Abstract
Purpose
To study whether the variation in maximum oblique muscle size accounts for individual variation in the Bielschowsky head tilt phenomenon (BHTP) in clinically diagnosed superior oblique (SO) palsy.
Methods
Seventeen subjects with clinically diagnosed early-onset or idiopathic SO palsy and 14 normal subjects were enrolled in the study. Magnetic resonance imaging (MRI) in coronal and sagittal planes was used for quantitative morphometry of inferior oblique (IO) and SO muscles. Maximum cross-sectional area of the SO and IO cross section at the mid-inferior rectus crossing were determined in central gaze and compared with paretic eye hypertropia on ipsilesional versus contralesional head tilt.
Results
Mean (±SD) maximum SO cross section was 18.1 ± 3.2 mm2 in normal subjects, 14.2 ± 6.8 mm2 ipsilesional to SO palsy, and 19.2 ± 4.5 mm2 contralesional to SO palsy. The ipsilesional SO cross section was significantly smaller than the contralesional (P = 0.004) and normal (P = 0.01) ones. The mean IO cross section was 18.3 ± 3.5 mm2 in normal subjects, 21.3 ± 7.9 mm2 ipsilesional to SO palsy (P = 0.43), and 22.0 ± 6.7 mm2 contralesional to SO palsy (P = 0.26). Hyperdeviation varied with head tilt by 20.1 ± 5.5° in subjects with SO atrophy, and 10.3 ± 5.6° in subjects without SO atrophy (P = 0.003). Although oblique muscle cross sections did not correlate with BHTP, subjects with clinically diagnosed SO palsy segregated into groups exhibiting normal versus atrophic SO size.
Conclusions
SO size does not account for the variation in BHTP in clinically diagnosed SO palsy, supporting the proposition that the BHTP is nonspecific for SO function.
Patients with early onset or idiopathic superior oblique (SO) palsy are heterogeneous. Only when orbital imaging shows a large asymmetry in cross-sectional areas of the SO muscles is actual muscle weakness1–4 likely. SO palsy may not necessarily be neuropathic, because abnormalities of the SO tendon,5–8 or of orbital pulleys may cause incomitant vertical strabismus mimicking SO palsy.9,10 For this reason, the gold standard for the diagnosis of SO palsy is ultimately radiographic. Nevertheless, much clinical literature on SO palsy is based on clinical, not radiographic, criteria. If clinical criteria are nonspecific for SO palsy, then some beliefs about SO palsy may benefit from reexamination.
The Bielschowsky head tilt phenomenon (BHTP) consists of a greater hypertropia during head tilt to the ipsilesional than contralesional shoulder in patients seated upright and is used as a clinical lateralizing test for SO palsy. The biomechanical basis of the BHTP is not fully understood, but probably includes loss of downward and intorsional torque of the palsied SO in compensatory ocular counterrolling (OCR).11 The BHTP is considered by many clinicians to be the defining clinical criterion for SO palsy.
Oblique muscles have both vertical and torsional actions. Contractility of the SO can be radiographically determined by evaluating the change in SO cross-sectional area during gaze shift from supraduction to infraduction. In patients with SO palsy, SO contractility is well correlated with maximum SO cross-sectional area in central gaze.12 Further, MRI evidence of SO muscle contractile change in vertical gaze shift resembles similar MRI findings during ocular counterrolling.13 During static ocular counterrolling,13 the posterior SO cross section was found to be greater during head tilt to the ipsilateral than the contralateral side, reflecting SO contraction to implement ocular torsion.2
Because changes in SO cross section due to vertical duction resemble changes associated with OCR, we sought to analyze whether variation in SO size accounts for variation in BHTP in SO palsy. Recognizing that maximum SO cross-sectional size in the central gaze is highly correlated with SO contractility,12 we supposed that the BHTP would also correlate with SO size if this diagnostic test directly reflects SO function.
