Abstract
The discovery of brain tumor-derived cells (BTSC) with the properties of stem cells has led to the formulation of the hypothesis that neural stem cells could be the cell of origin of primary brain tumors (PBT). In this review we present the most common molecular changes in PBT, define the criteria of identification of BTSC and discuss the similarities between the characteristics of these cells and those of the endogenous population of neural stem cells (NPCs) residing in germinal areas of the adult brain. Finally, we propose possible mechanisms of cancer initiation and progression and suggest a model of tumor initiation that includes intrinsic changes of resident NSC and potential changes in the microenvironment defining the niche where the NSC reside.
1. Primary brain tumors: an overview
Primary Brain Tumors (PBT) are a heterogeneous group of malignancies that originate and reside within the brain, in contrast to metastatic brain tumors that originate from a primary cancer outside the central nervous system (CNS) and spread to the brain. Gliomas are the most common group of PBT. According to the CBTRUS 2007–2008 report, the incidence of gliomas is 16.5 cases per 100,000 persons/year in the US. This translates to approximately 51,410 newly diagnosed cases in the US per year (CBTRUS, 2008). Gliomas represent a wide spectrum of malignancies ranging from slow growing to highly aggressive tumors. On the basis of their histological features, the World Health Organization (WHO) classifies gliomas into four grades: grade I (pilocytic astrocytoma), grade II (diffuse astrocytoma), grade III (anaplastic astrocytoma), and grade IV (glioblastoma multiforme, GBM) (Louis et al., 2007). The latter two grades are considered high-grade gliomas or “malignant gliomas” and are associated with poor prognosis. In particular, GBMs accounting for 50% of PBT have a 5 year survival rate less than 5% and a median survival rate of approximately 14 months (Stupp et al., 2005).
One of the first treatments for GBM consisted in surgical resection of the tumoral mass followed by focal external beam radiation (Salcman et al., 1980). Subsequently, several studies reported significant survival benefits of combining systemic chemotherapy with alkylating agents like nitrosurea (Steward et al., 2002), and oral alkylating agents such as temozolomide (Brada et al., 2001). In addition, alkylating agents such as bis-chloronitrosourea (BCNU, also known as carmustine) have been delivered in the affected brain region by placing a dime-sized biodegradable polymer wafers at the time of surgical resection of the GBM (Westphal et al., 2003). Despite the attempts to combine surgery, radiation and chemotherapy, high grade gliomas recur in more that 90% of cases, usually within 2 cm of the original site, and 10–20% may develop new lesions at distant sites (Brada et al.,2001). This enormous challenge in neuro-oncology has spurred the search for new findings that could account for the resilience of GBM cells to the most aggressive form of treatment and explain the high recurrence rate.
Recent developments in neuro-oncology have contributed to extend our knowledge of GBM and are likely to provide a critical framework for future therapeutic strategies. First, the classification of GBM into two categories, based on amplification and mutation of different genes (Ohgaki and Kleihues, 2007) and on the characterization of molecular pathways (Figure 1), has opened new venues to targeted therapies, based on the individual genetic signature of the tumors (Dey, 2009). Second, the existence of cancer stem cells (CSC) prompted focusing on searching for the identification of Brain Tumor Derived Stem Cells (BTSC). In this review, we will summarize important concepts pertinent to BTSC and analyze similarities between CSC derived from brain tumors and neural stem cells. We shall consider intracellular pathways modulating the proliferative and differentiative state of NSC and the extracellular signals affecting their growth and self-renewal. The overall idea is that recurrent GBM might arise from oncogenic transformation of endogenous NSC and changes in the local environment (i.e. niche).
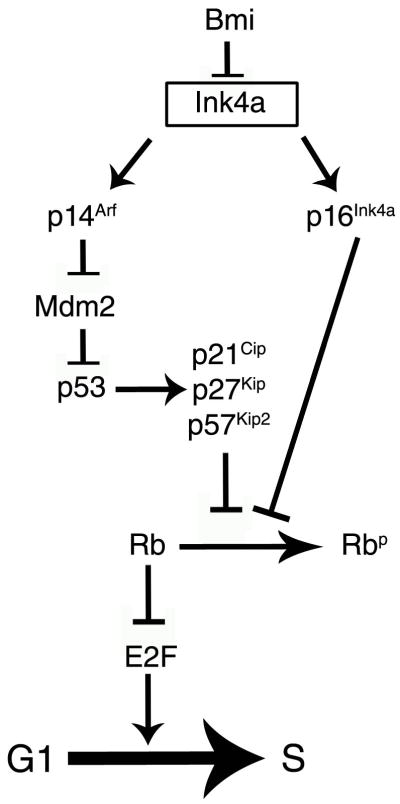
Figure 1. Schematic relationship of the most frequent genetic deletions, mutations and amplifications detected in glioblastomas.
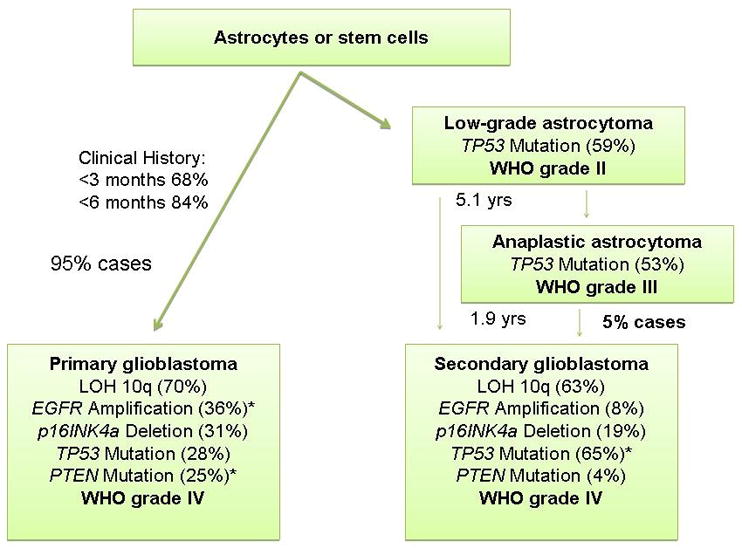
The relative frequency is listed in parenthesis.
