Abstract
Objective To determine whether BCG revaccination at 19 months of age reduces overall child mortality.
Design Randomised trial, with follow-up to age 5.
Setting A health project in Bissau, Guinea-Bissau, which maintains a health and demographic surveillance system in an urban area with 90 000 inhabitants.
Participants 2871 children aged 19 months to 5 years with low or no reactivity to tuberculin and who were not severely sick on the day of enrolment.
Intervention BCG vaccination or no vaccination (control).
Main outcome measure Hazard ratios for mortality.
Results 77 children died during follow-up. Compared with controls, the BCG revaccinated children had a hazard ratio of 1.20 (95% confidence interval 0.77 to 1.89). Two hundred and fifty children were admitted to hospital for the first time between enrolment and the end of the study, with an incidence rate ratio for BCG revaccinated children versus controls of 1.04 (0.81 to 1.33). The trial was stopped prematurely because of a cluster of deaths in the BCG arm of the study. This increase in mortality occurred at a time when many children had received missing vaccinations or vitamin A or iron supplementation; the hazard ratio for BCG revaccinated children compared with controls was 2.69 (1.05 to 6.88) in the period after these campaigns. Throughout the trial, the effect of BCG revaccination on mortality was significantly different (P=0.006) in children who had received diphtheria-tetanus-pertussis (DTP) booster vaccination before enrolment (hazard ratio 0.36, 0.13 to 0.99) and children who had not received the booster before enrolment (1.78, 1.04 to 3.04).
Conclusions There was no overall beneficial effect of being revaccinated with BCG. The effect of BCG revaccination on mortality might depend on other health interventions.
Trial registration Clinical Trials ICA4-CT-2002-10053-REVAC.
Introduction
Routine infant vaccines currently used in low income countries were not tested in randomised trials for their impact on overall child survival before their introduction.1 It has been assumed that the impact of a vaccine on mortality is proportional to the vaccine’s efficacy and the contribution of the target disease to overall mortality. The past 15 years of research on vaccines in low income countries, however, have shown that this assumption is not a tenable basis for vaccination policy1 2 3 4; vaccines might have important non-specific effects. This is best documented for standard measles vaccine. Numerous studies of measles vaccine have reported significant reductions in all cause mortality that could not be explained by the prevention of acute measles or its long term consequences.1 Furthermore, girls who received high titre measles vaccines had twice the mortality compared with girls who had received standard measles vaccine.2 3 As high titre measles vaccines are fully protective against measles infection, this suggests that non-specific effects are important for the total impact of a vaccine on mortality.
BCG vaccination might also have non-specific beneficial effects on overall mortality.4 When it was introduced in high income countries in the 1920s and 1930s, the vaccine was said to have a beneficial effect beyond protection against tuberculosis.5 A few of the well designed studies (randomisation, alternation) comparing individuals who were or were not vaccinated with BCG looked at the effect on mortality associated with tuberculosis and all cause mortality. All such studies, including general population studies6 7 and studies among contacts of people with tuberculosis,8 9 found lower mortality from other causes among those who received the BCG vaccination. Recently, several observational studies in low income countries have reported that BCG was associated with a reduction in mortality that could not be explained by prevention against tuberculosis, particularly in girls.10 11 As randomised studies of BCG in low income countries have not been carried out, we cannot be certain about the extent of these effects.
Diphtheria-tetanus-pertussis (DTP) vaccine has been associated with increased mortality, especially in girls.3 12 13 14 15 The excess female mortality after high titre measles vaccine3 and standard measles vaccine12 has been shown to be associated with receipt of DTP vaccine after the measles vaccine. Thus we started two trials of interventions designed to ameliorate these undesirable effects of DTP vaccine. Firstly, we provided BCG revaccination after DTP booster vaccination at 18 months, with the aim of inducing beneficial immune stimulation. Secondly, reported elsewhere,16 we administered measles vaccine early at age 4.5 months after the third dose of DTP vaccine to see whether this would reduce mortality before all children received their measles vaccination at 9 months.
