Abstract
OBJECTIVE
This study was designed to determine if angiopoietin (ANGPT)-1 and -2 are detectable in the circulation of nonhuman primates and women, and if these levels fluctuate in association with ovarian activity.
DESIGN
Prospective
SETTING
National Primate Research Center, medical center and infertility clinic.
PATIENTS
Adult, female rhesus monkeys; 15 women donating oocytes for infertility treatment.
INTERVENTIONS
Controlled ovarian stimulation with gonadotropins, removal of the corpus luteum and ovaries, oocyte retrieval and embryo transfer.
MAIN OUTCOME MEASURE
Circulating levels of ANGPT-1 and ANGPT-2.
RESULTS
Serum ANGPT-1 and ANGPT-2 levels were detectable and invariant in maintaining an ANGPT1:2 ratio >1 in: (a) macaques over the course of the natural menstrual cycle, during a controlled ovulation protocol and following removal of the corpus luteum or ovaries, and (b) women undergoing controlled ovarian simulation (COS). In contrast, the ANGPT1:2 ratio was markedly decreased (≪1) at mid-to-late gestation in macaques, and in the follicular fluid of women undergoing COS, due to increased levels of ANGPT-2.
CONCLUSIONS
The ovary and its dominant structures are not major contributors to circulating levels of ANGPT-1 or ANGPT-2. The physiologic importance of the rising levels of ANGPT-2 after the luteal-placental shift in pregnancy is unknown.
Keywords: angiopoietin-1, angiopoietin-2, angiogenesis, vascular permeability, ovarian hyperstimulation syndrome (OHSS)
INTRODUCTION
Angiogenesis, blood vessel stabilization and vascular degeneration within the ovary are essential components of the functional menstrual cycle (1-3). This sequence of vascular events allows for the extensive tissue remodeling necessary for follicular growth and atresia, ovulation and the subsequent development and regression of the corpus luteum. The regulation of these events is an intricate process, likely dependent on the activity of numerous angiogenic and angiolytic substances. Studies revealed that two such classes, the vascular endothelial growth factors (VEGFs)(1;4) and the angiopoietins (ANGPTs) (5-7) are present within both ovarian follicles and corpora lutea, and appear to play an integral role in endothelial cells and hence, blood vessel regulation within the ovary.
It is proposed that the VEGFs and ANGPTs work in concert with one another to control vascular growth, stabilization and regression (8). The VEGFs appear to be responsible for the formation, migration and proliferation of endothelial cells, as well as vascular tube formation. The ANGPTs appear to work through a single receptor tyrosine kinase, Tie-2; however, they exert opposing effects following receptor binding. ANGPT-1 recruits and interacts with periendothelial cells, providing stabilization and maintenance to those vessels stimulated and developed by VEGF. In contrast, ANGPT-2 appears to act as a natural antagonist to ANGPT-1, resulting in loosening of the supporting cell matrix and destabilization of existing vessels. In the presence of VEGF, this allows for further stimulation of endothelial cell proliferation and migration, promoting further angiogenesis. Acting in the relative absence of VEGF, ANGPT-2 appears to block the recruitment of periendothelial support cells, resulting in blood vessel destabilization and regression.
Evidence supporting the dynamic expression of ANGPT-1 and ANGPT-2 in the corpus luteum and/or ovulatory follicle of several species, including primates (6;7) and humans (9;10), is consistent with a role in ovarian vasculogenesis. The temporal expression of VEGF, ANGPT-1 and ANGPT-2 mRNA during the lifespan of the macaque corpus luteum was previously described (7). Levels of VEGF mRNA, primarily for the diffusible VEGF165 and 121 isoforms (11), are detectable in the developing corpus luteum during the early luteal phase; indeed, VEGF protein levels in luteal tissue are highest at this stage. There appears to be a divergence in the transcriptional versus translational regulation of VEGF, since mRNA levels continue to rise through the mid-late luteal phase, whereas protein levels decline to low levels. In contrast, mRNA levels for the ANGPTs, especially ANGPT-2, rise later in the luteal phase to peak at the late stages during luteal regression. To date, ANGPT-1 and -2 proteins have not been quantitated in the primate corpus luteum during the menstrual cycle. Nevertheless, intrafollicular injection of the ANGPTs demonstrated a critical role for these factors in controlling the health and subsequent fate of the preovulatory follicle in macaques. Injection of ANGPT-2, but not ANGPT-1, resulted in a dose-dependent inhibition of ovulation and prevented the development and function of the subsequent corpus luteum (12;13). The latter appeared secondary to the forced degeneration of the ovulatory follicle and resetting of the onset of the subsequent menstrual cycle.
