoagulant potential[9]. Although not experimentally shown in vivo, understanding the mechanisms of TF decryption may provide valuable knowledge that could ultimately allow therapeutic interventions in thrombosis.
Models of TF decryption
While many studies over the last two decades have focused on TF decryption, the molecular characterization of the two assumed populations of TF (cryptic/decrypted) and the mechanisms of their (inter-)conversion are still elusive[10]. Both cryptic and decrypted TF can bind FVIIa with similar affinity[9]. The resulting TF-FVIIa complexes have similar activity toward small peptide substrates but only the decrypted TF–FVIIa complex can proteolytically activate the macromolecular substrate FX[9]. Since FX docking involves domains from both TF and FVIIa[11], it was suggested that a conformational change between the cryptic and decrypted forms of TF constitutes the molecular basis for the substrate preference/activity of the tenase complex[12]. The experimental evidence for this conformational change in TF is mainly indirect; although, the use of a monoclonal antibody as a specific probe for the cryptic TF was recently reported[13] this requires further validation.
In the absence of tools for the direct detection and quantitation of cryptic and decrypted TF pools, decryption has been analyzed using FXa generation assays that mimic the initiation of coagulation. Phosphatidylserine (PS) exposure in response to multiple stimuli, such as cell lysis[9], calcium ionophores[12, 14] and apoptotic signals[15], has been considered as the most potent TF decrypting process. However, there is no direct experimental evidence of a TF-PS interaction that would translate into a TF conformational change to support decryption. PS may still contribute to the initiation of coagulation through direct interaction with FVIIa or FX and as such PS-induced decryption may not involve changes in TF at all. PS-independent mechanisms of TF decryption have also been postulated[16], such as TF self-association[12], association with specialized membrane domains[17, 18] and recently a redox switch of an exposed disulfide in the extracellular domain of TF[19].
PDI regulates the redox decryption of TF
According to the disulfide switch model, the shuffling between the reduced and oxidized states of the allosteric Cys186-Cys209 bond in TF extracellular domain induces a conformational change that supports TF decryption[13, 19, 20]. Accordingly, TF containing reduced cysteines is cryptic, while intramolecular cystine containing TF represents the decrypted, highly procoagulant form[19, 20]. TF labeling with maleimide-biotin and the effect of redox reagents on TF procoagulant activity support this model[19]. Furthermore, it was proposed that PDI regulates the redox switch of the allosteric disulfide, thus controlling TF coagulant and signaling functions[13, 20]. In this model, the oxidase or isomerase function of PDI is important to generate decrypted TF (Fig.1A). PDI accumulation at sites of vascular injury in multiple in vivo thrombotic models and the fibrin deposition defects in response to PDI inhibition support a role for PDI in the regulation of coagulation[20–22]. The redox switch model has been highly debated in recent publications[23–25], pointing that (1) there is no direct experimental evidence that the exposed disulfide would affect TF conformation and subsequent binding and activity of FVIIa; and (2) the redox agents used may have multiple effects on both TF and the cellular environment. First, while it is expected that a disulfide bond, even allosteric[26], would impose steric conformational restrictions in TF, its downstream effects in terms of FVIIa binding affinity and interaction with FX need to be determined. In contrast to a previous mutagenesis study which localized these cysteines in a domain that interact with both FVIIa and FX and thus important for the tenase function[27], a recent report indicates that TF mutants with impaired disulfide formation retain the specific procoagulant activity of the native TF[28]. Secondly, redox modulation of multiple cell types leads to changes in phospholipid dynamics at the plasma membrane[29], thus affecting coagulation[30]. The redox reagents may affect the regulatory oxidoreductase (PDI), the downstream substrate(s), –TF or other cellular components–, or both groups, complicating their use in redox-dependent decryption studies. Interestingly, the researchers supporting TF redox decryption also reported that one of the exposed TF cysteines may actually be S-nitrosylated[13] and/or glutationylated[20], and as such protected from subsequent oxidation. If that is the case then vicinal thiols oxidizing reagents, initially used to support the model[19], would not be able to induce the intramolecular disulfide during decryption, while they may still act on the regulatory oxidoreductase (PDI) or other cellular components. Furthermore it has been recently debated that the allosteric cysteines in TF are buried within the cryptic TF–FVIIa complex and as such PDI cannot change their redox status during decryption[25]. Further clarification of such inconsistencies is required to understand the full extent of direct redox modulation of TF.
