Abstract
Objective
High oxidative stress potentially leads to accelerated telomere shortening and consequent premature cell senescence, implicated in type 2 diabetes (T2D) development. Therefore, we studied the association of leukocyte telomere length (LTL) with the presence of T2D, as well as the effect on the patients’ LTL of plasma oxidative stress and of variation in UCP2, a gene involved in the mitochondrial production of reactive oxygen species.
Methods
Mean LTL was determined in 569 Caucasian, 103 South Asian and 70 Afro-Caribbean T2D patients aged from 24 to 92 years, 81 healthy Caucasian male students aged from 18 to 28 years and 367 healthy Caucasian men aged from 40 to 61 years by real-time PCR. Plasma total antioxidant status (TAOS) was measured in the T2D patients by a photometric microassay. The patients were also genotyped for the UCP2 functional variants −866G>A and A55V.
Results
Afro-Carribeans had 510 bp longer mean length compared to Caucasians (p < 0.0001) and 500 bp longer than South Asians (p = 0.004). T2D subjects displayed shorter age-adjusted LTL compared to controls [6.94(6.8–7.03) vs. 7.72(7.53–7.9), p < 0.001] with subjects in the middle and the lowest tertile of LTL having significantly higher odds ratios for T2D compared to those in the highest tertile [1.50(1.08–2.07) and 5.04(3.63–6.99), respectively, p < 0.0001]. In the patients, LTL was correlated negatively with age (r = −0.18, p < 0.0001) and positively with TAOS measures (r = 0.12, p = 0.01) after adjusting for age, while carriers of the UCP2 −866A allele had shorter age-adjusted LTL than common homozygotes [6.86(6.76–6.96) kb vs. 7.03(6.91–7.15) kb, p = 0.04].
Conclusion
The present data suggest that shorter LTL is associated with the presence of T2D and this could be partially attributed to the high oxidative stress in these patients. The association of the UCP2 functional promoter variant with the LTL implies a link between mitochondrial production of reactive oxygen species and shorter telomere length in T2D.
Keywords: Telomere length, Type 2 diabetes, Oxidative stress, UCP2 gene, Ethnic diversity
1. Introduction
Premature cell senescence has recently been postulated as an important cause and consequence of type 2 diabetes (T2D) and its complications [1]. The telomere shortening hypothesis is a widely accepted mechanism leading to senescence [2]. Telomeres are specialised DNA-protein structures at the end of all chromosomes, which preserve chromosome stability and integrity. In humans they consist of thousands of tandem repeats of the TTAGGG sequence [3]. Telomeres shorten with cell division and cells are triggered into senescence once mean length reduces below a critical value [2].
Telomere attrition is mainly caused by the “end-replication” problem, generated by the incapability of DNA polymerase to fully copy the very end of the lagging strand [4]. Another factor contributing to telomere attrition involves the processing of telomere ends to reconstitute 3′ single-strand overhangs, and telomere loss due to the fact that DNA repair mechanisms, particularly for single-stranded DNA damage, are less efficient in telomeric DNA than elsewhere in the genome. The resulting accumulation of single-strand breaks along the telomeres leads to DNA damage-dependent shortening during replication [5]. Hence, telomere shortening could serve as an indicator of replicative history and cumulative genomic damage of somatic cells. Telomeric DNA is particularly prone to oxidative damage at the GGG sequence. Exposure to free radicals or oxidants causes DNA damage including single-strand breaks and telomere erosion as shown with in vitro experiments [6–8]. Therefore, it is speculated that the rate of telomere shortening will be dependent on the balance between intracellular oxidative stress and antioxidant defence.
Prediabetes and metabolic syndrome are associated with increased oxidative stress [9,10]. It is well established that hyperglycaemia elicits an increase in reactive oxygen species (ROS) production, due to increased input of reducing equivalents into the mitochondrial electron transport chain. ROS overproduction is a trigger for pathways responsible for hyperglycaemia-induced cell damage [11,12].
Our hypothesis is that shorter telomeres eventually lead to senescent phenotypes in multiple cell types including beta cells, the consequent apoptosis of which hastens the onset of diabetes. These shorter telomeres can be either attributed to shorter length at birth in individuals predisposed to diabetes or to accelerated telomere loss during cell division caused by increased oxidative stress in prediabetic conditions, or both. In support of this, shorter telomeres have been observed in circulating epithelial progenitor cells in patients with metabolic syndrome [13] and in other conditions of high oxidative stress, such as smoking and obesity [14]. The data on T2D and telomere length though are scarce with only few small studies with up to 80 subjects showing that T2D patients have shorter telomeres than controls [15–17]. Whether this is due to oxidative stress, and to what degree, remains to be determined. Therefore, our aim was to examine the association of telomere length with the presence of T2D in a large cohort as well as the effect of plasma oxidative stress on the patients’ leukocyte telomere length.
In order to further enlighten the effect of ROS on telomere shortening, we also studied the effect of uncoupling protein 2 (UCP2) gene variation. This ubiquitously expressed protein is a plausible negative regulator of ROS production, since it dissipates the inner mitochondrial membrane electrochemical gradient that drives ATP synthesis and uncouples respiration from oxidative phosphorylation [18]. Decreased UCP2 expression results in increased ROS production, in vitro [19], while animal studies have shown that absence of UCP2 causes higher oxidative stress [20]. To maintain homeostasis, UCP2 expression is induced by elevated oxygen species concentration [21]. A common functional variant exists in the promoter of human UCP2 gene (−866G>A), with the A allele being associated with lower mRNA levels, while a non-synonymous SNP leading to an alanine to valine substitution has been indentified in exon 4 of the gene (A55V) [22]. A previous study from our laboratory, demonstrated that the UCP2 −866G>A variant interacts with smoking to increase oxidative stress in T2D patients [23]. Given this finding and the established function of UCP2, we hypothesised that these functional variants in the UCP2 gene will be associated with the stress-induced telomeric DNA damage and therefore with the telomere length of T2D patients, in whom oxidative stress is elevated.