Although considered a local, paracrine factor, circulating levels of free and total VEGF-A and its soluble VEGF receptors-1 and 2 are detectable in women and nonhuman primates during the menstrual cycle and controlled ovarian stimulation (COS) (14-16). During COS protocols in women, concentrations of free and total VEGF increased significantly following administration of hCG. Total VEGF remained detectable throughout early pregnancy, but free VEGF concentrations peaked during the midluteal phase and subsequently dropped to undetectable levels in the early first trimester. This decrease in free VEGF-A corresponded to an abrupt rise in sVEGFR-1 in the circulation (14). These findings suggest that alterations in the production and thus, circulating levels of VEGF and its soluble VEGF receptors (17;18) can serve as a marker for ovarian or placental function. Whether ANGPTs of ovarian or placental origin circulate in the blood has not been detailed. Based on our preliminary report of circulating ANGPT-2 and its soluble Tie-2 receptor in women during gestation (19), further studies were designed to determine if the ANGPTs are detectable in the circulation of nonhuman primates and women in natural or COS cycles, and whether these levels fluctuate in association with ovarian activity over the course of the reproductive cycle and in pregnancy.
MATERIALS/METHODS
Animal Protocols
Saphenous venous blood samples were collected from adult, female rhesus monkeys during the natural menstrual cycle, the periovulatory interval in a controlled ovulation protocol and during pregnancy, as previously described (14;20). The general care and housing of rhesus monkeys was provided by the Division of Animal Resources at the Oregon National Primate Research Center in accordance with the NIH Guide For The Care and Use of Laboratory Animals. All animal protocols were approved by the ONPRC Animal Care and Use Committee. Blood samples were collected from non-anesthetized animals.
Natural Cycles (14)
Saphenous venous blood samples were obtained from 10 female rhesus monkeys exhibiting regular menstrual cycles, from the first day of menses until the onset of the subsequent menstrual period.
Controlled Ovulation (COv) (20)
Daily serum samples were obtained from 6 female rhesus monkeys and treatment initiated when estradiol levels were between 80-120 pg/ml. On day 1 of treatment, 3 mg/kg of a GnRH antagonist (Antide; NICHD), 30 IU r-hFSH (Organon) and 15 IU r-hLH (Merck Serono) were administered at 0800, followed by a second dose of r-hFSH and r-hLH alone 8 hours later. On day 2 of treatment, 1.5 mg/kg of Antide, 30 IU r-hFSH and 15 IU r-hLH were administered at 0800, followed by ovulatory bolus of 1000 IU r-hCG (Merck Serono). Saphenous venous blood samples were obtained every 12 hours for 48-72 hours prior to and 36 hours following administration of hCG.
Lutectomy/Oophorectomy (12;21)
Daily venous blood samples were collected prior to (day -1), the day of (day 0) and after (day +1 and +2) surgical removal of the corpus luteum or ovaries at midluteal phase of the natural cycle.
Pregnancy
Venous blood samples were collected from 8 pregnant, rhesus monkeys from mid-to-late gestation; this interval was chosen based on our earlier data from human pregnancy (19). Pregnant monkeys for blood sampling were provided by the Assisted Reproductive Technologies (ART) Core, ONPRC. Briefly (22;23), in vitro fertilized eggs were cultured and two embryos transferred into recipient monkeys at 3-4 days following the midcycle peak in estradiol levels during the natural menstrual cycle. Gestational age was determined by the day of embryo transfer. Samples were obtained weekly and grouped based on a gestational age of 8-10, 11-14, 15-19 and 20-22 weeks (14).
Human Protocols
Patients (n=15) were recruited from a population of women under 35 years of age who were serving as oocyte donors within the infertility clinic, IVF New Jersey. The general consent form for oocyte donation and IVF provided patients’ approval for use of discarded materials (serum, follicular fluid) for research purposes. Discarded materials without patient identifiers were shipped to OHSU for analyses of ANGPT levels. The study for analyses of angiogenic factors at OHSU was approved by the Institutional Review Board.
Controlled Ovarian Stimulation (COS) in Women
The protocol, involving pituitary down-regulation with GnRH agonist (Lupron; TAP Pharmaceuticals, Deerfield, IL, USA), with a urinary gonadotropin (HMG) and recombinant human FSH mixture started after induced menses to promote multiple follicular development, was similar to that previously described (Gonal F, Serono, Rockland, MA; (19)). Dosage was individualized based on serial ultrasound results and serum estradiol levels. When ≥2 follicles were >17mm diameter, 10,000 IU hCG (Serono) was given IM, and transvaginal ultrasound-directed oocyte retrieval was performed 36 hours later. Venous blood samples were collected on the first day of COS onset (baseline levels prior to injection of endogenous gonadotropins), the day of the hCG bolus, and at oocyte retrieval, as well as follicular fluid from the lead (largest) follicle after oocyte removal. Samples were shipped frozen to ONPRC for analyses.
