Abstract
Consuming omega-3 fatty acids (ω-3 FA) during pregnancy and lactation is beneficial to fetal and infant development and might reduce the incidence and severity of preterm births by prolonging pregnancy. Consequently, supplementing maternal diets with large amounts of ω-3 FA is gaining acceptance. However, both over- and under-supplementation with ω-3 FA can harm offspring development. Adverse fetal and neonatal conditions in general can enhance age-related neural degeneration, shorten life span and cause other adult-onset disorders. We hypothesized that maternal over- and under-nutrition with ω-3 FA would shorten the offspring’s life span and enhance neural degeneration in old adulthood. To test these hypotheses, female Wistar rats were randomly assigned to one of the three diet conditions starting from day 1 of pregnancy through the entire period of pregnancy and lactation. The three diets were Control ω-3 FA (ω-3/ω-6 ratio ~ 0.14), Excess ω-3 FA (ω-3/ω-6 ratio ~ 14.5) and Deficient ω-3 FA (ω-3/ω-6 ratio ~ 0% ratio). When possible, one male and female offspring from each litter were assessed for life span and sensory/neural degeneration (n=15 litters/group). The Excess offspring had shorter life spans compared to their Control and Deficient cohorts (mean±SEM=506±24, 601±14 and 585±21 days, p≤0.004) when the study terminated on postnatal day 640. The Excess offspring had a higher incidence of presbycusis than the Control and Deficient groups (33.3, 4.3 and 4.5%, p=0.011) and a persistence of other sensory/neurological abnormalities and lower body weights in old adulthood. In conclusion, ω-3 FA over-nutrition or imbalance during pregnancy and lactation had adverse effects on life span and sensory/neurological function in old adulthood. The adverse outcomes in the Excess offspring were likely due to a “nutritional toxicity” during fetal and/or neonatal development that programmed them for life-long health disorders. The health implication is that consuming or administering large amounts of ω-3 FA during pregnancy and lactation seems inadvisable because of adverse effects on the offspring.
Keywords: Auditory brainstem response, Fish oil, Hearing loss, Lactation, Life span, Omega-3 fatty acids, Omega-6 fatty acids, Postnatal, Pregnancy, Prenatal
1. Introduction
Omega-3 fatty acids (ω-3 FA), especially docosahexanoic acid (DHA) and eicosapentanoic acid (EPA) from fish oil, have received much attention recently as pre- and postnatal treatments. Dietary ω-3 FA supplementation can increase infant birth weight and prolong pregnancy, thereby reducing the incidence and severity of preterm births and low birth weights [57,58,71,73]. The ω-3 FA in a mother’s milk or in fortified infant formulas can improve neurocognitive and visual development during the first year of life in comparison to infants who receive infant formula without ω-3 FA supplementation [7,17,42]. Thus, increasingly higher ω-3 FA doses are being recommended for pregnant women and nursing babies to advance the developmental health of preterm, low birth weight and even normal infants [4,46,58].
Not all research agrees with these conclusions, however, as there is burgeoning evidence that diets rich in ω-3 FA can be harmful to the fetus and neonate. Several human studies have reported decreased gestational length and/or fetal growth retardation [35,36,54,59,69,78] and increased infant morbidity [78] from high fish oil consumption by the mother during pregnancy. Adverse effects from high ω-3 FA consumption by infants drinking formulas fortified with ω-3 FA can include reduced body growth [20,47], reduced head circumference [20], decreased blood arachidonic acid (AA) levels [16,47] and decreased verbal skills [50,72]. A recent clinical trial reported that fish oil supplements taken by pregnant and lactating mothers may reduce infant body mass index [51].
Animal studies report adverse effects as well. Prenatal and/or postnatal dietary supplementation with large amounts of ω-3 FA or a high ω-3/ω-6 FA ratio can result in reduced birth weight, postnatal growth impairment, increased pre- and postnatal mortality, decreased brain sizes, decreased AA levels, abnormal brain structure/function [5,6,9,27,38,43,63,67,82] and abnormal retinal structure/function [49,85]. Studies using the auditory brainstem response (ABR), a measure of brain development and sensory function, found that high levels of dietary ω-3 FA supplementation in pregnant and lactating rats caused the offspring to have prolonged neural transmission times [28,38,70,76], delayed acoustic startle reflexes [38,70,76], reduced auditory acuity [27], and impaired brain myelination [38]. Harmful effects were similarly caused by dietary ω-3 FA deficiency. Such effects included impaired visual function [13,85], learning deficits, decreased brain weight and/or altered nerve FA composition [13,17,42,81,83] and ABRs indicating a faster aging auditory nervous system in old adulthood [12].
Adverse fetal and neonatal conditions can enhance age-related neural degeneration, shorten life span and cause other adult-onset disorders such as type II diabetes, obesity, hypertension and coronary heart disease. This is known as the Barker or “Fetal Programming” Hypothesis [10]. No one has investigated the possibility that nutritional toxicity from high levels of dietary ω-3 FA or a high ω-3/ω-6 ratio can cause fetal programming of adult-onset disorders, with the exception of studies by our group which found abnormal neurological function [29] and altered body fat composition in young adult rats born to dams receiving diets rich in ω-3 FA during pregnancy and lactation [45]. Only one animal study has investigated the life-long effects of prenatal essential FA deficiency and found a faster aging brain in old age as evidenced by the ABR [12]. Thus, further research to fill the gaps in knowledge about the potentially harmful long-term effects of perinatal ω-3 FA excess and deficiency is clinically important.
We hypothesized therefore that maternal consumption of diets that are excessively rich or deficient in ω-3 FA during pregnancy and lactation would shorten the offspring’s life span and enhance neural degeneration in old adulthood as evidenced by enhanced aging of the ABR. The ABR is a sensitive measure of brain and sensory development and function [23,24,30]. It is also an indirect measure of brain myelination [70]. Mother rats were treated during pregnancy and lactation because rat brain development during early postnatal life is similar to that occurring in the human brain during late gestation [65] and because human mothers may take ω-3 FA supplements during both pregnancy and lactation. In prior studies, our ω-3 FA excess condition caused postnatal growth retardation, abnormal ABRs in 24-day old rat offspring [27,28] and a persistence of some ABR abnormalities into young adulthood [29]. The current study followed these offspring into old adulthood to assess some long-term consequences of their treatments.
2. Methods
2.1. Diets and animal numbers
Wayne State University’s animal investigation committee approved the procedures for this study. Institutional and NIH guidelines were followed.
Our procedures are detailed elsewhere [27,28]. Briefly, female Wistar rats, 10 weeks of age, were mated individually with male Wistar rats (outbred Hsd:WI Wistar rats, Harlan Sprague Dawley, Inc., Indianapolis, IN 46250, USA). The presence of a sperm plug was designated as gestational day one. The females were then placed in separate polycarbonate cages (25 × 45 × 20 cm) and randomly assigned to one of the three diet conditions starting from day 1 of pregnancy through the entire period of pregnancy and lactation. The three diets were the Control ω-3 FA condition (ω-3/ω-6 ratio ~ 0.14), the Deficient ω-3 FA condition (ω-3/ω-6 ratio ~ 0% ratio) and the Excess ω-3 FA condition (ω-3/ω-6 ratio ~ 14.0). The Control diet contained 7% soybean oil. The Deficient ω-3 FA diet contained 7% safflower oil in place of soybean oil. The Excess ω-3 FA diet contained 7% menhaden oil (a type of fish oil) in place of soybean oil. Our rationale for these dose selections is detailed elsewhere [27,28]. We considered this an excess ω-3 FA diet because it had a ω-3/ω-6 ratio that was 100 times what is adequate for pregnant [64] and lactating rats and because our diet of 7% fish oil is consistent with other studies reporting adverse developmental effects [5,6,48].
All diets were formulated according to AIN-93G standards which have determined that the ω-3/ω-6 ratio ~ 0.14 and 7% oil composition is ideal for pregnant and lactating female rats [50]. Fish oil was selected for the Excess diet because of its use in clinical studies. The soybean and safflower oils in the Control and Deficient diets were selected because humans commonly consume them and they are used in animal studies. We used the naturally occurring fatty acid profiles of the fish, soybean and safflower oils; nothing was artificially altered. The diets were prepared by Dyets Inc (Bethlehem, PA 18017, USA). All three diets contained tertiary-butylhydroquinone (TBHQ) because this preservative restricts oxidation [33,34,64]. Diets were stored at refrigeration temperatures and fresh diet was provided twice weekly to further protect against oxidation. Each diet provided 3.96 kcal/g. Detailed composition of each diet, animal husbandry, maternal and offspring outcomes were recently published [27,28,45]. Weaning occurred on postnatal day 21 (PND 21). After PND 24, offspring were housed singly and switched to 5001 Rodent Diet (PMI Nutrition International, Richmond, IN 47374, USA).
Our initial studies started with the Control, Deficient and Excess groups having litter numbers of 23, 31 and 22, respectively [27–29]. Because of diminished financial resources, we culled our animal colony to15 litters/group for the current study by retaining the oldest litters. One male and one female offspring per litter were used, when possible. For the Control, Deficient and Excess litters, the number of male and female offspring were 12+13=25, 11+13=24 and 13+12=25 for the life span and body weight outcome variables (total n=74 offspring). The ABR outcome variables were assessed at ~16 months of age. By this age, the animal colony size had diminished because several animals died from natural causes.
Consequently, the ABR study had Control, Deficient and Excess litter numbers of 14, 13 and 9, respectively. The respective number of male and female offspring were 12+11=23, 9+13=22 and 8+7=15 for the ABR outcome variables (total n=60 offspring).
2.2. Life span, body weights and necropsies
For the life span portion of our study, animals were allowed to die naturally. The exception was when an animal appeared to be suffering to an unacceptable degree as determined by the veterinarian. This usually meant that the animal was too weak to eat or drink and was losing weight rapidly. At the first sign of significant weight loss (~10% decrease), an animal would be placed on soft food prepared by moistening the food pellets in water. Frequently, an animal would respond with weight stabilization and be maintained for weeks with this procedure. When an animal’s condition deteriorated to the point that it was clear that the animal would not survive more than a few days, it was humanely sacrificed by carbon dioxide inhalation followed by bilateral pneumothorax. Life span was measured as the animal’s age at the date of its sacrifice or the date of its natural demise. The life span study was terminated for any animal that reached the age of 21 months (640 days). By this age, virtually all Excess offspring had died and it was clear that the Deficient and Control offspring were not differing in their survival rates. The study was terminated at 21 months of age to conserve our limited financial resources. Animals still alive at 21 months of age were assigned an age of 640 days to enable subsequent data analyses by the Kaplan-Meier survival curves and Log Rank tests.
