Summary
We determine the calcium fluxes through inositol 1,4,5-trisphosphate receptor/channels underlying calcium puffs of Xenopus laevis oocytes using a simplified version of the algorithm of Ventura et al., 2005 [1]. An analysis of 130 puffs obtained with Fluo-4 indicates that Ca2+ release comes from a region of width ~ 450 nm, that the release duration is peaked around 18ms and that the underlying Ca2+ currents range between 0.12 and 0.95pA. All these parameters are independent of IP3 concentration. We explore what distributions of channels that open during a puff, Np, and what relations between current and number of open channels, I(Np), are compatible with our findings and with the distribution of puff-to-trigger amplitude ratio reported in Rose et al, 2006 [2]. To this end, we use simple “mean field” models in which all channels open and close simultaneously. We find that the variability among clusters plays an important role in shaping the observed puff amplitude distribution and that a model for which I(Np) ~Np for small Np and I(Np)~Np1/α (α>1) for large Np, provides the best agreement. Simulations of more detailed models in which channels open and close stochastically show that this nonlinear behavior can be attributed to the limited time resolution of the observations and to the averaging procedure that is implicit in the mean-field models. These conclusions are also compatible with observations of ~400 puffs obtained using the dye Oregon green.
1. Introduction
The term “puff” refers to a localized increase in fluorescence that arises as a consequence of the liberation of calcium ions from the endoplasmic reticulum into the cytosol through inositol (1,4,5)-trisphosphate receptor channels (IP3R’s). Puffs have been observed, in particular, in Xenopus laevis oocytes where the channels are preferentially located within a 6μm thick band a few micrometers away from the cell surface [3] and are thought to be condensed in clusters. Distances between channels within a cluster are thought to be of a few hundred nanometers [2, 4, 5], while inter-cluster distances are of a few micrometers.
Due to their internal localization in the cell, the Ca2+ current passing through these channels under physiological conditions cannot be measured by electrophysiological techniques. Instead, optical techniques provide an alternative approach to address this issue. Sun et al. [6] obtained estimates of the calcium flux associated with individual events in Xenopus laevis oocytes by integrating fluorescence profiles along a linescan in three dimensions to derive the signal mass as a function of time. Rates of signal mass rise corresponded to Ca2+ currents of 0.4 - 2.5 pA, though there was considerable variability in magnitudes and durations of Ca2+ flux between successive puffs and between different puff sites. Thul and Falcke [7] simulated the current and concentration profiles generated by Ca2+ release from the endoplasmic reticulum and compared these results with signal mass measurements in Xenopus laevis oocytes. They found that the release current was approximately proportional to the square root of the number of open channels in a cluster and depended linearly on the concentration of free Ca2+ in the lumen. The magnitudes of the Ca2+ currents estimated in that paper ranged from 0.015 pA (low Ca2+ concentration in the lumen, one open channel) to 0.8 pA (high Ca2+ concentration in the lumen, 36 open channels).
“Trigger” events, small Ca2+ signals that immediately precede puffs [2], were used in [4] to estimate the properties of Ca2+ fluxes during puffs observed in Xenopus laevis oocytes. To this end, a “forward method” was applied to generate numerically simulated images that were then compared to the experimental ones. The number (~25-35), single channel current (~0.4pA) and spatial distribution of the IP3R’s (uniformly distributed over a region of size ~300-800 nm in diameter) were chosen so as to reproduce the observed spread of fluorescence and ratio of puff-to-trigger amplitudes. Although the model of calcium release built in this way gave the correct ratio value, it was unable to reproduce the absolute value of the fluorescence (if a linear relationship between fluorescence and Ca2+-bound dye distribution was assumed [1, 8]). In the present paper we follow a complementary approach to estimate the properties of Ca2+ release during puffs. Namely, we use a “backward method” [9] which starts from the relationship between fluorescence and Ca2+-bound dye distribution and works its way back to obtain the underlying Ca2+ current amplitude and kinetics [1].
The quantal properties of Ca2+ release during puffs have recently been revealed using total internal reflection fluorescence (TIRF) microscopy [10]. In that paper puffs were observed in intact mammalian cells (human neuroblastoma SH-SY5Y cell line) using membrane-permeant forms of dye and caged IP3 [11]. The studies of [10] show that the amplitude (in fluorescence) of the observed events occurs in multiples of an elementary amplitude event. The largest amplitudes observed in these cells are of the order of 20 times the elementary amplitude, whereas in most puffs around 6 active IP3Rs are involved. The observations also show that channel openings are synchronized within 10-20ms, and that the subsequent time course is largely characterized by successive channel closings. Although that paper provides a detailed description of the kinetics of channel openings and closings, it does not give direct quantitative information on the single-channel IP3R Ca2+ current. An approximate estimate was made by comparison with fluorescent signals arising from Ca2+ flux through single nicotinic acetylcholine receptor channels [12]. The SH-SY5Y cell line provides an excellent system to study Ca2+ puffs, and the method of [10] employing TIRF microscopy gives cleaner records with better resolution than those obtained from Xenopus laevis oocytes using confocal microscopy. However, TIRF imaging is less readily applicable to Xenopus oocytes owing to the location of puff sites a few micrometers inward from the plasma membrane. We thus sought to derive as much quantitative data as possible from confocal images regarding local Ca2+ puffs in the Xenopus oocytes, in light of the extensive existing literature on this cell type and in order to make a more quantitative comparison between differing cell types.
Obtaining quantitative results for the calcium flux from the analysis of fluorescent images is not straightforward. Different approaches have been used, particularly for “sparks”, which are elementary events of Ca2+ release through ryanodine receptors (RyR’s) in skeletal and cardiac muscle. In some of these approaches, a detailed model of all the processes is proposed, a complete set of partial differential equations for all the species involved is solved numerically, and the obtained theoretical signal is compared with the experimental one to determine the magnitude of the ionic current [13-16]. Although the simulated signals reported in these works were comparable to the experimental ones and the magnitudes of the fluxes were in good agreement with the expected values, these methods have the great disadvantage that all the numerical parameters that characterize the processes that modulate calcium signals must be known in full detail. In Xenopus laevis oocytes, this information is not well known. To overcome this difficulty, in [17, 18], we explored the possibility of deriving simple mathematical descriptions directly from the experimental observations. This “data-driven” approach is at the basis of the algorithm to infer the calcium fluxes that underlie fluorescence images which we introduced in [1]. In the present paper, we modify this algorithm using an even simpler “data-driven” model to describe intracellular calcium dynamics which holds in the presence of localized calcium sources and which we tested extensively in [18]. We apply this version of the algorithm to IP3-evoked Ca2+ puffs in Xenopus laevis oocytes, in the presence of EGTA, and obtain information on the spatiotemporal dynamics of the underlying Ca2+ fluxes. In particular, we find that Ca2+ release comes from a region of width ~ 450 nm, that the release duration is peaked around 18ms and that the Ca2+ currents range between 0.12 and 0.95pA. Considering simple cluster models in which all channels open and close simultaneously, we infer what number and spatial organization of IP3Rs is compatible with the experimental observations analyzed in the present paper and with the puff-to- trigger amplitude distribution reported in [2]. We find that the variability among clusters plays an important role in shaping the observed puff amplitude distribution and that the observations are best fit by a model for which the current, I, scales linearly with the number of channels involved in the event, Np, for small Np and sublinearly for large Np. This simple “mean-field” model provides averaged information. Using a model that includes the stochastic dynamics of the individual channels it is possible to show that the nonlinear behavior of I(Np) can be attributed to the limited time resolution of the observations and the averaging procedure implicit in the mean-field models.
2. Materials and Methods
2.1. Experiments
Preparation of Xenopus oocytes
Xenopus laevis were anaesthetized by immersion in 0.17 % MS- 222 for 15 min and killed by decapitation in adherence with protocols approved by the UC Irvine Institutional Animal Care and Use Committee. Oocytes (Dumont stage V VI) were manually plucked and collagenase-treated (0.5 mg ml−1 for 30 min) before storage in Barth’s solution (composition (mM): NaCl, 88; KCl, 1; NaHCO3, 2.4; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; HEPES, 5; pH 7.4) containing 0.1 mg ml−1 gentamicin at 17 °C before use.
Microinjection of oocytes
Intracellular microinjections were performed using a Drummond microinjector. Oocytes were loaded with either Fluo-4 low affinity dextran (Kd= 4.07 μM: 11 oocytes) or Oregon green BAPTA-1 (KD= 0.17μM: 1 oocyte), together with caged IP3 (D-myo-inositol 1,4,5- trisphosphate, P4(5)-(1-(2-nitrophenyl)ethyl) ester. Final intracellular concentrations of were 25μM Fluo-4, 40μM Oregon green and 12.5 caged IP3; assuming 1 μl cytosolic volume. An EGTA buffer solution (5 mM EGTA together with 2.5 mM CaCl2; pH 7.25, with KOH) was then injected through a fresh micropipette to obtain final intracellular concentrations of 300 μM EGTA (for Fluo-4 experiments) or 135μM (for Oregon green experiments).