Hormone Assays
Serum concentrations of estradiol (E) and progesterone (P) were determined by the Endocrine Services Laboratory at the ONPRC (Roche Elecsys 2010 assay instrument) (12). ANGPT-1 and ANGPT-2 concentrations were determined using Quantikine immunoassays (R&D Systems, USA). Pooled samples were included in assays to confirm accuracy. Inter- and intra-assay variations were 18% and 11.5% for ANGPT-1 and 3% and 11% for ANGPT-2 (19), respectively.
Statistical Analyses
Mean differences over time or between experimental groups were evaluated by ANOVA or ANOVA on ranks followed by the Student-Newman-Keuls or Dunn’s test using the SigmaStat statistical software package (SPSS, Chicago, Il., USA) A significant difference was defined as P<0.05. Data are expressed as the mean ± the standard error of the mean (SEM).
RESULTS
Animal Studies
As illustrated in Figure 1, monkeys displayed the typical patterns and levels of circulating estradiol and progesterone during the follicular (11.6 ± 0.8 days) and luteal (17.2 ± 0.4 days) phases of the natural menstrual cycle. Both ANGPT-1 and ANGPT-2 were detectable in serum of adult, female macaques throughout the menstrual cycle. Due to normal variance in the lengths of the follicular and luteal phase of the menstrual cycle among the animals, the data were normalized to the midcycle LH surge (day 0), as well as to the day of onset of menstruation (first day 1). ANGPT-1 and -2 levels remained invariant throughout the early follicular phase after menses, at midcycle, as well as at the end of the luteal phase prior to the next menstrual period. At the time of the first menses, mean ANGPT-1 and -2 levels were 57 ng/ml and 9 ng/ml, respectively, resulting in an ANGPT1:2 ratio of 7.0 ± 1.6 (mean ± SEM). Likewise, ANGPT-1 and -2 levels at the time of the midcycle LH surge were comparable to those at menses, resulting in an ANGPT1:2 ratio of 5.8 ± 1.1.
Figure 1.
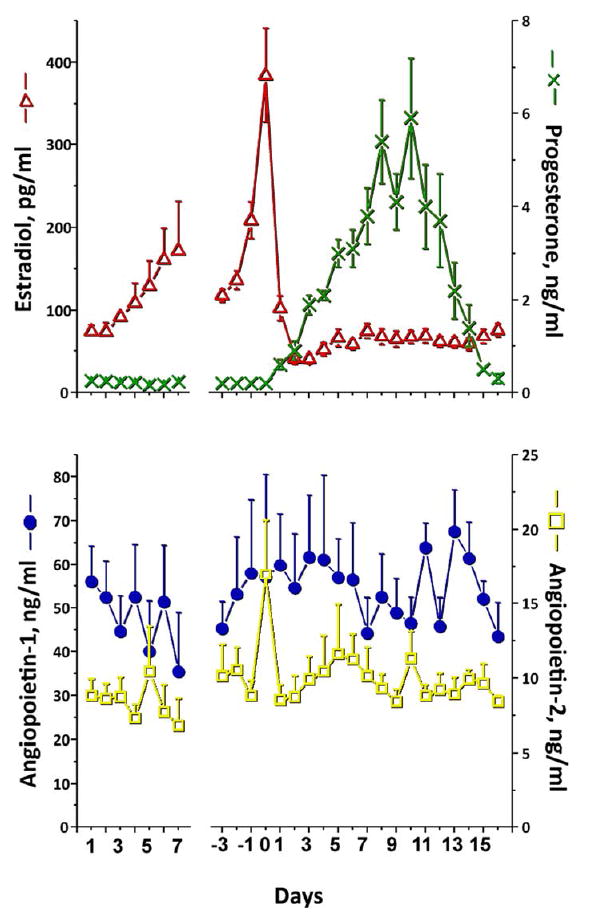
Levels and patterns of serum estradiol, progesterone (top panel), ANGPT-1 and -2 (bottom panel) in 10 adult rhesus monkeys during the menstrual cycle. Values (mean ± SEM) are normalized to first day of menstruation (first day 1), and to the day of the midcycle LH surge (day 0). There was no significant difference over time or between days for ANGPT-1 or -2 levels.
Since daily samples may not detect acute changes during the periovulatory interval (note the disparate value the day before the midcycle LH surge, Fig. 1), samples were obtained twice daily in macaques undergoing a COv protocol during this interval. The COv protocol (20) permits selection of the dominant follicle in the natural cycle, but then removes control of onset of ovulatory events from the pituitary by administration of a GnRH antagonist. Administration of an ovulatory bolus of hCG permits study of events at precise intervals in the periovulatory interval, with ovulation occurring in animals at ≥36 hours post-hCG injection. As was observed with the natural menstrual cycle, there were no significant changes in ANGPT-1 and -2 levels. ANGPT-1 and -2 levels were 36 +/- 6 and 7 +/- 1 ng/ml at the time of the ovulatory hCG bolus, resulting in an ANGPT1:2 ratio of 5.7 ± 0.9. The ANGPT1:2 ratio and levels remained unchanged for the 36-hour interval following exposure to hCG (data not shown).