The birth weights, neonatal weights and young adulthood weights have been previously published [27–29] and are described briefly here. From the age of three months onward, each animal was weighed at three-month intervals until 21 months of age (640 days). If an animal died before 21 months of age, its weight on the day of death was also recorded. Its death weight was also entered at each subsequent weighing session as a substitute for the missing data. This permitted us to perform a repeated measures analysis of variances on the body weight data.
While not a part of the original protocol, several animals (4 Control, 3 Deficient and 9 Excess offspring) that died in old age were given necropsies by a veterinarian to help ascertain the cause of death. The animals’ kidneys, livers and lungs were grossly and histologically inspected for pathology. Blood samples were collected by the veterinarian when it was clear these animals were approaching death. Blood samples were analyzed by the university’s Department of Laboratory Animal Resources. Mammary adenomas (tumors) were tallied for all animals. The choice of the organs surveyed was influenced by prior rat life span studies [26,52].
2.3. ABR procedure
2.3.1. ABR recordings
When possible, one male and one female pup/litter were randomly selected for testing in the current study. Using male/female littermate pairs allowed assessment of sex-dependent differences and controlled for within-litter effects by limiting the number of pups tested from any one litter. Rat offspring were initially ABR-tested on PND 24 and those test results were previously reported [27,28]. These same offspring were subsequently retested as young adults and those results were reported in another article [29]. These animals were old adults, aged 16 months (~480 days) at the time of the ABR testing reported in the current article. The rat ABR is fully mature and stable by approximately 70–100 days of age [30] and age-related hearing loss (presbycusis) usually does not occur in rats until about 17 months [23].
Our ABR procedure is detailed elsewhere [24,27]. Prior to ABR recording, each animal was given 100 mg/kg of the anesthetic ketamine (i.p.). Ketamine influences ABR latencies and amplitudes, but the effects are minor and the ABR quality is excellent [25]. Rectal temperature was monitored because temperature can influence the ABR [68] (Model 43TD, Yellow Springs Instruments Co., Yellow Springs, OH 45387, USA). A water-circulating heating pad was used to regulate and maintain normothermia by raising or lowering the temperature of the circulating water (Model TP500, Gaymar Industries, Orchard Park, NY 14127, USA).
The ABR was differentially recorded between two subcutaneous platinum E-2 needle electrodes. The active electrode was inserted at the vertex, the reference electrode below the left ear, and the ground electrode below the right ear. Evoked potentials were collected by a Bio-logic Navigator and amplified 300,000 times with a digital bandpass of 300–3000 Hz (Bio-logic Corp, Mundelein, IL 60060, USA). Electrode impedances ranged from 0–9 k´Ω. At least 256 responses were averaged. Recordings were made in an electrically shielded, double-walled sound attenuation chamber (Allotech, Inc., Raleigh, NC 27603, USA). Binaural, ‘open field’ tone pips in the ascending order of 2000 Hz, 4000 Hz, 8000 Hz and 16000 Hz were delivered through a TDH-39P headphone positioned in front of the animal with rise/fall time = 0.5 msec, plateau = 10.0 msec, polarity = alternating, repetition rate = 19.0/sec, and stimulus intensity = 15 to100 dB peSPL (Telephonics Corp, Farmingdale, NY 11735, USA).
2.3.2. ABR thresholds (hearing acuity)
ABR thresholds were determined by the method of limits [24,27]. Here, serial ABRs were gathered to a range of stimulus intensities starting at 100dB, then descending to 80, 60, 50, 40, 35, 30, 25, 20, and 15 dB as the ABR threshold was reached and passed. To establish ABR threshold more precisely, 2 and 3 dB changes in stimulus intensity levels were tested around the ABR’s threshold (as determined by visual detection) and multiple ABR traces (2 to 5) were collected at each near-threshold intensity level. Threshold was defined as the lowest intensity to elicit a reliably scored ABR component. An experimenter, who was ‘blind’ as to each animal’s treatment condition, scored the ABR thresholds. A second experimenter then checked the threshold scoring for reliability purposes.
2.3.3. ABR latency-intensity profiles
An ABR latency-intensity (L-I) profile can help determine if a subject’s hearing loss is a conductive hearing loss (CHL) or a sensorineural hearing loss (SNHL). A subject with a CHL would have an elevated ABR threshold and an L-I profile that is displaced upward and parallel to the normal curve. A subject with a SNHL will also have an elevated ABR threshold, however the P2 latencies will typically be normal or near normal in response to loud stimulus intensities but progressively curve upward from the normal range as the stimulus intensity decreases [24]. In rodents, one typically uses the ABR’s P2 wave to derive an animal’s latency-intensity (L-I) profile because the rodent’s P2 wave is the largest wave and the last wave to disappear as the sound stimulus is decreased [24]. P2 latency was measured at the positive peak of this waveform at each stimulus intensity level [32].
2.3.4. ABR amplitude-intensity profiles
The amplitude of the P2 waveform was used as an index of the ABR’s maximum amplitude because the rodent’s P2 wave is the largest wave and the last wave to disappear as the sound intensity is decreased. P2 amplitude was measured from the positive peak of wave P2 to the subsequent negative trough (labeled N2). This was done at each stimulus intensity level in order to derive amplitude-intensity (A–I) profiles. Whereas the ABR amplitude is a function of neural synchrony and the number of neural units firing, the ABR amplitude can provide diagnostic information on these neural functions.
2.3.5. ABR latencies (neural transmission times)
The ABR is a series of action potentials and postsynaptic potentials. The rat ABR is composed of four components (labeled P1 to P4) occurring within 6 msec of stimulus onset [23,25,27,30]. Although the neurogenerators of the rat’s ABRs have not been determined, in the mouse they reflect neural activity chiefly from the auditory nerve (P1), the cochlear nucleus (P2), the superior olivary complex (P3), and the lateral lemniscus and/or inferior colliculus (P4) [40]. The latency of each ABR component was measured as the time from the computer’s triggering of the earphone to a wave’s positive peak, including a 0.3 msec acoustic transit time between the earphone and the animal’s pinnae. Two experimenters, who were ‘blind’ as to each animal’s treatment condition, scored the latencies of ABR waves P1, P2, P3 and P4. When scorers disagree (rarely), the scores are averaged. The primary outcome variable was the P4 latency, a measure of neural transmission time along the auditory nerve and brainstem auditory pathway inclusively, elicited by the 100 dB stimuli. The secondary outcomes were the latencies of the individual ABR waves and the P1 to P4 interpeak latency (P1–P4 IPL). The P1–P4 IPL measures the brainstem portion of neural transmission by excluding the auditory nerve transmission time.
2.4. Data analyses
Kaplan-Meier survival curves and Log Rank tests were used to analyze the survival (life span) data [31]. The Log Rank test compares multiple survival curves simultaneously. Even though we predicted that the Excess and Deficient diet treatments would adversely affect offspring outcome, the use of directional probability levels were not necessary. The criterion for statistical significance was p≤0.05.
Analyses of variances (ANOVA) assessed statistical significance for the body weight and ABR variables. Because there was an interest in gender-dependent differences, male and female littermates were treated as individual units of measure. For the body weight variable, a three-way ANOVA was used to test the effects of Diet, Sex and Age. For the ABR variables, a three-way ANOVA was used to test for the effects of Diet, Sex and Tone Pip Frequency (or Intensity). Because Age and Tone Pip were within-subjects measures, the Greenhouse-Geisser adjustment was used with these variable’s main effects and interactions. Because each litter was sampled in a highly balanced manner, using only one male and one female from each litter with only rare exception, it was unnecessary to control for litter-dependent effects by using litter as a variable in the statistical design. If an ANOVA indicated a significant Diet treatment effect (p≤0.05), then univariate ANOVAs followed by Bonferroni tests were used to make planned pair-wise comparisons between treatment groups.
2.5. Time line
To summarize the time line: Treatment diets were given to dams from gestation day 1 to lactation/postnatal day 21 when pups were weaned. Offspring weights were collected at birth and on PND 7, 14 and 21 and again from the ages of 3 to 21 months at 3-month intervals. ABRs were collected on PND 24, ~174 and ~480. Life span assessment terminated on PND 640.
3. Results
3.1. Maternal and birthing outcomes
Maternal and birthing data were reported previously [27,28,45], thus these results will only be summarized here for the reader’s convenience. Briefly, there were no group differences in gestational length, maternal weight gain, food consumption during pregnancy and lactation, the number of pups per litter, litter and pup weights at birth, or age of teeth eruption. There was a trend for increased postnatal mortality between birth and weaning for the Excess offspring and their pinna detachment was delayed. On PND 24, we weighed the male/female littermate pairs that were channeled into the life span/ABR study. There was a significant effect for Diet group whereby the Excess male and female pups weighed significantly less than their Control and Deficient cohorts and the Deficient female pups weighed less than their Control cohorts.
