Abstract
The standard approaches to the treatment of acute myeloid leukemia (AML) have been predominantly based on cytarabine and anthracyclines. Yet, the outcomes associated with AML continue to be poor, especially for those patients who are older or carry higher-risk disease. In recent years, extensive research has led to the development and study of novel agents which target AML by diverse and varied mechanisms. Among these are targeted therapeutics such as kinase inhibitors and oligonuceotide constructs. These aim to suppress the production or activity of proteins, such as FLT3 and BCL2, among others, and thus disrupt related signaling cascades essential for leukemogenesis and proliferation. In addition, other agents like flavopiridol appear to target the myeloid blast by various mechanisms including suppression of cyclin dependent kinases and interference with nucleotide synthesis. Another class of novel therapies includes inhibitors of histone deacetylase, which cause growth arrest and apoptosis through histone acetylation and resultant conformational changes. Clinical trials are now studying these and other agents alone and in combination with traditional cytotoxic therapies, with some encouraging results. In this review, we aim to provide a summary of the preclinical and clinical investigations of selected promising agents currently under study.
Keywords: Acute myeloid leukemia, Flavopiridol, HDAC inhibitor, Targeted therapies, PARP, FLT3
Introduction
Acute myeloid leukemia (AML) is characterized by an arrest in differentiation and uncontrolled proliferation of myeloid precursors in the bone marrow. This underlying process leads to hematopoietic insufficiency, and when undifferentiated cells escape the marrow, to significant leukocytosis, with often devastating and life-threatening sequelae. Although the majority of patients under age 60 achieve a complete remission (CR) with traditional anthracycline- and cytarabine-based induction regimens, the long-term survival rates continue to be poor at approximately 30–40% 1, 2. The prognosis is even poorer for those with high-risk AML, such as those who are older, who had preceding myelodysplastic syndromes (MDS) or myeloproliferative disorders (MPD), or those with secondary AML from environmental exposures or prior chemotherapy. In such cases, a complete remission is achieved in less than 40% of cases, with survival rates of less than 10% 2, 3.
Novel therapies to improve these unsatisfactory outcomes are aimed at developing agents which target cell signaling and cycling, as well as those which interrupt DNA repair and replication. Some of these endeavors are in early phases of development and study, while others have shown promise in preclinical and clinical investigation. The ultimate goal will be to broaden the therapeutic potential of traditional induction regimens in AML by the rational incorporation of mechanistically novel agents. In the current review, we have selected these promising approaches to discuss below.
Flavopiridol
Flavopiridol is a semi-synthetic flavone derived from the stem bark of Amoora rohituka and Dysoxylum binectariferum, plants used in India as herbal medicine 4. It has been demonstrated to have strong activity against multiple cyclin dependent kinases, and arrests the cell cycle at the G2/M phase and delays the G1 to S phase progression 5. Flavopiridol also inactivates the cdk-9/cyclin T complex, also known as PTEF-b, resulting in inhibition of RNA polymerase II, and suppression of RNA and polypeptide synthesis. This transcriptional inhibition leads to a decrease in levels of proteins, such as cyclin D1, VEGF, MCL-1, and STAT-3, essential for cell cycling and survival 6–8. In addition, flavopiridol is active to a lesser degree on tyrosine kinases, such as the epidermal growth factor receptor (EGFR), protein kinase C (PKC)and Erk 5 (Table 1).
Table 1.
| Action of Flavopiridol | Impact on cell survival and proliferation |
|---|---|
| Inhibition of serine-threonine CDKs through non-cell cycle dependent and cycle dependent mechanisms |
Cell cycle arrest at the G1-S and G2-M checkpoints. |
| Decrease in the activty of VEGF | Inhibition of angiogenesis and cell growth. |
| Binding and inactivation of the CDK9/Cyclin T1 complex (PTEFb) |
Inhibition of the RNA polymerase II complex and resultant blockade of transcriptional elongation. |
| Binding to DNA and disruption of transcription |
Disruption of DNA binding to key transcription factors such as STAT3, leading to a decrease in the expression of the target proteins like Mcl-1. |
| Inhibition of tyrosine kinases e.g EGFR, Erk, etc. |
Inhibition of constitutive activation of receptors and downstream kinases, leading to a decrease in proliferation and survival. |
In preclinical studies, flavopiridol was active in diverse hematopoietic cell lines 9, 10. In AML, its novel mechanism of action and ability to target both cycling and non-cycling cells in vitro has rendered flavopiridol an intriguing candidate for combination with traditional cytotoxic therapies. When administered concomitantly with cytarabine and topotecan, S-phase dependent agents, it produces antagonistic effects through its propensity to induce cell cycle arrest 11. However, it was noted that when flavopiridol administration and withdrawal preceded cytarabine and topotecan, dormant surviving cells were allowed to re-enter the cell cycle and were thus further sensitized to the latter agents 7, 11.
Clinical trials based on the in vitro model findings are in progress. In these studies, flavopiridol is administered as an initial cytoreductive agent for 3 days, following which the remaining leukemic cells could be recruited into the cell cycle and thus be kinetically sensitized for cytotoxicity by the 72 hour continuous administration of cytarabine beginning on day 6 and mitoxantrone on day 9 12, 13. In a recent phase II study of this regimen (FLAM) in 62 patients with poor-risk AML, flavopiridol was directly cytotoxic, with 44% of patients experiencing ≥50% decrease in peripheral blasts by day 2 and 26% experiencing ≥80% decrease in blasts by day 3. CRs were achieved in 75% of patients with newly diagnosed secondary AML and those with first relapse after short CR. Rates of CR were significantly lower for those with refractory disease. Disease free survival (DFS) for all CR patients was 40% at 2 years 13. These results have recently been expanded to another cohort of 45 patients with newly diagnosed, poor-risk AML. Of these, 67% achieved CR and 40% underwent a myeloablative allogeneic bone marrow transplant (BMT) in first CR, translating into long-term survival 14.
Alternative dosing schedules of flavopiridol are also being studied. A “hybrid” bolus-infusion schedule of flavopiridol has been investigated in CLL with promising results. In this approach, a pharmacologically-modeled schedule of flavopiridol is administered, with a 30 minute bolus of roughly half of the total dose, followed by a 4 hr infusion of the remaining portion, in an attempt to overcome the observed effects of avid binding of flavopiridol by human plasma proteins 15, 16. This hybrid schedule of flavopiridol administration is currently being studied in a dose-escalation, phase I trial of patients with primary refractory and relapsed AML (clinicaltrials.gov, NCT00470197). Correlative in vivo pharmacodynamic studies demonstrate flavopiridol-induced suppression of target genes, including MCL-1, VEGF, E2F1, STAT-3, cyclin D1, and RNA polymerase II 17. Another ongoing study, a phase II trial comparing the hybrid infusion of flavopiridol with bolus administration of the drug in patients with newly diagnosed, poor-risk AML is currently recruiting (clinicaltrials.gov, NCT00795002).