2. Materials and methods
2.1. Subjects
2.1.1. University College London Diabetes and Cardiovascular disease Study (UDACS)
The UDACS is a cross-sectional sample of diabetes patients, according to the World Health Organization criteria [24], designed to study the association between common genetic variants and biochemical risk factors implicated in coronary heart disease (CHD) in patients with diabetes. It comprises of 1011 subjects consecutively recruited from the diabetes clinic at UCL Hospitals in 2001–2. Analyses were confined only to T2D patients (N = 742). Ethical approval was obtained from UCL/UCL Hospitals ethics committee and all subjects gave informed consent. The full characteristics of patients have been reported previously [25].
2.1.2. European Atherosclerosis Research Study II (EARS II)
The EARSII was carried out in 1993. Male students between the ages of 18 and 28 years whose fathers had a proven myocardial infarction before the age of 55 (cases, N = 407) and age-matched male controls (N = 415) were recruited from 14 university student populations of 11 European countries: Tallinn in Estonia, Helsinki and Oulu in Finland were designated Baltic; Glasgow, Belfast and Bristol were designated United Kingdom (UK); Aarhus in Denmark, Hamburg in Germany, Ghent in Belgium, and Zurich in Switzerland were designated Middle Europe; Lisbon in Portugal, Reus in Spain, Naples in Italy, and Athens in Greece were designated South Europe. Only the controls were considered in the present study. The study has been approved by ethics committees of collaborating centres and the subjects have given informed consent. Details of the study have been described previously [26].
2.1.3. Hypercoagulability and Impaired Fibrinolytic function MECHanisms predisposing to myocardial infarction (HIFMECH)
The HIFMECH study consists of 598 male survivors of a first myocardial infarction aged <60 years (excluding patients with familial hypercholesterolaemia and insulin-dependent diabetes mellitus) and 653 population-based control subjects of the same age and region recruited from four centers in Europe: Stockholm in Sweden and London in England were designated North Europe and Marseille in France and San Giovanni Retondo in Italy were designated South Europe. The study has been approved by ethics committees of collaborating centres and the subjects have given informed consent. Detailed description of the study can be found elsewhere [27].
2.2. Determination of plasma total antioxidant status (TAOS)
The UDACS plasma samples were collected within a 12-month period and stored immediately at −80 °C. A modification by Sampson of Laight's photometric microassay [28] was used to measure plasma TAOS. In this method, plasma TAOS is determined by its capacity to inhibit the peroxidase-mediated formation of the 2,2-azino-bis-3-ethylbensthiazoline-6-sulfonic acid radical. The assay is performed in a 96-well ELISA plate with 2.5 μL of plasma. The inter-assay coefficient of variation was 14% and the intra-assay 4.3%. In general terms, increased oxidative stress within a sample is negatively correlated with consumption of antioxidants and diminished antioxidant status within that sample.
In order to test whether TAOS is a valid measure of oxidative stress, we have previously examined the correlation between plasma TAOS and esterified F2-isoprostane and found this to be significant (r = −0.65; p = 0.003) [29]. Since the measures obtain by the two methods correlate, the inter-individual differences in TAOS and/or the correlation to other variables is feasible by using either method.
2.3. Determination of leukocyte telomere length
Leukocyte DNA was extracted by the salting-out method [30]. Telomere length was measured in these DNA samples using a validated quantitative PCR-based method as previously described [26]. Briefly, the relative telomere length was calculated as the ratio of telomere repeats to single-copy gene (SCG) copies (T/S ratio). For each sample the quantity of telomere repeats and the quantity of SCG copies were determined in comparison to a reference sample in a telomere and a SCG quantitative PCR, respectively. The raw data from each PCR was analysed using the comparative quantification analysis (Rotor-Gene 6000 software, Corbett Research Ltd., Cambridge, UK). All PCRs were performed on the Rotor-Gene 6000 (Corbett Research Ltd., Cambridge, UK). The coefficient of variation in repeated measurements was 5.6%. Using the linear regression line between measures obtained by both the PCR-based method and the conventional terminal restriction fragment (TRF) analysis for the same set of 32 samples, as previously described, we calculated the corresponding telomere length in bp from the T/S ratio measured in each subject [26].
2.4. Functional UCP2 single-nucleotide polymorphism (SNP) genotyping
The −866G>A promoter variant and the non-synonymous A55V variant of the UCP2 gene were genotyped using TaqMan Assay-by-Design platform (Applied Biosciences, ABI, Warrington, UK). Five μL reactions were performed on 384-well microplates and analysed using the ABI TaqMan 7900HT software (probe details available on request).
2.5. Statistical analysis
Statistical analysis was performed with SPSS statistical software (version 15.0 for Windows). Baseline characteristics were transformed to a normal distribution where appropriate. To compare telomere length in T2D cases and controls, mean values were adjusted to an age of 68 years (the mean age in the T2D cases) for all studies. Odds ratios were calculated from logistic regression models using telomere length both as a continuous variable and after dividing it into tertiles. The tertiles of telomere length were constructed using the combined case–control group with the age-adjustment made within the separate groups. Partial correlation coefficients were used to examine the association between telomere length and continuous classical risk factors after adjustment for age (when testing the correlation with age, telomere length was not age-adjusted). To examine the associations of age-adjusted telomere length and glucose with TAOS, the Spearman's rho correlation coefficient was calculated. Differences in telomere length between ethnic groups, tertiles of TAOS, categorical characteristics and UCP2 SNP genotypes were tested using analysis of covariance. All models used to test the differences in telomere length were adjusted for age. In each case of UCP2 SNP all three possible models (additive, dominant and recessive) were tested and the best fitted one was chosen by the higher R2 of the test. The differences in TAOS among the UCP2 SNP genotypes were tested with Kruskall–Wallis or Mann–Whitney U-tests. A nonparametric test for trend (performed with STATA, Version 9, StataCorp, TX, USA) was also used to examine these differences. Haplotypes were inferred after excluding individuals with missing values using THESIAS software (http://www.genecanvas.org). Haplotypes with frequency of less than 2% were excluded from the analysis. Results were presented as the geometric mean telomere length (95% CI) for two copies of each haplotype assuming a multiplicative effect and the mean TAOS (95% CI) assuming an additive effect. Statistical significance was taken as p < 0.05.