3.2. Life span and survival curves
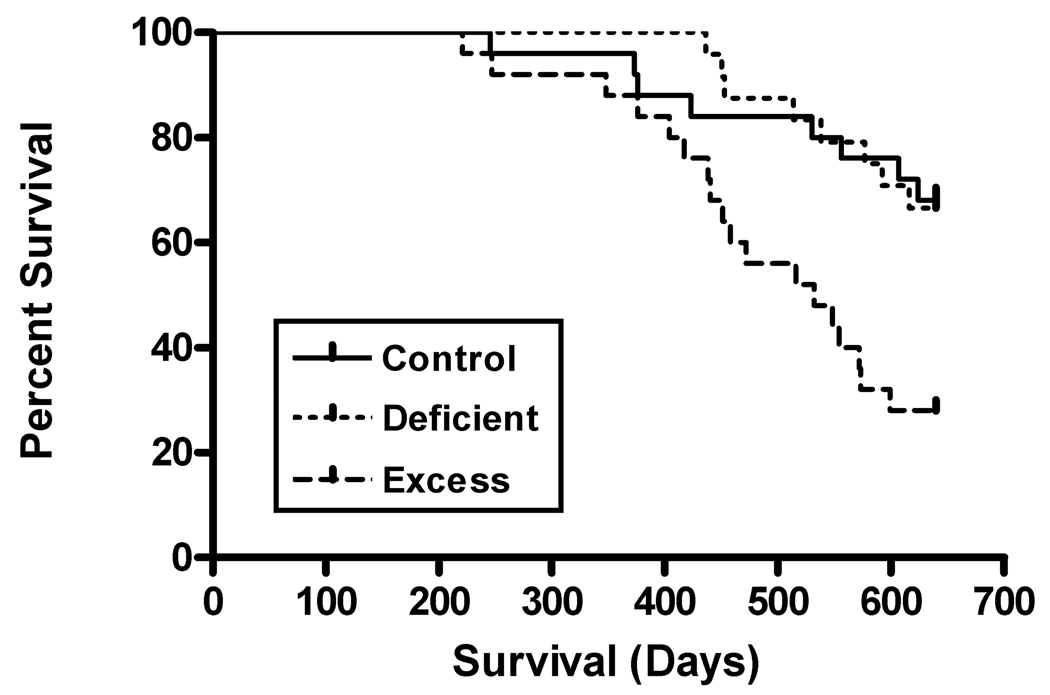
Fig. 1 shows the survival curves. The Log Rank test showed a significant Diet group difference: Chi Square (2)=13.351, p=0.001. Pairwise comparisons showed that the Excess offspring had shorter life spans than their Control and Deficient cohorts with respective mean±SEM life spans of 506±24, 601±14 and 585±21 days (p≤0.004). Control and Deficient offspring did not differ (p=0.992). When the study terminated at 21 months of age (640 days), the survival rate for the Excess offspring was only 28.0%. In contrast, survival rates for the Control and Deficient offspring were 68.0% and 66.7%. Females (597±13 days) lived longer than males (570±15 days) and had higher survival rates at the study’s end (76.9% vs. 55.1%): Chi Square (1)=4.822, p=0.028. This sex-dependent difference was consistent across diet groups.
Fig. 1. Survival curves.
Adult Excess offspring had a more rapid decline in their survival curve than their Control and Deficient cohorts.
3.3. Body weights and necropsies
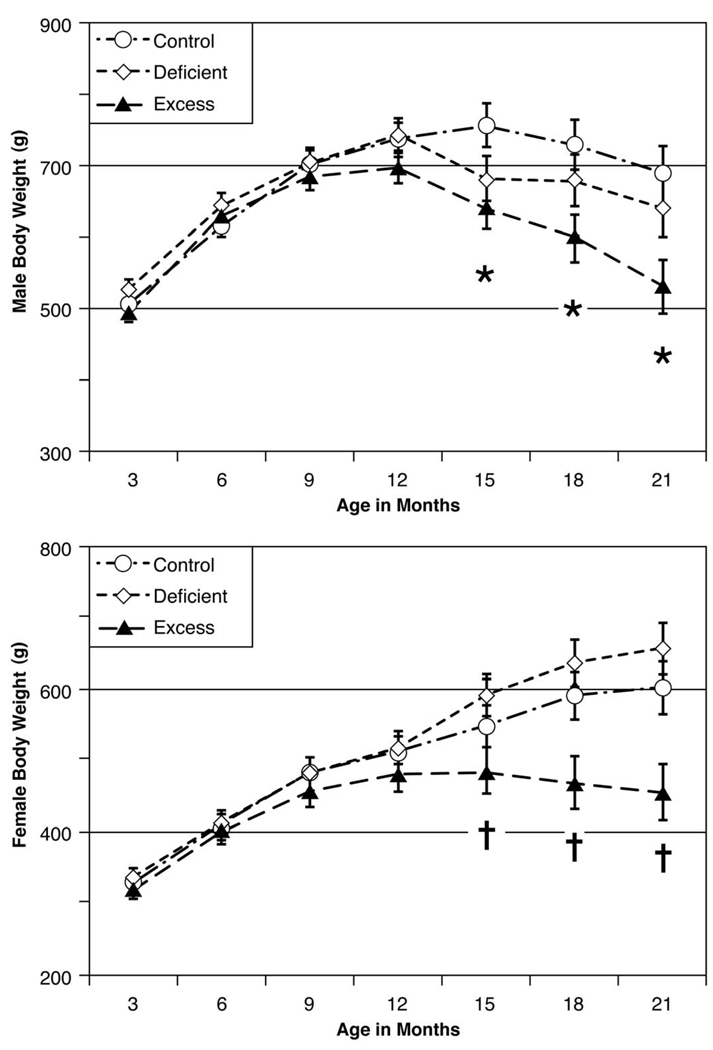
Fig. 2 shows the adult body weights as functions of Diet group and Age for the male and female offspring. There were significant effects for Diet group: F(2, 67)=6.506, p=0.003 and for Sex: F(1, 67)=100.702, p=0.000 but not for the Diet-by-Sex interaction. Post hoc comparisons indicated that the Excess offspring weighed significantly less than their Control and Deficient cohorts (p≤0.01) whereas the Control and Deficient offspring did not differ from each other (p=1.000). There were significant effects for Age: F(6, 402)= 88.587, p=0.000 and for the Age-by-Diet interaction: F(12, 402)=6.381, p=0.000 and the Age-by-Sex interaction: F(6, 402)=18.569, p=0.000 but not for the Age-by-Diet-by-Sex interaction.
Fig. 2. Body weights.
Upper panel: There were no group differences in male offspring body weights (mean±SEM) from 3 to 12 months of age. From 15 to 21 months of age, the Excess male offspring weighed significantly less than their Control male cohorts. Lower panel: There were no group differences in female offspring body weights from 3 to 12 months of age. From 15 to 21 months of age, the Excess female offspring weighed significantly less than their Control and Deficient female cohorts. *Excess males different from Control cohorts; †Excess females different Control and Deficient cohorts.
Fig. 2 shows that there were no significant Diet group differences in adult male offspring body weights from 3 to 12 months of age. From 15 to 21 months of age, however, the Excess male offspring weighed significantly less than their Control cohorts. From 15 to 21 months of age, the Deficient male offspring had intermediate body weights and did not differ significantly from their Control and Excess cohorts. Fig. 2 also shows that there were no significant Diet group differences in adult female offspring body weights from 3 to 12 months of age. From 15 to 21 months of age, however, the Excess female offspring weighed significantly less than their Control and Deficient cohorts. From 15 to 21 months of age, the Deficient female offspring curiously weighed more than their Control cohorts but not significantly.
Using data only from animals that died before the study’s termination at 21 months of age, death weights were analyzed for group differences. There were no significant Diet group differences in death weights. There was a significant Sex difference with the females weighing less than their male cohorts at the time of death: F(1, 28)=5.025, p=0.033. There was no significant Diet-by-Sex interaction. Even though there were no significant Diet group differences, a trend for lower death weights was observed for both males and females in the Excess group (Control=598±55g, Deficient=543±50g and Excess=499±37g for males; Control=480±46g, Deficient=466±57g and Excess=396±30g for females).
Mammary adenomas were uncommon in our colony of Wistar rats. Only two Deficient, one Excess and one Control offspring had such tumors. Seizures were uncommon with only four Deficient and one Excess offspring exhibiting seizure activity. Seizures always occurred at the end of their life span and were associated with anemia and kidney failure as evidenced by the animal’s pallid appearance, necropsies and blood analyses. Animals in all three groups had the same necropsy findings. Necropsies indicated chronic kidney disease as evidenced by chronic interstitial glomerulonephropathy (enlarged glomeruli, thickened glomerular capsules and basement membranes), thickened renal tubular basement membranes, tubular epithelial cell atrophy, collapsed ducts, proteinaceous tubular casts, dystrophic mineralization, interstitial infiltration by lymphoplasmacytic cells as well as increased fibroblasts and collagen in the interstitium. Consistent with kidney disease, blood analyses showed abnormally high blood urea nitrogen (BUN), creatinine and phosphate levels. Livers usually had multifocal macrovesicular and microvesicular lipidosis, portal fibrosis, periportal lymphoid infiltrates, micorgranulomas and microabcesses. Consistent with liver disease, blood analyses showed abnormally low albumin and hypoproteinemia as well as abnormally high alanine transaminase levels. Other blood results included abnormally high white blood cell counts, glucose and cholesterol levels as well as abnormally low hematocrit, red blood cell count and hemoglobin. Some lungs had severe chronic histiocytic pneumonia (alveolar macrophages, lymphocytic infiltrates).