Flavopiridol has been combined with other novel targeted therapies to enhance antileukemic efficacy. Among these are histone deacetylase inhibitors (HDIs), which allow for acetylation of histones with resultant conformational changes and transcription of genes that allow differentiation, growth arrest, and/or apoptosis 18. Interestingly, HDIs up-regulate the expression of MCL-1, an antiapoptotic member of the bcl-2 family 19, and p21, a cyclin dependent kinase (CDK) inhibitor 20, which together can limit the cytotoxic efficacy of these agents. Therefore, therapies that can down-regulate expression MCL-1 and p21, such as flavopiridol, may be synergistically efficacious in combination with HDIs. Indeed, the HDI-mediated decrease in induction of p21 appears to be interrupted by flavopiridol, leading to a potentiation of apoptosis in human leukemia cells 19–22. The HDI, suberoylanilide hydroxamic acid (vorinostat; SAHA), has been combined with flavopiridol in preclinical studies, with synergistic induction of apoptosis through mitochondrial damage, cell cycle dysregulation, and caspase activation 18. Currently, a phase I trial of SAHA and flavopiridol in patients with relapsed/poor prognosis acute leukemia or advanced MDS is underway and enrolling patients (clinicaltrials.gov, NCT 00278330).
Other HDI-related strategies
In view of their pleiotropic mechanisms of action, HDIs lend themselves particularly well to combination regimens involving other targeted agents, in addition to the one described above in the case of flavopiridol. HDIs have been broadly classified as pan-HDIs, such as the hydroxamates vorinostat, belinostat (PXD101), and panobinostat (LBH-589), which inhibit multiple HDAC classes (e.g. Class I and II), and those whose actions are primarily directed against a single class (e.g., Class I), such as SNDX-275 and MGCD0103. Aside from their capacity to modulate gene expression by altering chromatin structure, HDIs induce cell death through multiple other mechanisms, in some cases a consequence of acetylation of non-histone proteins. For example, in human leukemia cells, HDI lethality has been related to up-regulation of death receptors 23. Other postulated mechanisms of lethality include induction of oxidative damage 24, 25, acetylation of and interference with the function of chaperone proteins such as Hsp90 26, acetylation and disruption of the function of DNA repair proteins (e.g., Ku70) 27, up-regulation of pro-apoptotic proteins such as Bim 28, and disruption of cell cycle checkpoints 29. Finally, HDIs may act by interfering with the contribution of HDACs to co-repressor complexes responsible for the block to leukemic cell maturation 30. Initial results of clinical trials suggest that HDIs, including the HDIs vorinostat and the Class I-specific HDI MGCD0103, may have some single agent activity in refractory AML 31, 32.
However, because of their diverse mechanisms of action, attention has begun to focus on the capacity of HDIs to potentiate the antileukemic activity of other targeted agents. For example, mutant tyrosine kinases, including those implicated in AML such as FLT3 (see below), appear to be particularly dependent upon intact chaperone function for their maintenance. This raises the possibility that HDIs, at least those capable of inhibiting deacetylation of Hsp90, might enhance the activity of clinically relevant FLT3 inhibitors by down-regulating the expression of Hsp90. Indeed, the results of preclinical studies suggest that co-administration of pan-HDIs with FLT3 inhibitors results in a pronounced increase in antileukemic activity 33. Such findings support the concept of combining HDIs with tyrosine kinase inhibitors such as FLT3 inhibitors in refractory AML.
Another rational HDI combination strategy of potential relevance to AML involves the use of proteasome inhibitors. Preclinical studies indicate that HDIs interact synergistically with proteasome inhibitors such as bortezomib in diverse malignant hematopoietic cell types, including myeloid leukemia, CLL, and myeloma 34–36. The mechanisms underlying such interactions may be multi-factorial, including inhibition of NF-κB activation as well as disruption of aggresome formation, leading to ER stress 26. Notably, a regimen combining vorinostat with bortezomib has shown significant activity in patients with refractory multiple myeloma 37. Although proteasome inhibitors have relatively modest single agent activity in AML 38, the possibility that co-adminstration of proteasome and deactylase inhibitors may yield superior activity seems to be a plausible one. Consequently, Phase I trials of HDIs in combination with bortezomib are underway.
By promoting a more open chromatin structure, HDIs render transformed cells more susceptible to agents that interfere with DNA function and integrity. For example, pretreatment of breast cancer cells with vorinostat significantly potentiated the lethal effects of topoisomerase II inhibitors 39. Analogously, pretreatment of human leukemia cells with vorinostat sensitized them to the lethal effects of VP-16 and ara-C 40. A clinical trial combining vorinostat with cytotoxic chemotherapy (e.g., idarubicin and ara-C) is underway.
Over the last several years, attention has focused on a strategy combining HDIs with hypomethylating agents for the treatment of various malignancies, including AML. This is based on the concept that silencing of genes implicated in leukemogenesis may be overcome by hypomethylating agents such as the DNA methyltransferease inhibitors (DNMTIs) 5-azacytidine or deoxyazacytidine. Furthermore, reversal of silencing of such genes by DNMTIs combined with disruption of the activity of HDAC-associated co-repressor complexes (by HDIs) may allow full expression of genes responsible for cell differentiation and death. Multiple preclinical studies have shown synergistic induction of cell death by regimens combining HDIs and DNMTIs 41, including those involving leukemia cells 42. Based upon this rationale, multiple HDI/DNMTI trials are underway in AML and MDs e.g., 5-azacytidine and SNDX-275 or 5-deoxyazacytine and valproic acid), and initial results appear potentially promising, particularly in patients who present with high-risk disease 43. One key question remaining to be resolved is whether such regimens act through de-repression of cell death or differentiation-related genes, or more directly through cytotoxic actions.
New anti-FLT3 Targeted Agents
Despite an exciting rationale for the use of tyrosine kinase inhibitors (TKIs) in AML, the clinical results have so far been modest. The most advanced studies involve inhibitors of the FMS-like tyrosine kinase-3 (FLT3) receptor. Approximately a third of patients with a diagnosis of AML carry a FLT3 internal tandem duplication (ITD) mutation, which renders the kinase constitutively active in driving the proliferation of the leukemic blast 44. The preponderance of current data suggests that an ITD mutation is a significant, independent, negative prognostic predictor in AML, with disease-free and overall survival severely and adversely affected 45–47. Development of targeted therapy against FLT3 is rapidly evolving. A number of small molecule FLT3 inhibitors have been studied beyond phase I investigation in patients with AML, including two indolocarbazole derivatives, midostaurin (PKC412), and lestaurtinib, and have been reviewed elsewhere 48–57. In this review, we will focus on promising FLT3 inhibitors in earlier phases of clinical development.
Sorafenib, a multi-kinase inhibitor, was initially developed to inhibit the Raf-1 kinase pathway. It has since been demonstrated to be a potent inhibitor of multiple receptor tyrosine kinases, including FLT3 58, 59. Sorafenib has been approved for use in advanced renal cell and hepatocellular carcinomas, after improving survival parameters in clinical trials 60, 61. Targets of sorafenib, such as FLT3, c-KIT, NRAS, and Raf kinase, are frequently mutated in AML. Together, these mutations seem to promote proliferation and arrest of differentiation in hematopoietic progenitor cells 62. Preclinical studies in FLT3-driven leukemic cell lines, primary samples, and xenograft models have revealed that sorafenib suppresses FLT3 signaling and promotes apoptosis 63, 64.