To determine if the functional CL or ovary contributed to circulating levels of ANGPT-1 or -2, levels were monitored for an interval following excision of the CL or ovaries. Although serum progesterone levels declined 10-fold (p<0.05) within 24 hours, there was no change in circulating levels of ANGPT-1 or -2 following removal of the CL (Fig. 2) or following oophorectomy (not shown). ANGPT-1 and -2 levels were 35.7 ± 17.5 ng/ml and 7.5 ± 1 ng/ml just prior to oophorectomy, and 34.5 ± 3.3 ng/ml and 6.9 ± 1 ng/ml 48 hours later. Thus, an ANGPT1:2 ratio of 3-5 was maintained following both lutectomy and oophorectomy.
Figure 2.
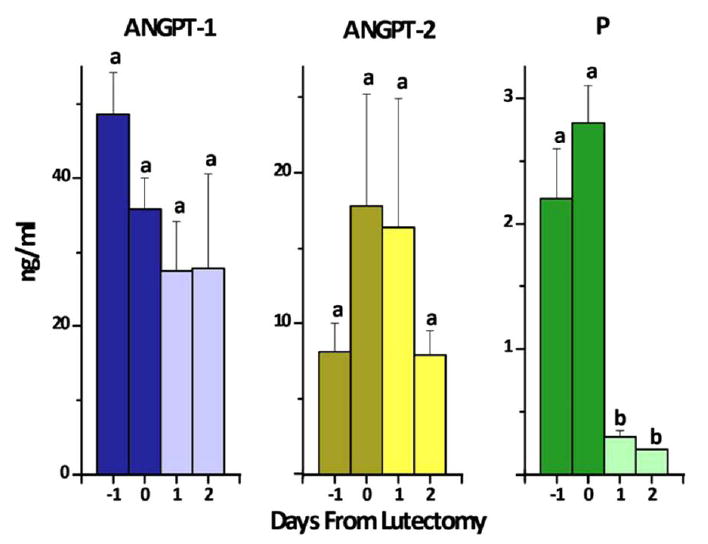
Serum levels of ANGPT-1, -2 and progesterone in female rhesus monkeys before (day -1), the day of (day 0) or after (day +1 and +2) lutectomy at midluteal phase of the menstrual cycle. Values (mean ± SEM, n=3) with different letters (a,b) differed, e.g., progesterone levels, after lutectomy.
The serum concentrations of ANGPT-1 and -2 at intervals from mid-to-late gestation in macaques are illustrated in Figure 3. Although ANGPT-1 levels did not change throughout these intervals, there was a significant (p=0.01) increase in the concentration of ANGPT-2. ANGPT-2 levels were 29 ± 10 ng/ml at 8-10 weeks, and increased to 155 ± 42 ng/ml at 15-19 weeks, with no further increase in ANGPT-2 levels in late gestation. This resulted in a marked decrease of the ANGPT1:2 ratio to 0.4 ± 0.1 at 8-10 weeks and 0.04 ± 0.1 at 15-19 weeks, which remained in late pregnancy.
Figure 3.
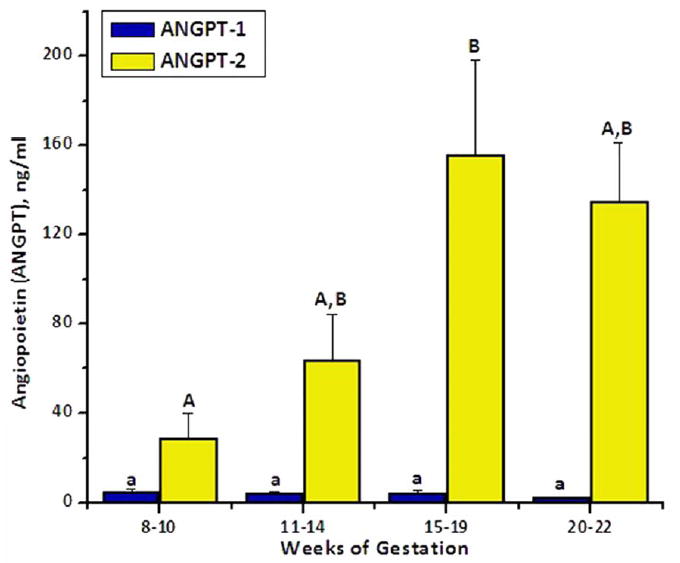
Levels and patterns of serum ANGPT-1 and -2 during intervals from mid-to-late gestation in pregnant macaques. Values (mean ± SEM, n=8) with different letters differed over time (e.g., ANGPT-2 at weeks 8-10 versus 15-19).
