Abstract
Human respiratory syncytial virus (RSV) is a ubiquitous respiratory virus causing serious lower respiratory tract disease in infants and young children worldwide. Studies have shown that RSV infection modulates chemokine expression patterns suggesting that particular cytokine expression profiles may be indicators of disease severity. In this study, we show that RSV F or G protein treatment of fully differentiated primary human bronchial epithelial (NHBE) cells induces apical and basolateral secretion of IL-8, IP-10, MCP-1, and RANTES. Purified RSV G (attachment) protein was shown to stimulate the secretion of IL-1α, and RANTES, while purified F (fusion) protein elicited the production of IL-8, IP-10, and RANTES. Studies with UV-inactivated RSV showed that treatment of NHBE cells induces apical IL-8, IP-10, and MCP-1 secretion independent of infection suggesting that RSV proteins alone modify the chemokine response pattern which may affect the early immune response prior to infection.
Keywords: RSV, chemokine, NHBE cells, human, bronchoepithelial
Introduction
Human respiratory syncytial virus (RSV) is the most important cause of serious lower respiratory tract disease mediated by virus infection in infants and young children and causes serious illness in the elderly and immune compromised individuals. RSV infection of host respiratory cells results in the expression and modulation of both cytokine and chemokine expression patterns [1, 2], and certain patterns of cytokine or chemokine expression in RSV-infected individuals may be an indicator of disease severity [3–6]. Dysregulation or inappropriate expression of cytokines or chemokines can modify the early innate response and negatively impact the development and magnitude of the adaptive immune response to RSV infection.
RSV primarily infects ciliated respiratory epithelial cells [7]. During this process, viral proteins are detected by pathogen-sensing pattern recognition receptors associated with the epithelium [8–10] evoking a complex signaling pathway that results in cell activation and regulation of expression of proinflammatory cytokines and chemokines [11–13]. Representative of prominent cytokines and chemokines induced in humans and animal models of RSV infection are CC chemokines (e.g. RANTES, MCP-1, MIP-1α, MIP-1β), CXC chemokines (e.g. IP-10, IL-8), and Th2-type cytokines [2, 13–15]. Evidence suggests that these chemokines are important in pathogenesis. Studies examining neutralizing antibody-depletion of RANTES or eotaxin in mice showed that that depletion was associated with less severe RSV disease and pulmonary eosinophilia [16, 17].
The presence of the G protein during RSV infection has been shown to inhibit early chemokine mRNA expression of MIP-1α, MIP-1β, MIP-2, MCP-1, and IP-10 by bronchoalveolar leukocytes in BALB/c mice [18]. Chemokines are important in recruiting immune cells to sites of infection and inflammation, thus RSV-mediated changes in the chemokine response may be a strategy to facilitate virus replication. A consequence of modified chemokine expression may be recruitment of inappropriate immune cells. Thymus- and activation-regulated chemokine (TARC) recruits Th2-type immune cells to sites of inflammation [19]. Recent studies have shown that RSV infection of BALB/c mice results in increased TARC expression in the lung, and that mice infected with a recombinant vaccinia virus expressing RSV G protein expressed significantly higher levels of TARC levels suggesting a feedback loop for TARC production and Th2-type cytokine expression [20].
Since the bronchial epithelium is recognized as an important source of chemokines fundamental to driving inflammation and the immune response to infection, and to better understand the chemokine response to RSV F or G proteins, we examined RSV infection and RSV F or G protein treatment of primary normal human bronchial epithelial (NHBE) cells grown at air-liquid interface. We show that RSV F and G proteins can induce apical and basolateral secretion of IL-1α, IL-8, IP-10, and RANTES early in infection. These findings are important for understanding the viral mechanisms that affect immunity and RSV disease pathogenesis.