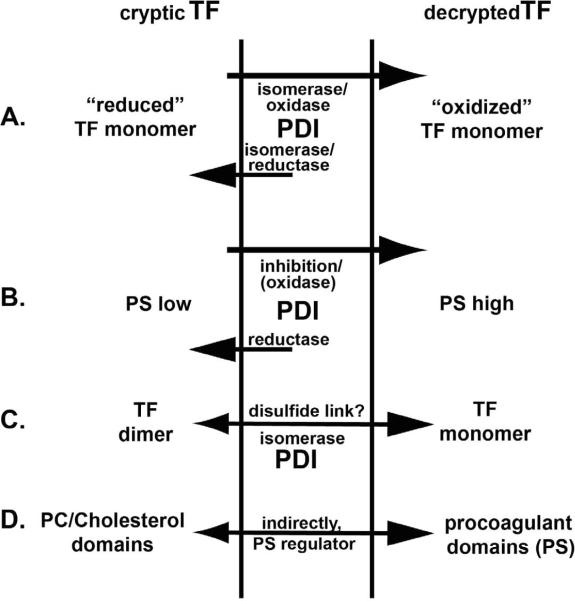
Figure 1.
Possible PDI contribution to 4 different models of TF decryption:
(A) disulfide switch model;
(B) PS exposure;
(C) TF self-association;
(D) TF association with lipid domains.
The depicted PDI functions are based on experimental (A, B) and theoretical (C, D) oxidoreductase control.
PDI modulation of lipid environment
Coagulation mainly occurs at the interface between a lipid bilayer and blood and is highly modulated by the lipid surface[31, 32]. It is generally accepted that phosphatidylcholine/cholesterol-rich lipid domains are poor coagulation surfaces, while anionic lipids, especially PS, enhance coagulation[33]. Although in resting conditions most of the PS is on the cytosolic side of the plasma membrane, cell activation may lead to PS externalization and increased membrane thrombogenicity. PS dynamics can be modulated by sulfhydryl modifying reagents[30], with oxidants enhancing the externalization while reducing agents accelerating the influx of PS[29]. Therefore we hypothesized that oxidoreductases may also affect the lipid environment and we recently reported that extracellular PDI modulation changes PS dynamics[34]. Using an endothelial cell model we showed that antibody–mediated PDI inhibition enhances both the tenase and prothrombinase activities. While a redox TF decryption could be partially responsible for the increased tenase activity, the prothrombinase enhancement is independent of TF and suggests changes in the lipid environment. PS exposure was confirmed by direct Annexin V binding and the dynamics of fluorescent analogs of PS. Our observations indicate that PDI reductase activity helps maintaining a low exposure of PS, while inhibition of PDI induces PS exposure (Fig. 1B). Thus, modulation of extracellular PDI affects coagulation at least in part through changes in lipid asymmetry of the plasma membrane. However, since annexin V could not completely inhibit the PDI-induced procoagulant effects, we cannot exclude the contribution of PS-independent mechanisms to the PDI-dependent regulation of coagulation. Further studies are necessary to assess the relative contribution of PDI-induced changes in lipid environment and TF redox switch to the initiation of coagulation.
PDI contribution to other decryption models
TF decryption has been suggested to correlate with TF self-association[12] as well as TF association with specialized membrane domains[17, 18]. Although preponderantly monomeric, TF may also form dimers and multimers[35, 36]. These complexes are sensitive to reducing agents[35] suggesting disulfide linkages and as such the monomer-multimer equilibrium may be regulated by oxidoreductases. In terms of TF decryption, the dimers were correlated with the cryptic and the monomer with the decrypted form of TF[12]. PDI isomerase or reductase function may control the isomerization of the intermolecular bridge into the intramolecular disulfides and subsequent monomerization of TF during decryption (Fig. 1C). So far it is not demonstrated that TF complexes observed during decryption[12] are homodimers and/or that TF homodimers have coagulant function, although they seem to enhance FVII autoactivation in solution[37]. Further work is needed to address the identity and involvement of TF complexes during decryption, whether these complexes are homodimers or the recently reported TF-PDI heterodimers [13].