There are different policies regarding BCG revaccination throughout the world.17 18 19 20 21 22 The World Health Organization (WHO) does not recommend BCG revaccination (www.who.int/immunization/wer7904BCG_Jan04_position_paper.pdf), but several countries still revaccinate teenagers. Though some studies have documented that PPD (tuberculin purified protein derivative or Mantoux test) and scar reactions get larger with revaccination,23 24 others have found no effect of revaccination on protection against pulmonary tuberculosis but found that it might improve protection against leprosy.17 24 Only one study has reported overall mortality after revaccination with BCG. In 1930-50 the Pasteur Institute in Alger conducted a large study with alternate allocation of 41 000 children. They found that vaccination at birth and revaccination with oral BCG at age 1 and 3 years was associated with a 27% reduction in mortality (95% confidence interval 22% to 31%) between 1 and 11 years.25 26 The impact on overall mortality of revaccination with intradermal BCG vaccination has not been examined. We assessed whether intradermal revaccination with BCG is associated with a reduction in childhood mortality between 19 months and 5 years.
Methods
Setting, study population, and routine data collection
The study was conducted in the Bandim Health Project’s study area, which covers six districts with about 90 000 inhabitants or around 30% of the population of the capital. The project has implemented a routine data collection system in this area. A census has been carried out regularly, and all houses have painted numbers and have been mapped. All residents have an identification number in the census files, and information on place of living can be retrieved from these files along with socioeconomic and demographic information. All houses are visited every month to register new pregnancies and births. All children are given a UNICEF road to health card at birth, which is used to trace children when they attend health centres or the hospital. All children are visited at home every three months until the age of 3 years and information collected on risk factors associated with child survival, including breastfeeding status, infections, admissions to hospital, vaccination status, and living with the mother. There are three health centres in the area.
Vaccination status—Data on vaccination status are gathered in two complementary ways. Firstly, all vaccinations given at the three health centres in the study area are registered on the day of vaccination. This covers more than 80% of the vaccinations received in the study area but does not account for those obtained outside the study areas, such as when the mother is travelling or if she lives close to another health centre outside the study area. Secondly, at the regular three monthly home visits, the field assistant brings a list on which previously registered vaccinations are already printed. The vaccination card is inspected and the field assistant adds newly received vaccinations to the list. Such data are obtained only from children whose vaccination card can be seen at the home visit and, importantly, only from surviving children.27
Survival status—Observations on deaths are obtained from the paediatric ward and through the three monthly home visits. Mothers from Bissau often travel to visit relatives living in other parts of the country. Most women earn their living in the cashew harvest, going to the rural areas from April to June, and about 20% of the mothers and their children are travelling at any given time. It is always possible, however, to obtain information on the survival status of a child; if a child dies in the rural areas, the family in the city will be informed immediately.
Study objective
We hoped to reduce a suspected negative effect of booster DTP vaccination at 18 months of age3 12 13 14 15 28 by revaccinating with BCG after DTP booster vaccination. Our main objective was to examine whether BCG revaccination would reduce child mortality by 30%.
Study design
When the trial started in July 2002 the vaccination programme in Guinea-Bissau comprised BCG and oral polio vaccine at birth; DTP and oral polio vaccine at age 6, 10, and 14 weeks; measles vaccine at 9 months; and booster doses of DTP and oral polio vaccine at 18 months. To prevent interference with the booster doses of DTP and oral polio vaccine, BCG revaccination was implemented at 19 months. We recruited children thought not to have tuberculosis and followed them for mortality and admissions to hospital until the age of 5 years. The analysis controlled for age and sex. We separately analysed children who were exposed to tuberculosis because they lived in the same house as a person with diagnosed tuberculosis.
In May 2006 the Ministry of Health organised a general measles vaccination campaign for all children under 15, when the mean age of children in the enrolled cohort was 5.4. We did not know about this campaign when we planned the BCG study. We have previously shown that measles vaccine has strong non-specific effects on child survival.1 3 As little follow-up was left and additional measles vaccination would make it impossible to measure any difference between BCG vaccinated children and controls, we decided to end follow-up in May 2006.
Intervention
Children enrolled in the study were randomised to receive BCG vaccine (Statens Serum Institut, Copenhagen, Denmark) or no vaccine. The BCG vaccine was given in the standard intradermal dose of 0.1 ml, as recommended by WHO for this age group. In all other respects the two groups were treated equally; they received the same clinical examination and treatment before enrolment, the same home visits to assess adverse events and tuberculin skin test reactions, and the same access to consultations and essential drugs.