In the present study a specific a priori hypothesis was tested. Therefore, we believe adjustment for multiple comparisons is not necessary. We and others [31] support that correction for multiple comparisons is too conservative in hypothesis-deriving analysis such as this and has also been suggested to lead to errors in interpretation [32].
3. Results
3.1. Study cohorts – ethnic/geographical diversity
3.1.1. UDACS T2D cases
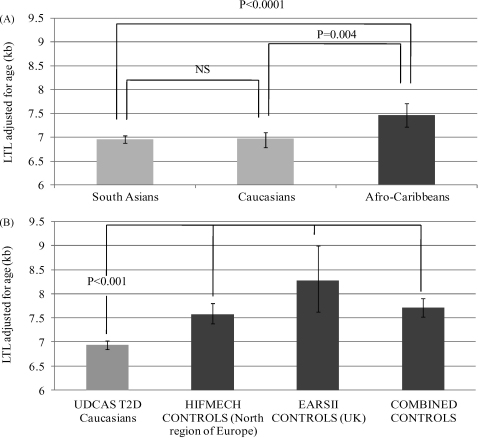
Leukocyte mean telomere length was successfully determined in 742 T2D patients. Significant differences were found among the three ethnicities, after adjusting for age (p < 0.0001). Afro-Carribeans had 510 bp longer mean length compared to Caucasians [7.46 (95%CI: 7.21–7.71) and 6.94 (95%CI: 6.80–7.03), p < 0.0001, respectively] and 500 bp longer than South Asians [6.96 (95%CI: 6.78–7.09), p = 0.004], while Caucasians’ and South Asians’ length did not differ significantly (p = 1.00) (Fig. 1A). Due to these ethnic differences and in order to allow the comparison with the Caucasian controls, we used only the 569 Caucasian T2D cases in all further analysis. The cases’ characteristics are presented in Table 1. These are typical of T2D patients with a mean age of 68 [24–92] years, a mean age of diabetes onset of 55 [13] years and the majority of them (59.4%) being males. The duration of diabetes was 9 [4–16] years and their BMI 29.3 (5.5) kg/m2.
Fig. 1.
(A) Ethnic differences in mean age-adjusted leukocyte telomere length (LTL) of the type 2 diabetes patients. (B) Case–control differences in mean age-adjusted leukocyte telomere length (LTL).
Table 1.
Characteristics of the Caucasian 2 diabetes cases and controls.
| UDACS T2D cases N = 569 | EARSII controls N = 81 | HIFMECH controls N = 367 | |
|---|---|---|---|
| Age (years), median [range] | 68 [24–92] | 21 [18–28] | 53 [40–61] |
| Age of T2D onset (years) | 55 (13) | ||
| Duration of T2D (years)a | 9 [4–16] | ||
| % Males (N) | 59.4 (338) | 100 (81) | 100 (367) |
| % Females (N) | 40.6 (231) | 0 (0) | 0 (0) |
| % Females >50 years (N) | 94.4 (218) | ||
| BMI (kg/m2)b | 29.3 (5.5) | 23.0 (2.4) | 25.6 (3.1) |
| SBP (mmHg)b | 140.5 (20.9) | 112.6 (9.8) | 127.1 (15.3) |
| DBP (mmHg)b | 79.3 (11.4) | 66.0 (7.6) | 82.8 (8.0) |
| Total cholesterol (mmol/l)b | 4.96 (1.09) | 4.06 (0.70) | 5.50 (0.96) |
| TG (mmol/l)b | 1.92 (1.08) | 0.93 (0.35) | 1.40 (0.60) |
| HDL (mmol/l)b | 1.28 (0.37) | 1.17 (0.20) | |
| LDL (mmol/l)c | 2.71 (0.94) | 2.38 (0.60) | |
| CRP (mg/l)b | 1.72 (1.49) | 0.59 (0.68) | 1.08 (1.38) |
| TAOS (%)a | 44.7 [35.4–52.4] | ||
| Glucose (mmol/l)b | 9.8 (4.3) | 5.20 (0.37) | |
| HBA1c (%)b | 7.65 (1.61) | ||
| % Hypertension (N) | 83.4 (472) | 0 (0) | 4.2 (15) |
| % Ex/current smokers (N) | 52.3 (291) | 38.3 (31) | 60.8 (223) |
| Hypoglycaemics | |||
| % None (N) | 11.2 (63) | ||
| % Insulin (N) | 13.1 (74) | 0 (0) | 0 (0) |
| % Oral (N) | 62.6 (353) | ||
| % Both (N) | 13.1 (74) | ||
| % BP lowering drug use (N) | 68.6 (387) | 0 (0) | 0 (0) |
| % Aspirin use (N) | 50.6 (285) | 0 (0) | 0 (0) |
| % Statin use (N) | 31.6 (177) | 0 (0) | 0 (0) |
| Telomere length (kb)d | 6.94 (6.80–7.03) | 8.27 (7.62–9.00) | 7.58 (7.39–7.81) |
TG: triglycerides, HDL: high density lipoprotein, LDL: density lipoprotein, BMI: body mass index, CRP: C-reactive protein, SBP: systolic blood pressure, DBP: diastolic blood pressure, TAOS: total antioxidant status.
Median [IQR] is presented.
Data were log-transformed and geometric mean (approx SD) is presented.