Human Studies
ANGPT-1 and -2 were detectable in the serum of women undergoing COS, as shown in Figure 4. However, as in monkeys (data not shown) mean concentrations were invariant throughout the stimulation cycle. ANGPT-1 and -2 levels were 33 ± 3 ng/ml and 1.5 ± 0.2 ng/ml at the time of baseline suppression prior to COS and remained unchanged at the time of the hCG bolus and at the time of oocyte retrieval. This resulted in an ANGPT1:2 ratio of 21-25. Conversely, the concentration of ANGPT-1, 1.0 ± 0.1 ng/ml, was markedly lower than ANGPT-2, 18.1 ± 4 ng/ml, in follicular fluid. Consequently, this resulted in a reversal of the ANGPT1:2 ratio to 0.07 ± 0.01.
Table 1 summarizes the observed ANGPT1:2 ratios among the study groups in macaques and women. Notably, the ANGPT1:2 ratio in the circulation remains >1 in macaques during the menstrual cycle, a controlled ovulation protocol, and following removal of the corpus luteum or ovaries. Likewise, the ANGPT1:2 ratio was >1 in serum of women undergoing controlled ovarian stimulation. However, the ANGPT1:2 ratio was markedly reduced (≪1) during macaque gestation and in human follicular fluid at the time of oocyte retrieval following COS.
Table 1.
Summary of the ratios of ANGPT 1:2 in serum of macaques and women during various protocols, plus in follicular fluid of women at follicle aspiration during COS protocols.1
| Sample | Time | ANGPT 1:2 Ratio2 |
|---|---|---|
| Macaque | ||
| Menstrual Cycle | Menses | 7.0 ± 1.6 |
| LH Surge | 5.8 ± 1.1 | |
| Controlled Ovulation Protocol | HCG Bolus | 5.7 ± 0.9 |
| Lutectomy | 48 hours following procedure | 3.2 ± 0.9 |
| Oophorectomy | 48 hours following procedure | 5.2 ± 1.3 |
| Pregnancy | 8-10 weeks gestation | 0.4 ± 0.1 |
| 11-14 weeks gestation | 0.2 ± 0.1 | |
| 15-19 weeks gestation | 0.04 ± 0.01 | |
| 20-22 weeks gestation | 0.02 ± 0.01 | |
| Human | ||
| Controlled Ovarian Stimulation | Baseline Suppression | 25.4 ± 4.1 |
| Following HCG Bolus | 21.9 ± 2.6 | |
| Oocyte Retrieval | 21.0 ± 2.2 | |
| Follicular Fluid (lead follicle) | 0.07 ± 0.01 | |
DISCUSSION
This report describes the first longitudinal study of the levels and temporal patterns of circulating ANGPT-1 and ANGPT-2 over the course of the reproductive cycle and pregnancy in primates. We were able to demonstrate that both ANGPT-1 and ANGPT-2 are present in macaque and human serum, as well as in human follicular fluid. Although detectable, the concentration of ANGPT-1 and -2 remained invariant throughout the natural menstrual cycle, following a controlled ovulation protocol and following the removal of the corpus luteum or ovaries in macaques. Moreover, circulating ANGPT levels remained unchanged throughout a controlled ovarian stimulation protocol in macaques (not shown) and women. The ANGPT1:2 ratio was maintained at greater than 1 among these study groups.
That circulating ANGPT levels did not change during the selection, maturation and ovulation/luteinization of the dominant follicle, nor during recruitment of multiple large antral follicles in COS protocols, suggests that the ovarian structures are not a primary source of these angioregulatory factors in the bloodstream. Also, since ANGPT levels did not change during the luteal phase, the corpus luteum must not contribute significantly to their presence in the circulation. This premise is supported by evidence that blood levels of ANGPT-1 and -2 did not change in the 24-48 hours following ablation of the corpus luteum or removal of the ovaries. Since ANGPT mRNA expression and protein can be detected in cells of the growing and ovulatory follicle (5;6), and corpus luteum (7;9;10), the ANGPTs of ovarian origin must be acting primarily, if not exclusively, as local factors. In addition, our observation that the ratio of ANGPT1:2 in follicular fluid can be very different from that in the blood indicates that circulating ANGPT levels typically do not reflect their synthesis or actions in the ovary.