2. Cancer stem cells, neural stem cells, and brain tumor stem cells
The search for new treatments for the cure of glioblastomas is strictly related to the search for the tumor cells of origin, since only the identification of these cells would guarantee complete eradication of the neoplasia. At least two main cellular mechanisms have been proposed: either de-differentiation of lineage-specified progenitors and mature astrocytes or transformation of the endogenous neural stem cell (NSC) population.
The concept of “de-differentiation” was based on the observation that PBT exhibit marked phenotypic heterogeneity, being composed of cells expressing both undifferentiated and differentiated markers. Indeed, both oligodendrogliomas and astrocytomas show characteristic expression of antigens that have been typically associated with their progenitors; for oligodendrogliomas NG2 and PDGF receptor alpha (Shoshan et al., 1999), Olig-1 and Olig-2 (Bouvier et al., 2003). In addition, it has been clearly shown that the tendency of gliomas to become more aggressive is associated with progressive “de-differentiation”, the expression of markers for undifferentiated cells and the down-regulation of markers of the differentiated state, such as glial fibrillary protein (GFAP) (Schiffer, 1986).
Since glial progenitors are quiescent in the adult brain (Noble and Mayer-Proschel, 1997), the probability that GBM could originate from de-differentiation of this adult progenitor population or from de-differentiation of astrocytes implies the acquisition of a proliferative phenotype possibly due to changes in the local environment (i.e. increased expression of growth factors) or in the cells (i.e. up-regulation of receptors, expression of receptor variants) (Fig. 2). However, an overwhelming literature favors the concept of BTSC generation from the oncogenic transformation of neural stem cells (NSC) residing in the subventricular zone (SVZ), rather than from lineage committed progenitors or de-differentiated astrocytes (experimental findings reviewed under forth heading below). The second hypothesis will be discussed extensively throughout the review.
Figure 2. Potential mechanisms of oncogenic transformation of neural stem cells.
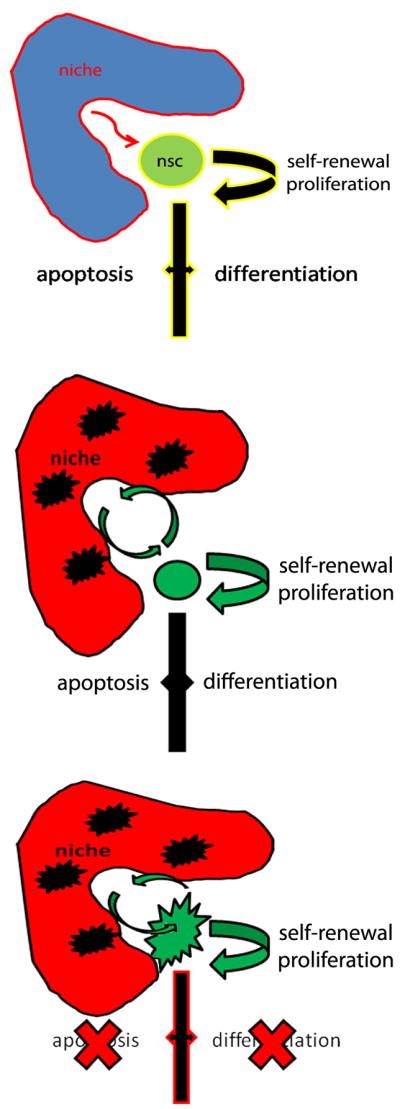
A: physiological conditions are characterized by a controlled response of the neural stem cell to signals derived from the niche and responsible for affecting proliferation, survival and differentiation. B: alterations of the niche may contribute to modifying the properties of the cells. C: intrinsic alterations of genes and microRNAs within the cells modify their properties and render them prone to mechanisms of amplification and de-differentiation.
The first report of neural stem cells in the adult brain occurred two decades after the identification of cells incorporated tritiated thymidine (Altman et al, 1967). The report of hormonally responsive growth of the ventral striatum in the brain of adult canaries was linked to the identification of new neurons generated from a rapidly cycling population of cells located within the subventricular zone (SVZ) (Goldman et al., 1983). The discovery of these neural stem cells (NSC) in the adult SVZ was validated by multiple groups (Doetsch et al., 1999, Mirzadeh et al., 2008, Johansson et al., 1999; Morrison et al., 1997). NSCs are pluripotent cells with the ability to differentiate into neurons, astrocytes and oligodendrocytes. They share cell surface markers with hematopoietic lineage cells (Uchida et al., 2000) and have the ability to grow in aggregates called neurospheres (Reynold et al. 1992) and the clonogenic property of self-renewal. NSCs also have the ability to engraft, migrate within the brain and differentiate into neuroblasts and other cell types when transplanted in the brain of nude mice (Tamaki et al., 2002; Liu et al., 2007).
In view of these discoveries, recent studies in cancer research have suggested that tumorigenesis occurs when a small group of cells with the properties of self-renewal and multipotentiality continues to proliferate and self-renew uncontrollably. The multipotential nature of the BTSC would explain the phenotypic heterogeneity of the tumors. The concept of cancer stem cells was originally proposed in the early 1990’s when it was suggested that few cells isolated from the blood of leukemia patients had the ability to proliferate and differentiate in vivo (Bonnet et al., 1993). Subsequently, CSC were identified in solid malignancies, including breast (Al-Haji et al., 2003), pancreas (Esposito et al., 2002), prostate (Dahlstrand et al.1992), head and neck (Prince, et al., 2007) and colon (Ricci-Vitiani et al., 2007) cancers.
A subpopulation of brain tumor derived cells shared several properties with the NSC (Hemmati et al., 2003; Ignatova et al 2002), hence the name Brain Tumor Stem Cells (BTSC), a concept that had been introduced earlier by Rosemblum et al (1982). The presence of BTSC in heterogenous tumors of neuroepithelial tissue was documented and reported by two independent groups (Singh et al, 2003 and 2004; Galli et al. 2004) and then confirmed by many others. Although the relationship of BTSC with tumor resistance and clinical outcome remains controversial, a better understanding of their properties and the potential mechanisms leading to their generation will be further discussed below.