Data were square root transformed and square of mean (approx SD) is presented.
Data were log-transformed and the geometric mean (95%CI) was then used to calculate the corresponding telomere length in kb. Telomere length presented is age-adjusted.
3.1.2. EARSII controls
The control subjects from two different studies were employed in an effort to cover the large age range of the cases (24–92 years). Leukocyte telomere length was measured in 396 controls from the EARSII study aged between 18 and 28 years recruited from 4 different European regions. In our previous article on EARSII [26] we observed significant differences in telomere length among the geographical regions of Europe. Therefore, we used for the present analysis only the 81 UK region controls to match the UDACS Caucasian patients which were recruited in the UK (Table 1).
3.1.3. HIFMECH controls
Leukocyte telomere length was also measured in 520 HIFMECH controls with an age range of 40–61 years recruited from the North and the South part of Europe. Again, due to the differences in telomere length found between the South and the North [8.27(4.14) kb vs. 7.99(4.51) kb, p = 0.02] we only used in the present analysis the 367 controls from the North of Europe matching to the UK Caucasian cases of UDACS (Table 1).
3.2. Case–control differences
The cases [6.94(6.80–7.03)] displayed shorter age-adjusted leukocyte telomere length compared to the EARSII [8.27(7.62–9.00), p < 0.001] and the HIFMECH controls [7.58(7.39–7.81), p < 0.001] as well as the two control samples combined [7.72(7.53–7.90), p < 0.001] (Table 1, Fig. 1B).
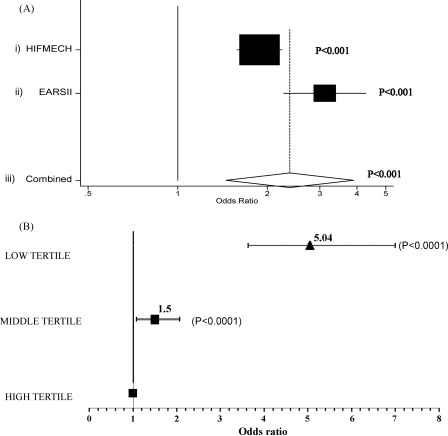
Decreasing age-adjusted telomere length was associated with a higher odds ratio for T2D when comparing the cases to the EARSII controls [2.96(2.16–4.06), p < 0.001], the HIFMECH controls [1.88(1.58–2.24), p < 0.001] and the combined controls [2.38(1.45–3.89), p < 0.001] (Fig. 2A). To better illustrate this effect we divided the age-adjusted telomere length in tertiles. Subjects in the middle and the lowest tertile of length had significantly higher odds ratios for T2D compared to the highest tertile [1.50(1.08–2.07) and 5.04(3.63–6.99) respectively, p < 0.0001], with the effect being more pronounced between lowest and highest tertiles (Fig. 2B).
Fig. 2.
(A) The odds ratio of type 2 diabetes for one standard deviation decrease in age-adjusted telomere length when comparing the cases with (i) the HIFMECH controls, (ii) the EARSII controls and (iii) the combined controls. (B) The odds ratio of type 2 diabetes for each tertile of age-adjusted telomere length when comparing the cases with the combined controls.
3.3. Telomere length determinants in T2D patients
3.3.1. Association with classical risk factors
As expected, there was a significant negative correlation with age (r = −0.18, p < 0.0001, Table 2 and Supplemental Fig. 1A). Telomere length was therefore age-adjusted in all further analyses.
Table 2.
Correlation coefficients of age-adjusted telomere length with classical risk factors in Caucasian type 2 diabetes patients.
| Correlation coefficient, r | p-Value | N | |
|---|---|---|---|
| Age (years) | −0.18 | <0.0001 | 569 |
| Age of onset (years) | −0.01 | 0.77 | 569 |
| Duration of diabetes (years) | 0.01 | 0.77 | 569 |
| BMI (kg/m2) | 0.02 | 0.64 | 563 |
| SBP (mmHg) | 0.05 | 0.27 | 566 |
| DBP (mmHg) | −0.03 | 0.51 | 566 |
| Total cholesterol (mmol/l) | 0.03 | 0.41 | 566 |
| TG (mmol/l) | −0.03 | 0.44 | 566 |
| HDL (mmol/l) | 0.04 | 0.40 | 566 |
| LDL (mmol/l) | 0.05 | 0.25 | 549 |
| CRP (mg/l) | −0.05 | 0.21 | 555 |
| TAOS (%) | 0.12, | 0.01 | 561 |
| Glucose (mmol/l) | −0.02 | 0.59 | 566 |
| HBA1c (%) | 0.01 | 0.77 | 563 |
TG: triglycerides, HDL: high density lipoprotein, LDL: density lipoprotein, BMI: body mass index, CRP: C-reactive protein, SBP: systolic blood pressure, DBP: diastolic blood pressure, TAOS: total antioxidant status.
Age-adjusted telomere length was not correlated with any of the classical clinical and biochemical factors measured in this study (Table 2). There was no significant difference in length between males and females, between patients with different smoking history, or between hypertensive and normotensive subjects. Interestingly, we did not find any significant association with the age of onset nor the duration of diabetes. The use of hypoglycaemic drugs, blood pressure lowering drugs, aspirin or statin was also not associated with length (Supplemental Table 1).