Notably, the ANGPTs were also detectable in the circulation during pregnancy, but there was a marked decrease in the ANGPT1:2 ratio by mid-to-late gestation in macaques. The reversal from a >1 ratio during the ovarian cycle to ≪1 in pregnancy was due to increasing levels of ANGPT-2, as ANGPT-1 concentrations remained unchanged during this interval. These results extend and support our earlier findings (19) of a significant rise in ANGPT-2 levels beginning 30 days after hCG administration in women following IVF-ET protocols that result in pregnancy. ANGPT-2 levels were similar between women who became pregnant during COS cycles versus those using donor eggs, suggesting again, that the corpus luteum is not the major source of ANGPT circulating in pregnancy. Moreover, since elevated levels occur after the luteal-placental shift (19) and continue in mid-to-late gestation (current study), it is most likely of placental origin (24;25). The recent observation that ANGPT-2 levels decline after parturition (26) supports this hypothesis. Our findings in monkeys and women are consistent with an evaluation of women with pre-eclampsia that reported elevated ANGPT-2 levels in healthy controls during pregnancy compared to nonpregnancy (26). In contrast, Loukovaara (27) et al, reported decreasing levels of ANGPT-2 from the first to third trimester in pregnant women with and without Type 1 diabetes mellitus. The reason for the discrepancy, e.g., assay or population differences, remains unknown.
The factors responsible for the marked change in ANGPT1:2 ratio during pregnancy are unclear. Presuming of placental origin, changes in ANGPT-1 versus -2 levels could reflect differential control of their expression/synthesis by local factors, or their differential production by tissue compartments. Notably, Albrecht and colleagues (28) observed diverse effects of androgen/estrogen on VEGF and ANGPT expression in the villous placenta of baboons leading to decreased ANGPT-1, without altering ANGPT-2, mRNA levels. Johnson et al (29) also observed time-dependent changes in estrogen-stimulation of ANGPT-1 versus -2 mRNA expression in the ovine endometrium. Others have emphasized a cell-specific manner of regulated expression, with ANGPT-1 mRNA and protein expression predominant in the baboon synctiotrophoblast and levels declining with advancing gestation; in contrast, ANGPT-2 expression was greatest in the villous stromal cells and did not decrease in late gestation (24). Dunk et al (25) noted that ANGPT-2 was expressed throughout the villous core in human term placenta, whereas ANGPT-1 was restricted to paravascular tissues in primary stem villi. These authors speculate that the different temporal and special expression of ANGPT-1 and -2 reflects different roles in the development of the trophoblast, independent of their well-established roles in angiogenesis.
The physiologic importance of elevated circulating ANGPT-2, and/or a significant decrease in the ANGPT1:2 ratio during gestation is unknown. Since the early rise in ANGPT-2 is not associated with increasing levels of soluble Tie-2 receptor (19), much of the ANGPT-2 circulating in pregnancy may not be bound and hence is free for bioactivity. In contrast, although total VEGF-A levels increased in the luteal phase and remained appreciable during the first trimester in pregnant women, the concentration of unbound/“free” VEGF declined after 30 days. This drop corresponded to a 20-40-fold increase in soluble VEGF-R1, but not soluble VEGF-R2, resulting in decreased levels of bioactive VEGF-A in the circulation (14). Based on proposed models of VEGF-A, ANGPT-1 and ANGPT-2 action in vascular development (8), the predominance of the endogenous Tie2 antagonist ANGPT-2 in the relative absence of VEGF-A would be expected to destabilize blood vessels. Since major vascular remodeling and growth occurs in the placenta (30), and circulating VEGF and ANGPTs are likely of placental origin, their presence in the maternal circulation may be secondary to their synthesis and actions in the fetal-placental unit (25;30). Whether there are other VEGF/ANGPT-responsive vascular beds in women that are influenced by the changes in circulating VEGF or ANGPTs during pregnancy requires further investigation.
Importantly, elevated levels of circulating VEGF-A (14;16), as well as VEGF in the follicle (18) and ascites fluid (31), are proposed to be a major etiologic factor in the vascular dysfunction associated with ovarian hyperstimulation syndrome. This role is purportedly due to the ability of VEGF to increase vascular permeability or “leakage” (32;33). Studies also proposed that the agonist ANGPT-1 (34), and its antagonist ANGPT-2 (35), can influence microvascular permeability within the context of promoting vessel stabilization or destabilization, respectively. Although the current study did not observe changes in ANGPT levels in women during COS cycles, none of these samples were collected from patients developing OHSS symptoms. It remains to be determined if the level or ratio of ANGPTs in the circulation or ovary is altered or plays a role in early- or late-onset OHSS (36). However, the low ANGPT1:2 ratio observed in follicular fluid from COS protocols would be consistent with “leaky” follicles, and warrants further investigation.
In summary, this study documents the presence of ANGPT-1 and ANGPT-2 in primate and human serum, as well as in human follicular fluid. The concentration of ANGPT-1 and ANGPT-2 remained invariant, and the ANGPT1:2 ratio was >1 over the course of the natural menstrual cycle, following a controlled ovulation protocol and following the removal of the corpus luteum or ovaries in macaques, as well as throughout a controlled ovarian stimulation protocol in women. These findings suggest that the ovary is not a major contributor to the ANGPTs in circulation. It is more likely that the ANGPTs of ovarian origin serve as local factors. Notably, circulating levels of ANGPT-2 markedly increased and thus, the ANGPT1:2 ratio decreased to ≪1 by mid-gestation in macaques. Although likely placental in origin, the physiologic importance of this is unknown. Further studies are needed to investigate the role of the ANGPTs in circulation during pregnancy and their local actions in the ovary during natural and COS cycles.