Materials and Methods
Cells, viruses and viral proteins
Normal human bronchial epithelial (NHBE) cells (Lonza, Walkersville, MD) from a single healthy 17 year old male donor were expanded, cryopreserved, and cultured in an air-liquid interface system as previously described [21]. The cells form a pseudo-stratified, highly differentiated model and exhibit a mucociliary phenotype which closely resembles the epithelial tissue of the human respiratory tract. The cells from the same donor were used in all assays, and for culture, the apical surface of the cells was exposed to a humidified 95% air/5% CO2 environment, and the basolateral medium was changed every two days. Recombinant RSV/A2 (6340WT) and recombinant RSV/A2 lacking the G gene (6340ΔG) were kindly provided by Dr. Peter Collins (NIH, NIAID, Bethesda, MD). Viruses were propagated in VeroE6 cells maintained in DMEM (Sigma-Aldrich Corp., St. Louis, MO, USA.) supplemented with 5% heat-inactivated (60°C) fetal bovine serum (FBS; Hyclone Laboratories, Salt Lake City, UT) as previously described [22]. Virus titers were determined by immunostaining plaque assay on VeroE6 cells with anti-RSV F protein monoclonal antibody (clone 131-2A) as previously described [23]. 6340WT was UV-inactivated (>170 J/m2) by exposure to ultraviolet light [24] on ice, and inactivated virus assayed by immunostaining plaque assay [25] to confirm inactivation. RSV F and G proteins were isolated and purified from RSV/A2-infected VeroE6 cells as previously described [26]. Purification was confirmed by Western blot with anti-G protein (131-2G) or anti-F protein (131-2A) monoclonal antibody as previously described [26].
NHBE cell infection
NHBE cells were washed 3 times with PBS to remove excess mucus secretion on the apical surface prior to infection and were infected with 6340WT or 6340ΔG at a multiplicity of infection (MOI) of 1, or apically mock infected with VeroE6 cell lysate (VCL). Viruses were allowed to adsorb for 1h at 37°C, the virus dilutions were removed by aspiration and washed again with PBS 3 times. NHBE cells were apically treated with the following conditions: purified RSV G protein (10 μg/mL), purified RSV F protein (10 μg/mL), or LPS (1 μg/mL; Sigma, St. Louis, MO). Bronchial epithelial basal medium (BEBM) (Lonza) was added to the apical surface of differentiated NHBE cells and were incubated for the indicated times post-infection or post-treatment at 37°C. RSV and mutant virus infection of NHBE cells was confirmed by immunostaining, immunoblot and qRT-PCR for RSV N gene expression as previously reported [25].
Luminex-based quantification of chemokines
MILLIPLEX MAP human chemokine immunoassay (Millipore, St. Charles, MO) was used for the detection of secreted chemokines from apical and basolateral NHBE cell supernatants using the Luminex® xMAP™ system according to the manufacturer protocol. All experiments were analyzed 3 – 6 times for accuracy. Briefly, beads coupled with biotinylated anti-IL-1α , anti-IL-8, anti-MCP-1, anti-MIP1α, anti-MIP1β, anti-IP-10, anti-RANTES monoclonal antibodies were sonicated, mixed, and diluted in PBS bead diluent. For the assay, beads were diluted 1:4 in bead diluent and incubated overnight at 4°C with apical or basolateral NHBE supernatant. After washing, beads were incubated with streptavidin-phycoerythrin for 1 hour at room temperature, washed, and resuspended in wash buffer. The assay was analyzed on a Luminex 200 instrument (Luminex Corporation, Austin, TX) using Luminex xPONENT 3.1 software. Additional analysis was performed using MILLIPLEX Analyst (Millipore).
Statistical analysis of data
Differences in chemokine expression in Luminex® analysis were evaluated by Student t test and considered significant when p < 0.05. Data are shown as means ± standard deviation (SD).
Results and Discussion
Accumulating evidence shows that during the acute phase of RSV infection several notable chemokines are expressed including IL-8, which attracts neutrophils, and RANTES, MCP-1, MIP-1α, MIP-1β, and IP-10, which attract monocytes and leukocytes to sites of inflammation [1, 13, 15, 19]. CC chemokines that include MCP-1, MIPs, RANTES and TARC, but neither Th1- or Th2-type cytokines correlate with severity of illness during RSV infection [27], and there is evidence that that the expression patterns of these and other chemokines are affected by RSV G protein expression [1, 2, 13]. The RSV components that trigger innate immunity are being discovered, and many of these features are linked to activation of early cell signaling events through pattern recognition receptors, e.g. Toll-like receptors (TLR) [12, 28–30]. In this regard, the two major RSV surface proteins, i.e. F and G protein, have been shown to interact with the TLR pathway [9, 12, 28, 31], and modify suppressor of cytokine signaling (SOCS) protein negative regulation of cytokine and chemokine expression [12, 31]. The RSV G protein appears to have a major role in modifying innate and adaptive immune responses at the level of cytokine and chemokine expression [1, 2, 13], and the G protein exhibits CX3C chemokine mimicry linked to G protein interaction with the fractalkine receptor (CX3CR1) [26], an effect that in part mediates immune modulation of CX3CR1+ immune cells that include NK and virus-specific T cells [32]. The immune modulatory features attributed to the G protein are likely important in facilitating RSV replication, but these features are also connected to disease pathogenesis. Studies examining the role of the G protein in enhanced pulmonary disease have shown that a formalin-inactivated RSV (FI-RSV) mutant virus lacking the G protein or lacking only the G protein CX3C motif does not prime for enhanced pulmonary disease following RSV challenge [33], and that BAL cells from mice vaccinated with FI-RSV G protein mutant viruses have modified chemokine transcription profiles showing increased expression of MIP-1α, MIP-1β, and MIP-2 mRNA expression early post-infection [33].