3.3.2. Association with plasma oxidative stress
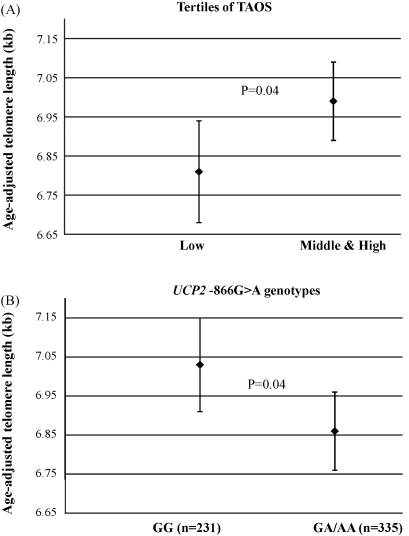
There was a significant positive correlation of the mean length with continuous TAOS measurements (r = 0.12, p = 0.01, Table 2 and Supplemental Fig. 1B). While TAOS measures were negatively correlated with glucose (r = −0.13, p = 0.002), as expected. To further examine this effect, TAOS measures were categorised in tertiles. Individuals in the low tertile had significantly shorter telomeres [6.81 (95%CI: 6.68–6.94) kb] compared to those in the middle and high tertiles of TAOS [6.99 (95%CI: 6.89–7.09) kb, p = 0.04, Fig. 3A].
Fig. 3.
(A) The mean age-adjusted telomere length in tertiles of plasma total antioxidant status (TAOS) measures in Caucasian type 2 diabetes patients. (The error bars represent 95% confidence intervals.) (B) The mean age-adjusted telomere length in Caucasian type 2 diabetes patients carrying or not the UCP2 −866G>A variant. (The error bars represent 95% confidence intervals.)
3.3.3. Association with UCP2 functional variants
Among the 569 Caucasian T2D patients, genotype was obtained in 566 (99.5%) for the −866G>A SNP and in 544 (95.6%) for the exon 4 variant A55V (Table 3). The genotype distribution for both the −866G>A and the A55V variants was as expected from Hardy–Weinberg equilibrium (χ2 = 0.50, p = 0.48 and χ2 = 0.06, p = 0.30, respectively).
Table 3.
Age-adjusted telomere length and plasma total antioxidant status (TAOS) measures by UCP2 functional SNPs in Caucasian type 2 diabetes patients.
| UCP2 SNP/haplotype | %Genotype/haplotype (N) | Telomere length (kb)a | p-Value | TAOS (%)b | p-Value |
|---|---|---|---|---|---|
| −866G>A (rs659366) | |||||
| GG | 40.8 (231) | 7.03 (6.91–7.15) | 0.11 | 45.6 [35.4–54.2] | 0.20 (0.08 for trend) |
| GA | 46.5 (263) | 6.86 (6.75–6.98) | 44.3 [35.7–51.7] | ||
| AA | 12.7 (72) | 6.84 (6.63–7.06) | 42.1 [34.1–48.7] | ||
| GA/AA | 59.2 (335) | 6.86 (6.76–6.96) | 0.04 | 43.9 [35.4–50.7] | 0.17 |
| A55V (rs660339) | |||||
| CC | 35.5 (193) | 7 (6.86–7.14) | 0.24 | 45.8 [35.3–54.6] | 0.05 (0.03 for trend) |
| CT | 48.4 (263) | 6.9 (6.79–7.02) | 44.7 [365.–52.8] | ||
| TT | 16.2 (88) | 6.8 (6.62–6.99) | 41.8 [32.1–48.5] | ||
| CT/TT | 64.5 (351) | 6.87 (6.78–6.97) | 0.15 | 44 [35.4–50.9] | 0.20 |
| −866G>A/A55Vc | |||||
| G/C | 63.0 | 6.98 (6.83–7.14) | 44.8 [42.4–47.2] | ||
| G/T | 4.0 | 6.89 (6.06–7.91) | 0.85 | 42.0 [30.1–53.9] | 0.03 |
| A/T | 33.0 | 6.79 (6.56–7.04) | 0.25 | 39.4 [35.6–43.1] | 0.65 |
All haplotypes with frequency <2% are excluded from the analysis.
Data were log-transformed and the geometric mean (95%CI) was then used to calculate the corresponding telomere length in kb.
median [IQR] is presented.
Data is presented for 2 copies of each haplotype.
As shown in Table 3, carriers of the −866A allele had ∼170 bp (2.4%) shorter mean length than the GG subjects (Fig. 3B) and there was a trend to lower TAOS towards in these −866A allele carriers. The effect of the −866A allele on TAOS was statistically significant among smokers of the present cohort, as previously shown [23]. The A55V amino acid change was associated with lower TAOS measures, but the effect on telomere length was not significant (Table 3). Haplotype analysis using these SNPs in combination did not detect a statistically significant effect on telomere length (Table 3).
4. Discussion
The present study is the largest to date to demonstrate that leukocyte telomere length is significantly shorter in T2D patients compared to controls over a wide age range, and that the shorter telomeres in patients can be partially attributed to high oxidative stress. We also report for the first time an association of shorter leukocyte telomeres in T2D patients with variation affecting the UCP2 gene expression.
The T2D patients had on average 780 bp shorter telomeres than healthy individuals, a difference which represents a biological age gap of approximately 24 years. This difference in leukocyte telomere length between T2D cases and controls is greater than the previously reported 500 bp difference in Caucasians [15] but smaller compared to the 3.1 kb difference found in an Asian Indian cohort [17]. The discrepancy of the effect size on telomeres might be due to the small sample size of these other studies but also, in the second case, might point to the increased tendency for diabetes in South Asians compared to Caucasians [17]. Nevertheless, the differences in telomere length of diabetes patients and controls, including the one observed here, are much greater than the ∼300 bp difference reported in other studies between coronary heart disease (CHD) patients and controls [33–36]. Moreover, in this study decreasing telomere length was associated with more than twofold higher risk for T2D. Having telomeres in the middle tertile of length was associated with 50% higher odds ratio for T2D, while having telomeres in the lowest tertile of length was associated with a fivefold higher odds ratio for T2D. These data suggest an important role of biological ageing as reflected by telomere shortenning in diabetes.