3.4. ABR thresholds (hearing acuity)
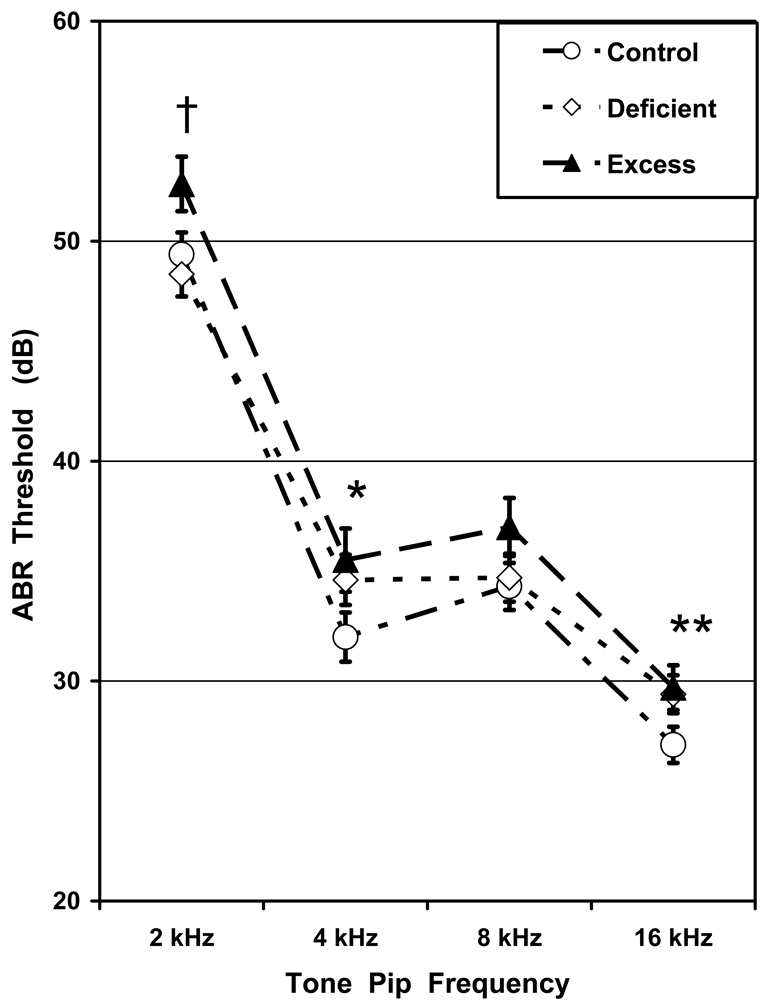
Fig. 3 shows ABR thresholds as functions of Diet group and Tone Pip Frequency. There was a significant effect for Diet group: F(2, 52)=4.293, p=0.019. Pair-wise comparisons showed that the Excess group had significantly higher (worse) ABR thresholds than the Control group at the tone pip frequencies of 2, 4 and 16 kHz (p<0.05) but missing significance at 8 kHz (p=0.12). The Excess group also had higher ABR thresholds than the Deficient group at 2 kHz. The Deficient group had a significantly higher ABR threshold than the Control group only at 16 kHz. There was an effect for Sex: F(1, 52)=7.681, p=0.008 but not for the Diet-by-Sex interaction. The sex difference was due to females having lower (better) ABR thresholds than their male cohorts by about 2.3 dB. There was a significant effect for Tone Pip Frequency, reflecting that rats have progressively better hearing acuity (lower thresholds) as the tonal frequency progressed from 2 to 16 kHz [24,27]: F(3, 156)=283.793, p=0.000. There were no significant interactions between Diet group or Sex and the Tone Pip Frequency factor.
Fig. 3. ABR thresholds.
Mean±SEM ABR thresholds as functions of Diet group and Tone Pip Frequency. The Excess offspring had elevated (worse) thresholds than their Control and Deficient cohorts: *Excess different from Control; †Excess different from Control and Deficient; **Excess and Deficient different from Control.
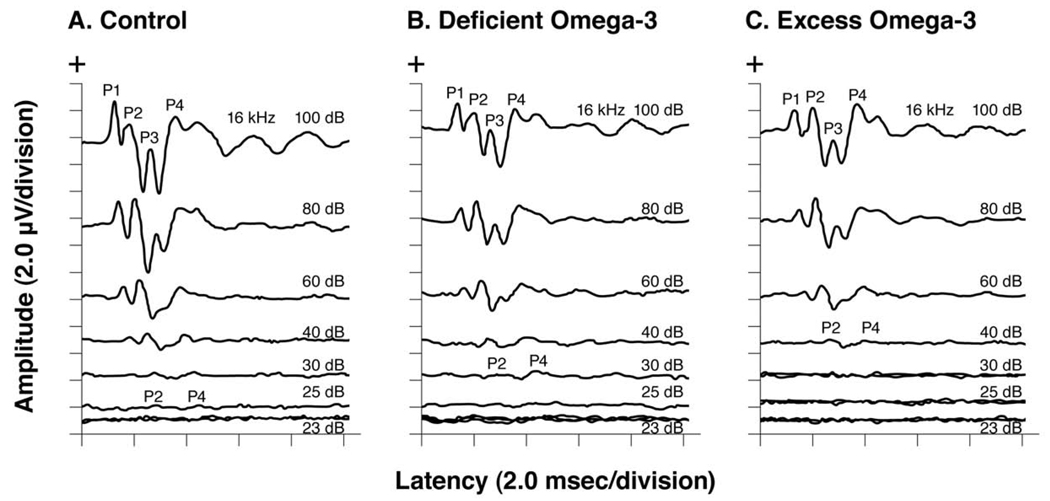
Fig. 4 shows serial ABRs elicited by 16 kHz tone pips of descending stimulus intensity from representative old adult offspring in the Control, Deficient and Excess diet groups. The Control (panel A) and Deficient (panel B) animals had their ABRs disappear below 25 and 30 dB respectively, whereas the Excess animal (panel C) had an ABR at 40 dB but not at 30 dB. The Excess animal therefore had elevated ABR threshold (technically defined as ≥2 standard deviations above the Control group’s mean), indicating hearing loss of ~10 to 15 dB.
Fig. 4. Serial ABRs.
Serial ABRs elicited by 16 kHz tone pips of descending stimulus intensities from representative old adult offspring in the Control, Deficient ω-3 FA and Excess ω-3 FA diet groups. The Control and Deficient offspring had ABRs at 25 and 30 dB, respectively. In contrast, the Excess offspring had an ABR at 40 dB but not at 30 dB, indicating an elevated (worse) ABR threshold of ~10–15 dB.
3.5. ABR latency-intensity (L-I) profiles
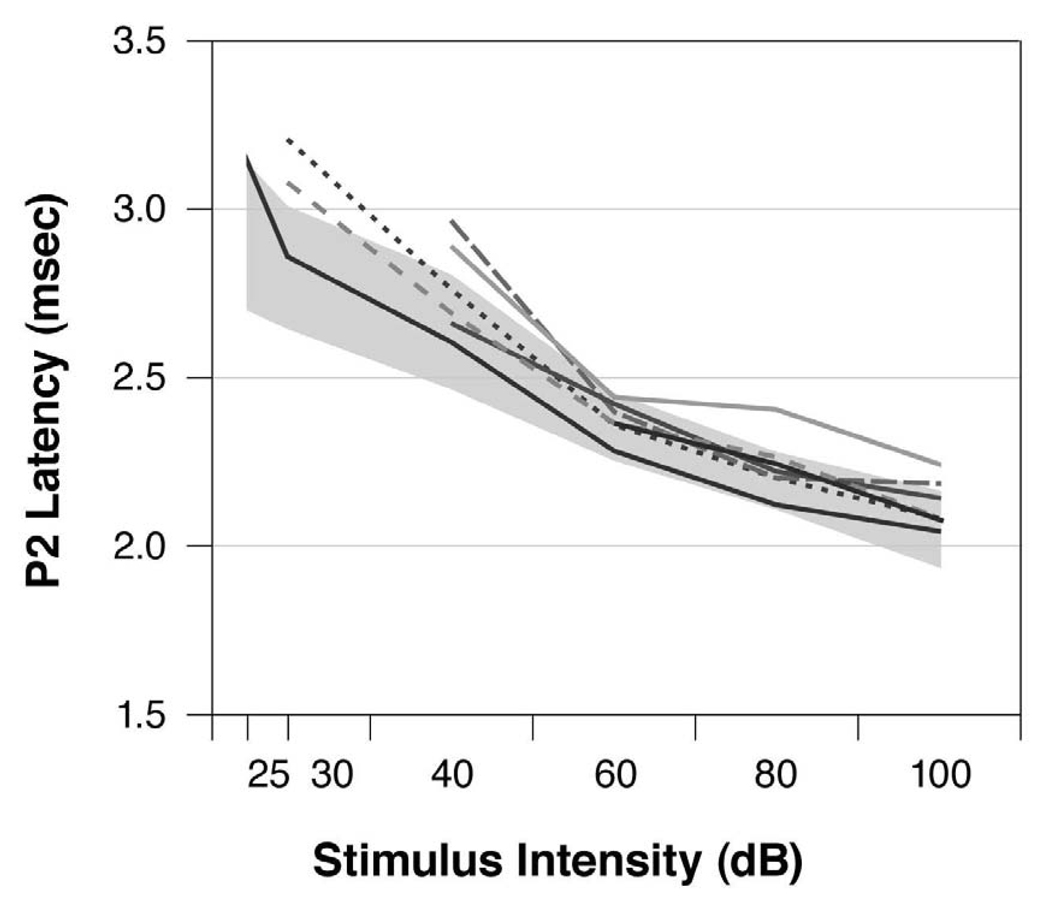
The L-I profile can diagnose whether a subject’s hearing loss is either CHL or SNHL. Fig. 5 shows the ABR’s P2 wave L-I profiles elicited by the 16 kHz tone pip condition for the seven animals (46.7%) in the Excess group with abnormal ABRs. The 16 kHz condition was chosen because age-related hearing loss is typically a high frequency hearing loss. The shaded region in Fig. 5 is the range of normalcy derived from Control data. This figure shows that the L-I profiles from five Excess offspring were typically within normal limits at the higher stimulus intensities but fell above the normal limits at the lower stimulus intensities. Thus, these five Excess offspring had L-I profiles consistent with SNHL, albeit mild SNHL. Therefore, 5 of 15 Excess offspring (33.3%) had some degree of SNHL. In contrast, only 1 of 23 Control (4.3%) and 1 of 22 Deficient offspring (4.5%) showed SNHL patterns: Chi Square (2)=9.111, p=0.011. Six of the Excess animals in Fig. 5 also had elevated ABR thresholds, defined as being ≥2 standard deviations (SD) above the Control group mean [24].
Fig. 5. L-I profiles.
ABR latency-intensity (L-I) profiles elicited by 16 kHz tone pips from the seven Excess offspring with abnormality. The shaded region is the range of normalcy for the P2 wave’s latency (mean±2 SD) as derived from the Control group data. Five of these Excess offspring had L-I profiles that fell above the range of normalcy at the lower stimulus intensities, suggesting sensorineural hearing loss (SNHL). Six of the Excess offspring had no ABRs at the lower stimulus intensity levels.