The role of RSV F or G proteins in apical or basolateral chemokine induction by normal human bronchial epithelial cells has not been investigated, thus we examined chemokine expression levels from fully differentiated primary NHBE cells following RSV infection or treatment with purified RSV F or G proteins. Contrary to previous studies that used a transformed type II alveolar cell line (A549) [34, 35], there was no increase in IL-1α secretion by NHBE cells during live RSV infection (6340WT) or following infection with a mutant virus lacking the G gene (6340ΔG) (Fig. 1 and 2). However, NHBE cells treated apically with purified RSV G protein secreted up to 676 pg/mL of IL-1α from the apical surface (Fig. 1B) and 120 pg/mL of IL-1α from the basolateral surface (Fig. 2B) by 18h post-treatment. These findings suggest that during RSV infection, G protein itself may induce IL-1α expression by human airway cells, a feature that may lead to upregulated cellular adhesion molecules, e.g. intercellular adhesion molecule-1 (ICAM-1, CD54)[36], and immune pathogenesis by enhancing immune cell adherence to infected airway cells.
Figure 1.

Apical chemokine secretion patterns of RSV-infected or RSV protein-treated NHBE cells. Cells were apically mock infected with Vero cell lysate (VCL), UV-inactivated 6340WT, or infected with 6340WT or 6340ΔG virus at MOI = 1. NHBE cells were apically treated with purified RSV F or G proteins (10 μg/mL) or LPS (1 μg/mL). At the indicated times post-treatment or post-infection, apical supernatants were analyzed for the level of IL-1α, IL-8, IP-10, MCP-1 or RANTES. Data are shown as means ± standard deviation (SD) and are representative of three independent experiments. Differences in chemokine expression were evaluated by Student t test and considered significant when p < 0.05. An asterisk (*) denotes differences from the control (Vero cell lysate, VCL or medium alone), and a dagger (†) denotes differences from 6340WT infection.
Figure 2.
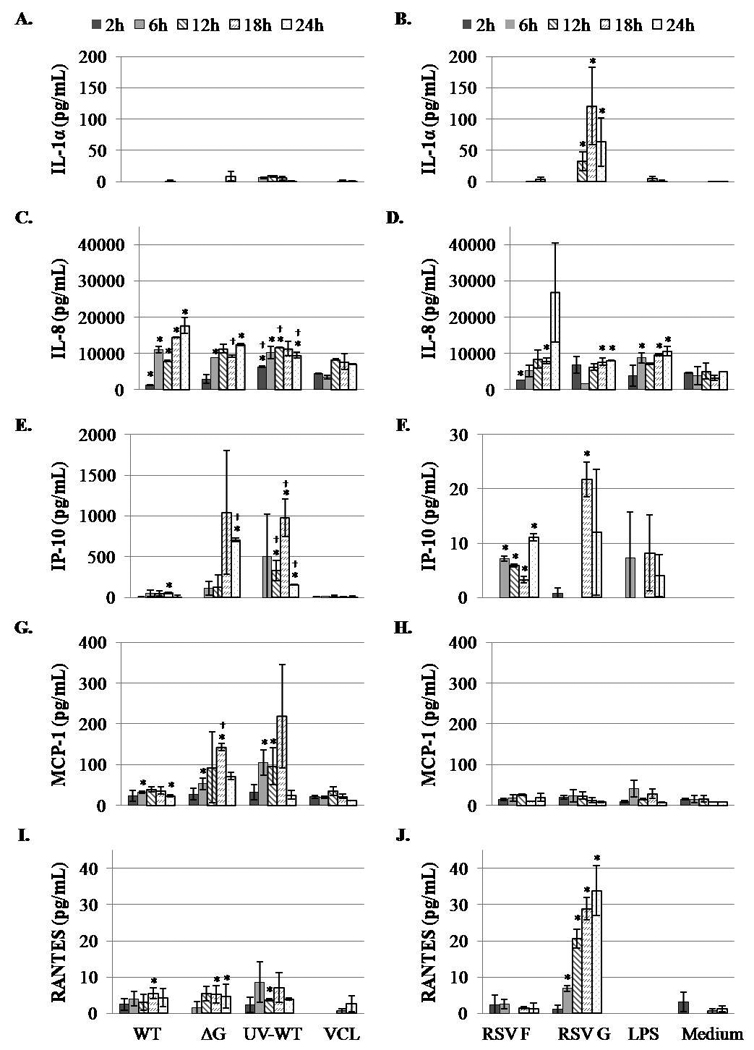
Basolateral chemokine secretion patterns of RSV-infected or RSV protein-treated NHBE cells. NHBE cells were apically mock infected with Vero cell lysate (VCL), UV-inactivated 6340WT, or infected with 6340WT or 6340ΔG at MOI = 1. NHBE cells were apically treated with purified RSV F or G proteins (10 μg/mL) or LPS (1 μg/mL). At the indicated times post-treatment or post-infection, basolateral media were analyzed for the presence of IL-1α, IL-8, IP-10, MCP-1 or RANTES. Data are shown as means ± standard deviation (SD) and are representative of three independent experiments. Differences in chemokine expression were evaluated by Student t test and considered significant when p < 0.05. An asterisk (*) denotes differences from the control (Vero cell lysate, VCL or medium alone), and a dagger (†) denotes differences from 6340WT infection.