Since PDI is not a transmembrane protein[38] it is unlikely to contribute directly to the partitioning of TF in lipid domains with different procoagulant potentials. However, by regulating phospholipid transporters at the plasma membrane, PDI may contribute to the formation of the procoagulant surface indirectly (Fig. 1D). Interestingly, we observed a punctated surface distribution of PDI on endothelial surface, suggesting an association with membrane microdomains. It would be interesting to evaluate whether TF decryption occurs in these specialized domains, but such endeavor will require generation of specific tools/antibodies for the cryptic and decrypted pools of TF.
PDI chaperone function and TF activity
Veersteg and Ruf proposed that PDI enhances the activity of the cryptic TF–FVIIa complex in reconstituted membranes through its chaperone activity and independent of the oxidoreductase control[39]. The PDI interaction with the cryptic tenase complex involved the TF macromolecular binding site around the allosteric disulfide and was sensitive to mutation of those cysteines. However, two subsequent reports have debated this hypothesis and even concluded that PS contamination of the PDI reagent may have been responsible for the observed effects[24, 40]. Unambiguous identification and functional modulation of PDI chaperone domains in controlled redox conditions are necessary to understand their contribution to coagulation.
In conclusion, PDI can have multiple regulatory implications for cellular TF decryption mainly through its oxidoreductase activity. Although experimental evidence exists for PDI regulation of coagulation through redox modification of TF and control of the phospholipid environment, further studies are needed to understand their relative contribution to the procoagulant functionality of TF. For the moment however, TF encryption is not fully decrypted.
Acknowledgements
Studies cited in this work that were performed in our laboratory were supported in part by American Heart Association grants 0615595Z (N.P.) and 0755765Z (C.L.), and National Institutes of Health RO1 GM037704 to F.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors report no conflicts of interest.
References
- 1.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 2.Santucci RA, Erlich J, Labriola J, Wilson M, Kao KJ, Kickler TS, Spillert C, Mackman N. Measurement of tissue factor activity in whole blood. Thromb Haemost. 2000;83:445–454. [PubMed] [Google Scholar]
- 3.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 4.Tang H, Ivanciu L, Popescu N, Peer G, Hack E, Lupu C, Taylor FB, Jr., Lupu F. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. Am J Pathol. 2007;171:1066–1077. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 6.Osterud B. Tissue factor expression by monocytes: regulation and pathophysiological roles. Blood Coagul Fibrinolysis. 1998;9(Suppl 1):S9–14. [PubMed] [Google Scholar]
- 7.Asakura H, Kamikubo Y, Goto A, Shiratori Y, Yamazaki M, Jokaji H, Saito M, Uotani C, Kumabashiri I, Morishita E, et al. Role of tissue factor in disseminated intravascular coagulation. Thromb Res. 1995;80:217–224. doi: 10.1016/0049-3848(95)00170-v. [DOI] [PubMed] [Google Scholar]
- 8.Westmuckett AD, Lupu C, Goulding DA, Das S, Kakkar VV, Lupu F. In situ analysis of tissue factor-dependent thrombin generation in human atherosclerotic vessels. Thromb Haemost. 2000;84:904–911. [PubMed] [Google Scholar]
- 9.Le DT, Rapaport SI, Rao LV. Relations between factor VIIa binding and expression of factor VIIa/tissue factor catalytic activity on cell surfaces. J Biol Chem. 1992;267:15447–15454. [PubMed] [Google Scholar]
- 10.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 11.Ruf W, Miles DJ, Rehemtulla A, Edgington TS. Cofactor residues lysine 165 and 166 are critical for protein substrate recognition by the tissue factor-factor VIIa protease complex. J Biol Chem. 1992;267:6375–6381. [PubMed] [Google Scholar]
- 12.Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89:3270–3276. [PubMed] [Google Scholar]
- 13.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990;87:6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greeno EW, Bach RR, Moldow CF. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Invest. 1996;75:281–289. [PubMed] [Google Scholar]