Acknowledgments
The authors acknowledge the valuable resources and skills provided by the animal care technicians and surgical department, Division of Animal Resources, and the assay technicians, Endocrine Services Laboratory, ONPRC, OHSU. A special thanks to Ms. Carol Gibbins and Traci Lea for the preparation and submission of this research article.
Financial Support: This study was supported by National Institute of Health U54 HD 018185 (Project 3) and RR00163.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hazzard TM, Stouffer RL. Angiogenesis in ovarian follicular and luteal development. In: Arulkumaran S, editor. Clinical Obstetrics & Gynaecology Angiogenesis in the Female Reproductive Tract. London: Bailliere Tindall; 2000. pp. 883–900. [DOI] [PubMed] [Google Scholar]
- 2.Fraser HM, Wulff C. Angiogenesis in the corpus luteum. Reprod Biol Endocrinol. 2003;1:88. doi: 10.1186/1477-7827-1-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser HM, Duncan WC. Vascular morphogenesis in the primate ovary. Angiogenesis. 2005;8:101–16. doi: 10.1007/s10456-005-9004-y. [DOI] [PubMed] [Google Scholar]
- 4.Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril. 2000;74:429–38. doi: 10.1016/s0015-0282(00)00670-1. [DOI] [PubMed] [Google Scholar]
- 5.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 6.Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999;5:1115–21. doi: 10.1093/molehr/5.12.1115. [DOI] [PubMed] [Google Scholar]
- 7.Hazzard TM, Christenson LK, Stouffer RL. Changes in expression of vascular endothelial growth factor and angiopoietin -1 and -2 in the macaque corpus luteum during the menstrual cycle. Mol Hum Reprod. 2000;6:993–8. doi: 10.1093/molehr/6.11.993. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 9.Wulff C, Wilson H, Largue P, Duncan WC, Armstrong DG, Fraser HM. Angiogenesis in the human corpus luteum: localization and changes in angiopoietins, Tie-2, and vascular endothelial growth factor messenger ribonucleic acid. J Clin Endocrinol Metab. 2000;85:4302–9. doi: 10.1210/jcem.85.11.6942. [DOI] [PubMed] [Google Scholar]
- 10.Sugino N, Suzuki T, Sakata A. Angiogenesis in the human corpus luteum: changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2005;90:6141–8. doi: 10.1210/jc.2005-0643. [DOI] [PubMed] [Google Scholar]
- 11.Tesone M, Stouffer RL, Borman SM, Hennebold JD, Molskness TA. Vascular endothelial growth factor (VEGF) production by the monkey corpus luteum during the menstrual cycle: isoform-selective mRNA expression in vivo and hypoxia regulated protein secretion in vitro. Biol Reprod. 2005;73:927–34. doi: 10.1095/biolreprod.105.039875. [DOI] [PubMed] [Google Scholar]
- 12.Xu F, Stouffer RL. Local delivery of angiopoietin-2 into the preovulatory follicle terminates the menstrual cycle in rhesus monkeys. Biol Reprod. 2005;72:1352–8. doi: 10.1095/biolreprod.104.037143. [DOI] [PubMed] [Google Scholar]
- 13.Xu F, Hazzard TM, Evans A, Charnock-Jones S, Smith S, Stouffer RL. Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception. 2005;71:239–48. doi: 10.1016/j.contraception.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Molskness TA, Stouffer RL, Burry KA, Gorrill MJ, Lee DM, Patton PE. Circulating levels of free and total vascular endothelial growth factor (VEGF)-A, soluble VEGF receptors-1 and -2, and angiogenin during ovarian stimulation in non-human primates and women. Hum Reprod. 2004;19:822–30. doi: 10.1093/humrep/deh132. [DOI] [PubMed] [Google Scholar]
- 15.Artini PG, Fasciani A, Monti M, Luisi S, D’Ambrogio G, Genazzani AR. Changes in vascular endothelial growth factor levels and the risk of ovarian hyperstimulation syndrome in women enrolled in an in vitro fertilization program. Fertil Steril. 1998;70:560–4. doi: 10.1016/s0015-0282(98)00221-0. [DOI] [PubMed] [Google Scholar]
- 16.Abramov Y, Barak V, Nisman B, Schenker JG. Vascular endothelial growth factor plasma levels correlate to the clinical picture in severe ovarian hyperstimulation syndrome. Fertil Steril. 1997;67:261–5. doi: 10.1016/S0015-0282(97)81908-5. [DOI] [PubMed] [Google Scholar]
- 17.Pau E, Alonso-Muriel I, Gomez R. Plasma levels of soluble vascular endothelial growth factor receptor-1 may determine the onset of early and late ovarian hyperstimulation syndrome. Hum Reprod. 2006;21:1453–60. doi: 10.1093/humrep/del005. [DOI] [PubMed] [Google Scholar]