3.6. ABR amplitude-intensity (A–I) profiles
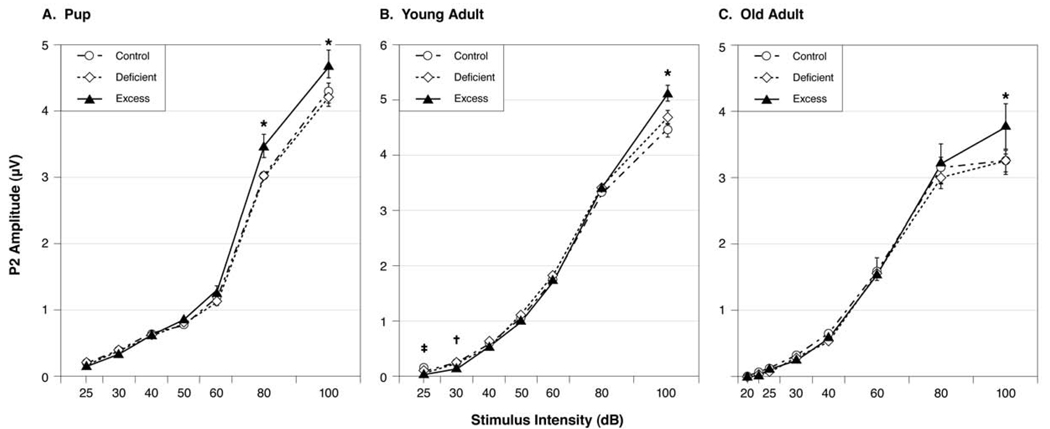
As young adults, the Excess offspring showed an A–I pattern of relatively small amplitude ABRs at the lowest stimulus intensities and relatively large amplitudes at the highest stimulus intensities [29]. To investigate if this pattern persisted into old adulthood, we measured the ABR’s P2-N2 amplitude as an index of the ABR’s amplitude for reasons described in the Methods section. Fig. 6A–C compares the A–I profiles for the P2-N2 wave across the three age categories. Fig. 6C shows the A–I profiles for the old adult offspring elicited by the 16 kHz tone pip condition. The 16 kHz tone pip condition was chosen because it was the most sensitive to group differences. There were no significant effects for Diet group or for the Diet-by-Sex interaction. There was a significant effect for Sex, indicating that females had higher ABR amplitudes than males: F(1, 54)= 8.986, p=0.004. There was a significant effect for Tone Pip Intensity, indicating that amplitudes decreased as tone pip intensity decreased: F(5, 270)=647.880, p=0.000. There were no significant interactions involving Tone Pip Intensity and Sex or Diet. Even though there was no significant Diet group differences, the Excess group in old age showed a similar A–I profile seen when they were young adults. Namely, the old adult Excess offspring had larger ABR amplitudes in response to the highest stimulus intensities (p=0.052, see Fig. 6C).
Fig. 6. A-I profiles at three ages.
ABR amplitude-intensity (A–I) curves for offspring in the three diet groups as pups (A), young adults (B) and old adults (C). At all three ages, the Excess offspring had abnormal growth in their ABR amplitudes (P2-N2 wave) as stimulus intensity increased, suggesting hyperacusis. Tone pip stimuli=4 kHz (pups), 8 kHz (young adult) or 16 kHz (old adults). *Excess greater than Control and Deficient at p<0.05, except as old adults where p=0.052. †Excess smaller than Control and Deficient (p<0.05). ‡Excess smaller than Control (p<0.05).
3.7. ABR maximum amplitudes
For maximum ABR amplitude, we assessed the largest waveform (the P2-N2 wave) as elicited by our highest stimulus intensity of 100 dB. These mean±SEM amplitudes as elicited by the 2, 4, 8 and 16 kHz tone pips for the Control, Deficient and Excess offspring are presented in Table 1. Despite the old adult Excess offspring having the largest ABR amplitudes across all four tone pip frequencies, there were no significant effects for Diet group, Sex or the Diet-by-Sex interaction. There was a significant effect for Tone Pip Frequency: F(3, 159)=27.149, p=0.000 and for the Frequency-by-Sex interaction: F(1, 159)=3.803, p=0.019 but not for any other interaction with the Frequency variable. As seen in Table 1, ABR amplitudes were largest in response to the 8 kHz tone pip condition, smallest to the 2 kHz condition.
Table 1.
The ABR’s P2-N2 amplitude (µV) as functions of diet group, offspring age and tone pip frequency (mean±SEM)
| Tone pip frequency | ||||
|---|---|---|---|---|
| Diet group and age | 2 kHz | 4 kHz | 8 kHz | 16 kHz |
| A. Pups (PND 24) | ||||
| Control (n=45) | 3.2±0.1 | 4.3±0.2 | 4.9±0.2 | 5.4±0.2 |
| Deficient (n=50) | 3.5±0.1 | 4.3±0.2 | 5.1±0.2 | 5.4±0.2 |
| Excess (n=42) | 3.8±0.1† | 4.7±0.2* | 5.5±0.2* | 6.0±0.2† |
| B. Young adults (PND 174) | ||||
| Control (n=44) | 3.5±0.1 | 3.9±0.2 | 4.5±0.2 | 4.1±0.2 |
| Deficient (n=61) | 3.5±0.1 | 4.1±0.1 | 4.7±0.1 | 4.1±0.1 |
| Excess (n=43) | 3.7±0.1 | 4.5±0.2* | 5.1±0.2* | 4.6±0.2† |
| C. Old adults (PND 480) | ||||
| Control (n=23) | 3.0±0.2 | 3.6±0.2 | 3.8±0.2 | 3.2±0.2 |
| Deficient (n=22) | 2.9±0.2 | 3.2±0.2 | 3.5±0.2 | 3.2±0.2 |
| Excess (n=15) | 3.1±0.2 | 3.6±0.2 | 3.9±0.2 | 3.6±0.2‡ |
PND=postnatal day
Larger than the Control group (p<0.05)
Larger than the Control and Deficient groups (p<0.05)
Larger than the Control group, but not quite significantly (p=0.052).
3.8. ABR amplitudes compared across age categories
We assessed our animals’ ABR amplitudes first as young adults [29] and now as old adults. We did not think to assess ABR amplitudes for our publications describing our animals as 24-day-old pups [27,28]. To see if the pattern of larger ABR amplitudes existed in the Excess offspring when they were pups, we decided to analyze the pup data. The results were consistent with our young adult [29] and old adult findings (present study). Specifically, the ABR’s P2-N2 amplitudes elicited by the highest tone pip intensity (100dB) were largest in the Excess offspring. There was a significant effect for Diet group: F(2, 131)=3.198, p<0.044; and for Tone Pip Frequency: F(3, 393)=430.933, p<0.000; but not for their interaction. Table 1 compares the amplitude data of the three diet groups as functions of age and tone pip frequency. This table shows that the Excess offspring had the largest amplitudes at all three ages and all four tone pip frequencies.
The Excess pups also showed the same A–I pattern seen when they were young adults and old adults. That is, the Excess pups had an abnormal growth in their P2-N2 amplitudes as tone pip intensity increased. Fig. 6A–C shows this pattern of exaggerated amplitude growth in the Excess offspring across all three age categories. For the pup data, there were significant effects for Tone Pip Intensity: F(6, 864)=1744.300, p<0.000; for Diet group: F(2, 144)=2.963, p=0.030; and for their interaction F(12, 864)=4.005, p=0.008. No other main effects or interactions were significant. Statistical results for the young adult [29] and old adult were already presented.
3.9. ABR latencies (neural transmission times)
For neural transmission times (P4 latencies), we assessed ABRs elicited by the highest stimulus intensity of 100 dB. There were no significant effects for Diet group, Sex or the Diet-by-Sex interaction. For example, the Control, Deficient and Excess offspring had similar mean±SEM P4 latencies of 3.96±0.04, 3.94±0.04 and 3.99±0.05 msec when data from all four tone pip stimuli conditions were combined. There was a significant effect for Tone Pip Frequency, indicating that P4 latency became gradually shorter (faster) as the tone pip frequency progressed from 2 to 16 kHz: F(3, 156)=45.955, p=0.001. No significant Diet or Sex effects were found for the secondary outcome variables of P1, P2 or P3 latencies or the P1–P4 IPL.
3.10. Body temperatures, weights and ages during ABR recordings
There were no significant body temperature differences during the ABR recording sessions for Diet group, Sex or the Diet-by-Sex interaction. The respective mean±SEM body temperatures for the Control, Deficient and Excess groups were 37.7±0.08, 37.6±0.09 and 37.5±0.10 °C. There were no significant age differences during the ABR recording sessions for Diet group, Sex or the Diet-by-Sex interaction. The respective mean±SEM ages for the Control, Deficient and Excess groups were 482±3, 483±3 and 474±3 days of age. There were significant differences in body weights during the ABR recording sessions for Diet group: F(2, 54)=4.620, p=0.014 and for Sex: F(1, 54)=59.963, p=0.000 but not for the Diet-by-Sex interaction. The respective mean±SEM body weights for the Control, Deficient and Excess males were 754±92, 779±55 and 696±114 grams. The respective mean±SEM body weights for the Control, Deficient and Excess females were 579±87, 613±60 and 581±82 grams.
4. Discussion
As hypothesized, maternal ω-3 FA over-nutrition during pregnancy and lactation (Excess group) shortened the adult offspring’s life span. The Excess group showed enhanced onset of presbycusis (age-related sensorineural hearing loss), a persistence of two sensory/neurological abnormalities from weanling and young adulthood into old adulthood in the form of raised ABR thresholds (hearing loss to soft sounds) and hyperacusis (impaired neural inhibition to loud sounds). Excess offspring weighed less than their Control and Deficient cohorts in old adulthood, despite all three groups weighing the same in young adulthood. Contrary to our hypothesis, maternal ω-3 FA Deficient condition had no effect on offspring life span and the Excess and Deficient groups did not show enhanced prolongation of neural transmission times in old age.
Although the Barker Hypothesis predicts shortened life span as one consequence of an adverse fetal and/or neonatal environment, this parameter is rarely studied. A small number of animal studies have found shortened life span resulting from prenatal drug [1,26,52], hypoxia [44] and protein under-nutrition [3]. The present study is the first to report shorter adult offspring life span from maternal dietary ω-3 FA excess during pregnancy and lactation.
Control and Deficient offspring life span data compare favorably with the normative data, whereas our Excess offspring were notably abnormal. Outbred Wistar rats have a median life span of ~760 days [53]. In contrast, our Excess offspring had a mean life span of 506 days and a median life span of 524 days, for an estimated 31–33% reduction in life span. This is large compared to the 3–22% life span reductions caused by other prenatal exposures [1,3,26,44,52]. Excess offspring had only a 28% survival rate at 640 days when our study was terminated. In contrast, Control and Deficient offspring had 68% and 67% survival rates at 640 days. Females had better survival statistics than males, which agrees with the literature [53].