Methods
Subjects
Subjects with clinically diagnosed congenital or idiopathic SO palsy, including presumably decompensated cases, were recruited from a prospective study of extraocular muscle function at Okayama University Hospital. The subjects agreed to participate and gave written informed consent according to a protocol conforming to the tenets of the Declaration of Helsinki. Diagnosis of SO palsy was based on clinical criteria including: ipsilesional hypertropia greater in the contralesional than the ipsilesional version, and greater during head tilt to the ipsilateral than the contralateral shoulder when seated upright (Bielschowsky head tilt test); a deficit in infraduction when the ipsilesional eye was adducted; and results of Hess screen testing performed by strabismologists confirming greater hypertropia in deorsumversion and V pattern. All participants underwent complete ophthalmic examinations, including measurement of heterophorias with prism and cover testing. The BHTP was defined quantitatively to be the difference in vertical phoria, as measured using alternate prism and cover test, between measurements made during head tilt 30° to the right shoulder, compared with the same degree of tilt to the left shoulder during fixation of a target 5 m distant. Fourteen normal volunteers were similarly examined.10
Magnetic Resonance Imaging
High-resolution, T1-weighted MRI was performed 1.5-T scanner and dual surface coils (Signa Horizon; General Electric, Milwaukee, WI), as described in detail elsewhere.9,14–16 Initially, an axial localizer scan was obtained at 3 mm thickness using a 256 × 192 matrix over a 10-cm2 field of view. Then, to image the SO muscle, sets of 17 contiguous 2-mm thick quasicoronal image planes were obtained perpendicular to the long axis of the orbit using a 256 × 256 matrix over an 8-cm2 field of view, giving 313-μm pixel resolution. To optimally image the inferior oblique (IO) muscle, we obtained sets of contiguous 2-mm-thick MRI image were obtained in the quasi-sagittal plane parallel to the long axis of the orbit at the same resolution. During imaging, subjects monocularly fixed a small central target. The fellow eye was occluded.10,17
Analysis
Cross sections of the SO and IO muscles were computed for each MRI image using NIH Image (W. Rasband, National Institutes of Health, Bethesda, MD; available by ftp from zippy.nimh.nih.gov or on floppy disc from NTIS, 5285 Port Royal Road, Springfield, VA 22161, part number PB95-500195GEI). The SO cross-sectional area was measured sequentially in image planes posterior to the globe–optic nerve junction to determine the plane containing maximum cross-sectional area.12 Because quasi-coronal image planes are perpendicular to the orbit, but not to the SO muscle, SO cross sectional area was trigonometrically corrected to obtain a true cross section perpendicular to the long axis of the SO muscle.12 Seventeen subjects clinically diagnosed with congenital or idiopathic SO palsy had an adequate MRI.10 Maximum SO cross sections of subjects with clinically diagnosed SO palsy were compared with those of 14 normal volunteers.10 Based on the maximum SO cross section, patients with SO palsy were classified into two groups: those exhibiting SO muscle atrophy and those without (Fig. 1).10
Figure 1.
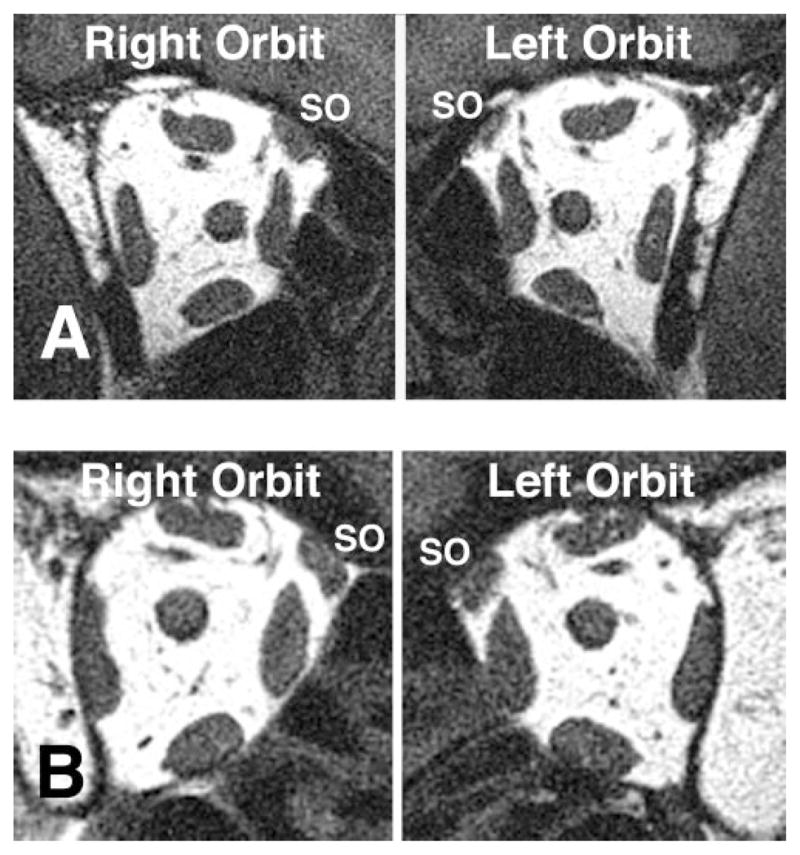
Orbital MRI in quasi-coronal planes 8 mm posterior to the globe–optic nerve injection in central gaze in two subjects with clinically diagnosed SO palsy. (A) Subject 1 with left SO palsy and SO atrophy manifested by smaller left than right SO cross-sectional area. (B) Subject 2 with right SO palsy without SO atrophy.