3. Brain Tumor Stem Cells: current identification methodology and its limitation
The identification of BTSC in glioblastoma was originally reported by several independent investigators using distinct experimental approaches (Ignatova et al., 2002; Singh et al, 2004, Galli et al., 2004). Singh et al (2004) isolated BTSC using cell sorting techniques based on the expression of the cell surface marker CD133 [see below for explanation of CD abbreviation], while Ignatova et al (2002) and Galli et al (2004) identified BTSC from tumor samples, based on their ability to form neurospheres in vitro.
The identification of CD133 immunoreactive cells in brain tumors and consequent characterization of them as BTSC (Singh et al, 2003) was based on the fact that the glycosylated epitope of the CD antigen AC133 appeared to be restricted to stem cells (Uchida, 2000). The abbreviation CD derives from “Cluster of Differentiation” or “Cluster of Designation” and refers to a protocol used for the identification and investigation of cell surface molecules on human cells. CD133, formerly known as AC133 or human prominin (PROML-1),is a member of a family of cell surface proteins with 5 transmembrane domains. It was originally discovered as the equivalent to mouse prominin, a pentaspan transmembrane glycoprotein of murine neuroepithelial stem cells located in plasma membrane protrusions (Fargeas, 2003). CD133+ cells in brain tumors were reported to be highly tumorigenic after xenotransplantation in non-obese diabetic/severe combined immunodeficient (SCID) mice (Singh, 2004). These cells lacked the expression of neural differentiation markers and were characterized by multipotential properties since they retained the ability to differentiate in vitro into neurons and glia and form tumors identical to the tumor of origin after transplantation (Galli, 2004). The CD133+ population was also characteristically resistant to the alkilating agent carmustine (BCNU) (Kang et al., 2007) and also several other chemotherapeutic agents, including temozolomide, carboplatin, paclitaxel (taxol), and etoposide (VP16) (Salmaggi, 2006; Eramo, 2006) thereby strengthening the relationship betweeen chemoresistance and BTSCs. Interestingly CD133+ cells showed remarkable recovery after cessation of chemotherapy compared to CD133- cells (Eramo, 2006). Finally, CD133+ cells were shown to be resistant to radiation therapy, since irradiated mice with glioma xenografts had a number of CD133+ cells that was 3 to 5 fold higher than untreated mice with xenografts (Bao et al., 2006).
Despite the recent surge of interest in CD133+ BTSC, the clinical significance of this subpopulation remains unclear. To date a correlation between CD133+ cells and clinical outcome has been reported by a few groups. For instance, it was reported than when compared with newly diagnosed GBM, recurrent GBM in the same patients had significantly higher level of CD133+ cells (Liu et al. 2006). More recently, Zeppernick and colleagues showed by multivariate analysis that both the proportion of CD133+ cells and their topological organization in clusters were significant prognostic factors for adverse progression-free survival and overall survival independent of tumor grade, extent of resection, or patient age in 95 gliomas of various grade and histology (Zeppernick, 2008). Furthermore, the proportion of CD133+ cells was an independent risk factor for tumor re-growth and time to malignant progression in WHO II and III (Zeppernick, 2008). The expression of CD133 was inversely correlated with patient survival, using flow-cytometry of cells isolated from 6 low-grade and 17 high grade glioma specimens (Rebetz et al. 2008). Finally, a perspective study on 44 consecutive patients with GBM supported the concept that presence of CD133+ cells should be regarded as an important prognostic factor of disease progression and poor clinical outcome (Pallini et al., 2008).
Although provocative and exciting, these results have been recently challenged by studies addressing the validity of the use of the neurosphere assay as criterium of identification (Cheng et al. 2009). One of the concerns was the fact that the assay could only retrospectively, but not prospectively infer the presence of BTSC. In addition, it was noted that the ability of human brain tumor cells to generate neurospheres is very variable among distinct tumor samples. (Chen, 2009).
In addition, experimental evidence within the past few years has clearly indicated that the absence of CD133 does not preclude the ability of tumor cells to generate heterogeneous tumors in vivo (Wang et al., 2008; Beier et al. 2007; Joo et al. 2008;Ogden et al. 2008).
Therefore, additional characterization of the functional role of CD133 expression in BTSC is needed to better understand the mechanism underlying brain tumor initiation, progression and possibly resistance to treatment.
4. Additional evidence supporting the hypothesis that glial tumors originate from neural stem cells
The studies discussed in the previous section suggested the possibility that NSCs could be regarded as cells of origin of glial tumors. Besides the expression of CD133 several additional markers were shared by BTSC and SVZ cells, including the intermediate filament protein nestin (Dahlstrand et al., 1992; Toda et al., 2001), the transcription factor Sox2 (Ellis et al., 2004;Gangemi et al., 2009; Favaro et al., 2009) and the RNA binding protein Musashi (Kong et al., 2008; Strojink et al., 2007).
Direct evidence for the importance of SVZ cells in the genesis of glial tumors was provided by studies in rodents using viral-mediated transfer of oncogenes (Holland et al., 2000; Marumoto et al., 2009). Two very powerful approaches were used to develop elegant models of gene delivery using viral vectors. One model included the use of avian viral vectors expressing activated forms of signaling intermediary (i.e. Ras mutant or Akt) in transgenic mice expressing the receptor for avian virus in specific cell populations (i.e. GFAP+; nestin+) (Holland et al., 2000). The infection of these transgenic mice with viral vectors targeting cells in the SVZ, resulted in the onset of spontaneous gliomas, thereby suggesting that cell-autonomous changes in this germinal zone are sufficient for oncogenic transformation (Holland et al., 2000). The other model employed the Cre-lox technology and lentiviral vectors expressing mutant Ras or constitutively active Akt were targeted to the GFAP+ population of cells residing within germinal zones (i.e. SVZ and hippocampus), of p53 heterozygous mice and will be discussed later (Marumoto et al., 2009). Additional evidence that SVZ cells may serve as cell of origin for glioblastomas was suggested by studies on the offspring of pregnant rats that were exposed to the alkylating agent N-ethyl-N-nitrosourea (ENU) during late-gestation (Zook et al., 2000). The rat pups were born and developed normally until 4 months of age when glial tumors spontaneously arose (Recht et al., 2003). A detailed morphological analysis of the SVZ in these developing young animals revealed progressive abnormalities of the proliferative and migratory behavior of SVZ cells preceding the frank onset of gliomas (Recht et al., 2003). Prenatal exposure to ENU, was also responsible for the induction of GBM in mice lacking the tumor suppressor gene p53 (Gil-Perotin et al., 2006), thereby supporting the concept that tumor initiation is the result of multiple hits to cells that are prone to changes in self-renewal and proliferation.