Confocal laser scanning microscopy
Confocal Ca2+ images were obtained using a custom-built linescan confocal scanner interfaced to an Olympus IX70 inverted microscope [19]. Recordings were made at room temperature, imaging in the animal hemisphere of oocytes bathed in normal Ringer’s solution (composition (mM): NaCl , 120; KCl, 2; CaCl2, 1.8; HEPES, 5; pH7.3). The laser spot of a 488 nm argon ion laser was focused with a 40X oil immersion objective (NA 1.35) and scanned every 8 or 2.6 ms along a 50 μm line (0.06 μm/pixel), in the experiments with Fluo-4 and every 2ms (with 0.15 μm/pixel), in the experiments with Oregon-green. Emitted fluorescence was detected (wavelengths > 510 nm) through a confocal pinhole providing lateral and axial resolutions of about 0.3 and 0.7 μm, respectively. The scan line was focused at the level of the pigment granules and images were collected through a coverglass forming the base of the recording chamber. Fluorescence signals are expressed as ratios (FR = F/F0) of the fluorescence (F) at each pixel relative to the mean resting fluorescence (F0) at that pixel prior to stimulation. IP3 was photoreleased from a caged precursor by delivering flashes of UV light, focused uniformly throughout a 200 μm spot surrounding the image scan line [20]. The amount of photo-released IP3 was controlled (in a linear manner) by varying flash duration (5-30 ms). Each flash consumes only a negligible fraction of the caged IP3 [20], thus it was possible to acquire numerous consistent responses using repeated flashes. Intervals of > 60 s were allowed between recordings to allow IP3Rs to recover from desensitization and for cytosolic [Ca2+] to return to basal levels.
Reagents
Fluo-4 dextran, Oregon green BAPTA-1 and caged IP3 were from Molecular Probes Inc. (Eugene, OR, USA); all other reagents were from Sigma Chemical Co. (St Louis, MO, USA).
2.2. Processing of experimental signals
The linescan images provide values of Ca2+-dependent fluorescence along a single spatial dimension as a function of time. Thus, we have a matrix of fluorescence values, F(xi,tj), at each point, xi along the line-scan and time, tj. Fluo-4 experiments yielded images of 1506 × 443 or 928 × 776 pixels. Oregon green experiments gave 907 × 321 pixel images. A typical Fluo-4 image, showing puffs evoked at several sites in response to a 20 ms photolysis flash, is shown in Fig. 1A.
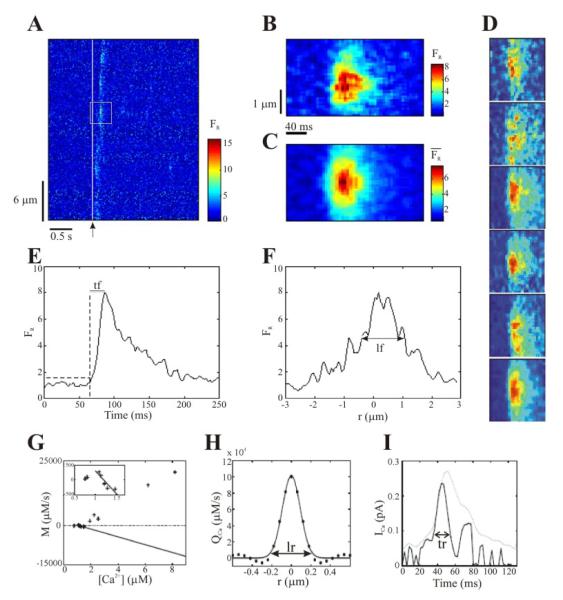
Figure 1.
A. Representative linescan image showing puffs evoked at multiple sites in a Xenopus laevis oocyte following photorelease of IP3 by a 20 ms flash of UV light delivered when marked by the arrow. The oocyte was loaded with EGTA to a final intracellular concentration of 300μM B. Spatiotemporal distribution of FR obtained from one of the puffs in A (marked by black box), shown on expanded spatial and temporal scales. C. Similar to B, illustrating the averaged fluorescence, derived from 3 puff events. D. Averaging procedure. Five raw puffs (first 5 images from top to bottom) and the one obtained using the averaging procedure (lowest image) as described in Materials and Methods. The mean values of puff amplitude, rising time and size are: 8±2, 15±3ms and 1.5±0.2μm for the raw puffs and: 6.5, 13ms and 1.26μm for the averaged one. E, F. Temporal and spatial profiles, respectively, of the puff shown in B, illustrates the measurements of tf and lf. G. M as a function of [Ca2+] for a representative puff. The filled line represents the extrapolated linear removal. Inset: enlarged view of the main plot for low [Ca2+]. H. Spatial profile of the source at the time of maximal signal. Circles: experimental data; solid line: Gaussian fit. lr is defined as shown. I. Solid line: current time course obtained from the integration of the Gaussian fit to QCa and the definition of tr. Dotted line: the corresponding fluorescence profile (times a constant) at r=0.
We process the signals according to the following steps:
- For each image, we compute the ratio:
where Fo(xi) is the average of F(xi,tj) over all the times before the flash occurs.(1) -
Puff identification
Puffs are identified using an automated threshold criterion [21]. First, the data set corresponding to each image is integrated over time and a spatial profile is obtained. Points from this profile for which the fluorescence value exceeds a given threshold are considered as puff site candidates. This selection gives disconnected sets of x values. From each set we keep the value, xk, of maximum fluorescence over that set. We then repeat the procedure, but working with the spatial average of the original signal. This gives “clusters” of t values from which we keep the times, tk, at which the maximum fluorescence occurs for each cluster. Then, we select a 40 × 50 pixel box around each point, (xk,,tk). To decide if a box contains a puff or not, we calculate its average fluorescence. If this value is above a given threshold (which we take as m+aσ, where m and σ are the mean and standard deviation of the fluorescence, FR and a is equal to 1 or 1.5, depending on the noise level of the data.), we consider that the box contains a puff. This procedure is rapid and efficient. An individual puff, captured from Fig. 1A, is shown in Fig. 1B. Puffs from Oregon green experiments are, on average, noisier than those from Fluo-4 experiments. The automated threshold criterion failed to detect several puffs, therefore we use a semi-automated criterion, as follows. We first determine an approximate puff location, (xk,,tk)app, bye eye inspection and then automatically refine the position of the puff by searching for the maximum fluorescence on a box of 11×61 pixels around (xk,,tk)app.
-
Puff averaging
The algorithm we use to infer the Ca2+ flux properties from the experimental data needs the image to be relatively smooth. To this end, we use averaged images, as the one in Fig. 1C, which we obtain in the following way. We first determine the amplitude A = max(FR), spatial spread lf and time to peak tf of the puffs, as explained below. Next, we compute the distance in parameter space between puffi and puffj as:
where a1=16, a2=400 and a3=1 are scaling factors. We then replace each puff, puffi, by its average with all other puffs, puffj, such that dij is smaller than a given tolerance. This average puff image is computed after the puff centers (i.e., the peak locations) are aligned. Typically between 2 and 5 puffs are used for each average. As expected, the resulting averaged puff has lower amplitude than the raw ones. However, the reduction of the puff amplitude is less than 20% and the distributions of the raw and averaged puffs amplitudes are very similar for Fluo-4 experiments (see Supplementary Information). Fig. 1.D shows an example of 5 Fluo-4 puffs that were averaged to obtain a smooth event. The amplitude, rising time and spatial extent of the 5 raw puffs were: A=9.09, 8.11, 8.9, 7.8 and 8.79 tf=10.4, 15.6,15.6, 17.1 and 18.2ms and lf=1.5, 1.5,1.44, 1.26 and1.62μm, those of the averaged puff are A=6.5, tf=13ms and lf=1.26μm. Finally, each of these averaged puffs is divided in two halves along the spatial coordinate (one half has x>0 and the other, x<0) and the values of FR with the same value of ∣x∣ and t are further averaged. With this procedure, each puff is replaced by a smoother one that approximately has its same properties. The total number of averaged puffs does not differ significantly from the total number of raw ones: only puffs that are very different from any other puff are left aside. In this way, we could obtain 117 averaged puffs out of 130 raw ones in the case of Fluo-4 experiments. In the case of Oregon green experiments we smoothed out the images by averaging the pixels over a 42ms×800nm region before applying the averaging procedure just described. In this way we obtained 406 averaged puffs after discarding those that qualified as out of focus (see Results). We call the resulting averaged fluorescence and the averaged puff amplitude. All the subsequent processing assumes that puffs have spherical symmetry and that the linescan passes through the center of the puff. In this way we can assume that a linescan provides complete information on the signal so that the spherical coordinate, r, (the distance from the puff center) is enough to fully describe the puff. We check the validity of this approximation in the Results Section.
2.3. Determination of puff duration and spatial spread
Typical temporal profiles of puffs at their center (r= 0) show that the signal rises rapidly to a maximum value and then decays at a slower rate (e.g. Fig. 1E). To characterize the duration of Ca2+ flux during a puff we determine the rise time or tf taken for the fluorescence signal to increase from a value 1.5 standard deviations above the mean basal value to the peak (Fig. 1E). A similar analysis is done to determine the spatial spread lf of the signals, defined as the full-width of the fluorescence signal at an amplitude of 60% of the peak at the time of the peak (Fig. 1F). As explained in the following section, we do not apply any deblurring to the image, which may result in a slight overestimation of the puff spatial spread.