The IO muscle follows a straight line from a nasally located origin to its pulley, conjoined with that of the inferior rectus (IR) muscle, but has a highly curved path between the IR pulley and scleral insertion. Maximum IO cross-sectional area was determined at the most temporal part of the straight path, which was in the quasi-sagittal image plane passing through the center of the IR muscle.12
Statistical analysis, including linear regression, was performed with commercial software (JMP 5.1; SAS Institute Inc., Cary, NC).
Results
A total of 28 orbits in 14 normal subjects, and 34 orbits in 17 patients with clinically diagnosed SO palsy were imaged. Mean age of the control subjects was 34.3 ± 15.4 years (±SD; range, 21–73), and that of subjects with SO palsy was 46.4 ± 17.9 years (range, 17–83).10
The mean maximum SO cross-sectional area in 28 normal orbits was 18.1 ± 3.2 mm2, with a 95% confidence interval of 16.8 to 19.3 mm2.10 Normal subjects exhibited bilaterally symmetrical maximum SO cross-sectional areas. Figure 2 shows a plot of maximum SO cross-sectional area for the left eye against that of the right eye, and includes a bivariate normal ellipse. A linear regression of right versus left SO cross-sectional areas in normal subjects yielded a slope of 1.00 that was significantly different from 0 (P < 0.0001) and accounted for 89% of the variance.
Figure 2.
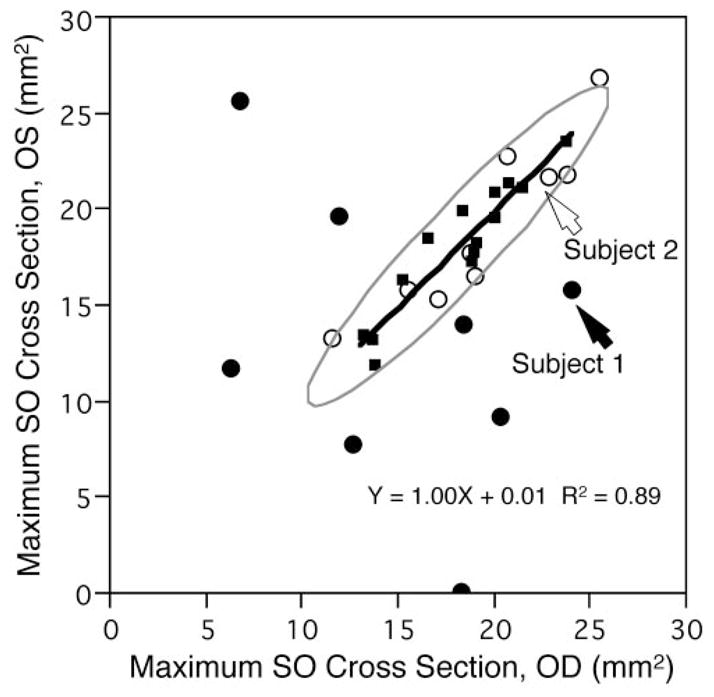
Comparison of maximum SO muscle cross-sectional area between right and left eyes. Ninety-five percent bivariate normal ellipse (gray) was computed from SO cross sections of 14 normal subjects. (○) Nine subjects clinically diagnosed with SO palsy but having almost normal SO cross-sectional area. (●) Eight subjects with clinically diagnosed SO palsy having subnormal SO cross-sectional area. (■) Data of 14 normal subjects. Solid line: linear regression through data points of the normal subjects with slope significantly differing from 0 (P < 0.0001).