- 18.Neulen J, Wenzel D, Hornig C, Wunsch E, Weissenborn U, Grunwald K, et al. Poor responder-high responder: the importance of soluble vascular endothelial growth factor receptor 1 in ovarian stimulation protocols. Hum Reprod. 2001;16:621–6. doi: 10.1093/humrep/16.4.621. [DOI] [PubMed] [Google Scholar]
- 19.Molskness TA, Stouffer RL, Burry KA, Gorrill MJ, Lee DM, Patton PE. Circulating levels of total angiopoietin-2 and the soluble Tie-2 receptor in women during ovarian stimulation and early gestation. Fertil Steril. 2006;86:1531–3. doi: 10.1016/j.fertnstert.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation (COv) of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod. 2003;18:2257–63. doi: 10.1093/humrep/deg467. [DOI] [PubMed] [Google Scholar]
- 21.Duffy DM, Chaffin CL, Stouffer RL. Expression of estrogen receptor a and b in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology. 2000;141:1711–7. doi: 10.1210/endo.141.5.7477. [DOI] [PubMed] [Google Scholar]
- 22.Wolf DP, VandeVoort CA, Meyer-Haas GR, Zelinski-Wooten MB, Hess DL, Baughman WL, et al. In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod. 1989;41:335–46. doi: 10.1095/biolreprod41.2.335. [DOI] [PubMed] [Google Scholar]
- 23.Weston AM, Zelinski-Wooten MB, Hutchison JS, Stouffer RL, Wolf DP. Developmental potential of embryos produced by in-vitro fertilization from gonadotrophin-releasing hormone antagonist-treated macaques stimulated with recombinant human follicle stimulating hormone alone or in combination with luteinizing hormone. Hum Reprod. 1996;11:608–13. doi: 10.1093/humrep/11.3.608. [DOI] [PubMed] [Google Scholar]
- 24.Babischkin JS, Suresch DL, Pepe GJ, Albrecht ED. Differential expression of placental villous angiopoietin-1 and -2 during early, mid and late baboon pregnancy. Placenta. 2007;28:212–8. doi: 10.1016/j.placenta.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunk C, Shams M, Nijjar S, Rhaman M, Qiu Y, Bussolat B, et al. Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development. Am J Pathol. 2000;156:2185–99. doi: 10.1016/S0002-9440(10)65089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirokoshi K, Maeshima Y, Kobayashi E. Elevated serum sFlt-1/Ang-2 ratio in women with preeclampsia. Nephron Clin Pract. 2007;106:c43–c50. doi: 10.1159/000101483. [DOI] [PubMed] [Google Scholar]
- 27.Loukovaara S, Immonen I, Koistinen R. Angiopoetic factors and retinopathy in pregnancies complicated with type1 diabetes. Diabetic Med. 2004;21:697–704. doi: 10.1111/j.1464-5491.2004.01235.x. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht ED, Babischkin JS, Pepe GJ. Regulation of placental villous angiopoietin-1 and -2 expression by estrogen during baboon pregnancy. Mol Reprod Dev. 2008;75:504–11. doi: 10.1002/mrd.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Effects of estradiol-17beta on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes. Endocrine. 2006;30:333–42. doi: 10.1007/s12020-006-0012-5. [DOI] [PubMed] [Google Scholar]
- 30.Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27:535–9. doi: 10.1016/j.placenta.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 31.McClure N, Healy DL, Rogers PAW, Sullivan J, Beaton L, Haning RV, Jr, et al. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. The Lancet. 1994;344:235–6. doi: 10.1016/s0140-6736(94)93001-5. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez C, Marti-Bonmati L, Novella-Maestre E. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab. 2007;92:2931–7. doi: 10.1210/jc.2007-0409. [DOI] [PubMed] [Google Scholar]
- 33.Ovarian Hyperstimulation Syndrome: Epidemiology Pathophysiology, Prevention and Management. New York: Cambridge University Press; 2006. [Google Scholar]
- 34.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 35.Nag S, Papneja T, Venugopalan R, Stewart DJ. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood-brain barrier breakdown. Lab Invest. 2005;85:1189–98. doi: 10.1038/labinvest.3700325. [DOI] [PubMed] [Google Scholar]
- 36.Mathur RS, Akande AV, Keay SD, Hunt LP, Jenkins JM. Distinction between early and late ovarian hyperstimulation syndrome. Fertil Steril. 2000;73:901–7. doi: 10.1016/s0015-0282(00)00492-1. [DOI] [PubMed] [Google Scholar]