Several chemokine receptors been shown to be involved in the host response to RSV infection, particularly CCR1 and CXCR1 [37, 38]. The ligands of CCR1 include MIP-1α, RANTES, and MCP [37], and an important ligand for CXCR1 is IL-8 [39–41]. RSV infection of NHBE cells was associated with significantly (P<0.05) increased levels of apical and basolateral IL-8 secretion relative to mock-infected cells throughout the duration of the study (Fig. 1C and 2C), and infection with 6340ΔG produced similar, albeit significantly (p<0.05) lower, apical IL-8 levels between 18h and 24h p.i. relative to 6340WT-infected cells (Fig. 1C). UV-inactivated 6340WT treatment of NHBE cells induced IL-8 levels which were similar to live infection between 6h and 24h pi (Fig. 1C). In contrast to IL-1α induction, RSV F or G proteins did not induce substantial apical IL-8 secretion (Fig. 1D); however, RSV F protein treatment induced detectable basolateral IL-8 secretion by 24h post-treatment that was not significantly different compared to mock-treated cells (Fig. 2D). These results suggest that F protein may not induce autocrine or paracrine IL-8 signaling among respiratory epithelial cells, but may contribute to the inflammatory response linked to neutrophil recruitment by IL-8.
IP-10 appears to be an important chemokine in the response to RSV infection [1, 2]. Nasopharyngeal aspirates from children less than 2 years of age showed increased levels of IP-10, as well as IL-8, MIP-1α, and MIP-1β during severe RSV infection [15]. In addition, RSV infection in mice leads to increased expression of IP-10 mRNA in total lung RNA [42]. In the studies reported here, 6340WT RSV infection of NHBE cells induced low levels of IP-10 secretion both apically and basolaterally (Fig. 1 and 2), however infection with 6340ΔG resulted in significantly (p<0.05) higher levels of IP-10 relative to 6340WT infection at 18h pi suggesting that G protein inhibits IP-10 expression. This observation is consistent with other findings in mice showing that infection with a RSV G protein deletion mutant virus results in increased early MIP-1α, MIP-1β, MIP-2, MCP-1, and IP-10 mRNA expression relative to wild type RSV infection [18]. Consistent with IL-8 secretion induced by infection (Fig. 1C and 2C), IP-10 is highly expressed following UV-inactivated 6340WT treatment (Fig. 1E and 2E) indicating that UV-inactivation may elicit an alternate route of chemokine stimulation compared to live virus infection. Purified RSV F or G protein treatment elicited low levels of apical and basolateral IP-10 secretion relative to mock-treated NHBE cells over the course of treatment (Fig. 1F and 2F).