- 16.Wolberg AS, Monroe DM, Roberts HR, Hoffman MR. Tissue factor de-encryption: ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul Fibrinolysis. 1999;10:201–210. [PubMed] [Google Scholar]
- 17.Dietzen DJ, Page KL, Tetzloff TA. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood. 2004;103:3038–3044. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- 18.Mandal SK, Iakhiaev A, Pendurthi UR, Rao LV. Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol. Blood. 2005;105:153–160. doi: 10.1182/blood-2004-03-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, May L, Liao P, Gross PL, Weitz JI. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol. 2009;29:863–869. doi: 10.1161/ATVBAHA.109.185678. [DOI] [PubMed] [Google Scholar]
- 23.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–3908. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kothari H, Sen P, Pendurthi UR, Rao LV. Bovine protein disulfide isomerase-enhanced tissue factor coagulant function: is phospholipid contaminant in it the real culprit? Blood. 2008;111:3295–3296. doi: 10.1182/blood-2007-12-129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach RR, Monroe D. What is wrong with the allosteric disulfide bond hypothesis? Arterioscler Thromb Vasc Biol. 2009;29:1997–1998. doi: 10.1161/ATVBAHA.109.194985. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt B, Ho L, Hogg PJ. Allosteric disulfide bonds. Biochemistry. 2006;45:7429–7433. doi: 10.1021/bi0603064. [DOI] [PubMed] [Google Scholar]
- 27.Rehemtulla A, Ruf W, Edgington TS. The integrity of the cysteine 186-cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–10299. [PubMed] [Google Scholar]
- 28.Kothari H, Rao LV, Pendurthi UR. Cryptic vs active tissue factor: Cystine186-cystine209 disulfide bond switch is not the answer. Blood. 2009;114:140. [Google Scholar]
- 29.Daleke DL. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol. 2008;15:191–195. doi: 10.1097/MOH.0b013e3282f97af7. [DOI] [PubMed] [Google Scholar]
- 30.Le DT, Rapaport SI, Rao LV. Studies of the mechanism for enhanced cell surface factor VIIa/tissue factor activation of factor X on fibroblast monolayers after their exposure to N-ethylmaleimide. Thromb Haemost. 1994;72:848–855. [PubMed] [Google Scholar]
- 31.Ruf W, Rehemtulla A, Morrissey JH, Edgington TS. Phospholipid-independent and -dependent interactions required for tissue factor receptor and cofactor function. J Biol Chem. 1991;266:2158–2166. [PubMed] [Google Scholar]
- 32.Fiore MM, Neuenschwander PF, Morrissey JH. The biochemical basis for the apparent defect of soluble mutant tissue factor in enhancing the proteolytic activities of factor VIIa. J Biol Chem. 1994;269:143–149. [PubMed] [Google Scholar]
- 33.Morrissey JH, Pureza V, Davis-Harrison RL, Sligar SG, Ohkubo YZ, Tajkhorshid E. Blood clotting reactions on nanoscale phospholipid bilayers. Thromb Res. 2008;122(Suppl 1):S23–26. doi: 10.1016/S0049-3848(08)70014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popescu NI, Lupu C, Lupu F. Inhibition of protein disulfide isomerase on endothelial cells enhances coagulation through phosphatidylserine exposure. J Thromb Haemost. 2009;7:21. [Google Scholar]
- 35.Paborsky LR, Tate KM, Harris RJ, Yansura DG, Band L, McCray G, Gorman CM, O'Brien DP, Chang JY, Swartz JR, et al. Purification of recombinant human tissue factor. Biochemistry. 1989;28:8072–8077. doi: 10.1021/bi00446a016. [DOI] [PubMed] [Google Scholar]
- 36.Roy S, Paborsky LR, Vehar GA. Self-association of tissue factor as revealed by chemical crosslinking. J Biol Chem. 1991;266:4665–4668. [PubMed] [Google Scholar]
- 37.Donate F, Kelly CR, Ruf W, Edgington TS. Dimerization of tissue factor supports solution-phase autoactivation of factor VII without influencing proteolytic activation of factor X. Biochemistry. 2000;39:11467–11476. doi: 10.1021/bi000986p. [DOI] [PubMed] [Google Scholar]
- 38.Hatahet F, Ruddock LW, Ahn K, Benham A, Craik D, Ellgaard L, Ferrari D, Ventura S. Protein Disulfide Isomerase: a Critical Evaluation of Its Function in Disulfide Bond Formation. Antioxid Redox Signal. 2009;11:2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 39.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 40.Persson E. Protein disulfide isomerase has no stimulatory chaperone effect on factor X activation by factor VIIa-soluble tissue factor. Thromb Res. 2008;123:171–176. doi: 10.1016/j.thromres.2008.04.012. [DOI] [PubMed] [Google Scholar]