Emerging data suggest that sorafenib is well tolerated as a single agent in high-risk AML, with some patients experiencing impressive clinical responses. Earlier studies revealed transient, but significant, decreases in bone marrow blasts, particularly in patients with FLT3-ITD mutations 65, 66. Sorafenib was subsequently employed on a compassionate use-basis in a limited number of FLT3-ITD AML patients both prior to and after allogeneic stem cell transplantation. Two of three patients with refractory disease, who were given sorafenib, were able to proceed to transplant after remissions, suggesting that sorafenib can effectively reduce leukemic burdens in patients awaiting stem cell transplantation. Additionally, prolonged complete molecular remissions were noted in the few patients given sorafenib after transplant in this study 67. A phase I/II trial in patients with newly diagnosed AML found that sorafenib, when combined with cyrtarabine- and idarubicin-based induction, produced complete remissions in the majority, 22 of 25 evaluated patients (88%). Eight of these patients had FLT3-ITD mutations, and the drug was noted to effectively suppress FLT3-phosphorylation in correlative studies 68. Other ongoing clinical trials are evaluating the safety and efficacy of sorafenib in combination with clofarabine, vorinostat, and various induction regimens (clinicaltrials.gov, NCT00516828, NCT00908167, NCT00893373, NCT00875745).
KW-2449, a promising multi-kinase inhibitor that effectively suppresses FLT3 phosphorylation, inhibited growth of leukemia cell lines and suppressed phosphorylation of FLT3 and its downstream target, STAT5. A phase I trial of KW-2449 demonstrated modest single agent clinical activity in 8 of 31 AML patients (26%), including 5 with FLT3 mutations 69. These responses were often transient decreases in blasts, likely due to transitory FLT3 inhibition. Correlative studies are defining optimal administration schedules to achieve the sustained target inhibition necessary for ideal clinical responses 70. KW-2449 is also an aurora kinase inhibitor 71, and it is possible that this action may contribute to the antileukemic activity of this compound.
AC220 is a receptor tyrosine kinase inhibitor (TKI), demonstrated to have potent and specific in vitro and in vivo activity against the FLT3 tyrosine kinase. A phase I study in relapsed or refractory AML is currently under way, with promising preliminary results. Eleven of 45 patients (24%) have experienced transient clinical responses, with 4 achieving CRs (2 patients with incomplete platelet recovery and 2 with incomplete platelet and neutrophil recovery). An additional 7 patients had partial responses. Of note, three of the responders were FLT3 mutants 72. These very promising results may be due to the exceptional potency and selectivity of AC220 when compared to other TKIs, as well as its ability to effectively suppress both wild-type and mutated FLT3 tyrosine kinases 73, 74.
Studies of AML cell lines have further identified an up-regulation of the serine/threonine kinase PIM (proviral integration site for Moloney murine leukemia), a downstream target of FLT3. PIM, currently under extensive investigation, appears to play an important mediating role in signaling cascades and is felt to directly suppress the pro-apoptotic BAD 75, 76. More recent investigation has revealed that PIM may be an integral component of FLT-3 signaling complex in FLT3-ITD cell lines, and that inhibitors of PIM appear to be preferentially cytotoxic to FLT3-ITD AML cell lines and primary patient samples. Furthermore, PIM inhibition appears to lead to a suppression of phosphorylation of STAT5 as well as Akt, and therefore may affect cell survival through these signaling pathways, in addition to its affect on BAD phosphorylation 77. Targeted agents against PIM are in early stages of development and study 78 (clinicaltrials.gov, NCT00848601), but may play an important role for the treatment in AML in the future.
Inhibitors of the PI3-K/Akt/mTOR Signal Transduction Pathways
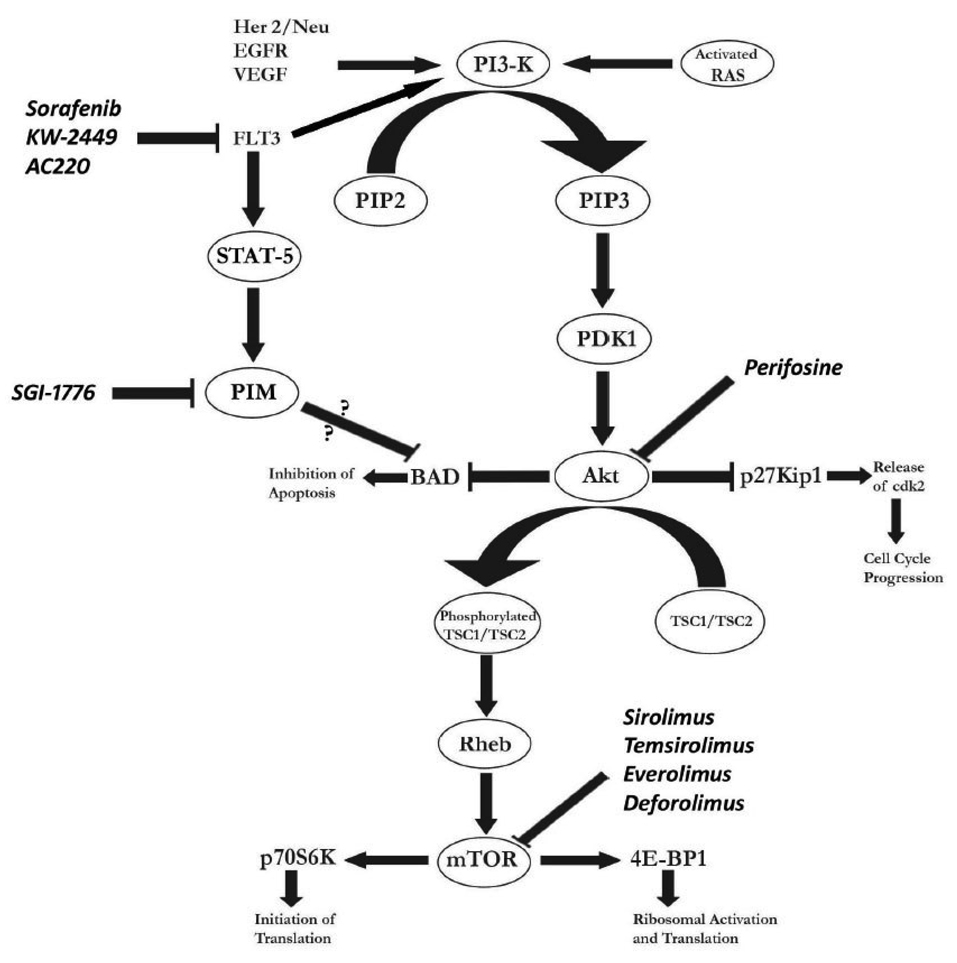
The phosphatidylinositol 3-kinase (PI3-K)/Akt/mammalian target of rapamycin (mTOR) signal transduction pathways are vital intracellular cascades which regulate translation, ribosomal biogenesis, cell cycling, and apoptosis. Its intricacies have been extensively reviewed elsewhere 79. In brief, PI3-K is activated when bound by a variety of receptor tyrosine kinases, such as FLT3, EGFR, and HER-2/neu (Figure 1). PI3-K converts phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3) at the inner surface of the membrane. Phosphoinositide-dependent kinase (PDK1) and Akt are then recruited to the membrane by PIP3. Akt, an important mediator in the intracellular cascade, is subsequently activated by PDK1 and acts on down-stream enzymes to stimulate proliferation and inhibit pro-apoptotic signals 80. As examples, it suppresses p27Kip1, a direct inhibitor of cdk-2, which is then free and able to promote transcription and resultant cell proliferation 81, and inhibits the pro-apoptotic bcl-2 antagonist of cell death (BAD) 82. Another target enzyme is tuberous sclerosis protein 2 (TSC2), which when phosphorylated, releases the protein Rheb to interact with and activate the mTOR kinase. mTOR, an important mediator, is involved in the progression from G1 phase to S when essential factors are available for cell division 80. mTOR’s targets include p70S6K, an activator of the ribosomal machinery and protein synthesis, and 4E-BP1, which promotes translation of RNA. Activation of these enzymes leads to enhanced synthesis of essential proteins in cell cycling and survival 80. Recent studies have also linked nucleophosmin (NPM) as an important mediator of mTOR dependent proliferation in oncogenesis 83.