One subject with SO palsy had no identifiable ipsilesional SO muscle, and that was considered to be a 0 cross-sectional area. In subjects with SO palsy, ipsilesional SO cross-sectional area (14.2 ± 6.8 mm2) averaged significantly smaller than the contralesional (19.2 ± 4.5 mm2; paired t-test, P = 0.004), and was also smaller than normal (unpaired t-test, P = 0.01). Contralesional SO cross-sectional area was not significantly different from normal (P = 0.33). As seen in Figure 2, maximum SO cross-sectional areas for eight cases of SO palsy exhibiting SO atrophy lay outside the normal 95% confidence ellipse. Five subjects whose maximum SO cross-sectional areas were included within or on the border of the ellipse, were classified as not exhibiting SO atrophy.
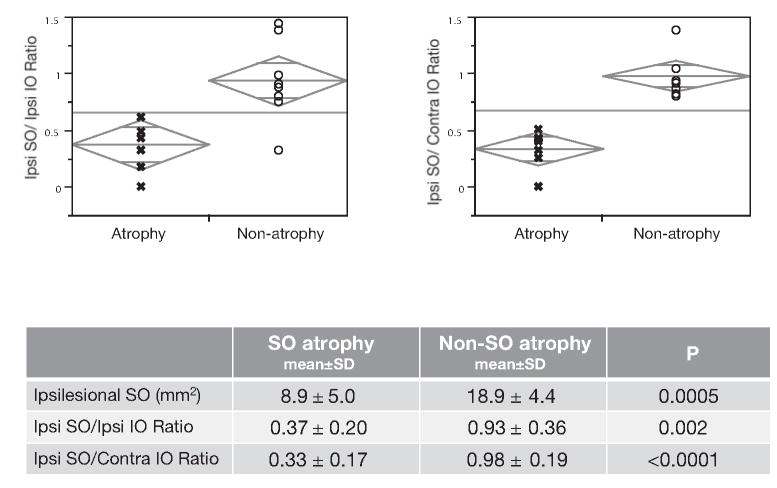
Mean maximum SO cross-sectional areas in all three subject groups are shown in Table 1. The mean maximum ipsilesional SO muscle cross-sectional area in subjects with SO muscle atrophy was significantly (Student’s t-test, P < 0.0001) subnormal at 8.9 ± 5.0 mm2, with a 95% confidence interval of 4.8 to 13.1 mm2, but was normal at 18.9 ±4.4 mm2 in subjects without SO atrophy (P = 0.55, 95% confidence interval 15.6–22.3 mm2).
Table 1.
Mean Maximum SO Muscle Cross-sectional Area
| Paretic Eye | Nonparetic Eye | |
|---|---|---|
| Control subjects | — — |
18.1 ± 3.2 (n = 28) |
| SO palsy | ||
| Atrophy | 8.9 ± 5.0 (n = 8) |
18.8 ± 4.8 (n = 8) |
| Nonatrophy | 18.9 ± 4.4 (n = 9) |
19.6 ± 4.4 (n = 9) |
Mean ± SD (mm2).
The cross-sectional area of the IO muscle was evaluated at the point at which the IO crossed the center of the IR muscle. The mean maximum IO cross-sectional area in five normal subjects in the quasi-sagittal image plane was 18.3 ± 3.5 mm2, with no significant differences ipsilesional (21.3 ± 7.9 mm2, P = 0.43) or contralesional (22.0 ± 6.7 mm2, P = 0.26) to SO palsy (Table 2). Mean ipsilesional IO cross-sectional areas in subjects with SO atrophy was 24.3 ± 10.0 mm2, with a 95% confidence interval of 16.0 to 32.7 mm2, with no significant differences compared to the group without SO atrophy at 17.8 ± 1.7 mm2, with a 95% confidence interval 16.3 to 19.4 mm2. Ipsi- and contralesional IO cross-sectional areas in subjects with SO atrophy were insignificantly larger than normal, but showed no significant variation relative to the side of the presumed lesion (Table 2).
Table 2.
Mean Maximum IO Muscle Cross-sectional Area
| Paretic Eye | Nonparetic Eye | |
|---|---|---|
| Control subjects | — — |
18.3 ± 3.5 (n = 5) |
| SO palsy | ||
| Atrophy | 24.3 ± 10.0 (n = 8) |
25.4 ± 8.3 (n = 7) |
| Nonatrophy | 17.8 ± 1.7 (n = 7) |
19.1 ± 3.3 (n = 8) |
Mean ± SD (mm2).