While increased MCP-1 levels in nasal secretions are associated with bronchiolitis and inflammation [27], RSV infection of human airway cells has previously been shown to mediate modest MCP-1 secretion but induce high levels of RANTES expressed in cell culture supernatants [43]. Contrary to these findings, 6340WT and 6340ΔG infection of NHBE cells resulted in high levels of apical MCP-1 secretion (ranging from 5.7 × 104 – 9.6 × 104 pg/mL for 6340WT and 6.4 × 104 – 1 × 105 pg/mL for 6340ΔG) that remained high from 2h to 24h post-infection (Fig. 1G). Furthermore, UV-inactivated 6340WT treatment of NHBE cells also elicited apical MCP-1 secretion to a level similar to that induced by live virus infection, but treatment with purified RSV F or G proteins had little effect on MCP-1 secretion relative to mock-treated cells (Fig. 1H and 2H). These results suggest that the RSV F and G proteins are not primary mediators of apically-expressed MCP-1. In contrast, basolateral secretion of MCP-1 was higher in 6340ΔG infected NHBE supernatant compared to 6340WT-infected NHBE cell supernatant indicating that the G protein has a role in modifying basolateral MCP-1 expression (Fig. 2G). It is possible that these differential effects may be linked to iNOS expression. RSV infection has been shown to induce iNOS gene expression in nasopharyngeal exudate cells obtained from infants during the acute phase of RSV bronchiolitis [44], and in human airway cell lines responding to RSV infection [45, 46]. Thus, it is possible that G protein induces cellular expression of nitrite components, e.g. NO, that has been shown to modulate the expression of MCP-1 in cultured human endothelial cells [47]. In addition, iNOS gene expression, initially induced by RSV G protein, may be further enhanced in a paracrine fashion by proinflammatory cytokines released by infection-activated inflammatory cells [44].
RANTES has been detected in nasopharyngeal and tracheal secretions of patients with severe RSV disease [16, 48, 49]. In this study, RSV infection of NHBE cells induced high levels of apical RANTES secretion ranging from 4 × 103 – 8 × 103 pg/mL for 6340WT, and 6.8 × 103 – 8.9 × 103 pg/mL for 6340ΔG (Fig. 1I), but lower basolateral RANTES secretion (Fig. 2I). Both purified RSV F and G protein treatment induced apical RANTES secretion; however, the G protein induced significantly (p<0.05) greater RANTES production from both apical and basolateral surfaces (Fig. 1J and 2J). The finding that 6340WT and 6340ΔG infection induces RANTES suggests the effect is contributed by features other than the G protein; however this finding contrasts the finding that purified G protein induces higher RANTES expression compared to F protein treatment. The data shows that F protein is a mediator of RANTES expression, but exogenous treatment with purified G protein may signal RANTES expression through a different pathway, e.g. TLR [12] than exogenous F protein treatment, or that associated with virus infection.
In conclusion, RSV, and specifically RSV F and G surface proteins, mediate induction of important CC and CXC chemokines in normal human bronchoepithelial cells. We show for the first time that RSV infection or treatment with purified RSV F or G proteins induces differential chemokine expression from the apical and basolateral surfaces in NHBE cells. The pattern, magnitude, and directional expression of the chemokines induced by RSV F and G proteins suggest a mechanism by which these viral proteins may contribute to immune-mediated pathogenesis associated with infection. These studies provide a better translational view of how chemokines are involved in the process of RSV infection, and suggest important targets for RSV disease intervention.
Acknowledgments
This work was supported by the Georgia Research Alliance, and by NIH grant RO1-AI069275-01.