Figure 1.
The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) and FMS-like tyrosine kinase 3 (FLT3) cascades in acute myeloid leukemia, and relevant targeted therapies. BAD—bcl-2 antagonist of cell death; CDK2—cyclin-dependent kinase 2; EGFR—epidermal growth factor receptor; PDK1—phosphoinositide-dependent kinase 1; PIP2—phosphatidylinositol-4,5-bisphosphate; PIP3—phosphatidylinositol-3,4,5-trisphosphate; TSC1, TSC2—tuberous sclerosis proteins 1 and 2; VEGF—vascular endothelial growth factor; PIM—Proviral integration site for Moloney murine leukemia virus.
Alterations in one or more components of the PI3-K/Akt/mTOR pathway have been noted in diverse neoplasms, including AML. Mutations of key enzymes can lead to increased constitutive signaling, with resultant survival and proliferation of malignant cells, and resistance to chemotherapy 84, 85. This survival can be suppressed by inhibiting the activity of PI3-K cascade, leading to the dephosphrylation of BAD and subsequent apoptosis 86, 87. Constitutive activation of the PI3-K/Akt signaling cascade is readily detectable in 50 to 70% of patients with AML 82, 88. Additionally, FLT3-ITD mutations lead to constitutive activation of the PI3-K/Akt cascade, promoting cell survival and proliferation 89. The mTOR pathway is also up-regulated, with targets, such as p70S6K and 4E-BP1, constitutively phosphorylated in the majority of AML samples 90. Dysfunction and down-regulation of the TSC1/TSC2 complex, a protein suppressor up-stream of mTOR, has also been linked to increased mTOR activity 91. Given the above observations, there is a burgeoning rationale for the therapeutic targeting of one or more members of the PI3-K/Akt/mTOR cascade for diverse malignancies, including AML (Table 2).
Table 2.
New Therapies in AML: Targeting the PI3-K/mTOR Cascade
| Target | Compound(s) | Mechanism |
|---|---|---|
| PI3-K | CAL-101 | Small molecule inhibitor of the delta isoform of the 110 kDa catalytic subunit of class IA PI3-K (clinicaltrials.gov, NCT00710528) |
| PI-103 | Small molecule inhibitor of PI3-K and mTOR 135. | |
| Akt | Perifosine | Decreases plasma membrane localization of Akt and its phosphorylation 136 (clinicaltrials.gov, NCT00391560). |
| GSK21110183 | Oral small molecule Akt inhibitor (clinicaltrials.gov, NCT00881946). |
|
| mTOR | Sirolimus (Rapamycin) Temsirolimus Everolimus (RAD 001) Deforolimus |
Directly suppresses mTOR when bound to FKBP12 79, 90, 93, 96, 137. |
| PIM | SGI-1776 | Small molecule inhibitor of tyrosine kinase (clinicaltrials.gov, NCT00848601). |
One member, the mTOR protein, is being extensively investigated for therapeutic potential in AML. Rapamycin (sirolimus), an antibiotic derived from the bacterial species streptococcus hygroscopicus, was initially approved, and has been extensively used as an immunosuppressant 92. However, it has been shown to also effectively inhibit mTOR when complexed with the FK506 binding protein 12 (FKBP12) 79. As a result, it has been employed to target the PI3-K/Akt/mTOR pathway in malignancies. An ester derivative of sirolimus, temsirolimus, and other mTOR inhibitors, such as everolimus (RAD001) and deforolimus, have also been studied as antineoplastic agents 93. These appear to have complex effects on the PI3-K/Akt/mTOR cascade. For example, some have found that temsirolimus and everolimus, in addition to their effects on mTOR signaling, additionally block the activity of Akt. This appears to be mediated through suppression of the newly discovered rictor/mTOR protein complex (mTORC2), which phosphorylates and activates Akt 94, 95.
Rapamycin has been demonstrated to effectively suppress leukemic cell lines and arrest the cell cycle at the G1 phase, which correlates with an up-regulation of the cdk inhibitor, p27kip1. The constitutive phosphorylation of down-stream targets of mTOR, p70S6K and 4E-BP1, was suppressed with the administration of rapamycin. A pilot clinical study of daily rapamycin in nine patients with refractory or relapsed AML produced 4 partial responses 90. Another small study of rapamycin in MDS-derived secondary AML in patients over the age of 65 demonstrated no clinical responses 96. A phase I/II study of temsirolimus in patients with hematologic malignancies included nine patients with AML and five with MDS. Of the latter, two patients achieved minor hematologic responses. The study also demonstrated that the phosphorylation of downstream targets of mTOR were effectively suppressed 97.
mTOR inhibitors are also being studied in combination with traditional cytotoxic therapies. In preclinical investigation, sirolimus dramatically increased the cytotoxicity of cytarabine and etoposide against AML blasts 85, 98. Multiple clinical trials are now under way to evaluate mTOR inhibitors in combination with traditional AML therapies for patients with poor risk AML (clinicaltrials.gov, NCT00235560, NCT00780104). Of these, the Eastern Cooperative Oncology Group is recruiting patients into a phase II randomized trial comparing three combination chemotherapy regimens for relapsed/refractory AML. One arm of this multi-center study will investigate the combination of sirolimus, mitoxantrone, etoposide, and cytarabine (clinicaltrials.gov, NCT00634244).
Bcl-2 Targeted Agents
Bcl-2, often up-regulated in AML, is a mitochondrial protein that impedes apoptosis. Patients with higher levels of bcl-2 expression have poorer prognoses, with lower rates of complete remission and worse survival, possibly due to the contribution of bcl-2 to chemotherapy resistance 99, 100. Therefore, suppressing bcl-2 has been pursued as a therapeutic approach, leading to the development of multiple potential therapeutic agents (Table 3).