Prediabetes [9] and diabetes [37] are characterised by increased oxidative stress. In the present patient cohort higher glucose levels were correlated with lower TAOS, which corresponds to higher plasma oxidative status. Plasma TAOS measures the antioxidant consumption, which is proportional to oxidative stress generation and represents the net effect of many different compounds and systemic interactions. A typical example of synergism between antioxidants is glutathione regenerating ascorbate [38] and subsequently ascorbate regenerating alpha-tocopherol [39]. Therefore, it is possible that plasma TAOS gives more biologically relevant information than that obtained from measuring plasma concentrations of individual antioxidants (e.g. alpha-tocopherol, ascorbate). Within the described sample no subject was known to take any form of vitamin supplements.
In an effort to reveal the factors determining the observed shorter telomere length in patients with diabetes we examined the correlation of length with classical risk factors and plasma oxidative stress as reflected by TAOS measures. As we hypothesised, telomere length was significantly correlated with measures of oxidative stress, with higher levels of systemic oxidative stress being associated with shorter telomeres in leukocytes. This observation in plasma is likely to be representative of the processes involved in the development and progress of T2D in other tissues, such as the pancreatic islets. Leukocyte telomere length has been shown to be representative of that in vascular wall cells [40] and thus inter-individual differences in leukocyte telomere length probably apply to those of other cell types like beta cells. Oxidative DNA damage and up-regulated DNA repair mechanisms have been observed in beta cell in T2D [41], and an inverse relationship between beta cell volume density and levels of DNA oxidation products has been reported [42]. Our data, coupled with these experiments, support the theory that hyperglycaemia-induced oxidative stress may accelerate local and systemic senescence process, as reflected by telomere dynamics.
Worthy of remark is that no other T2D risk factor was associated with the length of telomeres in patients, which suggests that plasma oxidative stress levels represent the oxidative burden conferred by all risk factors collectively and thus the only one found to significantly correlate with telomere length. The present observation of shortened telomeres in T2D subjects could also be attributed to increased cell turnover induced by chronic inflammatory responses involved in diabetes development and progression and therefore, markers of inflammation might be associated with telomere length [43]. To examine this possibility we looked at the correlation with C-reactive protein (CRP) levels, but it was not significant, confirming also the findings of Sampson et al. [15]. This either implies that a single plasma measure of CRP is not representative of the chronic inflammation underlying diabetes, or that, in diabetes, oxidative stress is such that it may over ride any effect of inflammation on telomeres. Cigarette smoking and obesity has been previously linked to telomere shortening, but no such association was detected here [14]. A possible reason for the discrepancy with our findings is that our study cohort consists of T2D patients who are under hyperglycaemia-induced oxidative stress, in contrast to the healthy subjects included in the study of Valdes et al., and this may mask the effect of obesity or smoking on telomeres. Men have been reported to have shorter telomeres than women of the same age [43]. In our sample, women had longer age-adjusted telomeres than men but this difference was not significant, possibly due to the fact that most of these women were postmenopausal. An oestrogen-responsive element exists in telomerase, the enzyme that replaces telomeric loss in progenitor cells [44]; thus hormonal changes may influence telomerase to maintain telomere length in the progenitor cells after menopause.
Interestingly, measures of diabetes severity such as the age of onset and the duration of the disease were not associated with differences in length. However, T2D in most cases has a silent onset; therefore the age of onset cannot be defined with precision in contrast to other diseases as CHD where specific overt symptoms as stable or unstable angina pectoris or myocardial infarction indicate the disease onset. In addition, we believe that duration of diabetes is not representative of the severity of diabetes in the present cohort, since we were unable to evaluate whether and for how long glycaemia was effectively controlled with treatment. Uziel et al. evaluated arterial and blood mononuclear cell telomere length in diabetic patients and found that glycaemic control attenuated the shortening, but in the present study it was not possible to examine this [16]. A better indicator of diabetes severity and worsening is probably the complications of the disease as kidney failure, blindness, and cardiovascular disease or the increasing need for hypoglycaemic treatment. Indeed, shorter telomeres were significantly associated with higher odds ratio for CHD in our patients (compared to the highest tertile of telomere length, OR for the lowest tertile of telomere length: 1.67 (1.04–2.69) and OR for the middle tertile of telomere length: 1.33 (0.82–2.16), p = 0.03). Records on other T2D complications were not available.
To examine in more depth the association of telomere length with oxidative stress in patients with diabetes, we evaluated in this study for the first time the effect of functional UCP2 variants. UCP2 is an established negative regulator of mitochondrial ROS overproduction [18] and its function has been linked to diabetes development [22]. Here we show that those carrying the functional promoter variant −866A of UCP2 have shorter telomeres. The minor allele −866A has been previously shown to interact with smoking to increase oxidative stress [23]. Considering this, and that in the present diabetes patients plasma oxidative stress was correlated with shorter telomere length, it is likely that the observed effect of the A allele on telomeres is due to its association with greater mitochondrial ROS production. In this case, such genetic variants are useful to provide a good indicator of the mitochondrial ROS effect on telomere length, since genetic predisposition to higher ROS is exerting its effect throughout life. On the other hand, it is believed that the association of UCP2 with T2D is caused by a pancreatic effect of UCP2 on insulin secretion. UCP2 is involved in the glucose-induced insulin secretion, through the uncoupling of ATP production from glucose metabolism. The uncoupling of the ATP production from glucose catabolism reduces the ATP production which in turn results in lower ATP/ADP ratio in the pancreatic cell and therefore decreased insulin secretion [45]. Thus, one cannot exclude the possibility of UCP2 being involved in cell senescence in T2D as reflected by the telomere dynamics here, through a pathway triggered by disturbed insulin secretion in addition or not to disturbed regulation of ROS.
A plausible mechanism of the situation in subjects who developed T2D may be that hyperglycaemia in the prediabetic state induces high oxidative stress, which in turn causes oxidative telomeric DNA damage and consequent shortened telomeres, which eventually lead to premature senescence. This theory is compatible with the beta cell failure in T2D and also the vascular endothelial and smooth muscle cell senescence, which promotes atherogenesis in hyperglycaemic patients.
