Abstract
A series of ethacrynic acid analogues, lacking the α,β-unsaturated carbonyl unit, was synthesized and subsequently evaluated for their ability to inhibit the migration of human breast cancer cells, MCF-7/AZ. Several of the analogues were already active in the low micromolar range, whereas ethacrynic acid itself shows no potential to inhibit the migration of these cancer cells. Preliminary studies show that the presence of one or more methoxy groups at the phenyl ring of ethacrynic acid is important in order for the ethacrynic acid analogues to demonstrate an inhibitory effect on the migration.
Ethacrynic acid (EA) (Fig. 1), a loop diuretic, is used to treat high blood pressure and the swelling caused by diseases like congestive heart failure, liver failure, and kidney failure.1,2 EA possesses an α,β-unsaturated carbonyl unit which, structurally, can be considered as a Michael acceptor, an active moiety often employed in the design of enzyme inhibitors. For example, EA is known to be a good glutathion S-transferase P1-1 (GSTP1-1) inhibitor.3 As GSTP1-1 belongs to the SH-enzyme family, it is more than likely that the α,β-unsaturated carbonyl unit of EA inhibits the enzyme by binding to the cysteinyl residue in the active site by means of a Michael-like addition.
Figure 1.
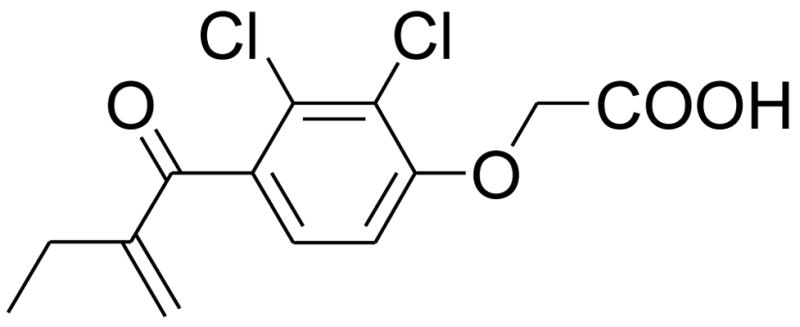
The chemical structure of ethacrynic acid (EA).
Several research groups have synthesized EA analogues to test their inhibitory properties on several enzymes. Jing et al. showed that EA also has anti-proliferative effects towards tumor cells but only at higher concentrations (60–100 μM).2,4–6
In another series of experiments, researchers could demonstrate that EA is cytotoxic towards primary chronic lymphocytic leukaemia (CLL) cells.7 However, EA is not ideal as a chemotherapeutic agent for cancer treatment due to its diuretic properties and relative lack of potency.8
An important aspect of cancer treatment is the prevention of metastases formation, because the metastatic spread of primary tumors to distant sites constitutes the most lethal aspect of cancer. Despite extensive research, the exact mechanisms of local invasion and the formation of metastases are still not entirely understood. The development and evaluation of biologically active compounds will give additional insight into this process and would therefore allow the development of new therapeutics that can effectively slow down or even prevent the metastatic process and consequently improve the treatment success of cancer patients.9
The metastatic process consists of a complex series of linked sequential steps, involving detachment of cells within a primary tumor and local area migration and invasion, followed by intravasation of invading tumor cells into the blood and lymphatic vessels. After circulating in the vascular system, tumor cells arrest and extravasate at distant sites where they form secondary tumors or metastases.10
Our results show that some analogues of EA, lacking the α,β-unsaturated carbonyl unit, are good to excellent inhibitors of the migration of human MCF-7/AZ breast cancer cells. Since migration is a prerequisite of invasion, these EA analogues could be lead compounds for drug development in order to prevent metastases formation.
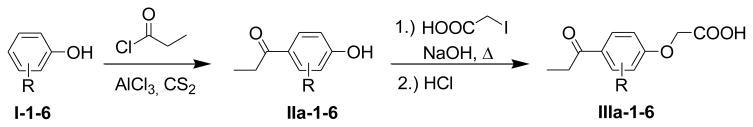
The preparation of the various ethacrynic acid analogues, lacking the α,β-unsaturated carbonyl unit (IIIa-1-6), was accomplished by a two-step reaction shown in Scheme 1. The Friedel-Crafts acylation reaction of the phenol I-1 or the substituted phenols I-2-6 (Fig. 2), respectively, with acyl chloride, was performed in the presence of powdered aluminum chloride (AlCl3) in carbon disulfide, to generate compounds IIa-1-6, respectively. To prevent cleavage of the methoxy groups of compounds I-4-6 by the Lewis-acid AlCl3, the reactions were carried out at 0°C. Additionally, at low temperatures, more of the para-acylated product was obtained with respect to the hydroxyl group. The obtained intermediates IIa-1-6 were treated with 2-iodocarboxylic acid in the presence of sodium hydroxide to give the corresponding sodium salts. Acidification of these salts with hydrochloric acid yielded compounds IIIa-1-6.