2.4. Inferring Ca2+ flux properties
Determination of free Ca2+ distribution
In order to determine the Ca2+-bound dye concentration, [CaB], we relate it to the fluorescence, assuming that the fluorescence in the absence of calcium, Fmin, is negligible for both indicator dyes; i.e., we assume that:
| (2) |
Based on the studies reported in [1], we do not apply any deblurring to the image since the procedure introduces too much noise and exaggerates errors if the scan line is offset with respect to the puff center. This can result in a slight overestimation of the puff spread and source size. In the case of experiments with Fluo-4, Fmax, the fluorescence of Ca2+-saturated dye, was determined by injecting saturating amounts of calcium from a pipette filled with 100 mM CaCl2 into oocytes, yielding a value of Fmax/Fo ≅ 48. For a total dye concentration [B]T = 25μM, we obtain [CaB] values around 0.5μM at rest, which corresponds to [Ca2+]basal ~ 80nM, assuming a KD=4.07 μM (with Oregon green, we obtain a value for [Ca2+]basal, which is in the same order of magnitude). This is a reasonable value for [Ca2+]basal, which we use to confirm that the approximation of Eq. 2 is valid for the experiments analyzed. Values of [CaB] are around 4μM at the peak fluorescence during puffs. Oregon green does not provide a fluorescence range as large as that of Fluo-4. In fact, Fmax/Fo is an order of magnitude smaller [6]. This results in noisier records experiments with puffs that are harder to detect. We analyzed Oregon green experiments mainly to validate the conclusions we obtained using Fluo-4 as the indicator. For the parameters that characterize Oregon-green we follow [22]; (Ddye = 20μm2s−1, kon = 15μM−1s−1, KD= 0.17μM) and take Fmax/Fo=1.6 ~ KD/[Ca2+]basal that gives current values within the range of those obtained in experiments with Fluo-4.
Discrete approximations, L and T, of the Laplacian and of the time derivative of [CaB] are computed numerically at every r and t. A first-neighbor smoothing procedure is applied to [CaB] before the derivation is performed [1].
Assuming that the dye, B, only reacts with free Ca2+ according to Ca2+ + B ⇔ CaB , that the total dye concentration, [B]T=[B]+[CaB] is uniformly distributed in space, that kon and koff are the reaction rates, and that CaB diffuses with coefficient Ddye, we compute the reaction term, , at each r and t as: R = T – DdyeL from which we obtain the free calcium concentration at every r and t:
| (3) |
We then compute discrete approximations of the Laplacian and time derivative of [Ca2+], using second and first difference order finite differences, respectively. A first-neighbor smoothing procedure is applied to [Ca2+] before the derivation is performed [1].
Determination of the Ca2+ current
Once the free [Ca2+] and its derivatives are obtained, we use the algorithm introduced in [1] to obtain the Ca2+ flux underlying the fluorescent puff image. The only difference with respect to the implementation discussed in [1] is that we assume that the sum of all the Ca2+ removal terms can be approximated by a linear function of [Ca2+]. As analyzed in [18], this assumption is valid in the vicinity of a localized Ca2+ source. Thus, we assume that:
| (4) |
with DCa the free diffusion coefficient of Ca2+, QCa the localized Ca2+ source and mR and bR parameters to be determined from the experiment [1, 18]. Briefly, by plotting M (which is obtained by finite differentiation of [Ca2+]) vs [Ca2+] we identify the region of low [Ca2+] (QCa=0) where the dependence is approximately linear from which we determine mR and bR. Extrapolation of this behavior to the source zone leads to the determination of QCa. Integration of QCa gives the Ca2+ current:
| (5) |
where γ= 1.92 × 10−4 pC/(μMμm3). This integral is very sensitive to the choice of dr because the spatial resolution of the experiments is at most of the order of rsource. To overcome this problem, we approximate QCa(r,t) by a Gaussian in r for each t. We choose rsource as the point for which two successive points of the Gaussian fitting differ by less than 0.01 (Fluo-4 experiments) or 1.5σ (Oregon green experiments).
As an example of how the method deals with the experimental data, Fig. 1G shows a plot of M as a function of [Ca2+] for a representative puff from a Fluo-4 experiment. The straight line corresponds to the sum of all the Ca2+ removal terms, mR [Ca2+] +bR, with mR and bR obtained by fitting the M vs [Ca2+] data points for low Ca2+ (see inset). Fig. 1H shows a spatial profile of QCa (black circles). The Gaussian fitting of the source is shown with a solid line in Fig. 1H. Integration of the Gaussian fitting using Eq. 5 gives the current time course, shown in Fig. 1I.
Determination of the duration of Ca2+ release and the spatial extent of the Ca2+ source
We determine the duration of the Ca2+ release (tr) from the temporal profile of the current, I (Fig 1I). We assume that the release starts and ends when the current, I, becomes equal to 1.5 times the standard deviation around the basal value.
The spatial extent of the source (lr), as explained before, is determined as twice the radius at which two successive points of the Gaussian fitting of the QCa spatial profile at the time of the Ca2+ flux peak differ by less than 0.01 (see Fig. 1H). Given that we work with the blurred image, the source size can be slightly overestimated. Therefore, the numbers we obtain should be considered as upper bounds of the actual values.
2.5. Puff models: Ca2+ distribution within an IP3R cluster
To interpret the experimental data, we perform a series of numerical simulations of the Ca2+ dynamics in a cytosolic medium with molecular buffers and localized Ca2+ sources, similar to the one introduced by Shuai et al. in [4]. The model includes the following species: cytosolic calcium (Ca2+), an immobile endogenous buffer (S), a cytosolic Ca2+ indicator (B) and an exogenous mobile buffer (EGTA) that react according to:
| (6) |
where X represents S, EGTA or B, and kon-X and koff-X are the forward and backward binding rate constants of the corresponding reaction, respectively. We do not include Ca2+ pumps because they act on a slower time scale than typical puff durations. We assume that the total concentration of Ca2+ dye, mobile and immobile buffer remain constant ([B]T, [EGTA]T and [S]T, respectively) and that the diffusion coefficient of their free and Ca2+ bound forms are equal. Furthermore, we assume that initially all concentrations are homogeneously distributed and in equilibrium among themselves with Ca2+ is at basal concentration, [Ca2+]basal. Therefore we can calculate [B], [EGTA] and [S] by subtracting the concentration of the Ca2+ bound forms to the total concentration. Given these assumptions, the set of partial differential equations that give the spatial and temporal evolution of the concentrations involved in the description is:
| (7.a) |
| (7.b) |
| (7.c) |
| (7.d) |
where DCa, Ddye and DEGTA are the diffusion coefficients of Ca2+, B and EGTA, respectively and the reaction terms are given by: . The Ca2+ entry through (open) IP3R’s is included in the boundary conditions [23]. Namely, we consider the cytosolic space as a parallelepiped of sides Lx, Ly and Lz. The plane z=0 represents the surface of the ER membrane, where there are Ns sources of (small) area δS centered at the positions . We assume no flux boundary conditions for all concentrations except for [Ca2+] at the location of the sources, where we consider the condition [23]:
| (8) |
where Jch is the Ca2+ flux through the sources. Namely, , where Ich is the single channel current (which is equal to zero if the channel is closed) and F is Faraday’s constant ().
The simulations are done using a forward Euler method in time and an explicit finite-difference formula in space with a 2nd order expression (first neighbors) for the Laplacian with grid sizes dx=dy=dz=0.02 μm and time step dt=0.3 μs. The values of the parameters used in the simulations are listed in Table 1 and coincide with those of [4].
Table 1.
Parameters values used to solve the puff model introduced in Section 2.5 taken from [4].
| Parameter | value | Units |
|---|---|---|
| Free Calcium: | ||
| DCa | 200 | μm2s−1 |
| [Ca2+]basal | 0.1 | μM |
| Calcium dye (Fluo4-dextran): | ||
| Ddye | 15 | μm2s−1 |
| kon-B | 150 | μM−1s−1 |
| koff-B | 300 | s−1 |
| [B]T | 25 | μM |
| Exogenous buffer (EGTA): | ||
| DEGTA | 200 | μm2s−1 |
| kon-EGTA | 5 | μM−1s−1 |
| koff-EGTA | 0.75 | s−1 |
| [EGTA]T | 40 | μM |
| Endogenous stationary buffer: | ||
| Kon-S | 400 | μM−1s−1 |
| Koff-S | 800 | s−1 |
| [S]T | 40 | μM |
To compare with experimental confocal signals we compute a weighted average of the Ca2+-bound dye concentration, [CaB], as [4]:
| (9) |
where ωx=ωy=0.0325 μm2 and ωz=0.231 μm2. Inserting it into Eq. 2 we obtain the fluorescence distribution of the simulations which we compare with the experimental data (at the puff center).
In all the simulations the number and location of the channels are chosen beforehand. In most cases, we assume that all the channels open and close at previously established times. We also use the method introduced in [24] to perform some simulations in which the stochastic dynamics of the individual IP3R’s is included. To this end, we use a very simple IP3R kinetic scheme:
| (10) |
where kCO=20 μM−1 s−1 [25] and kOI = 100s−1 (Ci kinetic model) or (Cd kinetic model). In the latter, has been chosen so that with [Ca2+]mouth the value of [Ca2+] at the mouth of an isolated open channel as predicted by numerical simulations of a single open channel with a 0.1pA. The scheme is similar to that of [26]. It does not include the IP3 binding and unbinding explicitly but assumes that the fractions of IP3R’s with or without IP3 bound are given by an equilibrium relationship. In order to make a sensible comparison with the experiments we smooth out the I(t) profile that we obtain with the stochastic simulations using a moving average with a window of 30 ms.
Verification of the algorithm with simulated data and sensitivity of the inferred currents on parameter uncertainties
As done in [1, 18], we have used numerically generated “images” to check that the simplified version of the algorithm that we use in this paper is capable of providing good estimates of the calcium flux. To this end, we simulated the simple model described by Eqs. 7 assuming that there is a single (spherical) Ca2+ source at the origin with radius varying from 0.05μm up to 1.0μm and active for a time between 1-15 ms. Applying the algorithm to the “images” obtained with these simulations, we not only obtained a very good value for the current, but we were also able to reproduce the time course and spatial spread of the source fairly well.
The effects of optical blurring owing to the microscope point-spread function, noise and finite resolution were also analyzed using simulated numerical data as described in [1]. Addition of noise at a magnitude comparable to that of the basal noise of averaged experimental records increased the errors with which the current amplitudes were determined (around 30%), but the time course remained well reconstructed (see Supplementary Material).