Excess and Deficient offspring had normal birth weights but were under-weight by the peri-weanling age of 24 days [27]. Weight differences dissipated by the young adult age of ~5.5 months [29]. The current study found that the Excess and Deficient offspring continued to have normal body weights prior to 15 months of age, when the Excess offspring began to lose weight.
Necropsies indicated that kidney failure was the major cause of deaths in all three diet groups. Kidney failure is a common cause of death in aged rats [53]. Thus, it was not unusual that most of our Excess offspring died from this disease. It was unusual however that our Excess offspring contracted kidney failure at a much younger age than the Control and Deficient offspring. We did not perform a detailed investigation of the kidney failures. Thus, we do not know if the kidney failures were secondary to infection, diabetes, hypertension, fewer cell numbers, abnormal renal cell membrane phospholipid content and function, or some other cause. It would be important for future studies to investigate this issue because it will guide patient management in adversely affected children.
In addition to kidney disease, some animals exhibited liver, lung and blood chemistry pathology. Such findings are common in aged rats [53]. Nonetheless, it would be important for future studies to determine the pathogenic basis of these pathologies and whether ω-3 FA excess (or imbalance) during pregnancy and lactation leads to early adult-onset of these disorders. Our Wistar rats had a low incidence of mammary adenomas. This contrasts with Long-Evans rats that have a high incidence in old age [26]. A few Excess offspring experienced seizures in old age, probably secondary to complications from kidney and liver disease.
Excess offspring had significantly higher (worse) ABR thresholds than Control offspring, indicating poorer auditory acuity to low intensity stimuli. Deficient offspring had ABR thresholds that were intermediate to the Excess and Deficient offspring. Similar results occurred in these animals as peri-weanling pups [27] and young adults [29]. Thus, there was a persistence of this abnormality throughout their life span. Testing ages and body temperatures were similar across diet groups during ABR recordings, eliminating these as confounding variables.
The ABR L-I profiles indicated that 33.3% of the Excess offspring had sensorineural hearing loss. This condition was not present when they were pups [27,28] or young adults [29]. Thus, they developed this condition in old age. The onset of SNHL in old age is called presbycusis. We saw this condition in less than 5% of the Control and Deficient animals. Thus, some Excess offspring had an early onset or a greater risk for this age-related hearing disorder. This finding is consistent with our hypothesis of enhanced sensory/neural degeneration in old age. Other Excess offspring had elevated ABR thresholds (hearing loss) without SNHL. We speculate that these latter animals had poor neural synchrony or a reduced number of neural units firing in response to low intensity sound stimuli. Either situation would cause reduced ABR amplitudes in response to low intensity stimuli and poorer ABR thresholds [29].
As pups (Fig. 6A) and young adults [29], our Excess offspring showed another ABR abnormality. Specifically, they had raised ABR thresholds in response to the lower tone pip intensities but abnormally large ABR amplitudes in response to the higher intensities. Such a pattern is consistent with a pathological condition known as hyperacusis. Hyperacusis is due to impaired neural inhibition [61]. As old adults, our Excess offspring showed a persistence of this pattern. Admittedly, the ABR evidence of hyperacusis in the old adult Excess offspring was not strong enough to achieve statistical significance. Failure to achieve statistical significance was due to smaller group sizes, the development of presbycusis in several Excess offspring, and some of the more adversely affected Excess offspring having died before their ABR test session.
One shortcoming for our study was population size. We had to cull our rat colony because of diminished financial resources. This was further complicated by the unexpectedly early and rapid dying of the Excess offspring. Our study originally scheduled the animals for ABR testing at 18 months (~550 days) of age because prior prenatal exposure studies indicated enhanced treatment effects in the ABR [12,23] and minimal death rates at this age [26]. We consequently had to reschedule the ABR testing to ~16 months of age, but by this time the Excess group was down to 60% of its population. Despite this loss in population and statistical power, the Excess treatment effects were strong enough to cause significant effects on life span, body weight, ABR thresholds and presbycusis as well as indicating a persistence of hyperacusis.
Another shortcoming was a failure to produce strong effects in the Deficient offspring. Evidently their mothers had adequate nutritional stores to compensate for the ω-3 FA diet deficiency. In retrospect, we should have placed these dams on their treatment diet several weeks before mating in an attempt to deplete their ω-3 FA stores.
A high omega-3 FA diet for a rat pregnancy study has both precedence and relevance. A study by the American Institute of Nutrition recommended that pregnant and lactating rats be given the AIN-93G diet which contains 7% oil [64]. Like our study, other rat pregnancy and lactation studies have used diets with fish oil content of 7% or higher [5,6,48]. Our Excess condition had non-significant effects on birthing variables [27,28]. In contrast, reduced birth weights were seen in some human [35,36,54–56,69,78] and rat studies [56] following high marine oil consumption during pregnancy. Thus, our Excess condition was less fetotoxic. It is recommended that preclinical developmental and reproductive toxicology studies use a broad range of ω-3/ω-6 FA ratios [2]and a high dose that is 10 to 100 times the human dose in order to test the drug’s safety factor, to compensate for the rat’s different nutritional needs or insensitivity to drugs, and to reveal morbidities not apparent at lower dose levels [8]. Large doses are consumed voluntarily by [35,36,51,54,59,69,78] or given clinically to pregnant women to reduce preterm deliveries and promote infant development [59,75,80] and given for child therapy [74]. The current study was a preliminary study designed to explore what life-long morbidities are possible in extreme conditions. Future studies should evaluate more moderate doses and offspring tissue peroxidation levels to better determine how much dietary fish oil is too much.
All 3 diets had polyunsaturated fatty acids (PUFA). Hence some oxidation is inevitable in studies such as ours. It is unlikely that oxidation of the diets during maternal consumption was a confounding variable, however. Addition of the preservative TBHQ substantially inhibits oxidation [33] and is a standard ingredient in PUFA rodent diets [5,6,48,64]. We used the standard procedures of storing the diets in air-tight bags in a darkened refrigerated room and replacing them twice weekly to further guard against oxidation. The decreased growth in the Excess offspring occurred postnatally, not prenatally. It is likely therefore that a major factor in their postnatal growth retardation was the consumption of maternal milk which had significantly high levels of ω-3 FA and low levels of ω-6 FA compared to Control [45]. When women were given 200 mg DHA daily from gestation week 21 until the third month of lactation, their infants had lower body weights [51]. With this small amount of DHA and the lack of opportunity for its oxidation, the reduced postnatal weights cannot be attributed to oxidation effects. Finally, there is precedence that a maternal diet rich in certain nutrients can be harmful. Although beneficial in moderation, over-supply of prenatal Vitamin A [77], iron [66] calories and fat [15,86,87] have harmful effects on the offspring. Likewise ω-3 FA in moderation is beneficial to fetal and neonatal development, but increasing evidence indicates that its over-supply can be harmful.
The current study is the fourth in a series of articles describing our animals’ progress across their life span, first as pups [27,28] then as young adults [29] and now as old adults. To summarize these articles, we found a mixture of transient, permanent, recurring and adult-onset effects: (A) As a transient effect, the Excess offspring showed delayed neural transmission times as pups [28] but not as young adults [29] or as old adults. (B) As permanent effects, the Excess offspring showed elevated ABR thresholds (hearing loss) and abnormally large ABR amplitudes in response to high stimulus intensities (hyperacusis) as pups, as young adults [29] and as old adults. In a related study, we also found a permanent change in the Excess offspring’s body fatty acid profiles in that their ω-3/ω-6 FA ratio was significantly higher than their Control and Deficient cohorts as adults [45]. (C) As a recurring effect, the Excess offspring had low body weight as weanling pups [28], normal weights as young adults [29] and a recurrence of low body weights as old adults. (D) As adult-onset effects, the Excess offspring had a shortened life span possibly caused by early-onset kidney and liver failure and a significant proportion of the Excess offspring had an early onset or increased risk for presbycusis. The Deficient offspring were often normal or intermediate between their Control and Excess cohorts on these outcome variables.
There are several mechanisms by which ω-3 FA excess or ω-3/ω-6 FA imbalance could have produced adverse effects on our offspring: (A) A relative excess of ω-3 FA will lower AA concentrations in blood, brain and other tissues through competitive displacement [37]. Lowered AA concentrations impair fetal and infant growth [19,21,37] and alter cell membrane, organ, brain and sensory functioning [37]. Consistent with this notion, our Excess offspring had decreased AA body composition levels as adults [45]. (B) Excess ω-3 FA consumption can cause oxidative stress and subsequent cell apoptosis [22]. (C) Over-nutrition can cause epigenetic and hormonal changes in the fetus and adult-onset health disorders [15,60,62,86,87]. (D) Exposure of the oocyte to an environment high in ω-3 FA results in perturbed mitochondrial distribution, metabolism and calcium levels, adversely affecting embryo morphology and development [84]. (E) A diet rich in ω-3 FA can decrease the milk yield of lactating rats [41] and alter the fatty acid composition of maternal milk [45], resulting in postnatal growth retardation.
It may seem puzzlingly that some studies report beneficial effects from pre- and postnatal ω-3 FA supplementation, while others report harmful or even no effects. Studies reporting beneficial effects usually focus on visual [11,79] or cognitive functions [7,39]. Studies reporting adverse effects usually focus on auditory [27–29,38,70,76] or motor functions [14,82,83]. One possibility is that ω-3 FA excess or imbalance has differential effects, benefiting some functions while harming or not influencing others [37]. Other explanations concern dose-dependency and population differences in metabolism: (A) Harmful effects are associated with ω-3 FA mega-dosing or an imbalanced ω-3/ω-6 FA ratio. (B) Populations that consumed high levels of marine oils for generations may have adapted to such diets and are less prone to adverse effects.