Table 3.
New Therapies in AML: Targeting BCL-2 and Anti-Apoptotic Pathways
| Target | Compound(s) | Mechanism |
|---|---|---|
| BCL-2 | Oblimersen | Antisense oligonucleotide which binds to BCL-2 mRNA, leading to degradation of the complex 101, 104. |
| Obatoclax ABT-263 AT-101 |
Small molecule inhibitors which suppress BCL-2 by binding to its BH3-binding groove 138–140. (clinicaltrials.gov, NCT00684918). |
|
| XIAP | AEG-35156 | Antisense oligonucleotide which binds to XIAP mRNA, leading to degradation of the complex 107, 108. |
Antisense oligonucleotides are short sequences of single-stranded deoxyribonucleotides that complement and bind specific coding regions on mRNA, forming DNA-mRNA complexes which are subsequently degraded. In this manner, the ultimate translation of the targeted protein is prevented. Oblimersen (Genasense), a phosphorothioate, 18-base oligonucleotide, was found in preclinical studies to effectively suppress bcl-2 mRNA expression 101. A Phase I trial of oblimersen combined with FLAG (fludarabine, cytarabine, and GCSF) salvage therapy in relapsed/refractory AML yielded a 29% CR rate, as well as evidence of decreased Bcl-2 mRNA and protein expression 102. In the setting of newly diagnosed AML in older patients, the combination of oblimersen with traditional cytarabine/anthracycline based regimens yielded a 48% CR rate 103. These results affirmed the safety of combining this agent with traditional regimens. Unfortunately, a randomized, phase III trial of older patients failed to show improved outcomes for those receiving the combination with oblimersen 104.
Another anti-apoptotic protein is XIAP (X-linked suppressor of apoptosis), which binds and inhibits the caspases 3, 7 and 9, essential down-stream mediators of the apoptotic cascade. Like bcl-2, XIAP is over-expressed in AML, may be involved in leukemic cell survival and drug resistance, and when highly expressed, linked to poor clinical outcomes 105. Inhibitors of XIAP have been shown to activate downstream caspases and promote apoptosis in AML cell lines 106. AEG35156 is a 19-base, antisense phosphorothioate, which effectively suppressed XIAP mRNA and protein levels in preclinical models 107. A phase I/II trial of AEG35156 in combination with re-induction therapy was recently completed in refractory/relapsed AML patients. In the phase I portion of the study, 24 patients were treated with escalating doses of AEG35156 and one achieved a CR. In the subsequent phase II trial, 32 patients were treated with the highest planned dose, and of these, 15 (47%) achieved a CR/CRp. Importantly, this regimen was not efficacious in patients with multi-refractory AML. However, of 11 patients who were refractory to single induction regimen, 10 (91%) experienced a CR/CRp. XIAP mRNA levels from patient blasts were quantified by RT-PCR, and their suppression was detected 108, 109.
PARP Inhibitors
Poly ADP-ribosylation is known to occur after single or double-stranded DNA damage, a process of post-translational modification of histones and other nuclear proteins by PARP (poly ADP ribosylation polymerase). The PARP superfamily consists of multiple nuclear proteins, of which PARP-1 and PARP-2 appear to play a central role in repairing DNA damage. PARP binds DNA by the zinc-finger motif of its N-terminal, recruiting other essential enzymes, and bringing about base excision repair (BER) 110–112. Increased PARP activity is one of the mechanisms by which tumor cells avoid apoptosis caused by DNA damaging agents 113, 114, and thus has been considered as a target for anti-neoplastic therapy. Inhibition of PARP sensitizes tumor cells to cytotoxic agents which induce DNA damage that would be normally repaired through the BER system 115, 116.
The promise of clinical activity for PARP inhibitors was increased by the recent demonstration of prolonged survival in breast cancer patients with metastatic triple-negative disease 117. Although in earlier phases of investigation and development, PARP inhibition is also being actively investigated in AML 118. One agent, ABT-888, a potent inhibitor of PARP-1 and -2, has been demonstrated to potentiate the cytotoxic effects of temozolamide, platinum agents, cyclophosphamide, and radiation 119. ABT-888 has since been studied in an early phase study, and demonstrated proof of target inhibition of PARP in tumor biopsies and peripheral blood samples 120. A phase I clinical trial of ABT-888 in combination with topotecan and carboplatin in patients with high-risk MDS or relapsed/refractory AML is currently recruiting patients (clinicaltrials.gov, NCT 00588991).
MEK1/2 Inhibitors
The Ras/Raf/MEK1/2/ERK1/2 pathway, referred to as the mitogen-activated protein kinase (MAPK) pathway is frequently dysregulated in cancer, including hematologic malignancies such as AML 121, 122. The Raf family (Raf-1, A-Raf, B-Raf) signals downstream to phosphorylate the mitogen-associated/extracellular regulated kinases 1/2 (MEK1/2), which in turn phosphorylate extracellular regulated kinases 1 and 2 (ERK1/2) on threonine and tyrosine residues. ERK1/2 is involved in phosphorylation of multiple substrates implicated in cell survival and proliferation. These include p90RSK1, which activates the CREB transcription factor, and, following nuclear translocation, the Fos and Elk1 transcription factors 123. In addition, ERK1/2 modulates the expression, in some cases through phosphorylation, of multiple Bcl-2 family members and components of the apoptotic apparatus, including Bcl-2, Bim, Bad, survivin, and caspase-9 124. Thus, this pathway has become a major target for therapeutic intervention. In addition to inhibitors of upstream components of the pathway, including Ras and Raf, attention has recently focused on inhibitors of MEK1/2.
In preclinical studies, MEK1/2 inhibitors such as PD98059 and PD184352 have been shown to inhibit the growth and survival of AML cells, and to sensitize them to retinoids and standard chemotherapeutic agents 125. MEK1/2 inhibitors have also been shown to enhance the antileukemic activities of other targeted agents, including Mdm2 126 and Bcl-2 antagonists 127. The first MEK1/2 inhibitor to enter the clinic, PD325901 (Pfizer), has not been tested in AML, but plans are underway to evaluate several newer MEK1/2 inhibitors in this disease, including AZD6244 (Astra Zeneca), AS703026 (EMD Serono), and GSK1120212 (Glaxo-Smith-Kline). Finally, in view of evidence that simultaneous interruption of the Ras/Raf/MEK1/2/ERK1/2 and PI3K/Akt/mTOR pathways markedly increases transformed cell lethality 128, combination of MEK1/2 with PI3K or mTOR inhibitors represents an intriguing future possibility for the treatment of AML.