To test the performance of the algorithm in cases where the dimensions of the Ca2+ source are smaller than the pixel resolution, we applied it to numerically generated images obtained with a 0.05μm source and for which the data was saved every 0.1μm at 1ms time-steps (which correspond to typical experimental pixel spacing and time resolutions). Using a Gaussian fitting interpolation to QCa to compute the integral in Eq. 5, and choosing rsource as the point for which the signal reaches half of the maximum value, we obtained a current that was 20% higher than the input value of the simulation.
The algorithm assumes the a priori knowledge of several parameters: the Ca2+-dye reaction constants and the dye and free calcium diffusion coefficients. The dissociation constant, KD=koff/kon=4.07μM (for Fluo-4), is taken from the manufacturer’s data, but the values of the individual rate constants (kon = 100μM−1s−1 and koff = 400s−1, for Fluo-4) and the cytosolic diffusion coefficients (DCa= 220μm2s−1, Ddye=50μm2s−1) are less certain. To evaluate the sensitivity of our results to changes in these parameters we generated images numerically using a model with three mobile buffers and one immobile buffer as in [1], and then applied the algorithm using values of DCa, Ddye and kon different from those of the simulations. We explored 114 sets of parameter values taken within the ranges defined in Table 2 and compared the maximum value of the current, Ii, obtained for each set, i, with the one used to generate the numerical image (Is=1pA). Defining the relative errors as ΔE(Ii ) = Ii / Is −1 (if Ii> Is) andΔE(Ii) = Is / Ii −1 (if Ii< I), and equivalent expressions for DCa, Ddye and kon, we obtained that the errors in the reconstructed current were more sensitive to errors in Ddye and kon than in DCa. Furthermore, the relative error in the current decreased much more when the error in kon decreased than when the errors in the other parameters decreased. Thus, having the correct values for kon first and then, for Ddye, seems crucial to obtain good estimates of the underlying current. If we kept the subset for which the relative errors in DCa, Ddye and kon are less than 0.5, the relative error of the current is 0.17 ± 0.03 (mean ± standard deviation, n=23). For the values used in this study this is reached when Ddye varies in the range (25-75) μm2/s, DCa varies between (160-480) μm2/s and kon remains between 25 and 180 (μMs)−1.
Table 2.
Range of parameters values for the sensitivity analysis.
| Parameter | Actual value | Range of variation |
|---|---|---|
| DCa | 319 μm2s−1 | (80 - 560) μm2s−1 |
| Ddye | 50 μm2s−1 | (12.5 - 250) μm2s−1 |
| kon | 100 μM−1s−1 | (10 - 1000) μM−1s−1 |
An extended description of these procedures and numerical examples is given in the Supplementary Material.
2.6. Inferring the distribution of the number of channels that open during a puff
We propose very simple models to relate the distribution of open channels with the current distribution and then choose the one that gives the best fit of the observations. As done in [27], we assume that the number of channels that open during a puff is equal to the number of IP3R’s with IP3 bound in the cluster and that they all open and close simultaneously (see Supplementary Section). In this way, the distribution of observed currents, fI(I), depends on the probability that there are Np IP3R’s with IP3 bound in a cluster, P(Np), and on the relationship between the current, I, and Np (the number of channels that open during a puff). We investigate the possibility of reproducing the observed distribution assuming that P(Np) is a Poisson distribution:
| (11) |
where m is the mean number of IP3R’s with IP3 bound in a cluster. The underlying assumption here is that all clusters have approximately the same number of IP3R’s and that they have similar sensitivities to their agonists so that the mean number of channels that open during a puff is the same for all clusters. As discussed later, we cannot fit the observed fI(I) very well using Eq. 11. We then relax the assumption that all clusters are similar. For simplicity, we assume that there are discrete cluster populations, each of them characterized by a different mean number of channels, mi, with IP3 bound:
| (12) |
where M is the number of distinct cluster populations and λi is the fraction of clusters that belong to each population.
Based on previous works, we first analyze if I(Np) can be approximated by the expression:
| (13) |
with α=1 or α=2. Taking α=1 corresponds to assuming that the Ca2+ current is the same through each open channel, regardless of the number of IP3R’s that are open [4]. The α=2 case corresponds to the results of [7] where the effect of the local [Ca2+] depletion in the lumen of the ER owing to the opening of several closely packed IP3R’s was analyzed. Taking into account this observation and the ability to fit the experimentally determined fI(I) (see Supplementary Information), we also explore the possibility that I(Np) is a nonlinear function such that I and Np scale differently depending on Np. For the sake of simplicity, we approximate I(Np) by expressions of the form IoNp1/α with different values of α depending on Np. Here we only present the results that correspond to the case with α=1 for small Np and α=2 for large Np:
| (14) |
Given that Np2 >1, Io1 corresponds to a single IP3R current, while Io2 is only a fitting parameter. We can fit the data from Fluo-4 experiments with Np1=Np2= Npt so that I(Np) is continuous. However, it is not differentiable, which can result in discontinuities in the current distribution function. Since this is only an approximation, we prefer to leave a gap of current values for which we do not determine the current distribution (see Supplementary Information). Something similar occurs for Oregon green experiments. The region for which we do not determine I(Np) is relatively small and corresponds to values of I for which fI(I) is also small.
From all possible combinations of P(Np) and I(Np) that can be obtained using Eqs. 11-12, we present here the results of assuming that P(Np) is given by Eq. 11 and I(Np) by Eq. 13 (both with α=1 and α=2) and that P(Np) is given by Eq. 12 and I(Np) by Eq. 14. In the first case the distribution of puff currents, fI(I), reads:
| (15) |
where Γ is the gamma function, which, for integer arguments, n, satisfies Γ(n)=(n-1)! In the second case, given that most of the data that we obtained for Fluo-4 experiments is concentrated in the region with two apparent peaks (see Fig.3A), we only analyze a model with M=2. We also assume that, if there are more populations, their contribution to fI(I) is negligible in the region where most of the data is concentrated (I<Im = 0.57pA). The current distribution then reads:
| (16) |
where we approximate f1 and f2 by:
| (17) |
with I1* = Io1 Np1 and I2*=Io2 Np21/2. We then use Eq. 15 (both with α=1 and α=2) or Eqs.16-17 to approximate the observed distribution of puff current amplitudes (Fig. 3A) and extract the best values of the unknown parameters from the fitting. In the latter, we actually approximate fI ≅ λ1f1 for I<I* and fI ≅ λ2f2 for I>I* with I* = Io1 Npt =Io2 Npt1/2 , and Np1 = Np2 = Npt (i.e., with no current gap) to do the fitting. We follow a similar approach in the case of Oregon green experiments but leaving a gap in the values of I (fI ≅ λ1f1 for I<I(Np1) and fI ≅ λ2f2 for I> I(Np2)) (see Supplementary Information).
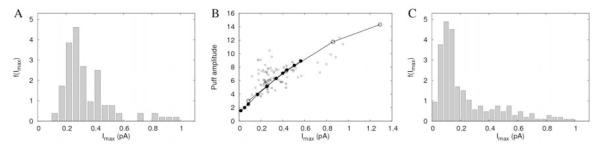
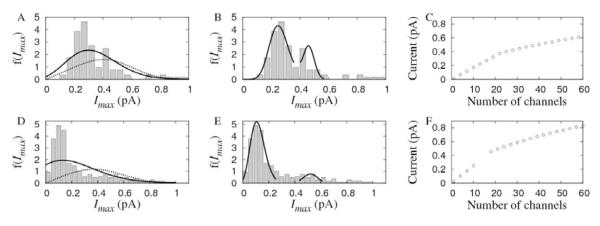
Figure 3.
A. Maximum current value distribution from 105 averaged puffs of Fluo-4 experiments. B. Dependence of puff amplitude on the maximum current during release (Fluo-4 experiments). Black circles correspond to simulations of the model described in Section 2.5 in which the number of open channels was Np=1, 3, 5, 10, 15, 20, 25, 30, 40 and 50 and the single IP3R current Ich was given by Eq. 13 while white circles correspond to simulations with Np=1, 3, 5, 10, 15 and a constant IP3R current of Ich =0.086 pA. All channels open during 18 ms. C. Similar to A but for 364 puffs of Oregon green experiments.
3. Results
We present here the analysis of a series of linescan images containing 130 identified puffs of experiments done using Fluo 4-dextran, which yielded 117 averaged signals. The underlying currents were determined for 105 of these averaged events, using the algorithm described in Section 2.4 with parameter values koff = 400s−1, kon = 100μM−1s−1 and Ddye= 50μm2/s for the dye (low affinity Fluo 4-dextran; KD = 4.07μM), and DCa=220μm2s±1 [28] for free calcium. We also analyze puffs coming from experiments done with Oregon-green BAPTA-1. In this case, we identified 440 puffs. After averaging and discarding those that qualified as out of focus (see later), we were left with 406 puffs, for 364 of which we could compute the underlying currents. Some of the currents obtained in this case were larger than the largest value (~1pA) obtained for experiments with Fluo-4. Most of these large currents corresponded to relatively wider puffs coming from images with at least two puffs that were very close to one another. Oregon-green records are noisier and the spatial resolution is worse than Fluo-4 ones. Furthermore, the parameter values of Oregon green are not as well characterized. For all these reasons, the only events recorded with Oregon green included in the present analysis have currents below 1pA, leaving 266 puffs that we analyze to verify some of the conclusions drawn from Fluo-4 images. To a large extent all the analysis is done in terms of the distributions of puff amplitudes and maximal Ca2+ currents, Imax, that underlie each averaged puff. The latter is computed, using current bins of size Δ=0.05pA for Fluo-4 and Δ=0.04 pA for Oregon green experiments, as:
| (18) |
where N(Imax) is the number of puffs for which Imax falls within each bin, and Ntotal is the total number of puffs that are analyzed. The amplitude distributions of raw and averaged puffs (f(A) and , respectively, are computed similarly, using bin sizes Δ=0.71 and 0.73, respectively, for Fluo-4 experiments and a bin size Δ=0.025 for Oregon green. The puff amplitude distribution, the time course and spatial extent of puffs and the underlying Ca2+ currents are presented in Section 3.1-3.3. A scaling relationship between current and the number of open channels during a puff is presented in Section 3.4. In order to validate our interpretation of the experimental results, we complement our analysis with numerical simulations of Ca2+ release from clusters of IP3R’s in Section 3.5.