5. Extrinsic signals modulating proliferation, self-renewal and differentiation of endogenous neural stem cells
We have previously mentioned that the ability of tumor-derived cells to proliferate and form neurospheres in vitro is directly linked to the ability to form tumor in vivo (Galli et al., 2004) thereby suggesting that the same molecular pathways modulating proliferation and self-renewal in physiological conditions, might also affect tumorigenesis (Fig. 2A). Pathways that have been involved in glioma formation, based on their effect on the behavior of endogenous NSCs include the mitogens EGF and PDGF, the morphogen Shh, Notch. Based on the distinctive pattern of expression of receptors and ligands within endogenous NSC and their niche and on the presence of genetic variants and co-expression in CD133+ cells within GBM, it is likely that different pathways may play a differential role in tumor initiation or progression or both.
The two mitogens EGF and PDGF, for instance, both signal via activation of tyrosine kinase receptors, and modulation of cell-proliferation (Ekstran et al., 1991; Wikstrand et al., 1997).
PDGF and PDGFR are primarily expressed in low grade tumors, but not in CD133+ cells (Martinho et al, 2008). Based on these findings and on the detection of tumors in over-expression studies conducted in rodents (Shih et al., 2004; Assanah et al., 2006), it has been proposed that PDGF might play an important role in tumor initiation, but may be not involved in mechanism of recurrence or resistance to treatment.
In contrast EGFR is detected on tumor cells, including CD133+ BTSCs (Li et al., 2009; Murat et al., 2008). Because proliferation of these cells might occur independently of the presence of ligand, (Kelly et al., 2009). It has been proposed that BTSC cells may be selected on the basis of a proliferative advantage and constitutive mechanism of activation of mitogenic pathways. Consistent with this explanation, specific EGFR genetic variants lacking exons 2 to 7 have been identified in GBM. The most commont variant of EGFR in GBM is composed only of exons 1 and 8 and encodes a constitutively active form of the receptor.
Of the extracellular signals involved in the modulation of the self-renewal properties of NSCs, Shh and its downstream effectors Gli have been reported in GBMs (Ruiz I Altaba et al., 2004; Stecca et al., 2005; Ehtesham et al., 2007; Becher et al., 2008). Intriguingly, Shh has also been detected in CD133+ of GBM (Clement et al., 2007). In physiological conditions, Shh has been shown to modulate the properties of NSCs (Ahn and Joyner 2005). The discovery of active Shh signaling pathways in glioma (Xu et al., 2008) and the detection of these molecules in CD133+ cells has led to the hypothesis that treatment with Shh inhibitors could be an effective form of treatment. Indeed, treatment with cyclopamine decreases tumor growth (Bar et al., 2007). However, it is important to note that downstream Shh signaling molecules (i.e. Gli) have been observed only in low grade gliomas, rather than in more aggressive glioblastomas (Becher et al., 2008) and the growth of these cells appears to be dependent on the presence of the ligand (Ehtesham et al., 2007). Together these studies suggest a dual role of Shh. During the initiation phase Shh expression within the micro-environmental niche might play an instrumental role for the induction of a hyperproliferative phenotype. If these changes in the niche are not accompanied by additional mutations within the cells, the NSC cell may retain the ability, at least in part, to differentiate and activate apoptotic pathways (Fig. 2B). However, intrinsic changes that might alter the cellular responsiveness to extracellular signals might render a population of cells independent from extracellular control. Thus the two events: intrinsic transformation of NSCs and modifications of the extracellular niche might contribute to the induction and/or recurrence of brain tumors (a schematic representation is shown in Fig 2).
An additional signal that has been shown to potentiate the effect of Shh on NSC is the activation of the Notch pathway. The potential role of Notch in GBM has been suggested by gene expression profiling studies (Margareto et al., 2007) and by the detection of increased levels of Notch and its signaling intermediates in patient-derived samples and cell lines (Kanamori et al., 2007). Treatment of animals with Notch inhibitors decreases angiogenesis and tumor formation (Paris et al., 2005), thereby supporting the role of this pathway in maintaining the glioma cells in an undifferentiated proliferative state. An additional link between Shh and Notch in GBM has been recently proposed (Zhao et al., 2009). It was previously shown that N-Myc is downstream of Shh activation (Hatton et al., 2007; Oliver et al., 2003) and modulates the levels of the Notch ligand Dll3 (Zhao et al., 2009). The modulation of Dll3 expression by N-Myc is dependent on the activity of Huwe1, a ubiquitin ligase that is mutated in a small percentage of GBMs. In physiological conditions Huwe 1 is active in cortical progenitors and this allows for the degradation of N-Myc and the down-regulation of Dll3. In pathological conditions, in contrast, mutations that interfere with Huwe1 activity allow the accumulation of N-Myc and its downstream target gene Dll3, thereby potentiating the synergism between Shh and Notch.
6. Cell-autonomous alterations of differentiation and self-renewal within neural stem cells as predisposition to cancerous transformation
A critical property of tumor cells is the loss of the ability to properly respond to the environmental cues due to changes that are intrinsic to the cells. Our current understanding of the mechanisms regulating proliferation and clonogenic properties of the cells has recently expanded from the analysis of genetic deletions and amplifications, mainly involving cell cycle regulators, to the discovery of powerful networks of epigenetic modulators, especially microRNAs. It is anticipated that these new discoveries will have a tremendous impact in shaping new concepts of tumor-based signature therapy.