In conclusion, this is the first study to investigate the long-term consequences of maternal ω-3 FA over-nutrition. We found that a maternal diet rich in ω-3 FA, when taken during pregnancy and lactation, had permanent harmful effects on offspring life span and auditory function. These effects were likely due to a nutritional toxicity that programmed the offspring for life-long health disorders. As emphasized by others, caution should be exercised in taking too much ω-3FA or a high ω-3/ω-6 FA ratio during pregnancy, lactation and infant formula feeding [9,18,35,43,56]. Children pre- or postnatally exposed to excess ω-3 FA or a high ω-3/ω-6 FA ratio should be assessed and managed for possible sensory, neurodevelopmental and adult-onset health disorders. Such children may be at risk for auditory processing disorders (APD), leading to the co-morbidities of speech and language delays, learning disabilities and attention deficit disorders. There is tentative evidence of decreased verbal skills in such children [50,72]. They may also be at risk for presbycusis, hypertension, type II diabetes, early-onset kidney and liver failure and shorter life spans in adulthood. Thus, there is a need for long-term follow-up of such children, exploring the pathogenic bases of these disorders, and pursuing intervention strategies.
Acknowledgements
Supported by grants from the Gerber Foundation and the National Institute of General Medical Sciences (GM58905), neither of which contributed to the design, analyses or writing of this study. We thank Dr. Karen Rossman, DVM (Department of Laboratory Animal Resources, Wayne State University) for collecting and analyzing tissue and blood samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
There were no conflicts of interest.
References
- 1.Abel EL, Church MW, Dintcheff BA. Prenatal alcohol exposure shortens life span in rats. Teratology. 1987;36:217–220. doi: 10.1002/tera.1420360209. [DOI] [PubMed] [Google Scholar]
- 2.Rockville, MD: United States Department of Health and Human Services; Agency for Healthcare Research and Quality, Effects of omega-3 fatty acids on child and maternal health. 2005 AHRQ Publication No 05-E0.
- 3.Aihie Sayer A, Dunn R, Langley-Evans S, Cooper C. Prenatal exposure to a maternal low protein diet shortens life span in rats. Gerontology. 2001;47:9–14. doi: 10.1159/000052764. [DOI] [PubMed] [Google Scholar]
- 4.Akabas SR, Deckelbaum RJ. Summary of a workshop on n-3 fatty acids: current status of recommendations and future directions. The American journal of clinical nutrition. 2006;83:1536S–1538S. doi: 10.1093/ajcn/83.6.1536S. [DOI] [PubMed] [Google Scholar]
- 5.Amusquivar E, Herrera E. Influence of changes in dietary fatty acids during pregnancy on placental and fetal fatty acid profile in the rat. Biol Neonate. 2003;83:136–145. doi: 10.1159/000067963. [DOI] [PubMed] [Google Scholar]
- 6.Amusquivar E, Ruperez FJ, Barbas C, Herrera E. Low arachidonic acid rather than alpha-tocopherol is responsible for the delayed postnatal development in offspring of rats fed fish oil instead of olive oil during pregnancy and lactation. The Journal of nutrition. 2000;130:2855–2865. doi: 10.1093/jn/130.11.2855. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. The American journal of clinical nutrition. 1999;70:525–535. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous, Detection of toxicity to reproduction for medicinal purposes S5A; International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 1993. http://www.ich.org/ [Google Scholar]
- 9.Arbuckle LD, Rioux FM, Mackinnon MJ, Hrboticky N, Innis SM. Response of (n−3) and (n−6) fatty acids in piglet brain, liver and plasma to increasing, but low, fish oil supplementation of formula. The Journal of nutrition. 1991;121:1536–1547. doi: 10.1093/jn/121.10.1536. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 11.Birch EE, Birch DG, Hoffman DR, Uauy R. Dietary essential fatty acid supply and visual acuity development. Invest Ophthalmol Vis Sci. 1992;33:3242–3253. [PubMed] [Google Scholar]
- 12.Bourre JM, Durand G, Erre JP, Aran JM. Changes in auditory brainstem responses in alpha-linolenic acid deficiency as a function of age in rats. Audiology. 1999;38:13–18. doi: 10.3109/00206099909072997. [DOI] [PubMed] [Google Scholar]
- 13.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. The Journal of nutrition. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 14.Bouwstra H, Dijck-Brouwer DJ, Decsi T, Boehm G, Boersma ER, Muskiet FA, Hadders-Algra M. Relationship between umbilical cord essential fatty acid content and the quality of general movements of healthy term infants at 3 months. Pediatric research. 2006;59:717–722. doi: 10.1203/01.pdr.0000215013.19164.57. [DOI] [PubMed] [Google Scholar]
- 15.Buison A, Lu H, Guo F, Jen K-LC. High-fat feeding of different fatty acids during pregnancy and lactation: effects on maternal metabolism. Nutrition Research. 1997;17:1541–1554. [Google Scholar]
- 16.Carlson SE. Arachidonic acid status of human infants: influence of gestational age at birth and diets with very long chain n−3 and n−6 fatty acids. The Journal of nutrition. 1996;126:1092S–1098S. doi: 10.1093/jn/126.suppl_4.1092S. [DOI] [PubMed] [Google Scholar]
- 17.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–449. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- 18.Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH. Effect of vegetable and marine oils in preterm infant formulas on blood arachidonic and docosahexaenoic acids. The Journal of pediatrics. 1992;120:S159–S167. doi: 10.1016/s0022-3476(05)81251-x. [DOI] [PubMed] [Google Scholar]
- 19.Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH, Tolley EA. Long-term feeding of formulas high in linolenic acid and marine oil to very low birth weight infants: phospholipid fatty acids. Pediatric research. 1991;30:404–412. doi: 10.1203/00006450-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year growth of preterm infants fed standard compared to marine oil n−3 supplemented formula. Lipids. 1992;27:901–907. doi: 10.1007/BF02535870. [DOI] [PubMed] [Google Scholar]
- 21.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1073–1077. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho SH, Choi YS. Lipid peroxidation and antioxidant status is affected by different vitamin E levels when feeding fish oil. Lipids. 1994;29:47–52. doi: 10.1007/BF02537090. [DOI] [PubMed] [Google Scholar]
- 23.Church MW, Abel EL, Kaltenbach JA, Overbeck GW. Effects of prenatal alcohol exposure and aging on auditory function in the rat: preliminary results. Alcoholism, clinical and experimental research. 1996;20:172–179. doi: 10.1111/j.1530-0277.1996.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 24.Church MW, Blakley BW, Burgio DL, Gupta AK. WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J Assoc Res Otolaryngol. 2004;5:227–237. doi: 10.1007/s10162-004-4011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Church MW, Gritzke R. Effects of ketamine anesthesia on the rat brain-stem auditory evoked potential as a function of dose and stimulus intensity. Electroencephalography and clinical neurophysiology. 1987;67:570–583. doi: 10.1016/0013-4694(87)90060-5. [DOI] [PubMed] [Google Scholar]
- 26.Church MW, Holmes PA, Tilak JP, Hotra JW. Prenatal cocaine exposure influences the growth and life span of laboratory rats. Neurotoxicology and teratology. 2004;26:429–441. doi: 10.1016/j.ntt.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Church MW, Jen K-LC, Stafferton T, Hotra JW, Adams BR. Reduced auditory acuity in rat pups from excess and deficient omega-3 fatty acid consumption by the mother. Neurotoxicology and teratology. 2007;29:203–210. doi: 10.1016/j.ntt.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church MW, Jen KL, Dowhan LM, Adams BR, Hotra JW. Excess and deficient omega-3 fatty acid during pregnancy and lactation cause impaired neural transmission in rat pups. Neurotoxicology and teratology. 2008;30:107–117. doi: 10.1016/j.ntt.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Church MW, Jen KL, Jackson DA, Adams BR, Hotra JW. Abnormal neurological responses in young adult offspring caused by excess omega-3 fatty acid (fish oil) consumption by the mother during pregnancy and lactation. Neurotoxicology and teratology. 2009;31:26–33. doi: 10.1016/j.ntt.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Church MW, Williams HL, Holloway JA. Postnatal development of the brainstem auditory evoked potential and far-field cochlear microphonic in non-sedated rat pups. Brain research. 1984;316:23–31. doi: 10.1016/0165-3806(84)90005-1. [DOI] [PubMed] [Google Scholar]
- 31.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. 7th ed., Editoin Edition. New York, USA: Wiley; 1999. [Google Scholar]
- 32.Davis H. Brain stem and other responses in electric response audiometry. Ann Otol Rhinol Laryngol. 1976;85:3–14. doi: 10.1177/000348947608500103. [DOI] [PubMed] [Google Scholar]
- 33.Fritsche KL, Johnston PV. Rapid autoxidation of fish oil in diets without added antioxidants. The Journal of nutrition. 1988;118:425–426. doi: 10.1093/jn/118.4.425. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez MJ, Gray JI, Schemmel RA, Dugan L, Jr., Welsch CW. Lipid peroxidation products are elevated in fish oil diets even in the presence of added antioxidants. The Journal of nutrition. 1992;122:2190–2195. doi: 10.1093/jn/122.11.2190. [DOI] [PubMed] [Google Scholar]
- 35.Grandjean P, Bjerve KS, Weihe P, Steuerwald U. Birthweight in a fishing community: significance of essential fatty acids and marine food contaminants. International journal of epidemiology. 2001;30:1272–1278. doi: 10.1093/ije/30.6.1272. [DOI] [PubMed] [Google Scholar]
- 36.Grandjean P, Weihe P. Neurobehavioral effects of intrauterine mercury exposure: potential sources of bias. Environmental research. 1993;61:176–183. doi: 10.1006/enrs.1993.1062. [DOI] [PubMed] [Google Scholar]
- 37.Hadders-Algra M. Prenatal long-chain polyunsaturated fatty acid status: the importance of a balanced intake of docosahexaenoic acid and arachidonic acid. Journal of perinatal medicine. 2008;36:101–109. doi: 10.1515/JPM.2008.029. [DOI] [PubMed] [Google Scholar]