3.1. Puff amplitude distribution
We show in Figs. 2A,B the averaged puff amplitude distributions for Fluo-4 and Oregon green experiments, respectively. In the case of Fluo-4, the distribution is similar to that of raw puffs but is slightly shifted to the left due to the averaging procedure. The magnitude of the shift is less than 12% and the mean values of the raw and averaged data are 7.5 and 6.7, respectively. Taking this correction into account, both distributions do not statistically differ (p<0.25, Kolmogorov-Smirnov (K-S) test [29], see Supplementary material). The time course and spatial spread of the raw and averaged puffs are also very similar (data not shown). We further validate the conclusions that we can draw from the analyses of averaged puffs in the next Section. Thus, from now on we will show results that were obtained using averaged puffs, unless otherwise noted.
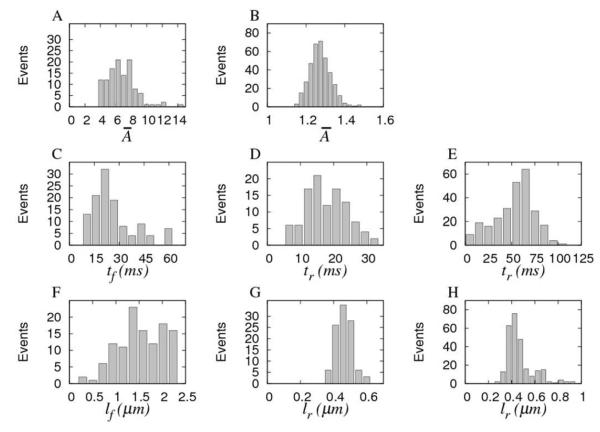
Figure 2.
A,B. Normalized distributions of peak fluorescence amplitude of averaged puffs for Fluo-4 (n = 117) and Oregon green (n=406) experiments. C. Distribution of rise times tf for Fluo-4 experiments. D, E Distributions of Ca2+ flux durations tr for Fluo-4 and Oregon green experiments, respectively. F-H: Similar to C-E, but for puff (lf) and Ca2+ source (lr) sizes.
Fluo-4 experiments were done for various flash durations. This means that the amount of photoreleased IP3 may be different for different puffs. We analyzed the puff amplitude cumulative distribution function (CDF) for events evoked by flash durations ranging between 8 and 30ms. The distributions overlapped, revealing that there is no apparent dependence of the measured amplitude on the amount of photoreleased IP3 (the distributions do not differ with p<0.1 according to the K-S test, see Supplementary material), in agreement with [2, 11]. Thus, all further results were obtained after pooling data with various flash durations, in the case of Fluo-4 experiments.
The amplitude distribution, f(A) obtained from the experiments may be affected by two problems. Firstly, very faint puffs are hard to detect, so that low amplitude events may be underestimated. Secondly, puff amplitudes may be underestimated if the linescan does not go through the center of the puff. Given that out of focus events should give rise to relatively wider and dimmer images than in focus ones, we compared the cumulative amplitude distribution, , obtained using all observed puffs and the one obtained after having discarded the widest dimmest images and they do not differ with p<0.1 according to the K-S test. We kept all averaged puffs in the case of Fluo-4 experiments and discarded the widest dimmest in the Oregon green case.
3.2. Time course and spatial extent of puffs and underlying Ca2+ fluxes
Fig. 2C shows the distribution of puff rise times, tf, for Fluo-4 experiments. Rise times range between 8 and 60ms, with a mean value around 25ms. On the other hand, the corresponding distribution of release flux durations, tr (Fig. 2D) has a mean around 18ms, with a range from 5 to 33 ms. We could expect similar distributions for tf and tr. However, we found that the fluorescence rise time is longer than the underlying Ca2+ release. This discrepancy may arise from the procedure used to compute both magnitudes (see Materials and Methods). In this procedure we use 1.5 times SD from baseline as a threshold criterion: since current profiles are generally noisier than puff profiles, the threshold value could be shielding the beginning of the event in the case of the fluxes. For the sake of comparison, we show in Fig. 2E the distribution of tr obtained for Oregon green experiments. The distribution of flux durations obtained from Oregon green experiments is spread over a wider range, although all durations remain within the same order of magnitude, 41±17ms. The difference in the duration of flux duration may be due to the extra smoothing procedure we needed to apply to reduce the noise in Oregon green experiments.
Fig. 2F shows the observed distribution of fluorescence spread, lf, during puffs from Fluo-4 experiments. It can be seen that puffs show a great variability in size ranging from 0.3 to 2.2μm. The marked cutoff at lf >2.2μm is artifactual, resulting from the choice of box dimensions used to capture the puffs to avoid the overlap of neighboring puffs. Corresponding estimates of the spatial extent of the underlying Ca2+ sources, lr, are plotted in Fig. 2G, showing a tight distribution with a mean 450nm. This value, however, should be considered as an upper bound of the actual size. The distribution of source sizes obtained from Oregon green experiments is mainly concentrated around ~400nm (470±120nm) as shown in Fig. 2H. However, there is a large size tail comprising 15% of the events. The discrepancy with respect to Fluo-4 experiments may be due to the different spatial resolutions of both types of experiments. The extra smoothing procedure that we needed to apply to the records coming from Oregon green experiments may also be the reason for the difference in the distributions of lr.
In order to test to what extent the determined values of tr and lr are compatible with the observed tf and lf values, we numerically simulated Eqs. 7 assuming that there was a single (spherical) Ca2+ source at the origin, that released Ca2+ with a constant current, Imax, that remains on during a time, tr. We performed a series of simulations varying tr, Imax, and the source spatial extent, lr, compatible with the ones obtained from the analysis of Fluo-4 experiments. We then computed the fluorescence distribution according to Eq. 2 using the blurred version of the numerically determined Ca2+-bound dye distribution, Eq. 9. We compared this numerically determined fluorescence with the experimental puff image for which we obtained similar values of tr, Imax, and lr as those used in the simulations, and we obtained values that differed in less than 20% (not shown). We also performed a numerical simulation using Imax= 0.4pA, lr=230nm, and tr=18ms, which correspond to the mean values of these quantities over all the puffs that we analyzed. The numerically determined puff, in this case, has a spatial spread of 1.3μm, which is within the size values of the observed puffs. These comparisons provide both a validation of the algorithm and of the puff averaging procedure.
3.3. Magnitudes of Ca2+ currents during puffs
We show in Fig. 3A the distribution of inferred peak Ca2+ currents, f (Imax) underlying the averaged puffs of type I experiments. Current amplitudes vary between 0.12 and 0.95 pA, with a mean of 0.34 ± 0.2 pA (n=105). Fig. 3B shows the dependence of the averaged puff amplitudes, as a function of current amplitude, Imax, for Fluo-4 experiments. The relationship between A and Imax, is nonlinear. The saturation of A with increasing Imax is also compatible with the results of Thul and Falcke [7]. In that paper the authors simulated the currents and concentration profiles generated by the release of Ca2+ from the endoplasmic reticulum through clusters of IP3R’s. Their Fig. 7, for example, shows that, at the center of the cluster, [Ca2+] is an increasing nonlinear function of the number of open channels, which saturates and eventually starts to decrease as the number of open channels increases. This happens for all the simulations, which correspond to different ratios of mobile to immobile buffer concentrations in the reticulum. In agreement with [7], the current is also an (increasing) nonlinear function of the number of open channels (see their Fig. 7). We discuss later the causes of this behavior. We show in Fig. 3C the distribution of inferred currents for Oregon green experiments.
Figure 7. Stochastic puff model.
A: Puff duration (tf) as a function of puff amplitude for experimental observations in Xenopus laevis oocytes (open circles) and stochastically simulated puffs obtained using model Cd (black squares and error bars correspond to mean values and standard deviations, respectively). B: Duration of the Ca2+ release as a function of the maximum Ca2+ current for stochastically simulated puffs obtained using model Cd (black circles) and Ci (open circles). C: Maximum released current (open circles) and averaged current (mean with black dots and standard deviation with vertical lines) released during the events as function of the number of channels in the cluster, Np, obtained with simulations that use the Cd kinetic model. Superimposed in black, curves that correspond to the I~Np and I~Np1/2 scalings for Np< 22 and Np> 22, respectively. In B and C, the maximum released current was obtained from a smoothed version of the simulated I(t).