Conclusion and Future Directions
AML therapy continues to be a daunting challenge. Survival has not changed significantly for years, and new strategies are needed. Over the last decade, investigators have evaluated multiple approaches in targeting the survival, cycling, and proliferation of AML blasts. Attempts at impeding DNA repair, interrupting up-regulated signaling cascades, and targeting epigenetic modulation are ongoing as investigational approaches. Some agents, such as flavopiridol have already demonstrated promise in serially designed clinical trials. Others, such as those targeting individual signaling proteins, are in earlier phases of investigation and development. Additionally, in this review, we have chosen not to include discussion on certain emerging therapies in AML, such as hypomethylating agents and tipifarnib. These promising approaches merit detailed and wide-ranging discussion beyond the scope of our review, and we refer the reader to extensive reviews in the literature 129–132. Future directions for therapeutic exploitation in AML may include immuno-modulation with vaccines, investigating the leukemic microenvironment, targeting leukemic stem cells, and targeting oncogenic fusion proteins or transcription factors implicated in leukemogenesis (e.g. AML-ETO, MLL etc).
It is now clear that mutation or upregulation in one pathway does not account for AML transformation. Blasts rely on multiple dysregulated pathways to emerge and survive, and to ultimately develop resistance to therapy. Therefore, pursuing several molecular lesions in a concurrent or serial fashion may be a promising approach to targeted therapy. This pursuit has been advanced by a better understanding of the nature of defects underlying AML. These have been described as either class I mutations, compromising of alterations in genes for integral components of signal transduction and promoting increased survival and proliferation, or class II inactivating mutations, leading to chromosomal aberrations which target core binding factors with resultant disruption of differentiation 133, 134. Finally, targeted agents should also be considered for and could be incorporated into maintenance regimens after induction therapy, particularly for those patients with minimal residual disease. All in all, it is hoped that the ongoing progress in expanding novel therapies will soon yield useful adjuncts to the therapy of AML and significantly improve its currently poor prognosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Karp JE, Smith MA. The molecular pathogenesis of treatment-induced (secondary) leukemias: foundations for treatment and prevention. Semin Oncol. 1997;24:103–113. [PubMed] [Google Scholar]
- 3.Bao T, Smith BD, Karp JE. New agents in the treatment of acute myeloid leukemia: a snapshot of signal transduction modulation. Clin Adv Hematol Oncol. 2005;3:287–296. [PubMed] [Google Scholar]
- 4.Klasa RJ, List AF, Cheson BD. Rational approaches to design of therapeutics targeting molecular markers. Hematology Am Soc Hematol Educ Program. 2001:443–462. doi: 10.1182/asheducation-2001.1.443. [DOI] [PubMed] [Google Scholar]
- 5.Sedlacek HH. Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol. 2001;38:139–170. doi: 10.1016/s1040-8428(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 6.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA Polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 7.Karp JE, Ross DD, Yang W, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res. 2003;9:307–315. [PubMed] [Google Scholar]
- 8.Melillo G, Sausville EA, Cloud K, Lahusen T, Varesio L, Senderowicz AM. Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res. 1999;59:5433–5437. [PubMed] [Google Scholar]
- 9.Byrd JC, Shinn C, Waselenko JK, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 10.Decker RH, Dai Y, Grant S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Differ. 2001;8:715–724. doi: 10.1038/sj.cdd.4400868. [DOI] [PubMed] [Google Scholar]
- 11.Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res. 1997;57:3375–3380. [PubMed] [Google Scholar]
- 12.Karp JE, Passaniti A, Gojo I, et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin Cancer Res. 2005;11:8403–8412. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 13.Karp JE, Smith BD, Levis MJ, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13:4467–4473. doi: 10.1158/1078-0432.CCR-07-0381. [DOI] [PubMed] [Google Scholar]
- 14.Karp JE, Blackford A, Smith BD, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2009 doi: 10.1016/j.leukres.2009.11.007. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum W, Klisovic RB, Johnson A, et al. Final results of a dose escalation study of flavopiridol in acute leukemias using a novel treatment schedule. Blood, ASH Annual Meeting Abstracts. 2007 part 1;110:Abstract 890. [Google Scholar]
- 16.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resar L, Hillion J, Alino K, Rudek M, Karp JE. Flavopiridol down-regulates genes involved in cell cycle regulation and tumor progression in adults with refractory or poor-Risk acute leukemia. Blood. 2008;112:351. [Google Scholar]
- 18.Almenara J, Rosato R, Grant S. Synergistic induction of mitochondrial damage and apoptosis in human leukemia cells by flavopiridol and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) Leukemia. 2002;16:1331–1343. doi: 10.1038/sj.leu.2402535. [DOI] [PubMed] [Google Scholar]
- 19.Inoue S, Walewska R, Dyer MJ, Cohen GM. Downregulation of Mcl-1 potentiates HDACi-mediated apoptosis in leukemic cells. Leukemia. 2008;22:819–825. doi: 10.1038/leu.2008.1. [DOI] [PubMed] [Google Scholar]
- 20.Rosato RR, Almenara JA, Yu C, Grant S. Evidence of a functional role for p21WAF1/CIP1 down-regulation in synergistic antileukemic interactions between the histone deacetylase inhibitor sodium butyrate and flavopiridol. Mol Pharmacol. 2004;65:571–581. doi: 10.1124/mol.65.3.571. [DOI] [PubMed] [Google Scholar]
- 21.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 22.Ma Y, Cress WD, Haura EB. Flavopiridol-induced apoptosis is mediated through up-regulation of E2F1 and repression of Mcl-1. Mol Cancer Ther. 2003;2:73–81. [PubMed] [Google Scholar]
- 23.Nebbioso A, Clarke N, Voltz E, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 24.Ruefli AA, Ausserlechner MJ, Bernhard D, et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci U S A. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63:3637–3645. [PubMed] [Google Scholar]
- 26.Bali P, Pranpat M, Bradner J, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 27.Chen CS, Wang YC, Yang HC, et al. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res. 2007;67:5318–5327. doi: 10.1158/0008-5472.CAN-06-3996. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci U S A. 2005;102:16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu L, Burgess A, Fairlie DP, Leonard H, Parsons PG, Gabrielli BG. Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell. 2000;11:2069–2083. doi: 10.1091/mbc.11.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed-Inderbitzin E, Moreno-Miralles I, Vanden-Eynden SK, et al. RUNX1 associates with histone deacetylases and SUV39H1 to repress transcription. Oncogene. 2006;25:5777–5786. doi: 10.1038/sj.onc.1209591. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Manero G, Assouline S, Cortes J, et al. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bali P, George P, Cohen P, et al. Superior activity of the combination of histone deacetylase inhibitor LAQ824 and the FLT-3 kinase inhibitor PKC412 against human acute myelogenous leukemia cells with mutant FLT-3. Clin Cancer Res. 2004;10:4991–4997. doi: 10.1158/1078-0432.CCR-04-0210. [DOI] [PubMed] [Google Scholar]