Another very interesting finding of the present study is the ethnical diversity in telomere length among T2D patients. These data confirm the recent observation of Hunt et al. [46] that Africans have longer telomeres than Caucasians, but also show that South Asians have similar telomere length to Caucasians. Hunt et al. showed in their study [46] that the rate of age-dependent telomere shortening was faster in Africans than in Caucasians. Such evidence supports the theory that Africans are more likely to be born with longer telomeres and is in accordance with previously reported evidence of a high degree of genetic determination in telomeric length [47].
In previous studies of ours and others, family history of CHD has been associated with shorter telomere length [26,48], hence family history of T2D might also be partly expressed through inherited short telomeres. This theory could not be examined as no such data were available in the present study. The ethnic differences seen in telomere length though imply an important genetic component in its determination. Taking all these into account one could speculate that oxidative stress is having an effect on the length of telomeres, but the inter-individual variability is, to a large degree, determined by the age of the individual and the telomere length at birth [47]. The role of genetic predisposition to shorter telomeres in subjects prone to develop diabetes with regard to environmental factors needs further investigation.
Limitations of our study need to be considered. The possibility that an unmeasured factor, such as other inflammation markers or lifestyle factors might confound the observed effects of oxidative stress or genetic variation on telomere length cannot be excluded. The absence of data on T2D complications, other than CHD, and the increasing need for hypoglycaemic treatment did not allow us to examine the association of the disease severity and telomere length. Moreover, this study does not include any data on telomere loss over time, as all measurements were taken at a certain time point and therefore the rate of telomere attrition in diabetes patients could not be addressed. Finally, no definite conclusions can be made on whether the observed shorter telomeres in patients are a cause or a consequence of diabetes since this is a case–control study.
5. Conclusion
This study showed that T2D is associated with shorter telomeres in Caucasians and moreover provided suggestive evidence for a link between systemic oxidative stress and mitochondrial production of ROS with the shorter telomeres in T2D patients. Thus, telomere length might be of use as a marker of cell oxidative damage and biological age, providing a valuable tool in the management of T2D. A prospective evaluation of T2D risk in relation to leukocyte telomere length could shed light on the question of whether telomere shortening is a cause or a consequence of diabetes, and provide an insight to the potential of using telomere length for the risk assessment of T2D development. Finally, in vitro investigation of the UCP2 down-regulation will establish the role of this gene in determining the intracellular oxygen species levels, the oxidative-induced telomere loss and the consequent cell senescence.
Acknowledgments
We acknowledge the British Heart Foundation for funding Klelia D. Salpea (FS/06/053), Jackie A. Cooper and Steve E. Humphries (RG2005/014). Financial support for UDACS was provided by the Diabetes UK.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atherosclerosis.2009.09.070.
Appendix A. Supplementary data
(A) The correlation of telomere length (T/S ratio) with age in Caucasian type 2 diabetes patients. (B) The correlation of age-adjusted telomere length (T/S ratio) with plasma total antioxidant status (TAOS) in Caucasian type 2 diabetes patients.
References
- 1.Sampson M.J., Hughes D.A. Chromosomal telomere attrition as a mechanism for the increased risk of epithelial cancers and senescent phenotypes in type 2 diabetes. Diabetologia. 2006;49:1726–1731. doi: 10.1007/s00125-006-0322-4. [DOI] [PubMed] [Google Scholar]
- 2.Allsopp R.C., Harley C.B. Evidence for a critical telomere length in senescent human fibroblasts. Exp Cell Res. 1995;219:130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn E.H. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 4.Olovnikov A.M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 5.Richter T., von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42:1039–1042. doi: 10.1016/j.exger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Petersen S., Saretzki G., von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 7.Serra V., Grune T., Sitte N., Saretzki G., von Zglinicki T. Telomere length as a marker of oxidative stress in primary human fibroblast cultures. Ann N Y Acad Sci. 2000;908:327–330. doi: 10.1111/j.1749-6632.2000.tb06666.x. [DOI] [PubMed] [Google Scholar]
- 8.Matthews C., Gorenne I., Scott S. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 9.Su Y., Liu X.M., Sun Y.M. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract. 2008;62:877–882. doi: 10.1111/j.1742-1241.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 10.Hansel B., Giral P., Nobecourt E. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa T., Edelstein D., Du X.L. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 13.Satoh M., Ishikawa Y., Takahashi Y. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 14.Valdes A.M., Andrew T., Gardner J.P. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 15.Sampson M.J., Winterbone M.S., Hughes J.C., Dozio N., Hughes D.A. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 16.Uziel O., Singer J.A., Danicek V. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Exp Gerontol. 2007;42:971–978. doi: 10.1016/j.exger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Adaikalakoteswari A., Balasubramanyam M., Mohan V. Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabet Med. 2005;22:1151–1156. doi: 10.1111/j.1464-5491.2005.01574.x. [DOI] [PubMed] [Google Scholar]
- 18.Casteilla L., Rigoulet M., Penicaud L. Mitochondrial ROS metabolism: modulation by uncoupling proteins. IUBMB Life. 2001;52:181–188. doi: 10.1080/15216540152845984. [DOI] [PubMed] [Google Scholar]