The algorithm to infer the Ca2+ current associated with an event relies heavily on the assumption that the linescan goes through the puff center. As analyzed in [1], the Ca2+ flux is underestimated if this assumption does not hold. In [30] an algorithm to go from the distribution of observed amplitudes to the distribution of actual (in focus) amplitudes in the case of sparks was introduced. As mentioned by the authors, the algorithm gives a real amplitude distribution only under the assumption that all the signals (sparks) generate Gaussian fluorescence distributions with the same standard deviation, an assumption that most likely does not hold for puffs. The issue of to what extent the observed puff amplitude distributions, fA(A), reflect that of the underlying currents that generate the observations, fI(I), was addressed in [31], where the authors introduced the observation function, g(I,h) to relate A, I and the line-scan offset, h. Unfortunately, there is no method to obtain this observation function directly from the experiments. Furthermore, both the methods of [30] and [31] use statistical arguments to go from the observed to the real distribution, something that is not good for our purposes since our algorithm works with each puff individually, for which we would need to know the offset in each case. Therefore, as done before, taking into account that out-of-focus events should give rise to relatively wider and dimmer images than in-focus ones, we compared (in the case of Fluo-4 experiments) the cumulative current distribution, , obtained using all the determined currents (a total of 105) and the one obtained using the currents that corresponded to the 51 narrowest puffs. The Kolmogorov statistics, T≡supx ∣F1(x)-F2(x)∣, determined that both distributions did not differ with an 80% statistical significance [29]. In addition, using “numerical simulated images” as described in the above section, we compared 32 puffs coming from both distributions and the numerically generated fluorescence distribution differed by less than 20% with respect to the experimental ones, independently if they were narrow and intense or not. We then assume that out of focus events are not affecting the current distribution that we could obtain significantly in the case of Fluo-4 experiments. We thus work with the distribution function obtained using all the 105 currents that we could obtain in this case. For Oregon green experiments we discard the information coming from the 8 widest, dimmest events.
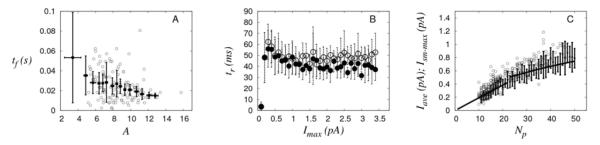
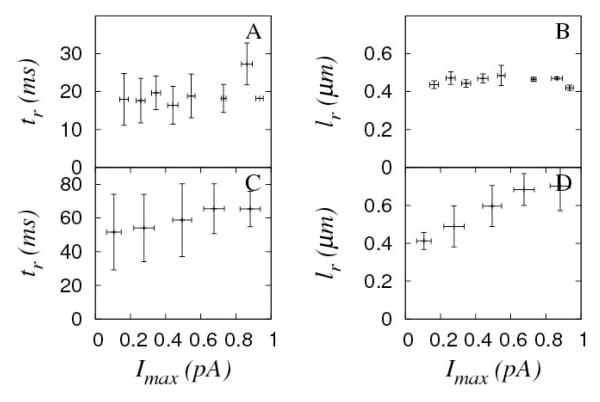
Fig. 4 shows the flux duration and the size of the underlying Ca2+ sources as functions of the inferred maximum current, Imax for Fluo-4 and Oregon green experiments. None of these parameters showed any appreciable dependence on the underlying current, although a weak increasing dependence may be observed in Fig. 4C. We arrive at the same conclusion if we only analyze a subset of puffs, e.g., those with amplitudes larger than the value at which f(A) has its maximum [31]. The discarded events in the latter comprised around 20% of the total number of puffs, in the case of Fluo-4 experiments. Oregon green experiments show a much larger variability both in tr and lr. Although the size of the source seems to increase with the current I>0.6 pA, the data in that region are not sufficient to draw a definitive conclusion (Fig. 2H and 3C).
Figure 4.
Dependence of Ca2+ flux duration (tr) and of Ca2+ source size (lr) on the maximum current, Imax for Fluo-4 (A,B) and Oregon green (C,D) experiments. Standard deviations and bin sizes of current around mean values are represented with error bars.
3.4. Relationship between current and number of open channels
We show in Fig. 5A the current distribution of Fig. 3A (Fluo-4 experiments) with bars and the results of approximating it by Eq. 15 (dashed curves) with α=1 and α=2. We show a similar plot in Fig. 5D but for Oregon green experiments. For Fluo-4, the fitting gives Io=0.086pA and m=4 for α=1 and Io= 0.45pA and m=0.72 for α=2. For Oregon green, Io=0.19pA and m=1.2 for α=1 and Io=0.14pA and m=13 for α=2. None of the theoretical distributions seems to be a good representation of the experimental data. Furthermore, in the α=2 case, the mean number of open channels, m, and the single channel current, Io, for Fluo-4 experiments are quite unrealistic.
Figure 5.
Current distribution functions for Fluo-4 (A, B) and Oregon green (D,E) experiments. Bars: experimental data. Lines: Theoretical expressions. A: Theoretical expression given by Eq. 15 with α=1, Io=0.086pA, m=4 (solid line) and with α=2, Io=0.45pA and m=0.72 (dashed curve). B: Theoretical expression given by Eq. 16-17 with I1*=0.36pA, I2*=0.4pA, λ1=0.71, α1=1, Io1=0.017pA, m1=15, λ2=0.27, α2=2, Io2=0.08pA and m2=33. D. Theoretical expression similar to A, but with α =1, Io=0.19pA, m=1.22 (solid line) and with α =2, Io=0.56pA and m=0.32 (dashed curve). E: Theoretical expression similar to B but with λ1=0.71, α1=1, Io1=0.025pA, m1=4.72, λ2=0.11, α2=2, Io2=0.107pA and m2=23.6. C, F: Current vs Np relationship given by Eq. 14 with Io1=0.017pA, Io2=0.08pA and Npt≈22 in C and with Io1 = 0.025 pA, Io2 = 0.107pA, in F.
We show in Fig. 5C the result of approximating the observed Fluo-4 distribution by Eqs.16-17 (up to Im). The fitting gives Io1 = 0.017 pA, m1=15, Io2 = 0.08pA, m2 = 33, λ1= 0.71, λ2= 0.27 and I*=0.38pA. As expected, each population of IP3R clusters is characterized by a different mean number of channels that open during a puff, m. We show the corresponding function I(Np) (Eq.15) in Fig. 5C. The current that guarantees the continuity of I(Np) which we used for the fitting, I*=0.38pA, practically coincides with the value at which the current distribution has a minimum. Small variations in the value I* that we used did not alter the parameter values of the theoretical distribution too much. We show similar plots for Oregon green experiments in Figs.5E-F. The parameters in this case are: Io1 = 0.025 pA, m1=4.72, Io2 = 0.107pA, m2 = 23.6, λ1= 0.71, and λ2= 0.11. In this case we kept a gap (the region between 0.26 and 0.43pA where I switches from the ~ Np to the ~ Np1/2 scaling) to do the fitting. We observe that a change of scaling from I ~Np to I ~Np1/2 and a single channel current Io1~0.02 pA are compatible with the observations from Oregon green experiments. The distribution defined by Eqs.16-17 seems to approximate the observed current distribution better than Eq.15. However, it involves many more parameters and it is not evident which distribution is the most likely.
We also tried to fit the data assuming a single scaling relationship between I and Np for the whole range of observed currents (α1 = α2= 1), but two cluster populations. Namely, we considered that I(Np) was given by Eq. 13 with α=1 and that P(Np) was given by Eq. 12 with M=2. The values obtained in this way for Fluo-4 experiments were very unreasonable (e.g., Io = 0.0038pA and m2 = 118) and we do not show these results here. It is also possible to fit the distribution assuming that P(Np) is given by Eq. 12 with M=2 and that I(Np) is given by Eq. 13 with α=1, but with a different value of I0 for each population. In particular, for the second population we obtain I02=0.008pA and m2 = 57, which are unrealistic numbers. For this reason, we do not discuss these models here.
3.5. Numerical simulations and comparison with other experimental observations
One may argue that the quality of the fit is better using Eqs.16-17 than using Eq.15 (with α=1) only because there are more fitting parameters in the former model. To choose between them, we first applied the Akaike (AIC) and Bayesian (BIC) information criteria (in the case of Fluo-4 experiments). The AIC criterion favored the model given by Eqs.16-17 while the BIC criterion favored the other one. Taking this result into account and considering that the puff amplitude and current distributions may not be a faithful representation of the whole set of events that occur in the cell given that very dim puffs could go undetected, we decided to compare the predictions of the models by means of numerical simulations. To this end, we simulated the Ca2+ distribution in the presence of different numbers of open IP3R’s and different choices of the single channel current as described in Sec. 2.5. Based on the results of Fig. 2G, we distributed the open channels randomly over a 0.5 μm × 0.5 μm square, as shown in Fig. 6B. We picked the channel locations among the nodes of a square grid of 20nm sides and discarded the configurations for which the inter-channel distance was less than 56nm. For the single channel current, we either chose a constant value, Ich=Io=0.086pA, (the one we obtained from the fitting of Fluo-4 experiments under the assumption that fI(I) is given by Eq. 15 with α=1) or a value that depended on the total number of open channels, Np, so that NpIch was given by Eq. 14 (the relationship that underlies Eq. 17).
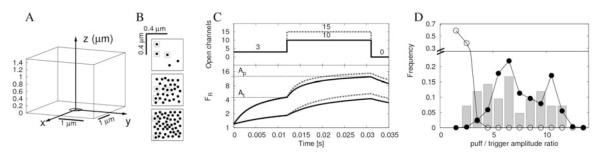
Figure 6.