Genes involved in regulating Cell Cycle: Rb pathway and p53
A critical cell cycle regulator that is often mutated during glial tumor progression is p53. Loss of p53 per se does not induce spontaneous glial tumors (Donehower et al., 1992; Philipp-Staheli et al., 2004), but periventricular areas of cellular hyperplasia in the adult SVZ (Gil-Perotin et al., 2006). These hyperplastic regions were highly reminiscent of the “microtumors” around the ventricles that were described at the early stages of glioma formation in rats prenatally exposed to mutagens (Lantos and Pilkington, 1979). Transformation of adult-derived p53−/− SVZ cells occurred only when mutant Ras was transduced (Gil-Perotin et al., 2006). These findings were recently supported by in vivo studies in p53 heterozygous mice (Marumoto et al., 2009). Using loxP engineered lentiviral vectors and GFAP-dricen Cre recombinase, a recent study elegantly shows that overexpression of mutant Ras or Akt is capable of inducing glial tumors only in GFAP+ SVZ cells, but not in cortical GFAP+ astrocytes, suggesting that additional intrinsic features of the SVZ cells play a critical role (Marumoto et al., 2009). Therefore, GBM formation in the absence of p53 involves the synergism with other pathways including increased PDGF signaling (Hesselager et al., 2003), prenatal exposure to ENU (Oda et al., 1997; Leonard et al., 2001 Katayama et al., 2005) or Ras activation (Reilly et al, 2000).
Altered expression of the retinoblastoma protein in contrast (Hilton et al., 2002 and 2004) has been associated with decreased survival and worse prognosis. In addition, the expression of several other genes within the Rb pathway have been found to be altered in GBMs. Genes encoding for positive regulators of the cell cycle (i.e. cyclins; E2F1, etc) have been shown to be amplified (Tamiya et al., 2001), while cell cycle inhibitors, including p27Kip1, p16 Ink4a/p14Arf have been shown to be down-regulated (Arifin et al., 2006; Hidaka et al., 2005). It was originally proposed that decreased p16INK4a levels were due to epigenetic regulation of expression, due to repression mediated by the polycomb protein Bmi (Valk-Lingbeek et al., 2004). Because the INK4A/Arf locus is genetically upstream of p53 (Fig. 3), it was proposed that Bmi predominantly acted by creating a proliferative advantage for cancer cell and facilitating self-renewal by repressing p16Ink4a (Bringmann et al., 2005). However recent studies have extended this concept and identified several additional genes whose expression is regulated by Bmi (Abdouh et al., 2009). They included several downstream effectors of the Notch pathway, transcription factors of the homeobox HOX family and additional genes involved in proliferation and survival. Since Bmi is part of the Polycomb Repressive Complex PRC1 that is able to recognize marks induced by the PRC2 complex, which includes the enzyme EZH2, it is not surprising that inhibition of Bmi or EZH2 reduced the clonogenic potential of the glioblastoma derived cells and increased survival after xenograph (Abdouh et al., 2009).
Figure 3.
Schematic representation of the cell cycle regulatory networks as discussed in the text.
Pluripotency genes and the microRNA network
Because self-renewal is a critical property that has been related to the tumorigenic properties of stem cells, several studies have focused on the regulatory networks modulating it. Sox2 is a transcription factor that is expressed in stem cells, NSC and in a population of mature neurons (Ellis et al., 2004). The function of Sox2 is cell context specific (reviewed Pevny and Nicolis 2009) and has been shown to modulate self-renewal and pluripotency of NSC (Fong et al., 2008). With Oct4, Nanog and Klf4 or c-Myc, Sox 2 is capable of inducing reprogramming of fibroblasts into induced pluripotent stem cells (Takahashi and Yamanaka, 2006). Therefore, it is not surprising that cMyc, Oct4 and Sox2 have all been implicated as regulators of self-renewal and clonogenic properties in NSC (Babaie et al., 2007; Wang et al., 2008; Fong et al., 2008). The detailed functional analysis of these critical transcription factors has uncovered the existence of important regulatory circuitry for Oct4 (Babaie et al., 2007), Sox2 (Fong et al., 2008) and Klf4 (Jiang et al., 2008). It was recently reported that Sox2 is expressed in all gliomas samples and both immunotherapy as well as silencing constructs specific for Sox2 have been proposed as therapeutic strategies (Schmitz et al., 2007; Gangemi et al., 2009). Indeed silencing of Sox2 decreases proliferation in vitro and counteracts tumor formation in nude mice after transplantation (Gangemi et al., 2009). Similarly Oct4 is expressed in gliomas (Du et al., 2009) and silencing approaches have been successful in decreasing proliferation and reducing tumor size (Du et al., 2009). One of the potential explanations for such remarkable effects on gliomas formation and expansion was recently provided by the identification of a network of regulatory microRNA, such as the cluster containing miR-371/372/373, that epigenetically control the levels of gene products involved in maintenance of stem cell properties (Laurent et al., 2008; Suh et al., 2004). An example is the microRNA miR-302, which is a down-stream target for Oct4 and Sox2 and has been shown to modulate the levels of cyclin D expression (Card et al., 2008). Finally the recent identification of miR-145 as Oct4 target involved in the repression of Sox2, Oct4 and Klf4 (Xu et al. 2009), has uncovered complex mechanisms of feedback regulation that might lead to a progression from undifferentiated, pluripotent state characterized by self-renewal to a differentiated and quiescent state (Chivukula and Mendell, 2009).
7. Concluding Remarks
BTSC can be defined as a small subpopulation of cancer cells with striking similarities to NSC including self-renewal, multi-potency and relative quiescence. It is becoming evident that BTSC are crucial players in PBT recurrence and treatment resistance. Thus, specifically targeting these cells might provide a novel tool over brain tumor progression and recurrence. Novel therapies targeting this small group of cells together with the tumor bulk might provide better success in treating this fatal disease.
In this review we discuss the hypothesis that tumor derived stem cells originate from a population of endogenous neural stem cells, rather than de-differentiation of committed progenitors or mature glial cells. We present recent advances in the field of epigenetic and genetic characterization of NSC cells in physiological conditions and during transformation provide a new framework for the identification of novel therapies. The overall idea is that the behavior of endogenous NSC in the adult brain is tightly regulated by ligand-activated signaling pathways allowing the coordination between production of new cells and their elimination due to differentiation or apoptosis. Transformation occurs when NSC cells begin accumulating genetic abnormalities, including the presence of gene deletions (i.e. p53), amplifications (i.e. Gli or Myc) or precise genetic variants (i.e. EGFRVIII) as well as epigenetic alterations (i.e. changes in DNA methylation, histone variants and micro-RNA). These events render the cells resilient to environmental signals and therapeutic management. Only a detailed characterization of the precise changes occurring within each tumor would allow the identification of targeted therapeutic agents.