- 34.Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood. 2003;102:3765–3774. doi: 10.1182/blood-2003-03-0737. [DOI] [PubMed] [Google Scholar]
- 35.Dai Y, Chen S, Kramer LB, Funk VL, Dent P, Grant S. Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res. 2008;14:549–558. doi: 10.1158/1078-0432.CCR-07-1934. [DOI] [PubMed] [Google Scholar]
- 36.Catley L, Weisberg E, Kiziltepe T, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–3449. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badros A, Burger AM, Philip S, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortes J, Thomas D, Koller C, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10:3371–3376. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 39.Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN. Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem. 2004;92:223–237. doi: 10.1002/jcb.20045. [DOI] [PubMed] [Google Scholar]
- 40.Shiozawa K, Nakanishi T, Tan M, et al. Preclinical studies of vorinostat (suberoylanilide hydroxamic acid) combined with cytosine arabinoside and etoposide for treatment of acute leukemias. Clin Cancer Res. 2009;15:1698–1707. doi: 10.1158/1078-0432.CCR-08-1587. [DOI] [PubMed] [Google Scholar]
- 41.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 42.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 45.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 46.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 47.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 48.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 49.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 50.Levis M, Smith BD, Beran M, et al. A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: Clinical response correlates with successful FLT3 inhibition. Blood. 2005;106:121a. [Google Scholar]
- 51.Fathi AT, Levis M. Lestaurtinib: A multi-targeted FLT3 inhibitor. Expert Rev. Hematol. 2008;2:17–26. doi: 10.1586/17474086.2.1.17. [DOI] [PubMed] [Google Scholar]
- 52.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 53.Levis M, Smith BD, Beran M, et al. A Randomized, Open-Label Study of Lestaurtinib (CEP-701), an Oral FLT3 Inhibitor, Administered in Sequence with Chemotherapy in Patients with Relapsed AML Harboring FLT3 Activating Mutations: Clinical Response Correlates with Successful FLT3 Inhibition. Blood. 2005;106:121a. [Google Scholar]
- 54.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 55.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 56.Stone RM, Fischer T, Paquette R, et al. Phase IB Study of PKC412, an Oral FLT3 Kinase Inhibitor, in Sequential and Simultaneous Combinations with Daunorubicin and Cytarabine (DA) Induction and High-Dose Cytarabine Consolidation in Newly Diagnosed Patients with AML. Blood. 2005;106:121a. [Google Scholar]
- 57.Deangelo DJ, Stone RM, Heaney ML, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 59.Ng R, Chen EX. Sorafenib (Bay 43-9006): review of clinical development. Curr Clin Pharmacol. 2006;1:223–228. doi: 10.2174/157488406778249325. [DOI] [PubMed] [Google Scholar]
- 60.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 61.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 62.Christiansen DH, Andersen MK, Desta F, Pedersen-Bjergaard J. Mutations of genes in the receptor tyrosine kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2005;19:2232–2240. doi: 10.1038/sj.leu.2404009. [DOI] [PubMed] [Google Scholar]
- 63.Auclair D, Miller D, Yatsula V, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 65.Delmonte J, Kantarjian HM, Andreef M, et al. Update of a phase I atudy of sorafenib in patients with refractory/relapsed acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. (ASH Annual Meeting Abstracts 2007);110: Abstract 893. [Google Scholar]
- 66.Pratz KW, Cho E, Karp J, et al. Phase I dose escalation trial of sorafenib as a single agent for adults with relapsed and refractory acute leukemias. J Clin Oncol. 2009;27:15s. (suppl; abstr 7065) 2009. [Google Scholar]
- 67.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 68.Ravandi F, Cortes J, Faderl SH, et al. Combination of sorafenib, idarubicin, and cytarabine has a high response rate in patients with newly diagnosed acute myeloid leukemia (AML) younger than 65 years. Blood. (ASH Annual Meeting Abstracts 2008);112(Abstract):768. [Google Scholar]
- 69.Cortes J, Roboz GJ, Kantarjian H, et al. A phase I dose escalation study of KW-2449, an oral multi-kinase inhibitor against FLT3, Abl, FGFR1 and Aurora in patients with relapsed/refractory AML, ALL and MDS or resistant/intolerant CML. Blood. 2008:112. (Abstract 2967) [Google Scholar]
- 70.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113:3938–3946. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiotsu Y, Kiyoi H, Ishikawa Y, et al. KW-2449, a novel multi-kinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315l-mutated BCR/ABL translocation. Blood. 2009;114:1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- 72.Cortes J, Ghirdaladze D, Foran JM, et al. Phase 1 AML study of AC220, a potent and selective second generation FLT3 receptor tyrosine kinase inhibitor. Blood. (ASH Annual Meeting Abstracts 2008);112 (Abstract 767) [Google Scholar]
- 73.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.James J, Pratz KW, Stine A, et al. Clinical pharmacokinetics and FLT3 phosphorylation of AC220, a highly potent and selective inhibitor of FLT3. Blood. (ASH Annual Meeting Abstracts 2008);112 (Abstract 2637) [Google Scholar]
- 75.Kim KT, Baird K, Ahn JY, et al. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood. 2005;105:1759–1767. doi: 10.1182/blood-2004-05-2006. [DOI] [PubMed] [Google Scholar]
- 76.Kim KT, Levis M, Small D. Constitutively activated FLT3 phosphorylates BAD partially through pim-1. Br J Haematol. 2006;134:500–509. doi: 10.1111/j.1365-2141.2006.06225.x. [DOI] [PubMed] [Google Scholar]
- 77.Fathi AT, Swinnen I, Rajkhowa T, et al. PIM: An integral component of FLT3 signaling and a potential therapeutic target in acute myeloid leukemia. (ASH Annual Meeting Abstracts 2009), (Abstract no.1735) [Google Scholar]
- 78.Chen LS, Redkar S, Bearss D, Wierda WG, Gandhi V. Pim kinase inhibitor, SGI-1776, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2009;114:4150–4157. doi: 10.1182/blood-2009-03-212852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol. 2006;7:285–294. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 80.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 81.Cappellini A, Tabellini G, Zweyer M, et al. The phosphoinositide 3-kinase/Akt pathway regulates cell cycle progression of HL60 human leukemia cells through cytoplasmic relocalization of the cyclin-dependent kinase inhibitor p27(Kip1) and control of cyclin D1 expression. Leukemia. 2003;17:2157–2167. doi: 10.1038/sj.leu.2403111. [DOI] [PubMed] [Google Scholar]
- 82.Martelli AM, Nyakern M, Tabellini G, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 83.Sandsmark DK, Zhang H, Hegedus B, Pelletier CL, Weber JD, Gutmann DH. Nucleophosmin mediates mammalian target of rapamycin-dependent actin cytoskeleton dynamics and proliferation in neurofibromin-deficient astrocytes. Cancer Res. 2007;67:4790–4799. doi: 10.1158/0008-5472.CAN-06-4470. [DOI] [PubMed] [Google Scholar]
- 84.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 85.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 86.Zhao S, Konopleva M, Cabreira-Hansen M, et al. Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD and promotes apoptosis in myeloid leukemias. Leukemia. 2004;18:267–275. doi: 10.1038/sj.leu.2403220. [DOI] [PubMed] [Google Scholar]
- 87.Bertrand FE, Spengemen JD, Shelton JG, McCubrey JA. Inhibition of PI3K, mTOR and MEK signaling pathways promotes rapid apoptosis in B-lineage ALL in the presence of stromal cell support. Leukemia. 2005;19:98–102. doi: 10.1038/sj.leu.2403560. [DOI] [PubMed] [Google Scholar]