Simulation of paired trigger-puff events considering 3-channel triggers. A: Geometry of the cytosolic space: a cuboid of dimensions 2.7 × 2.7 × 1.5 μm. The plane z=0 represents the surface of the ER and the circle at the center a cluster of IP3R’s. B: Three examples of IP3R distributions within clusters (from top to bottom, Np=5, 30 and 50). The width of the cluster is constant (0.5 □m). In the figure, the triggering channels are surrounded by boxes. C. Results of the simulation of four paired trigger-puff events. The upper plot shows the number of open channels as a function of time. Three trigger channels open at t=0. At t=0.012s a puff begins with a total of Np channels simultaneously open. We show results with Np=10 (solid lines) and Np=15 (dotted lines). The lower panel shows the corresponding time evolution of the fluorescence signal, FR. The upper traces correspond to simulations in which the single channel current was Ich=Io=0.086pA, independent of Np, while the other two correspond to NpIch=I, with I given by Eq. 13. We also show the puff and trigger amplitudes obtained in these simulations in the lower panel. D. Comparison of the distributions of puff-to-trigger amplitude ratios obtained from the simulations and from the experiments. The bar plot shows the experimental distribution reported by Rose et al in [2] (the data points were directly captured from their Fig.4F). The circles correspond to simulations in which the distribution of open channels was assumed to be given by Eq. 11 with m=4 (white circles) or was assumed to be given by the superposition of two Poisson distributions that eventually leads to Eq. 16 (black circles).
First we checked whether the two models could reproduce the puff amplitude to current relationship displayed in Fig. 3B. To this end we performed the simulations assuming that all the channels became open at the same time and remained open for 18 ms. We then computed the blurred version of the Ca2+-bound dye distribution given by Eq. 9 and the corresponding fluorescence amplitude with Eq. 2. The results are displayed in Fig. 3B (open circles for the model with constant Ich=Io=0.086pA and solid circles for the model for which NpIch is given by Eq. 14). We used Np=1,3,5,10,15 for the model with constant Ich which gives a total current between 0.086pA and 1.29pA. Instead, the total current varies between 0.017 and 0.57pA (Np =1,3,5,10,15,20,25,30,40,50) when we use the model for which NpIch is given by Eq.14. Given that there is no noise added to the simulations, based on this comparison we conclude that both models give good representations of the observations.
We then investigated what puff-to-trigger amplitude distribution they gave rise to comparing them with the results reported in [2]. It is worth mentioning that the puff-to-trigger amplitude distribution should not be affected much by offsets of the linescan. To this end, we performed a series of simulations where a certain number - 1, 2 or 3 - of triggering channels opened at t=0 while the rest opened at t=12ms. All the channels closed at t=31ms. A total of Np channels were open during the puff, which was chosen to last for 19ms. One of the triggering channels was always placed at the center of the cluster. The rest of the channels that opened during the trigger, and those that gave rise to the puff, were distributed randomly over a 0.5 μm × 0.5 μm region. In Fig. 6B we show three examples of the spatial distribution of the channels during a puff with Np = 5, 30 and 50 open channels. We obtained the best results for 3-channel triggers. In Fig. 6C we show traces of the fluorescence ratio, FR, for four different simulations of paired trigger-puff events with a 3-channel trigger. The upper traces correspond to simulations in which the single channel current was Ich=Io=0.086pA, independent of Np, while the other two correspond to NpIch=I, with I given by Eq. 14. We calculated the amplitude of the puff as FR at t=0.031s and the amplitude of the trigger as FR at t=0.012s. In this way we found the puff-to-trigger amplitude ratio for different numbers of open channels during the puff. Assuming that the distribution of open channels is given by Eq. 11 with m=4 or by Eq.12 with M=2, m1=15, m2 = 33, λ1= 0.71 and λ2= 0.27 (parameters that were obtained from the fitting of Fluo-4 experiments assuming that fI(I) was given by Eq. 15 with α=1 and Eqs.16-17, respectively) we could further calculate the expected distribution of puff-to-trigger amplitude ratios for both cases. In Fig. 6D we show these distributions together with the experimental distribution reported in [2] (bar plot). We observe that the model that underlies Eqs.16-17 is able to reproduce the experimental puff-to-trigger amplitude distribution fairly well, if we assume that the trigger corresponds to the opening of three channels. The simple Poisson model described by Eq. 11 with m=4 and α=1 fails to reproduce these observations.
4. Discussion
We have applied a “backward method” to infer the properties of Ca2+ release during puffs observed in Xenopus Laevis oocytes. Backward methods start from the image and perform a series of computations to determine the underlying Ca2+ current. Previous works have relied on forward methods in which a model of intracellular Ca2+ dynamics is used to generate numerically simulated images that are then compared with experimental ones. Whether forward or backward, most methods that have been used to infer information on the amplitude and kinetics of the Ca2+ current that underlies an image require a working model of the cytosolic Ca2+ dynamics. This implies the knowledge of a large number of parameters, not all of which can be extracted from the analyzed experiments. The method that we have used requires a minimum of a priori assumptions and constructs the model of Ca2+ dynamics from the observations themselves. Using our method we determined the kinetics and amplitude of the calcium current and the size of the calcium release region. Previous estimates of puff calcium currents obtained directly from experiments were based on signal mass calculations that used a rough estimate of how much calcium was bound to the dye compared to endogenous buffers [6]. These calculations gave currents that ranged between 0.4 and 2.5pA. Forward modeling efforts used puff currents that ranged from 0.015pA to 0.8pA [7] or concluded that a good correspondence with experiments could be obtained by assuming the synchronous opening of 25-35 IP3Rs and a single channel current between 0.2 and 0.5pA [4].The application of our method to over 500 puffs gave currents between 0.12 and 0.95pA. The study also showed that the release duration is peaked around 18ms and that the size of the release region ~450 nm does not change much from site to site. This implies that larger puffs correspond to larger densities of channels within the cluster, a result that has implications on signaling since the distance between channels affects inter-channel communication and the ability of signals to propagate. The relatively narrow distribution of source dimensions does not prevent puffs from showing a great variability in extent with sizes ranging from 0.3 to 2.2μm. Numerical simulations in which all channels opened and closed simultaneously showed that having a relatively invariant size of the Ca2+ release region is consistent with having puffs of different sizes and that the averaging procedure that we apply to the experimental data does not affect this value significantly. This value, however, could be slightly overestimated (due to blurring) and should be considered as an upper bound for the actual size of the calcium release region. Nevertheless, it is consistent with the estimate of IP3R cluster sizes suggested in [4] (300-800 nm) and with the one determined in [10], which is bigger than those presented in [32, 33] (~100 nm).
We studied whether there was a correlation between the duration and the amplitude of the calcium released. As shown in Figs. 4A and 4C and in agreement with [33], the duration of the release and the maximum current amplitude are relatively independent, although a weak increasing dependence can be observed in Fig. 4C. The increasing behavior seems to be contradictory with the inhibitory role that a large [Ca2+] can exert on IP3R’s. This apparent contradiction can be understood in terms of the stochastic model described in Sec. 2.5 some of which results we show in Fig. 7. Fig. 7A is a plot of puff duration as a function of puff amplitude for the experiments (open circles) and for the stochastic simulations performed with the Cd IP3R kinetic model (black squares and error bars) and a 0.1pA single channel current. Simulations performed with the Ci kinetic model give similar results. In both the experiments and in the stochastic simulations, the puff duration decreases with the amplitude. This occurs because the larger the maximum current the shorter is the time to achieve the peak current so that tf is shorter too. For example, a simulated puff with a 3pA maximum current achieves its peak current at 6.5ms and tf=10.6ms while another puff with a maximum current of 0.9pA achieves its peak current at 16.6ms and tf=29.8ms. This different behavior may be understood in terms of the spatial organization of the channels involved. The stochastic simulations have been done assuming that the spatial extent of the IP3R clusters is constant regardless of the number of channels that they contain. Thus, the simulated puffs that involve more open channels and have larger amplitudes occur in clusters where the mean distance between available channels is shorter so that CICR occurs on a faster time-scale and the peak current is achieved within a shorter time. This decreasing behavior does not mean that a similar relationship holds between release duration and Ca2+ current. This is apparent in Fig. 7B where we show the release duration as a function of the maximum Ca2+ current for the stochastically simulated puffs obtained using model Cd (black circles) and Ci (open circles). The maximum current in this case is computed over a smoothed out version of the simulated current that is obtained applying a 20 ms moving average. We observe that the mean value of the release duration increases very rapidly as a function of the maximum current and then reaches a plateau. Thus, the apparent independence observed in the experiments may be attributed to the finite time resolution of the observations. In agreement with this, a nonlinear increasing dependence between puff duration and maximum number of open channels was observed in experiments with a higher time resolution in which the puff duration is roughly equivalent to release duration [10].