Acknowledgments
Patrizia Casaccia acknowledges the support of NIH-NINDS R01NS42925-07 and R01NS052738-04
Isabelle Germano acknowledges the support of NIH/NCI 1RO1 CA129489-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 Sustains Human Glioblastoma Multiforme Stem Cell Renewal. J Neurosci. 2009;29:8884–8896. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult NSCs responding to Sonic hedgehog. Nature. 2005;437(7060):894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Pro Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic Examination of the Effects of Enriched Environment on the Rate of Glial Multiplication in the Adult Rat Brain. Nature. 1964;204:1161 – 1163. doi: 10.1038/2041161a0. [DOI] [PubMed] [Google Scholar]
- Alonso MM, Fueyo J, Shay JW, Aldape KD, Jiang H, Lee OH, Johnson DG, Xu J, Kondo Y, Kanzawa T, Kyo S, Bekele BN, Zhou X, Nigro J, McDonald JM, Yung WK, Gomez-Manzano C. Expression of transcription factor E2F1 and telomerase in glioblastomas: mechanistic linkage and prognostic significance. J Natl Cancer Inst. 2005;97(21):1589–600. doi: 10.1093/jnci/dji340. [DOI] [PubMed] [Google Scholar]
- Arifin MT, Hama S, Kajiwara Y, Sugiyama K, Saito T, Matsuura S, Yamasaki F, Arita K, Kurisu K. Cytoplasmic, but not nuclear, p16 expression may signal poor prognosis in high-grade astrocytomas. J Neuro-oncol. 2006;77(3):273–7. doi: 10.1007/s11060-005-9037-5. [DOI] [PubMed] [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26(25):6781–90. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–77. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB. Glioma stem ce;;s promote radioresistance. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, Katz AM, Edgar M, Kenney AM, Cordon-Cardo C, Blasberg RG, Holland EC. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68(7):2241–9. doi: 10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- Blum R, Nakdimon I, Goldberg L, Elkon R, Shamir R, Rechavi G, Kloog Y. E2F1 identified by promoter and biochemical analysis as a central target of glioblastoma cell-cycle arrest in response to Ras inhibition. Int J Cancer. 2006;119(3):527–38. doi: 10.1002/ijc.21735. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Bouvier C, Bartoli C, Aguirre-Cruz L, Virard I, Colin C, Fernandez C, Gouvernet J, Figarella-Branger D. Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J Neurosurg. 2003;99:344–50. doi: 10.3171/jns.2003.99.2.0344. [DOI] [PubMed] [Google Scholar]
- Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse, Ann. Oncol. 2001;12:259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, Hulsman D, Leung C, Arsenijevic Y, Marino S, van Lohuizen M. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005;19(12):1438–43. doi: 10.1101/gad.1299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, Archer TK. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28(20):6426–38. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBTRUS. Statistical report: Primary Brain Tumors in the United States, 2000–2004. 2008. [Google Scholar]
- Cheng JX, Liu BL, Zhang X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treatment Reviews. 2009;35:403–408. doi: 10.1016/j.ctrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Cheshire SH, Kalani MS, Yashar M, Lim M, Ailles L, Huhn SL, Weissman IL. A Neurosurgeon’s guide to stem cells, cancer stem cells, and brain tumor stem cells. Neurosurgery. 2009;65:237–250. doi: 10.1227/01.NEU.0000349921.14519.2A. [DOI] [PubMed] [Google Scholar]
- Chivukula RR, Mendell JT. Abate and switch: miR-145 in stem cell differentiation. Cell. 2009;137(4):606–8. doi: 10.1016/j.cell.2009.04.059. [DOI] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i, Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007 Jan 23;17(2):165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human center nervous system tumors. Cancer Research. 1992;52:5334–5341. [PubMed] [Google Scholar]
- Dey M, Ulasov IV, Lesniak MS. Virotherapy against malignant glioma stem cells. Cancer Letters. 2009 doi: 10.1016/j.canlet.2009.04.045. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y, Cao X, Ling EA, Hao A. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia. 2009;57(7):724–33. doi: 10.1002/glia.20800. [DOI] [PubMed] [Google Scholar]
- Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, Thompson RC, Cooper MK. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26(39):5752–61. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26(2–4):148–65. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Eramo A, Ricci-Vititani L, Zeuner A, Pallini R, Lotti F, Sette G. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- Esposito I, Kleeff J, Bischoff SC, Fuscher L, Collecchi P, Iorio M, Bevilacqua G, Buchler MW, Friess H. The stem cell factor-c-kit system and mast cells in human pancreatic cancer. Lab Invest. 2002;82:1481–1492. doi: 10.1097/01.lab.0000036875.21209.f9. [DOI] [PubMed] [Google Scholar]
- Fargeas CA, Huttner WB, Corbeil D. Nomenclature of prominin-1 (CD133) splice variants-an update. Tissue Antigens. 2007;69:602–606. doi: 10.1111/j.1399-0039.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri ALM, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis S. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nature Neuroscience advance. 2009 doi: 10.1038/nn.2397. online publication Published online. [DOI] [PubMed] [Google Scholar]
- Ferletta M, Uhrbom L, Olofsson T, Pontén F, Westermark B. Sox10 has a broad expression pattern in gliomas and enhances platelet-derived growth factor-B--induced gliomagenesis. Mol Cancer Res. 2007 Sep;5(9):891–7. doi: 10.1158/1541-7786.MCR-07-0113. [DOI] [PubMed] [Google Scholar]
- Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26(8):1931–8. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda e, Orfanelli U, Cipelletti B, Gritti A, DeVitis Simona, Fiocco Roberta, Foroni c, Dimenco F, Vescovi A. Isolation and Characterization of Tumorigenic, Stem-like Neural Precursors from Human Glioblastoma. Cancer Researc. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27(1):40–8. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- Gil-Perotin S, Verdugo JM, Li J, Marin-Husstege M, Soriano-Navarro M, Zindy F, Roussel M, Casaccia-Bonnefil P. Loss of p53 induces changes in the behaviour of subventricular zone cells: implications for the genesis of glial tumors. J Neurosci. 2006;26:1107–16. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Pro Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton BA, Knoepfler PS, Kenney AM, Rowitch DH, de Alborán IM, Olson JM, Eisenman RN. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66(17):8655–61. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser K, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T, Hama S, Shrestha P, Saito T, Kajiwara Y, Yamasaki F, Sugiyama K, Kurisu K. The combination of low cytoplasmic and high nuclear expression of p27 predicts a better prognosis in high-grade astrocytoma. Anticancer Res. 2009 Feb;29(2):597–603. [PubMed] [Google Scholar]