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.Tripp RA. Pathogenesis of respiratory syncytial virus infection. Viral Immunol. 2004;17:165–181. doi: 10.1089/0882824041310513. [DOI] [PubMed] [Google Scholar]
- 2.Tripp RA, Oshansky C, Alvarez R. Cytokines and respiratory syncytial virus infection. Proc Am Thorac Soc. 2005;2:147–149. doi: 10.1513/pats.200502-014AW. [DOI] [PubMed] [Google Scholar]
- 3.Hornsleth A, Loland L, Larsen LB. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J Clin Virol. 2001;21:163–170. doi: 10.1016/s1386-6532(01)00159-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZM, Mao JH, Du LZ, Tang YM. Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr. 2002;91:914–922. doi: 10.1080/080352502760272588. [DOI] [PubMed] [Google Scholar]
- 5.Bont L, Heijnen CJ, Kavelaars A, et al. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14:144–149. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Harb M, Bell F, Finn A, et al. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J. 1999;14:139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monick MM, Yarovinsky TO, Powers LS, et al. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem. 2003;278:53035–53044. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]
- 9.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Wei W, Yun Y. Upregulation of TLR7 and TLR3 gene expression in the lung of respiratory syncytial virus infected mice. Wei Sheng Wu Xue Bao. 2009;49:239–245. [PubMed] [Google Scholar]
- 11.Hippenstiel S, Opitz B, Schmeck B, Suttorp N. Lung epithelium as a sentinel and effector system in pneumonia--molecular mechanisms of pathogen recognition and signal transduction. Respir Res. 2006;7(97) doi: 10.1186/1465-9921-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshansky CM, Krunkosky TM, Barber J, Jones LP, Tripp RA. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a toll-like receptor pathway. Viral Immunol. 2009;22:147–161. doi: 10.1089/vim.2008.0098. [DOI] [PubMed] [Google Scholar]
- 13.Oshansky CM, Zhang W, Moore E, Tripp RA. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol. 2009;4:279–297. doi: 10.2217/fmb.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon JS, Kim HH, Lee Y, Lee JS. Cytokine induction by respiratory syncytial virus and adenovirus in bronchial epithelial cells. Pediatr Pulmonol. 2007;42:277–282. doi: 10.1002/ppul.20574. [DOI] [PubMed] [Google Scholar]
- 15.Bermejo-Martin JF, Garcia-Arevalo MC, De Lejarazu RO, et al. Predominance of Th2 cytokines, CXC chemokines and innate immunity mediators at the mucosal level during severe respiratory syncytial virus infection in children. Eur Cytokine Netw. 2007;18:162–167. doi: 10.1684/ecn.2007.0096. [DOI] [PubMed] [Google Scholar]
- 16.Tekkanat KK, Maassab H, Miller A, Berlin AA, Kunkel SL, Lukacs NW. RANTES (CCL5) production during primary respiratory syncytial virus infection exacerbates airway disease. Eur J Immunol. 2002;32:3276–3284. doi: 10.1002/1521-4141(200211)32:11<3276::AID-IMMU3276>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Matthews SP, Tregoning JS, Coyle AJ, Hussell T, Openshaw PJ. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J Virol. 2005;79:2050–2057. doi: 10.1128/JVI.79.4.2050-2057.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripp RA, Jones L, Anderson LJ. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J Virol. 2000;74:6227–6229. doi: 10.1128/jvi.74.13.6227-6229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11:35–42. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- 20.Monick MM, Powers LS, Hassan I, et al. Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17. J Immunol. 2007;179:1648–1658. doi: 10.4049/jimmunol.179.3.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krunkosky TM, Fischer BM, Martin LD, Jones N, Akley NJ, Adler KB. Effects of TNF-alpha on expression of ICAM-1 in human airway epithelial cells in vitro. Signaling pathways controlling surface and gene expression. Am J Respir Cell Mol Biol. 2000;22:685–692. doi: 10.1165/ajrcmb.22.6.3925. [DOI] [PubMed] [Google Scholar]
- 22.Tripp RA, Dakhama A, Jones LP, Barskey A, Gelfand EW, Anderson LJ. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. J Virol. 2003;77:6580–6584. doi: 10.1128/JVI.77.11.6580-6584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 24.Stadnyk AW, Gillan TL, Anderson R. Respiratory syncytial virus triggers synthesis of IL-6 in BALB/c mouse alveolar macrophages in the absence of virus replication. Cell Immunol. 1997;176:122–126. doi: 10.1006/cimm.1996.1075. [DOI] [PubMed] [Google Scholar]