Scheme 1.
Synthetic pathway for the synthesis of EA analogues.
Figure 2.
Several substituted phenols I-1-6.
The results from the in vitro cytotoxicity assay11,12 (Table 1) show, that 24 h treatment of MCF-7/AZ cells with compounds IIIa-1, IIIa-2, and IIIa-3 at concentrations of 25μM, 20μM, and 10μM, respectively, results in a 20% reduction of cell viability. Compound IIIa-1 is not substituted at the phenyl ring, whereas compound IIIa-2 possesses a methyl substituent in ortho-position to the phenolic oxygen atom and compound IIIa-3 possesses a methyl group in meta-position with respect to the phenolic oxygen atom.
Table 1.
The inhibitory effect of ethacrynic acid (EA) and EA analogues, lacking the α,β-unsaturated carbonyl unit (IIIa-1-6), on the migration of human MCF-7/AZ breast cancer cells and their respective observed cytotoxicities expressed in OD80/IC20 values.
| Code | Structure | Inhibition (%) | OD80/IC20 (μM) |
|---|---|---|---|
| EA | 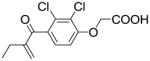 |
none | >100 |
| IIIa-1 | none | 25 | |
| IIIa-2 | none | 20 | |
| IIIa-3 | none | 10 | |
| IIIa-4 | 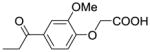 |
38 | >100 |
| IIIa-5 | 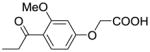 |
29 | >100 |
| IIIa-6 | 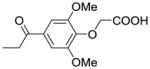 |
52 | >100 |
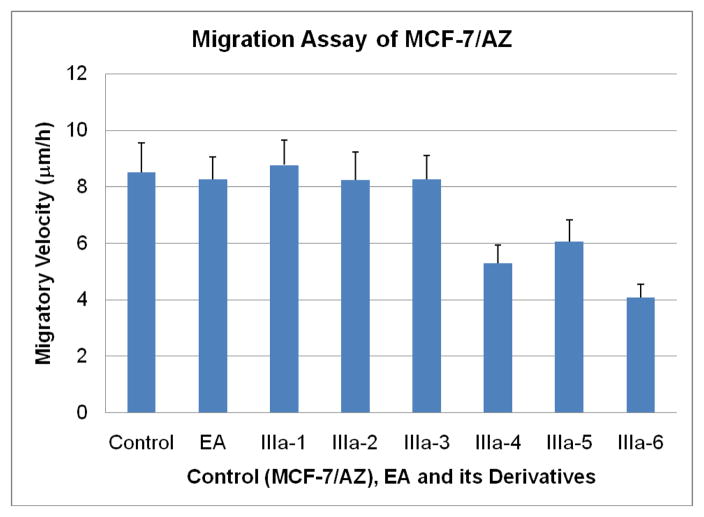
In contrast, no significant cytotoxic effect could be observed for compounds IIIa-4, IIIa-5, and IIIa-6, at concentrations lower or equal to 100μM. These three compounds have in common that their phenyl rings are substituted with either one (IIIa-4, IIIa-5) or two (IIIa-6) methoxy groups. The in vitro wound healing assay13,14 shows very interesting and promising results (Fig. 3). Compounds IIIa-1, IIIa-2, and IIIa-3 show no substantial effect on the migration of the breast cancer cells. In stark contrast to these results, compounds IIIa-4, IIIa-5, and IIIa-6 show a significant inhibition of the migration of the cancer cells. Compound IIIa-4 inhibits the migration of the human MCF-7/AZ breast cancer cells by 38%, compound IIIa-5 by 29% and compound IIIa-6 even by 52%.
Figure 3.
Migration assay of the human breast cancer cell line, MCF-7/AZ, in the absence (control) or presence of ethacrynic acid (EA) and its analogues (IIIa-1-6).
The data from the wound healing assay suggest that electron donating groups, attached to the aromatic system of the EA analogues, have a positive effect on the potency of the corresponding compounds to inhibit the migration of human MCF-7/AZ breast cancer cells. Additionally, these compounds don’t show any observable cytotoxicity at concentrations up to 100 μM.
Electron donating groups, like the methoxy groups, increase the electron density of the aromatic system by donating lone pair electrons into the aromatic system by resonance, thereby increasing its reactivity towards electrophiles. On the other hand, methyl groups are weak activators, they can only donate electron density into the aromatic system through the σ-bond, resulting in only a slight increase in reactivity towards electrophiles.
It can be speculated that the strong increase of electron density in the aromatic system is responsible for the anti-migratory properties of compounds IIIa-4, IIIa-5, and IIIa-6. In further investigations, other electron donating substituents will be attached to the phenyl ring. These new compounds will subsequently be evaluated for their effect on the migratory capacity of human MCF-7/AZ breast cancer cells.