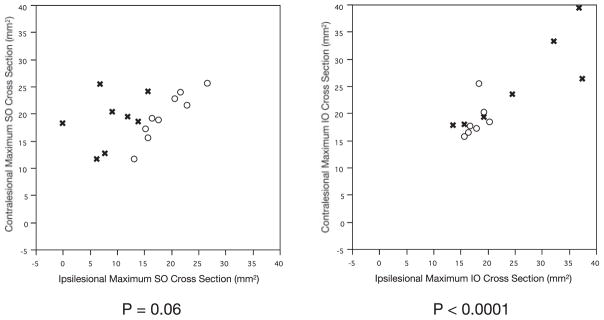
For subjects with SO palsy, there was no significant correlation between ipsi- and contralesional maximum SO cross section (P = 0.06), but there was a highly significant (P < 0.0001) positive correlation between maximum ipsi- and contralesional IO cross sections (Fig. 3). The bilateral symmetry of IO cross section, even in subjects with SO atrophy, was evident from a significant correlation (P = 0.01) between ipsi- and contralesional IO cross sections. There was no hypertrophy of ipsilesional IO in subjects with SO atrophy. In contrast, the poor correlation between ipsi- and contralesional SO cross-sectional areas in subjects with SO atrophy is evident in Figure 3, left.
Figure 3.
Relation of ipsi- to contralesional maximum SO and IO muscle cross-sectional area. Groups with clinically diagnosed SO palsy either exhibited SO atrophy (x) or nonatrophy (○).
We defined the unilateral BHTD to be the difference in hyperdeviation between the head-upright and the ipsilesional head-tilt position. Table 3 summarizes the hyperdeviation with head upright in subjects with and without SO atrophy, the BHTP, and the unilateral BHTD. Subjects with SO atrophy showed nearly double the hyperdeviation by all these measures than did those without SO atrophy (P ≤ 0.02).
Table 3.
Hyperdeviation in Subjects with SO Palsy
| Hyperdeviation in Upright Position | BHTP | Unilateral BHTD | |
|---|---|---|---|
| Atrophy (n = 8) | 13.1 ± 3.7 | 20.1 ± 5.5 | 7.0 ± 4.5 |
| Nonatrophy (n = 9) | 7.6 ± 5.0 | 10.3 ± 5.6 | 2.7 ± 2.4 |
| P | 0.02 | 0.003 | 0.02 |
Mean ± SD (degrees). Unilateral BHTD, unilateral Bielschowsky head tilt difference (difference in hypertropia between ipsilesional head tilt and upright head position). Statistical analysis comparing groups with versus without atrophy was performed by Student’s t-test.
We considered the ratio of maximum ipsilesional SO to IO cross-sectional area, and the ratio of ipsilesional SO to contralesional IO cross-sectional area (Fig. 4). Both ratios were nearly unity in subjects without SO atrophy, but were markedly smaller in subjects with SO atrophy (P < 0.01).
Figure 4.
Ipsilesional maximum SO muscle cross-sectional area (ipsilesional SO), size ratio of ipsilesional SO to ipsilesional IO muscle cross-sectional area (Ipsi SO/Ipsi IO), and size ratio of ipsilesional SO to contralesional IO muscle cross-sectional area (Ipsi SO/Contra IO) in subjects with clinically diagnosed SO palsy who did, or did not, exhibit SO muscle atrophy by MRI. All mean values were significantly larger in the group without SO atrophy. Statistical analyses comparing groups with versus those without atrophy were performed by Student’s t-test.
The ipsilesional maximum SO cross-sectional area did not correlate significantly with hyperdeviation with head upright, BHTP, or unilateral BHTD. Similarly, the ipsilesional maximum IO cross-sectional area did not correlate significantly with hyperdeviation with head upright, BHTP, or unilateral BHTD.
Neither hyperdeviation with head upright nor BHTP correlated with the ratio of maximum ipsilesional SO to ipsilesional IO cross-sectional aarea, but there was a significant (R2 = 0.279, P = 0.04) linear correlation of this ratio with unilateral BHTD.
DISCUSSION
The present study demonstrated that BHTP does not correlate with maximum SO or IO cross-sectional area. Since for vertical duction, the SO cross sectional area strongly correlated with SO contractility,12 the present findings infer that BHTP is not directly related to SO contractility and that IO size is also not a determinant of BHTP.