- 25.Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol. 1999;73:7099–7107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 27.Welliver RC, Garofalo RP, Ogra PL. Beta-chemokines, but neither T helper type 1 nor T helper type 2 cytokines, correlate with severity of illness during respiratory syncytial virus infection. Pediatr Infect Dis J. 2002;21:457–461. doi: 10.1097/00006454-200205000-00033. [DOI] [PubMed] [Google Scholar]
- 28.Murawski MR, Bowen GN, Cerny AM, et al. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awomoyi AA, Rallabhandi P, Pollin TI, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 31.Moore EC, Barber J, Tripp RA. Respiratory syncytial virus (RSV) attachment and nonstructural proteins modify the type I interferon response associated with suppressor of cytokine signaling (SOCS) proteins and IFN-stimulated gene-15 (ISG15) Virol J. 2008;5:116. doi: 10.1186/1743-422X-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol. 2006;176:1600–1608. doi: 10.4049/jimmunol.176.3.1600. [DOI] [PubMed] [Google Scholar]
- 33.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. J Virol. 2003;77:9831–9844. doi: 10.1128/JVI.77.18.9831-9844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel JA, Kunimoto M, Sim TC, et al. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:602–609. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- 35.Chang CH, Huang Y, Anderson R. Activation of vascular endothelial cells by IL-1alpha released by epithelial cells infected with respiratory syncytial virus. Cell Immunol. 2003;221:37–41. doi: 10.1016/s0008-8749(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 36.Arnold R, Konig W. ICAM-1 expression and low-molecular-weight G-protein activation of human bronchial epithelial cells (A549) infected with RSV. J Leukoc Biol. 1996;60:766–771. doi: 10.1002/jlb.60.6.766. [DOI] [PubMed] [Google Scholar]
- 37.Thomas LH, Friedland JS, Sharland M. Chemokines and their receptors in respiratory disease: a therapeutic target for respiratory syncytial virus infection. Expert Rev Anti Infect Ther. 2007;5:415–425. doi: 10.1586/14787210.5.3.415. [DOI] [PubMed] [Google Scholar]
- 38.Morrison PT, Sharland M, Thomas LH, et al. Chemokine-receptor upregulation and disease severity in respiratory syncytial virus infection. Clin Immunol. 2008;128:85–93. doi: 10.1016/j.clim.2008.03.460. [DOI] [PubMed] [Google Scholar]
- 39.Roebuck KA, Carpenter LR, Lakshminarayanan V, Page SM, Moy JN, Thomas LL. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-kappaB. J Leukoc Biol. 1999;65:291–298. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- 40.Openshaw PJ. Potential therapeutic implications of new insights into respiratory syncytial virus disease. Respir Res. 2003;3 Suppl 1:S15–S20. doi: 10.1186/rr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson TR, Graham BS. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23:S46–S57. doi: 10.1097/01.inf.0000108192.94692.d2. [DOI] [PubMed] [Google Scholar]
- 42.Culley FJ, Pennycook AM, Tregoning JS, Hussell T, Openshaw PJ. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J Virol. 2006;80:4521–4527. doi: 10.1128/JVI.80.9.4521-4527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker S, Reed W, Henderson FW, Noah TL. RSV infection of human airway epithelial cells causes production of the beta-chemokine RANTES. Am J Physiol. 1997;272:L512–L520. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- 44.Tsutsumi H, Takeuchi R, Ohsaki M, Seki K, Chiba S. Respiratory syncytial virus infection of human respiratory epithelial cells enhances inducible nitric oxide synthase gene expression. J Leukoc Biol. 1999;66:99–104. [PubMed] [Google Scholar]
- 45.Kao YJ, Piedra PA, Larsen GL, Colasurdo GN. Induction and regulation of nitric oxide synthase in airway epithelial cells by respiratory syncytial virus. Am J Respir Crit Care Med. 2001;163:532–539. doi: 10.1164/ajrccm.163.2.9912068. [DOI] [PubMed] [Google Scholar]
- 46.Kilani MM, Mohammed KA, Nasreen N, Tepper RS, Antony VB. RSV causes HIF-1alpha stabilization via NO release in primary bronchial epithelial cells. Inflammation. 2004;28:245–251. doi: 10.1007/s10753-004-6047-y. [DOI] [PubMed] [Google Scholar]
- 47.Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76:980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- 48.Sheeran P, Jafri H, Carubelli C, et al. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis. 2001;184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]