We analyzed to what extent the determined current distribution, fI(I), was compatible with various underlying distributions of number of open channels, P(Np), and relationships between I and Np. In particular, we compared in detail two models. In the first one, P(Np) was given by Eq. 11 (a Poisson distribution) and I= Io Np, so that the current distribution was given by Eq. 15. In the second one, P(Np) was the superposition of two Poisson distributions (Eq.12 with M=2) and I was given by Eq. 14, so that the current distribution was given by Eqs.16-17. By fitting the experimental data we obtained the various parameters of the models. In particular, the single channel current was Io=0.086pA in the first model and, in the second one, Io1 = 0.017pA when only one channel was open, while it decreased nonlinearly with the number of open channels (giving I~Np1/2) when this number was large enough. The nonlinear scaling and the single channel current of the latter agrees with the estimates of [7]. In order to choose between the models, we first applied two information based criteria (AIC and BIC) and each of them favored a different model. In order to overcome this difficulty and the one derived from the possible inaccuracy of the experimental puff amplitude distribution in the low amplitude region, we performed a series of numerical simulations of intracellular Ca2+ dynamics in the vicinity of a cluster of IP3R’s in which the number and location of the channels that opened during the event were chosen a priori. In some simulations all the channels opened simultaneously and, in others, a few channels (a “trigger”) opened first and were subsequently followed by the rest. We repeated all the simulations for both types of single channel current. We could reproduce the experimentally determined amplitude vs current relationship reasonably well in both cases (Fig. 3B), but for very different values of the number of open channels. When we tried to reproduce the puff-to- trigger amplitude distribution of [2], the model that corresponds to Eqs.16-17 performed much better than the one obtained using Eq. 15 with α=1. We concluded that the model of Eqs.16-17 gives a better description of the experimental observations than the model of Eq. 15 with α=1. This implies, on one hand, that the variability among clusters plays an important role in shaping the puff amplitude distribution that is observed experimentally, which agrees with the findings of [10]. Another distinctive feature of the model that underlies Eqs.16-17 is the nonlinear scaling between the current and number of open channels. This feature could be attributed to luminal Ca2+ depletion as discussed in [7]. However, we must remember that the two models that we are comparing here assume that all channels open and close simultaneously. In that respect, they are mean-field models that give “averaged” information. When we drop this simplification and allow the individual channels to open and close stochastically as described in Section 2.5, the experimental observations may be reproduced with the same intra-cluster spatial organization as the one considered in the mean-field models analyzed in Sec. 3.5 but with a different combination of single channel current and number of available channels. In particular, the stochastic model reproduces the observations using any of the two kinetic IP3R models, a fixed single channel current, Ich ~0.1pA (similarly to the averaged model that underlies Eq. 15) and a number of available channels, Np, within the range of the averaged model that underlies Eqs.16-17, as shown in Fig. 7C. This figure displays the results of 474 stochastic simulations performed with the Cd kinetic model for each value of Np. We show with open circles the maximum released currents as functions of the number of available channels for the stochastic simulations. In order to compare these results with those of the mean field models we computed the average of the Ca2+ current released during the whole puff duration for each simulation. We plot the mean (black dots) and standard deviation (vertical lines) of this average as functions of the number of available channels. From the average, which can be approximated as I~Np for small Np and as I~Np1/2 for large Np, as shown in Fig. 7C, it is possible to compute an effective single channel current, Ieff= Iave/Np that is of the same order of magnitude as that of the model that underlies Eqs.16-17. This is an indication that the nonlinear relationship between current and number of available channels that we inferred from the observations could be a consequence of having used a mean field model. We will explore the limitations of mean field models elsewhere in more detail. In any case, even if the mean field model that underlies Eqs. 16-17 may not necessarily provide an accurate description of all intra-cluster properties, it can still be preferred, due to its simplicity, over more detailed models as the building block with which to describe more global signals, such as waves.
Finally, we caution that the quantitative information that we extract from the observations is highly sensitive to uncertainties in a priori assumptions of some parameters.. With the aid of simulated models, we have found that good estimates (error less that 20%) of the current amplitudes can be obtained if the uncertainties in the dye diffusion coefficient and the on and off rate constants of the calcium-dye reaction are less than 50%. These results and others [14], [34] point to the necessity of having a good determination of these parameter values for each experimental condition to get reliable results.
Supplementary Material
Acknowledgements
We acknowledge useful conversations with Ian Parker and Jian-Wei Shuai. L.B and S.P.D. are members of the Carrera del Investigador Científico (CONICET). This research is supported by UBA (UBACyT X208), PICT 17-21001 and PICT06 928 of ANPCyT (Argentina), Santa Fe Institute, CONICET (PIP 5131), and the U.S. National Institutes of Health (GM48071 and GM 65830).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ventura AC, Bruno L, Demuro A, Parker I, Dawson SP. A model-independent algorithm to derive Ca2+ fluxes underlying local cytosolic Ca2+ transients. Biophys J. 2005;88:2403–21. doi: 10.1529/biophysj.104.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose HJ, Dargan S, Shuai J, Parker I. ‘Trigger’ events precede calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4024–32. doi: 10.1529/biophysj.106.088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callamaras N, Parker I. Radial localization of inositol 1,4,5-trisphosphate-sensitive Ca2+ release sites in Xenopus oocytes resolved by axial confocal linescan imaging. J Gen Physiol. 1999;113:199–213. doi: 10.1085/jgp.113.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuai J, Rose HJ, Parker I. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4033–44. doi: 10.1529/biophysj.106.088880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swillens S, Dupont G, Combettes L, Champeil P. From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc Natl Acad Sci U S A. 1999;96:13750–5. doi: 10.1073/pnas.96.24.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun XP, Callamaras N, Marchant JS, Parker I. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J Physiol. 1998;509(Pt 1):67–80. doi: 10.1111/j.1469-7793.1998.067bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thul R, Falcke M. Release currents of IP(3) receptor channel clusters and concentration profiles. Biophys J. 2004;86:2660–73. doi: 10.1016/S0006-3495(04)74322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith GD, Pearson JE, Keizer J. Modeling Intracellular Calcium Waves and Sparks. In: Fall CP, Marland ES, Wagner JM, Tyson JJ, editors. Computational cell biology. Springer; New York: 2002. [Google Scholar]
- 9.Rios E, Brum G. Ca2+ release flux underlying Ca2+ transients and Ca2+ sparks in skeletal muscle. Front Biosci. 2002;7:d1195–211. doi: 10.2741/A834. [DOI] [PubMed] [Google Scholar]
- 10.Smith IF, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith IF, Wiltgen SM, Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demuro A, Parker I. “Optical patch-clamping”: single-channel recording by imaging Ca2+ flux through individual muscle acetylcholine receptor channels. J Gen Physiol. 2005;126:179–92. doi: 10.1085/jgp.200509331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blatter LA, Huser J, Rios E. Sarcoplasmic reticulum Ca2+ release flux underlying Ca2+ sparks in cardiac muscle. Proc Natl Acad Sci U S A. 1997;94:4176–81. doi: 10.1073/pnas.94.8.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rios E, Stern MD, Gonzalez A, Pizarro G, Shirokova N. Calcium release flux underlying Ca2+ sparks of frog skeletal muscle. J Gen Physiol. 1999;114:31–48. doi: 10.1085/jgp.114.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soeller C, Cannell MB. Estimation of the sarcoplasmic reticulum Ca2+ release flux underlying Ca2+ sparks. Biophys J. 2002;82:2396–414. doi: 10.1016/S0006-3495(02)75584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baylor SM, Hollingworth S, Chandler WK. Comparison of simulated and measured calcium sparks in intact skeletal muscle fibers of the frog. J Gen Physiol. 2002;120:349–68. doi: 10.1085/jgp.20028620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventura AC, Bruno L, Dawson S. Ponce. Probing a reduced equation for intracellular calcium dynamics. Physica A: Statistical Mechanics and its Applications. 2004;342:281–287. [Google Scholar]
- 18.Ventura AC, Bruno L, Dawson SP. Simple data-driven models of intracellular calcium dynamics with predictive power. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74:011917. doi: 10.1103/PhysRevE.74.011917. [DOI] [PubMed] [Google Scholar]
- 19.Parker I, Callamaras N, Wier WG. A high-resolution, confocal laser-scanning microscope and flash photolysis system for physiological studies. Cell Calcium. 1997;21:441–52. doi: 10.1016/s0143-4160(97)90055-5. [DOI] [PubMed] [Google Scholar]
- 20.Callamaras N, Parker I. Caged inositol 1,4,5-trisphosphate for studying release of Ca2+ from intracellular stores. Methods Enzymol. 1998;291:380–403. doi: 10.1016/s0076-6879(98)91024-2. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–17. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baran I. Characterization of local calcium signals in tubular networks of endoplasmic reticulum. Cell Calcium. 2007;42:245–60. doi: 10.1016/j.ceca.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Smith GD, Dai L, Miura RM, Sherman A. Asymptotic Analysis of Buffered Calcium Diffusion near a Point Source. SIAM Journal on Applied Mathematics. 2001;61:1816–1838. [Google Scholar]
- 24.Solovey G, Fraiman D, Pando B, Dawson S. Ponce. Simplified model of cytosolic Ca2+ dynamics in the presence of one or several clusters of Ca2+ -release channels. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:041915. doi: 10.1103/PhysRevE.78.041915. [DOI] [PubMed] [Google Scholar]
- 25.De Young GW, Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992;89:9895–9. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groff JR, Smith GD. Calcium-dependent inactivation and the dynamics of calcium puffs and sparks. J Theor Biol. 2008;253:483–99. doi: 10.1016/j.jtbi.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Fraiman D, Pando B, Dargan S, Parker I, Dawson SP. Analysis of puff dynamics in oocytes: interdependence of puff amplitude and interpuff interval. Biophys J. 2006;90:3897–907. doi: 10.1529/biophysj.105.075911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–5. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 29.Conover WJ. Practical nonparametric statistics. Wiley; New York: 1999. [Google Scholar]
- 30.Rios E, Shirokova N, Kirsch WG, Pizarro G, Stern MD, Cheng H, Gonzalez A. A preferred amplitude of calcium sparks in skeletal muscle. Biophys J. 2001;80:169–83. doi: 10.1016/S0006-3495(01)76005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izu LT, Wier WG, Balke CW. Theoretical analysis of the Ca2+ spark amplitude distribution. Biophys J. 1998;75:1144–62. doi: 10.1016/s0006-3495(98)74034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falcke M. On the role of stochastic channel behavior in intracellular Ca2+ dynamics. Biophys J. 2003;84:42–56. doi: 10.1016/S0006-3495(03)74831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuai JW, Jung P. Stochastic properties of Ca(2+) release of inositol 1,4,5-trisphosphate receptor clusters. Biophys J. 2002;83:87–97. doi: 10.1016/S0006-3495(02)75151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuai J, Parker I. Optical single-channel recording by imaging Ca2+ flux through individual ion channels: theoretical considerations and limits to resolution. Cell Calcium. 2005;37:283–99. doi: 10.1016/j.ceca.2004.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.