- 38.Haubner LY, Stockard JE, Saste MD, Benford VJ, Phelps CP, Chen LT, Barness L, Wiener D, Carver JD. Maternal dietary docosahexanoic acid content affects the rat pup auditory system. Brain research bulletin. 2002;58:1–5. doi: 10.1016/s0361-9230(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 39.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 40.Henry KR. Auditory brainstem volume-conducted responses: origins in the laboratory mouse. Journal of the American Auditory Society. 1979;4:173–178. [PubMed] [Google Scholar]
- 41.Herrera E, Lopez-Soldado I, Limones M, Amusquivar E, Ramos MP. Lipid metabolism during the perinatal phase, and its implications on postnatal development. Int J Vitam Nutr Res. 2006;76:216–224. doi: 10.1024/0300-9831.76.4.216. [DOI] [PubMed] [Google Scholar]
- 42.Innis SM. The role of dietary n−6 and n−3 fatty acids in the developing brain. Developmental neuroscience. 2000;22:474–480. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 43.Innis SM, de La Presa Owens S. Dietary fatty acid composition in pregnancy alters neurite membrane fatty acids and dopamine in newborn rat brain. The Journal of nutrition. 2001;131:118–122. doi: 10.1093/jn/131.1.118. [DOI] [PubMed] [Google Scholar]
- 44.Janicke B, Coper H. The effects of prenatal exposure to hypoxia on the behavior of rats during their life span. Pharmacol Biochem Behav. 1994;48:863–873. doi: 10.1016/0091-3057(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 45.Jen K-LC, Church MW, Wang C, Moghaddam M, Dowhan L, Laja F, Sherman J. Perinatal n−3 fatty acid imbalance affects fatty acid composition in rat offspring. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 46.Jensen CL. Effects of n−3 fatty acids during pregnancy and lactation. The American journal of clinical nutrition. 2006;83:1452S–1457S. doi: 10.1093/ajcn/83.6.1452S. [DOI] [PubMed] [Google Scholar]
- 47.Jensen CL, Prager TC, Fraley JK, Chen H, Anderson RE, Heird WC. Effect of dietary linoleic/alpha-linolenic acid ratio on growth and visual function of term infants. The Journal of pediatrics. 1997;131:200–209. doi: 10.1016/s0022-3476(97)70154-9. [DOI] [PubMed] [Google Scholar]
- 48.Joshi S, Rao S, Golwilkar A, Patwardhan M, Bhonde R. Fish oil supplementation of rats during pregnancy reduces adult disease risks in their offspring. The Journal of nutrition. 2003;133:3170–3174. doi: 10.1093/jn/133.10.3170. [DOI] [PubMed] [Google Scholar]
- 49.Koutz CA, Wiegand RD, Rapp LM, Anderson RE. Effect of dietary fat on the response of the rat retina to chronic and acute light stress. Exp Eye Res. 1995;60:307–316. doi: 10.1016/s0014-4835(05)80112-5. [DOI] [PubMed] [Google Scholar]
- 50.Lauritzen L, Jorgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast-fed infants. Reprod Nutr Dev. 2005;45:535–547. doi: 10.1051/rnd:2005044. [DOI] [PubMed] [Google Scholar]
- 51.Lucia Bergmann R, Bergmann KE, Haschke-Becher E, Richter R, Dudenhausen JW, Barclay D, Haschke F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? Journal of perinatal medicine. 2007;35:295–300. doi: 10.1515/JPM.2007.085. [DOI] [PubMed] [Google Scholar]
- 52.Martin JC, Martin DD, Radow B, Day HE. Life span and pathology in offspring following nicotine and methamphetamine exposure. Exp Aging Res. 1979;5:509–522. doi: 10.1080/03610737908257225. [DOI] [PubMed] [Google Scholar]
- 53.Masoro EJ. Mortality and growth characteristics of rat strains commonly used in aging research. Exp Aging Res. 1980;6:219–233. doi: 10.1080/03610738008258359. [DOI] [PubMed] [Google Scholar]
- 54.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsen SF, Grandjean P, Weihe P, Videro T. Frequency of seafood intake in pregnancy as a determinant of birth weight: evidence for a dose dependent relationship. Journal of epidemiology and community health. 1993;47:436–440. doi: 10.1136/jech.47.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsen SF, Hansen HS, Jensen B. Fish oil versus arachis oil food supplementation in relation to pregnancy duration in rats. Prostaglandins Leukot Essent Fatty Acids. 1990;40:255–260. doi: 10.1016/0952-3278(90)90046-n. [DOI] [PubMed] [Google Scholar]
- 57.Olsen SF, Hansen HS, Sorensen TI, Jensen B, Secher NJ, Sommer S, Knudsen LB. Hypothesis: dietary (N-3)-fatty acids prolong gestation in human beings. Prog Clin Biol Res. 1987;242:51–56. [PubMed] [Google Scholar]
- 58.Olsen SF, Secher NJ. A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br J Nutr. 1990;64:599–609. doi: 10.1079/bjn19900063. [DOI] [PubMed] [Google Scholar]
- 59.Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, Grant A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003–1007. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 60.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 61.Phillips DP, Carr MM. Disturbances of loudness perception. J Am Acad Audiol. 1998;9:371–379. quiz 399. [PubMed] [Google Scholar]
- 62.Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- 63.Rao SS, Kale AA, Joshi SR, Mahadik SP. Sensitivity of fetus and pups to excess levels of maternal intakes of alpha linolenic acid at marginal protein levels in Wistar rats. Reprod Toxicol. 2007;24:333–342. doi: 10.1016/j.reprotox.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. The Journal of nutrition. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 65.Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rioux FM, LeBlanc CP. Iron supplementation during pregnancy: what are the risks and benefits of current practices? Appl Physiol Nutr Metab. 2007;32:282–288. doi: 10.1139/H07-012. [DOI] [PubMed] [Google Scholar]
- 67.Roegge CS, Widholm JJ, Engeseth NJ, Wang X, Brosch KO, Seegal RF, Schantz SL. Delayed spatial alternation impairments in adult rats following dietary n−6 deficiency during development. Neurotoxicology and teratology. 2005;27:485–495. doi: 10.1016/j.ntt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Rossi GT, Britt RH. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalography and clinical neurophysiology. 1984;57:143–155. doi: 10.1016/0013-4694(84)90173-1. [DOI] [PubMed] [Google Scholar]
- 69.Rump P, Mensink RP, Kester AD, Hornstra G. Essential fatty acid composition of plasma phospholipids and birth weight: a study in term neonates. The American journal of clinical nutrition. 2001;73:797–806. doi: 10.1093/ajcn/73.4.797. [DOI] [PubMed] [Google Scholar]
- 70.Saste MD, Carver JD, Stockard JE, Benford VJ, Chen LT, Phelps CP. Maternal diet fatty acid composition affects neurodevelopment in rat pups. The Journal of nutrition. 1998;128:740–743. doi: 10.1093/jn/128.4.740. [DOI] [PubMed] [Google Scholar]
- 71.Sattar N, Berry C, Greer IA. Essential fatty acids in relation to pregnancy complications and fetal development. Br J Obstet Gynaecol. 1998;105:1248–1255. doi: 10.1111/j.1471-0528.1998.tb10002.x. [DOI] [PubMed] [Google Scholar]
- 72.Scott DT, Janowsky JS, Carroll RE, Taylor JA, Auestad N, Montalto MB. Formula supplementation with long-chain polyunsaturated fatty acids: are there developmental benefits? Pediatrics. 1998;102:E59. doi: 10.1542/peds.102.5.e59. [DOI] [PubMed] [Google Scholar]
- 73.Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101:469–479. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 74.Sorgi PJ, Hallowell EM, Hutchins HL, Sears B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr J. 2007;6:16. doi: 10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spong CY, Thom EA, Harper M. Omega-3 fatty acid supplementation to prevent preterm birth in high risk pregnancies. 2009 NCT00135902 http://clinicaltrials.gov/ct2/show/NCT00135902.
- 76.Stockard JE, Saste MD, Benford VJ, Barness L, Auestad N, Carver JD. Effect of docosahexaenoic acid content of maternal diet on auditory brainstem conduction times in rat pups. Developmental neuroscience. 2000;22:494–499. doi: 10.1159/000017481. [DOI] [PubMed] [Google Scholar]
- 77.Sulik KK, Cook CS, Webster WS. Teratogens and craniofacial malformations: relationships to cell death. Development. 1988;103 Suppl:213–231. doi: 10.1242/dev.103.Supplement.213. [DOI] [PubMed] [Google Scholar]
- 78.Thorsdottir I, Birgisdottir BE, Halldorsdottir S, Geirsson RT. Association of fish and fish liver oil intake in pregnancy with infant size at birth among women of normal weight before pregnancy in a fishing community. Am J Epidemiol. 2004;160:460–465. doi: 10.1093/aje/kwh239. [DOI] [PubMed] [Google Scholar]
- 79.Uauy R, Birch E, Birch D, Peirano P. Visual and brain function measurements in studies of n-3 fatty acid requirements of infants. The Journal of pediatrics. 1992;120:S168–S180. doi: 10.1016/s0022-3476(05)81252-1. [DOI] [PubMed] [Google Scholar]
- 80.van Goor SA. The effect of high docosahexaenoic acid (DHA)-fish oil and arachidonic acid (AA) supplementation during pregnancy and lactation on long-chain polyunsaturated fatty acids (LCP) status of mother and child and on the neurological development of the baby. 2008 ISRCTN58176213 http://www.controlled-trials.com/ISRCTN58176213/
- 81.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 82.Wainwright PE, Jalali E, Mutsaers LM, Bell R, Cvitkovic S. An imbalance of dietary essential fatty acids retards behavioral development in mice. Physiol Behav. 1999;66:833–839. doi: 10.1016/s0031-9384(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 83.Wainwright PE, Xing HC, Mutsaers L, McCutcheon D, Kyle D. Arachidonic acid offsets the effects on mouse brain and behavior of a diet with a low (n−6):(n−3) ratio and very high levels of docosahexaenoic acid. The Journal of nutrition. 1997;127:184–193. doi: 10.1093/jn/127.1.184. [DOI] [PubMed] [Google Scholar]
- 84.Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab. 2008;294:E425–E434. doi: 10.1152/ajpendo.00409.2007. [DOI] [PubMed] [Google Scholar]
- 85.Weisinger HS, Vingrys AJ, Sinclair AJ. The effect of docosahexaenoic acid on the electroretinogram of the guinea pig. Lipids. 1996;31:65–70. doi: 10.1007/BF02522413. [DOI] [PubMed] [Google Scholar]
- 86.White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. The Journal of nutrition. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]