Due to the relative lack of potency and its diuretic properties, EA is not suitable as a drug in cancer treatment. Several of the analogues mentioned above demonstrate anti-migratory activity towards the migration of human MCF-7/AZ breast cancer cells at non-toxic concentrations. Due to the considerable difference in structure of the new analogues versus EA, it can be speculated that these analogues do not possess a diuretic nature. These trends are therefore worth to be studied further with an eye to drug development.
In summary, we have synthesized EA analogues, lacking the α,β-unsaturated carbonyl unit, with enhanced potency, relative to EA, towards the inhibition of migration of human MCF-7/AZ breast cancer cells (Table 1 and Fig. 3). Differences in potency among the various analogues may simply be due to the presence of different substituents at the phenyl ring of the EA analogues. Further studies with other EA analogues and various cancer cell lines are underway.
Acknowledgments
We thank New Mexico Tech for supplying the start-up funds for Dr. I. Janser. The biomedical evaluation of this work was supported by the US National Institutes of Health (RR-16480) under the INBRE program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Zhao G, Yu T, Wang R, Wang X, Jing Y. Bioorg Med Chem. 2005;13:4056. doi: 10.1016/j.bmc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Zhao G, Liu C, Wang R, Song D, Wang X, Lou H, Jing Y. Bioorg Med Chem. 2007;15:2701. doi: 10.1016/j.bmc.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 3.Iersel ML, Ploemen JP, Struit I, Van Amersfoort C, Keyzer AE, Schefferlie JG, Van Bladeren PJ. Chem Biol Interact. 1996;102:117. doi: 10.1016/s0009-2797(96)03739-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhao GS, Wang XB. Curr Med Chem. 2006;13:1461. doi: 10.2174/092986706776872934. [DOI] [PubMed] [Google Scholar]
- 5.Aizawa S, Ookawa K, Kudo T, Asano J, Hayakari M, Tsuchida S. Cancer Sci. 2003;94:886. doi: 10.1111/j.1349-7006.2003.tb01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Liu G, Li H, Zhang Y, Song D, Li C, Wang R, Liu B, Liang W, Jing Y, Zhao G. J Med Chem. 2010 doi: 10.1021/jm9011565. Publication Date (Web): January 7, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Twentyman PR, Lambert E, Muller M, Rees JK. Leukemia. 1992;6:726. [PubMed] [Google Scholar]
- 8.Jin G, Lu D, Yao S, Wu CCN, Liu JX, Carson DA, Cottam HB. Bioorg Med Chem. 2009;17:606. doi: 10.1016/j.bmcl.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christofori G. Nature. 2006;25:444. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 10.Fidler IJ. Nat Rev Cancer. 2003;3:453. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 11.The cytotoxicities of ethacrynic acid and its analogues, lacking the α,β-unsaturated carbonyl unit, were tested in accordance to Sigstedt et al.12 Mitochondrial dehydrogenase activities were measured by an MTT-reagent. Cells were seeded in 96-well plates at an initial density of 1.5 × 104 cells in 200 μL of the appropriate culture medium, supplemented with 5% fetal bovine serum (FBS), 100 IU/mL penicillin, 100 μg/mL streptomycin. After 24 h of incubation, at 37 C in a humidified atmosphere containing 5% CO2, cells were treated with eight different concentrations (1, 5, 10, 20, 40, 60, 80, and 100 μM) of EA and various EA analogues in culture medium. After 24 h of incubation, 100 μL medium was removed prior to the addition of the MTT reagent. Three independent experiments were carried out for each concentration, to determine the mean optical density (OD) referring to cell viability. OD80/IC20 values were determined from the respective graphs. These values represent the concentrations of the analogues, required for 20% inhibition in vitro or leaving approximately 80% of the cells viable.
- 12.Sigstedt SC, Hooten CJ, Callewaert MC, Jenkins AR, Romero AE, Pullin MJ, Kornienko A, Lowrey TK, Van Slambrouck S, Steelant WF. Int J Oncol. 2008;32:1085. [PubMed] [Google Scholar]
- 13.Cells were grown in 6-well plates in the appropriate medium until confluence and then washed twice with phosphate buffered saline (PBS). A gap of approximately 1500 μm (the wound) was created in the cell monolayer and images were captured. Subsequently, 3 mL of medium, in the presence or absence of ethacrynic acid or EA analogues, was added. The respective concentrations of these solutions were determined in the 24 h MTT assay. After 24 h of cell migration in order to close the wound, images were once again captured, and compared to the respective previous images in order to quantify the migration rate, which is expressed as migratory velocity (μm/h). At least three independent experiments were performed.
- 14.Van slambrouck S, Hilkens J, Bisoffi M, Steelant WFA. Int J Oncol. 2009;35:693. doi: 10.3892/ijo_00000381. [DOI] [PMC free article] [PubMed] [Google Scholar]