- 88.Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, Mapkinase and p53 pathways. Leukemia. 2005;19:586–594. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- 89.Brandts CH, Sargin B, Rode M, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- 90.Recher C, Beyne-Rauzy O, Demur C, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105:2527–2534. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 91.Xu Z, Wang M, Wang L, et al. Aberrant expression of TSC2 gene in the newly diagnosed acute leukemia. Leuk Res. 2009;33:891–897. doi: 10.1016/j.leukres.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 92.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 93.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 94.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 96.Callera F, Lopes CO, Rosa ES, Mulin CC. Lack of antileukemic activity of rapamycin in elderly patients with acute myeloid leukemia evolving from a myelodysplastic syndrome. Leuk Res. 2008;32:1633–1634. doi: 10.1016/j.leukres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 98.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–4268. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campos L, Rouault JP, Sabido O, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–3096. [PubMed] [Google Scholar]
- 100.Karakas T, Miething CC, Maurer U, et al. The coexpression of the apoptosis-related genes bcl-2 and wt1 in predicting survival in adult acute myeloid leukemia. Leukemia. 2002;16:846–854. doi: 10.1038/sj.leu.2402434. [DOI] [PubMed] [Google Scholar]
- 101.Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12:193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 102.Marcucci G, Byrd JC, Dai G, et al. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood. 2003;101:425–432. doi: 10.1182/blood-2002-06-1899. [DOI] [PubMed] [Google Scholar]
- 103.Marcucci G, Stock W, Dai G, et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol. 2005;23:3404–3411. doi: 10.1200/JCO.2005.09.118. [DOI] [PubMed] [Google Scholar]
- 104.Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part 1. 2007;25:7012. [Google Scholar]
- 105.Lacasse EC, Kandimalla ER, Winocour P, et al. Application of XIAP antisense to cancer and other proliferative disorders: development of AEG35156/ GEM640. Ann N Y Acad Sci. 2005;1058:215–234. doi: 10.1196/annals.1359.032. [DOI] [PubMed] [Google Scholar]
- 106.Carter BZ, Gronda M, Wang Z, et al. Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells. Blood. 2005;105:4043–4050. doi: 10.1182/blood-2004-08-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tamm I. AEG-35156, an antisense oligonucleotide against X-linked inhibitor of apoptosis for the potential treatment of cancer. Curr Opin Investig Drugs. 2008;9:638–646. [PubMed] [Google Scholar]
- 108.Schimmer AD, Estey E, Borthakur G, et al. Phase 1/2 Trial of AEG35156 in combination with idarubicin and cytarabine in patients with relapsed or refractory AML. JCO. 2009 doi: 10.1200/JCO.2009.21.8172. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carter BZ, Mak DH, Morris S, et al. Pharmacodynamic study of phase 1/2 trial of the XIAP antisense oligonucleotide ( AEG35156) in combination with chemotherapy in patients with relapsed/refractory AML. Blood, 2008 ASH Annual Meeting Abstracts. 2008;112:678. [Google Scholar]
- 110.Ame JC, Rolli V, Schreiber V, et al. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–17868. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 111.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 112.Schreiber V, Ame JC, Dolle P, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028–23036. doi: 10.1074/jbc.M202390200. [DOI] [PubMed] [Google Scholar]
- 113.Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 114.de Murcia JM, Niedergang C, Trucco C, et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci U S A. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park SY, Cheng YC. Poly(ADP-ribose) polymerase-1 could facilitate the religation of topoisomerase I-linked DNA inhibited by camptothecin. Cancer Res. 2005;65:3894–3902. doi: 10.1158/0008-5472.CAN-04-4014. [DOI] [PubMed] [Google Scholar]
- 116.Masutani M, Nozaki T, Nakamoto K, et al. The response of Parp knockout mice against DNA damaging agents. Mutat Res. 2000;462:159–166. doi: 10.1016/s1383-5742(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 117.O'Shaughnessy J, Osborne C, Pippen J, et al. Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): Results of a randomized phase II trial. J Clin Oncol. 2009;27:18s. (suppl; abstr 3) [Google Scholar]
- 118.Gaymes TJ, Shall S, Farzaneh F, Mufti GJ. Poly ADP ribose polymerase (PARP) inhibitors induce apoptosis alone or synergistically with histone deacetylase inhibitors in primary acute myeloid leukemic patient cells. Blood. (ASH Annual Meeting Abstracts 2008)112: Abstract 2974. [Google Scholar]
- 119.Donawho CK, Luo Y, Penning TD, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 120.Kummar S, Kinders R, Gutierrez ME, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–2711. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 122.Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia. 1997;11:479–484. doi: 10.1038/sj.leu.2400617. [DOI] [PubMed] [Google Scholar]
- 123.Steelman LS, Abrams SL, Whelan J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 124.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. Embo J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milella M, Kornblau SM, Estrov Z, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kojima K, Konopleva M, Samudio IJ, Ruvolo V, Andreeff M. Mitogen-activated protein kinase kinase inhibition enhances nuclear proapoptotic function of p53 in acute myelogenous leukemia cells. Cancer Res. 2007;67:3210–3219. doi: 10.1158/0008-5472.CAN-06-2712. [DOI] [PubMed] [Google Scholar]
- 127.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 128.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martinelli G, Iacobucci I, Paolini S, Ottaviani E. Farnesyltransferase inhibition in hematologic malignancies: the clinical experience with tipifarnib. Clin Adv Hematol Oncol. 2008;6:303–310. [PubMed] [Google Scholar]
- 130.Karp JE, Lancet JE. Tipifarnib in the treatment of newly diagnosed acute myelogenous leukemia. Biologies. 2008;2:491–500. doi: 10.2147/btt.s3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gore SD. Combination therapy with DNA methyltransferase inhibitors in hematologic malignancies. Nat Clin Pract Oncol. 2005;2 Suppl 1:S30–S35. doi: 10.1038/ncponc0346. [DOI] [PubMed] [Google Scholar]
- 132.Plass C, Oakes C, Blum W, Marcucci G. Epigenetics in acute myeloid leukemia. Semin Oncol. 2008;35:378–387. doi: 10.1053/j.seminoncol.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilliland DG. Molecular genetics of human leukemias: new insights into therapy. Semin Hematol. 2002;39:6–11. doi: 10.1053/shem.2002.36921. [DOI] [PubMed] [Google Scholar]
- 134.Pedersen-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 135.Park S, Chapuis N, Bardet V, et al. PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML. Leukemia. 2008;22:1698–1706. doi: 10.1038/leu.2008.144. [DOI] [PubMed] [Google Scholar]
- 136.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 137.Rizzieri DA, Feldman E, Dipersio JF, et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 138.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 139.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schimmer AD, Brandwein J, O’Brien SM, et al. A phase I trial of the small molecule pan-Bcl-2 family inhibitor obatoclax mesylate (GX15-070) administered by continuous infusion for up to four days to patients with hematological malignancies. Blood. (ASH Annual Meeting Abstracts 2007)110: Abstract 892. [Google Scholar]