We might have compared functional measures such as BHTP or unilateral BHTD, with oblique muscle contractility as determined from cross-sectional area change in infraduction and supraduction. However, multipositional MRI was not available for the current subjects, and maximum SO cross sectional area in central gaze is known to correlate strongly with SO contractility.12
The present study demonstrates that BHTP, a vertical strabismus phenomenon, does not correlate with SO size and presumably not with SO function. However, there is evidence that SO size correlates with ocular torsion in response to head tilting, OCR. Hamasaki et al.18 reported that OCR gain, the mean ratio of paretic eye torsion to the head tilt angle in SO palsy, correlates positively with SO size. They observed that paretic eye OCR gain in SO muscle atrophy is significantly decreased during ipsilesional head tilting, in comparison with comparable gain in patients with a clinical diagnosis of SO palsy who did not exhibit SO atrophy. Biomechanical simulations indicate that the SO muscle itself has too little vertical action to be responsible for the large hypertropia typical of SO palsy; other innervational and mechanical changes must be invoked to account quantitatively for the BHTP.19 The acute effect of trochlear neurectomy in monkeys is only a small and incomitant hypertropia. Only after binocular visual experience does the hypertropia increase to resemble human SO palsy.20 It is most likely that the large-magnitude BHTP observed in human SO palsy is due to contraction of the superior rectus (SR) and relaxation of the IR muscles of the involved eye. There may be excessive SR innervation to compensate for weak SO intorsion during ipsilesional head tilt, leading to excessive supraduction by the SR21 or corresponding excessive IR inhibition. This hypothesis may explain how an increased OCR gain can cause a prominent BHTP, even in cases without SO atrophy, but also in atrophy cases as in our present study demonstrated.22 Kommerell and Klein23 reported evidence that OCR gain can be influenced by an adaptive phenomenon in a patient with SO palsy.
Recent experimental data support the expectation of SO atrophy in true SO palsy. In experimental SO palsy produced by intracranial trochlear neurectomy in monkeys, ipsilesional SO atrophy was evident both by MRI and by histomorphometry (Demer JL, et al. IOVS 2008;49:ARVO E-Abstract 4495). This SO atrophy in acquired SO palsy, while significant, was not profound, because whereas the global layer fibers atrophied to 20% of normal size, the remaining half of the SO fibers in the orbital layer maintained normal size (Demer JL, et al. IOVS 2008;49:ARVO E-Abstract 4495). In the context of this experimental finding, we reiterate the contention of Demer et al.24 that there are “masquerading” SO palsies. The three-step test, which includes the Bielschowsky head tilt test, is not specific for deficient contractility of the SO muscle belly. Kushner25,26 has emphasized that the three-step test may lead to erroneous diagnoses. Masquerading conditions could include primary mechanical abnormalities of the SO tendon in the presence of a normally innervated SO belly, primary abnormalities of the rectus pulleys, and the central mechanisms postulated by Guyton,22 that can create the BHTP in the presence of normal SO function. While in some clinical situations the distinction between true and masquerading SO palsy may not alter treatment of strabismus, this distinction may be clinically important in determining the neurologic significance of vertical strabismus exhibiting the BHTP. For example, the finding of SO atrophy in acquired hypertropia having the BHTP suggests an acquired lesion along the trochlear nerve, which perhaps deserves neurologic investigation. Conversely, the same presentation of hypertropia with MRI evidence of normal SO and contractility may suggest no further consideration of a trochlear nerve lesion.
Acknowledgments
Supported in part by Grants-in-Aid 15790990 and 19592023 from the Ministry of Education, Science, Sports, Culture and Technology of Japan. JLD is supported by United States Public Health Service NIH-NEI Grant EY08313, and is Leonard Apt Professor of Ophthalmology.
The authors thank Kazushi Kinugasa, Director of the National Agency for Automotive Safety and Victims’ Aid, Okayama Ryogo Center, Okayama, Japan, who provided the MRI facility and equipment.