- Hilton DA, Penney M, Pobereskin L, Sanders H, Love S. Histological indicators of prognosis in glioblastomas: retinoblastoma protein expression and oligodendroglial differentiation indicate improved survival. Histopathology. 2004 Jun;44(6):555–60. doi: 10.1111/j.1365-2559.2004.01887.x. [DOI] [PubMed] [Google Scholar]
- Hilton DA, Penney M, Evans B, Sanders H, Love S. Evaluation of molecular markers in low-grade diffuse astrocytomas: loss of p16 and retinoblastoma protein expression is associated with short survival. Am J Surg Pathol. 2002;26(4):472–8. doi: 10.1097/00000478-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–7. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler VA. Human cortical glial tumors contain neural stem-like cells expressing atrogliala and neuronal marlers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Joo KM, Kim SY, Jiin X. Clinical and biological implications of CD133 positive and CD133 negative cells in glioblastomas. Lab invest. 2008;88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106(3):417–27. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant ccancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837–847. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- Katayama K, Ueno M, Yamauchi H, Nagata T, Nakayama H, Doi K. Ethylnitrosourea induces neural progenitor cell apoptosis after S-phase accumulation in a p53-dependent manner. Neurobiol Dis. 2005;18:218–225. doi: 10.1016/j.nbd.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes and Development. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Kelly JJ, Stechishin O, Chojnacki A, Lun X, Sun B, Senger DL, Forsyth P, Auer RN, Dunn JF, Cairncross JG, Parney IF, Weiss S. Proliferation of human glioblastoma stem cells occurs independently of exogenous mitogens. Stem Cell. 2009;27(8):1722–1733. doi: 10.1002/stem.98. [DOI] [PubMed] [Google Scholar]
- Kirla RM, Haapasalo HK, Kalimo H, Salminen EK. Low expression of p27 indicates a poor prognosis in patients with high-grade astrocytomas. Cancer. 2003;97(3):644–8. doi: 10.1002/cncr.11079. [DOI] [PubMed] [Google Scholar]
- Kong DS, Kim MH, Park WY, Suh YL, Lee JI, Park K, Kim JH, Nam DH. The progression of gliomas is associated with cancer stem cell phenotype. Oncol Rep. 2008;19(3):639–43. [PubMed] [Google Scholar]
- Lantos PL, Pilkington GJ. The development of experimental brain tumours. A sequential light and electron microscope study of the subependymal plate. I. Early lesions (abnormal cell clusters) Acta Neuropathol (Berl) 1979;45:167–175. doi: 10.1007/BF00702668. [DOI] [PubMed] [Google Scholar]
- Laurent LC, Chen J, Ulitsky I, Mueller FJ, Lu C, Shamir R, Fan JB, Loring JF. Comprehensive microRNA profiling reveals a unique human embryonic stem cell signature dominated by a single seed sequence. Stem Cells. 2008;26(6):1506–16. doi: 10.1634/stemcells.2007-1081. [DOI] [PubMed] [Google Scholar]
- Leonard JR, D’Sa C, Klocke BJ, Roth KA. Neural precursor cell apoptosis and glial tumorigenesis following transplacental ethyl-nitrosourea exposure. Oncogene. 2001;20:8281–8286. doi: 10.1038/sj.onc.1205024. [DOI] [PubMed] [Google Scholar]
- Li L, Dutra A, Pak E, Labrie JE, 3rd, Gerstein RM, Pandolfi PP, Recht LD, Ross AH. EGFRvIII expression and PTEN loss synergistically induce chromosomal instability and glial tumors. Neuro Oncol. 2009 Feb;11(1):9–21. doi: 10.1215/15228517-2008-081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yan X, Zeng Z, Tunici P, Ng H, Abdulkair IR. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67–75. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Act Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margareto J, Leis O, Larrarte E, Idoate MA, Carrasco A, Lafuente JV. Gene expression profiling of human gliomas reveals differences between GBM and LGA related to energy metabolism and notch signaling pathways. J Mol Neurosci. 2007;32(1):53–63. doi: 10.1007/s12031-007-0008-5. [DOI] [PubMed] [Google Scholar]
- Martinho O, Longatto-Filho A, Lambros MB, Martins A, Pinheiro C, Silva A, Pardal F, Amorim J, Mackay A, Milanezi F, Tamber N, Fenwick K, Ashworth A, Reis-Filho JS, Lopes JM, Reis RM. Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br J Cancer. 2009;101(6):973–82. doi: 10.1038/sj.bjc.6605225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto T, Tashiro A, Friedmann-Morvinsli D, Scadeng M, Soda Y, Gage FH, Verma IM. Development of a novel mouse glioma model using lentiviral vectors. Nat med. 2009;15(1):110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alverez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ. Regulatory Mechanisms Review in Stem Cell Biology. Cell. 1997:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, de Tribolet N, Regli L, Wick W, Kouwenhoven MC, Hainfellner JA, Heppner FL, Dietrich PY, Zimmer Y, Cairncross JG, Janzer RC, Domany E, Delorenzi M, Stupp R, Hegi ME. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008 Jun 20;26(18):3015–24. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Proschel M. Growth factors, glia and gliomas. J Neurooncol. 1997;35:193–209. doi: 10.1023/a:1005898228116. [DOI] [PubMed] [Google Scholar]
- Oda H, Zhang S, Tsurutani N, Shimizu S, Nakatsuru Y, Aizawa S, Ishikawa T. Loss of p53 is an early event in induction of brain tumors in mice by transplacental carcinogen exposure. Cancer Res. 1997;57:646–650. [PubMed] [Google Scholar]