- 19.Duval C., Negre-Salvayre A., Dogilo A. Increased reactive oxygen species production with antisense oligonucleotides directed against uncoupling protein 2 in murine endothelial cells. Biochim Biol CellBiochem Cell Biol. 2002;80:757–764. doi: 10.1139/o02-158. [DOI] [PubMed] [Google Scholar]
- 20.Blanc J., Alves-Guerra M.C., Esposito B. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 21.Echtay K.S., Roussel D., St-Pierre J. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 22.Wang H., Chu W.S., Lu T. Uncoupling protein-2 polymorphisms in type 2 diabetes, obesity, and insulin secretion. Am J Physiol. 2004;286:E1–7. doi: 10.1152/ajpendo.00231.2003. [DOI] [PubMed] [Google Scholar]
- 23.Stephens J.W., Dhamrait S.S., Mani A.R. Interaction between the uncoupling protein 2−866G>A gene variant and cigarette smoking to increase oxidative stress in subjects with diabetes. Nutr Metab Cardiovasc Dis. 2008;18:7–14. doi: 10.1016/j.numecd.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Stephens J.W., Hurel S.J., Cooper J.A. A common functional variant in the interleukin-6 gene is associated with increased body mass index in subjects with type 2 diabetes mellitus. Mol Genet Metab. 2004;82:180–186. doi: 10.1016/j.ymgme.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Salpea K.D., Nicaud V., Tiret L., Talmud P.J., Humphries S.E. The association of telomere length with paternal history of premature myocardial infarction in the European Atherosclerosis Research Study II. J Mol Med (Berl, Germany) 2008;86:815–824. doi: 10.1007/s00109-008-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juhan-Vague I., Morange P.E., Aubert H. Plasma thrombin-activatable fibrinolysis inhibitor antigen concentration and genotype in relation to myocardial infarction in the north and south of Europe. Arterioscl Thromb Vasc Biol. 2002;22:867–873. doi: 10.1161/01.atv.0000015445.22243.f4. [DOI] [PubMed] [Google Scholar]
- 28.Sampson M.J., Gopaul N., Davies I.R., Hughes D.A., Carrier M.J. Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25:537–541. doi: 10.2337/diacare.25.3.537. [DOI] [PubMed] [Google Scholar]
- 29.Stephens J.W., Gable D.R., Hurel S.J. Increased plasma markers of oxidative stress are associated with coronary heart disease in males with diabetes mellitus and with 10-year risk in a prospective sample of males. Clin Chem. 2006;52:446–452. doi: 10.1373/clinchem.2005.060194. [DOI] [PubMed] [Google Scholar]
- 30.Bolla M.K., Haddad L., Humphries S.E., Winder A.F., Day I.N. High-throughput method for determination of apolipoprotein E genotypes with use of restriction digestion analysis by microplate array diagonal gel electrophoresis. Clin Chem. 1995;41:1599–1604. [PubMed] [Google Scholar]
- 31.Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology (Cambridge, MA) 1990;1:43–46. [PubMed] [Google Scholar]
- 32.Perneger T.V. Adjusting for multiple testing in studies is less important than other concerns. BMJ (Clin Res Ed) 1999;318:1288. doi: 10.1136/bmj.318.7193.1288a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samani N.J., Boultby R., Butler R., Thompson J.R., Goodall A.H. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 34.Brouilette S., Singh R.K., Thompson J.R., Goodall A.H., Samani N.J. White cell telomere length and risk of premature myocardial infarction. Arterioscl, Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 35.Brouilette S.W., Moore J.S., McMahon A.D. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee M., Brouilette S., Stevens S., Shetty K.R., Samani N.J. Association of shorter telomeres with coronary artery disease in Indian subjects. Heart (British Cardiac Society) 2009;95:669–673. doi: 10.1136/hrt.2008.150250. [DOI] [PubMed] [Google Scholar]
- 37.Orie N.N., Zidek W., Tepel M. Increased intracellular generation of reactive oxygen species in mononuclear leukocytes from patients with diabetes mellitus type 2. Exp Clin Endocrinol Diabetes. 2000;108:175–180. doi: 10.1055/s-2000-7740. [DOI] [PubMed] [Google Scholar]
- 38.Packer J.E., Slater T.F., Willson R.L. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 39.Stocker R., Hunt N.H., Weidemann M.J., Clark I.A. Protection of vitamin E from oxidation by increased ascorbic acid content within Plasmodium vinckei-infected erythrocytes. Biochim Biophys Acta. 1986;876:294–299. doi: 10.1016/0005-2760(86)90287-0. [DOI] [PubMed] [Google Scholar]
- 40.Wilson W.R., Herbert K.E., Mistry Y. Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur Heart J. 2008;29:2689–2694. doi: 10.1093/eurheartj/ehn386. [DOI] [PubMed] [Google Scholar]
- 41.Tyrberg B., Anachkov K.A., Dib S.A. Islet expression of the DNA repair enzyme 8-oxoguanosine DNA glycosylase (Ogg1) in human type 2 diabetes. BMC Endocr Disorders. 2002;2:2. doi: 10.1186/1472-6823-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakuraba H., Mizukami H., Yagihashi N. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 43.Bekaert S., De Meyer T., Rietzschel E.R. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 44.Kyo S., Takakura M., Kanaya T. Estrogen activates telomerase. Cancer Res. 1999;59:5917–5921. [PubMed] [Google Scholar]
- 45.Gable D.R., Stephens J.W., Cooper J.A., Miller G.J., Humphries S.E. Variation in the UCP2–UCP3 gene cluster predicts the development of type 2 diabetes in healthy middle-aged men. Diabetes. 2006;55:1504–1511. doi: 10.2337/db05-1645. [DOI] [PubMed] [Google Scholar]
- 46.Hunt S.C., Chen W., Gardner J.P. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graakjaer J, Pascoe L., Der-Sarkissian H. The relative lengths of individual telomeres are defined in the zygote and strictly maintained during life. Aging Cell. 2004;3:97–102. doi: 10.1111/j.1474-9728.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- 48.Brouilette S.W., Whittaker A., Stevens S.E. Telomere length is shorter in healthy offspring of subjects with coronary artery disease: support for the telomere hypothesis. Heart (British Cardiac Society) 2008;94:422–425. doi: 10.1136/hrt.2007.139675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The correlation of telomere length (T/S ratio) with age in Caucasian type 2 diabetes patients. (B) The correlation of age-adjusted telomere length (T/S ratio) with plasma total antioxidant status (TAOS) in Caucasian type 2 diabetes patients.