Footnotes
Disclosure: R. Kono, None; H. Okanobu, None; H. Ohtsuki, None; J.L. Demer, None
References
- 1.Horton JC, Tsai RK, Truwit CL, Hoyt WF. Magnetic resonance imaging of superior oblique muscle atrophy in acquired trochlear nerve palsy (letter) Am J Ophthalmol. 1990;110:315–316. doi: 10.1016/s0002-9394(14)76358-5. [DOI] [PubMed] [Google Scholar]
- 2.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906–913. [PubMed] [Google Scholar]
- 3.Ozkan S, Arlbal ME, Sener EC, Sanac AS, Gurcan F. Magnetic resonance imaging in evaluation of congenital and acquired superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1997;34:29–34. doi: 10.3928/0191-3913-19970101-07. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Yagasaki T, Kora T, Awaya S. Comparison of muscle volume between congenital and acquired superior oblique palsies by magnetic resonance imaging. Jpn J Ophthalmol. 1998;42:466–470. doi: 10.1016/s0021-5155(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 5.Guyton DL. Exaggerated traction test for the oblique muscles. Ophthalmology. 1981;88:1035–1040. doi: 10.1016/s0161-6420(81)80033-4. [DOI] [PubMed] [Google Scholar]
- 6.Plager DA. Traction testing in superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1990;27:136–140. doi: 10.3928/0191-3913-19900501-08. [DOI] [PubMed] [Google Scholar]
- 7.Plager DA. Tendon laxity in superior oblique palsy. Ophthalmology. 1992;99:1032–1038. doi: 10.1016/s0161-6420(92)31854-8. [DOI] [PubMed] [Google Scholar]
- 8.Sato M. Magnetic resonance imaging and tendon anomaly associated with congenital superior oblique palsy. Am J Ophthalmol. 1999;127:379–387. doi: 10.1016/s0002-9394(98)00329-8. [DOI] [PubMed] [Google Scholar]
- 9.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic rectus muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 10.Kono R, Okanobu H, Ohtsuki H, Demer JL. Displacement of the rectus muscle pulleys simulating superior oblique palsy. Jpn J Ophthalmol. 2008;52(1):36–43. doi: 10.1007/s10384-007-0492-8. [DOI] [PubMed] [Google Scholar]
- 11.Bielschowsky A. Lectures on motor anomalies of the eye. II. Paralysis of individual eye muscles. Arch Ophthalmol. 1935;13:33–59. [Google Scholar]
- 12.Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology. 2003;110:1219–1229. doi: 10.1016/S0161-6420(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 13.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. doi: 10.1152/jn.01157.2004. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–212. [PubMed] [Google Scholar]
- 15.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 16.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gaze. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- 17.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management. Philadelphia: Saunders; 1999. pp. 84–98. [Google Scholar]
- 18.Hamasaki I, Hasebe S, Ohtsuki H. Static otolith-ocular reflex reflects superior oblique muscle disorder. Am J Ophthalmol. 2006;142:849–850. doi: 10.1016/j.ajo.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Robinson DA. Bielschowsky head-tilt test–II. Quantitative mechanics of the Bielschowsky head-tilt test. Vision Res. 1985;25:1983–1988. doi: 10.1016/0042-6989(85)90023-9. [DOI] [PubMed] [Google Scholar]
- 20.Shan X, Tian J, Ying HS, et al. Acute superior oblique palsy in monkeys: I. Changes in static eye alignment. Invest Ophthalmol Vis Sci. 2007;48:2602–2611. doi: 10.1167/iovs.06-1316. [DOI] [PubMed] [Google Scholar]
- 21.Kushner BJ. Multiple mechanisms of extraocular muscle “overaction”. Arch Ophthalmol. 2006;124:680–688. doi: 10.1001/archopht.124.5.680. [DOI] [PubMed] [Google Scholar]
- 22.Guyton DL. Ocular torsion reveals the mechanisms of cyclovertical strabismus: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2008;49:847–857. doi: 10.1167/iovs.07-0739. [DOI] [PubMed] [Google Scholar]
- 23.Kommerell G, Klein U. Adaptive changes of the otolith-ocular reflex after injury to the trochlea. Neuroophthalmology. 1986;6:101–107. [Google Scholar]
- 24.Demer JL, Miller MJ, Koo RY, Rosenbaum AL, Bateman JB. True versus masquerading superior oblique palsies: Muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G, editor. Update on Strabismus and Pediatric Ophthalmology. Boca Raton, FL: CRC Press; 1995. pp. 303–306. [Google Scholar]
- 25.Kushner BJ. Errors in the three-step test in the diagnosis of vertical strabismus. Ophthalmology. 1989;96:127–132. doi: 10.1016/s0161-6420(89)32933-2. [DOI] [PubMed] [Google Scholar]
- 26.Kushner BJ. Ocular torsion: rotations around the “WHY” axis. J AAPOS. 2004;8(1):1–12. doi: 10.1016/j.jaapos.2003.09.004. [DOI] [PubMed] [Google Scholar]