- Ogden AT, Waziri AE, Lochhead RA. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–514. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Genetic Pathways to primary and secondary glioblastomas. The American Journal of Pathology. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, Wickramasinghe R, Scott MP, Wechsler-Reya RJ. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci U S A. 2003;100(12):7331–6. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallini R, Ricci-Vitiani L, Banna GL, Signore M, Lombardi D, Todaro M, Stassi G, Martini M, Maira G, Larocca LM, De Maria R. Cancer stem cell analysis and clinical outcome in patietns with glioblastoma multiforme. Clin Cancer Res. 2008;15:8205–12. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]
- Paris D, Quadros A, Patel N, Delle Donne A, Humphrey J, Mullan M. Inhibition of angiogenesis and tumor growth by beta and gamma-secretase inhibitors. Eur J Pharmacol. 2005;514(1):1–15. doi: 10.1016/j.ejphar.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebetz J, Tian D, Persson A, Widegren B, Salford LG, Englund E, Gisselsson D, Fan X. Glial progenitor-like phenotype in low grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS One. 2008;3:e1936. doi: 10.1371/journal.pone.0001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht L, Jang T, Savarese T, Litofsky NS. Neural stem cells and neuro-oncology: quo vadis? J Cell Biochem. 2003;88:11–9. doi: 10.1002/jcb.10208. [DOI] [PubMed] [Google Scholar]
- Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expression of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Rosemblum ML, Gerosa M, Dougherty DV, Reese C, Barger GR, Davis RL, Levin VA, Wilson CB. Age-related chemosensitivity of stem cells from human malignant brain tumors. Lancet. 1982;1:885–887. doi: 10.1016/s0140-6736(82)92154-7. [DOI] [PubMed] [Google Scholar]
- Ruiz i, Altaba A, Stecca B, Sánchez P. Hedgehog--Gli signaling in brain tumors:stem cells and paradevelopmental programs in cancer. Cancer Lett. 2004;204(2):145–57. doi: 10.1016/S0304-3835(03)00451-8. [DOI] [PubMed] [Google Scholar]
- Salcman M. Survival in glioblastoma: Historical perspective. Neurosurgery. 1980;7:435–439. doi: 10.1227/00006123-198011000-00001. [DOI] [PubMed] [Google Scholar]
- Schiffer D, Giordana MT, Germano I, Mauro A. Anaplasia and heterogeneity of GFAP expression in gliomas. Tumori. 1986;72:163–70. doi: 10.1177/030089168607200208. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK, Fussel M, Bachmann M, Rieber EP, Weigle B. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer. 2007;6(8):1293–301. doi: 10.1038/sj.bjc.6603696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64(14):4783–9. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- Shoshan Y, Nishiyama A, Chang A, Mork S, Barnett GH, Cowell JK, Trapp BD, Staugaitis SM. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci U S A. 1999;96:10361–6. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, Nakashima S, Kunisada T, Iwama T. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J Biol Chem. 2008;283(16):10958–66. doi: 10.1074/jbc.M704205200. [DOI] [PubMed] [Google Scholar]
- Stecca B, Ruiz i, Altaba A. Brain as a paradigm of organ growth: Hedgehog-Gli signaling in neural stem cells and brain tumors. J Neurobiol. 2005;64(4):476–90. doi: 10.1002/neu.20160. [DOI] [PubMed] [Google Scholar]
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- Strojnik T, Røsland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68(2):133–43. doi: 10.1016/j.surneu.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Eng J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, Kim VN, Kim KS. Human embryonic stem cells express a unique set of microRNAs. Dev Biol. 2004;270(2):488–98. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tamaki S, Eckert K, He D, Sutton R, Doshe M, Jain G, Tushinski R, Reitsma M, Harris B, Tsukamoto A, Gage F, Weissman I, Uchida N. Engraftment of sorted/expanded human central nervous system stem cells from fetal brain. J Neurosci Res. 2002;69:976–986. doi: 10.1002/jnr.10412. [DOI] [PubMed] [Google Scholar]
- Tamiya T, Mizumatsu S, Ono Y, Abe T, Matsumoto K, Furuta T, Ohmoto T. High cyclin E/low p27Kip1 expression is associated with poor prognosis in astrocytomas. Acta Neuropathol. 2001;101(4):334–40. doi: 10.1007/s004010000261. [DOI] [PubMed] [Google Scholar]
- Thon N, Damianoff K, Hegermann J, Grau S, Krebs B, Schnell O, Tonn JC, Goldbrunner R. Presence of pluripotent CD133(+) cells correlates with malignancy of gliomas. Mol Cell Neurosci. 2008 doi: 10.1016/j.mcn.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Toda M, Iizuka Y, Yu W, Imai T, Ikeda E, Yoshida K, Kawase T, Kawakami Y, Okano H, Uyemura K. Expression of the neural RNA-binding protein Musashi1 in human gliomas. 2001;34:1–7. doi: 10.1002/glia.1034. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous sytem stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118(4):409–18. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Wang J, Sakariassen PO, Tsinkakovsky O. CD133 negative glioma cells form tumors in nude rats and give raise to CD133 positive cells. Int J Cancer. 2008a;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, Hjelmeland AB, Rich JN. bc-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3(11):e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M, Hilt DC, Bortey E, Delvault P, Olivares R, Warnke PC. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers ( Gliadel wagers) in patients with primary with malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–4140. [PubMed] [Google Scholar]
- Xu Q, Yuan X, Liu G, Black KL, Yu JS. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26(12):3018–26. doi: 10.1634/stemcells.2008-0459. [DOI] [PubMed] [Google Scholar]
- Zaidi HA, Kosztowski T, DiMeco F, Quinones-Hinojosa A. Origins and clinical implications of the brain tumor stem cell hypothesis. J Neurooncol. 2009;93:49–60. doi: 10.1007/s11060-009-9856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14(1):123–9. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- Zhao X, D’Arca D, Lim WK, Brahmachary M, Carro MS, Ludwig T, Cardo CC, Guillemot F, Aldape K, Califano A, Iavarone A, Lasorella A. The N-Myc-DLL3Cascade is Suppressed by the Ubiquitin Ligase Huwe1 to Inhibit Proliferation and Promote Neurogenesis in Developing Brain. Developmental Cell. 2009;17:210–221. doi: 10.1016/j.devcel.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook BC, Simmens SJ, Jones RV. Evaluation of ENU-induced gliomas in rats: nomenclature, immunochemistry, and malignancy. Toxicol Pathol. 2000;28(1):193–201. doi: 10.1177/019262330002800124. [DOI] [PubMed] [Google Scholar]