Outcomes
Our primary outcome was survival between 19 months and 5 years. Secondary outcomes included admission to hospital, BCG scarring, tuberculin reactivity, and malaria morbidity. As reported elsewhere we followed a subgroup of the study cohort for malaria infection.29 In another subgroup, we collected blood samples to measure changes in cytokine profile after BCG revaccination. We report the results for overall mortality, admission to hospital, and adverse events.
Sample size
Based on data from 1990-8, we expected an annual mortality of 4.0% between the ages of 18 months and 4 years. With a significance level of 5%, a power of 80%, and 1:1 allocation of children to revaccination and control groups, we needed 3750 child years in each group with 150 deaths in the control group to document a 30% reduction in mortality. Assuming recruitment of 3000 children over a two year period with an average follow-up of three years, and no more than a 20% loss to follow-up, we expected to have at least 7500 child years of follow-up in the trial.
Enrolment
At 18-19 months of age, all children registered as living in the study area were assessed for BCG scar and tuberculin reaction. Tuberculin reactivity was measured with the Mantoux method, with an intradermal injection of 2 tuberculin units (RT23, Statens Serum Institute) and subsequent reading within 48-72 hours of bulae formation with the ball point technique. Children with a tuberculin reaction of 15 mm or more were referred to an experienced clinician to be examined for possible tuberculosis infection. If the child was considered to need tuberculosis prophylaxis or tuberculosis treatment according to a diagnostic scoring system this was supplied as currently recommended by the local tuberculosis programme.30
A field worker contacted the mothers/guardians of the children during the morning. They explained the study and filled in a questionnaire on background factors and vaccination status. They documented major risk factors for childhood mortality, including exposure to tuberculosis, recent infections, access to malaria drugs, ethnic group, mother’s education, and recent drug consumption. The mother was asked to bring the child to the health centre in the afternoon. When the study started in July 2002 four districts and two health centres participated. From the end of April 2003, two more districts and the third health centre in the area were included.
Inclusion criteria were residence in the study area, a Mantoux test reaction of less than 15 mm, and being sufficiently healthy to be vaccinated according to the clinician. Exclusion criteria were a reaction of 15 mm or more and not being sufficiently healthy to be vaccinated according to the clinician. Children did not have to have documentation of previous BCG vaccination or previous booster DTP vaccination to be included. We assumed that essentially all children had received BCG during the first year of life, and we were testing the effect of a general introduction of BCG revaccination in the community.
Informed consent
In the afternoon, the mothers/guardians of children presenting at the local health centre received an oral and a written explanation of the study from a physician. The physician performed a medical examination. Clinical examination and treatment was independent of consent and randomisation group. The clinical examination included anthropometrics and assessment of vaccination status from the vaccination card. Children who were missing doses of oral polio, DTP, or measles vaccines were advised to complete vaccinations. Children who were ill were not randomised. They received treatment according to local standards and were told to return when fully recovered.
Randomisation
The data manager, who was not involved in the recruitment of children, prepared bags of envelopes containing printed allocation numbers indicating to which of the two groups the child should be allocated. Allocation numbers could not be seen by the physician informing and obtaining consent from the mother/guardian.
If consent was obtained and if the clinician considered the child fit for participation, the mother or guardian was asked to pick an allocation number defining the randomisation group. This procedure has been used in several other trials in the study area to emphasise to the mother that she is participating in a randomised trial. Randomisation was even between the two groups, with block randomisation with 40 envelopes per bag.
Masking
There was no placebo for BCG and no “control” vaccine was given.
Adverse events
At the beginning of the study, we followed a group of 400 revaccinated and 400 control children weekly during the first month after enrolment to examine morbidity and to monitor possible adverse effects of the vaccination. Vaccination site, tuberculin reactivity, morbidity, and hospital visits were assessed after two and six months. We classified pustules ≥10 mm in diameter as a large local reaction to BCG injection compared with the normal local reaction to BCG (pustule, spontaneous drainage, and scarring).31 32
We did not expect that Koch-like reactions would be common in this age group. We did plan, however, that if too many adverse reactions were seen among children with a positive tuberculin test result (1-14 mm), we would include only children with negative results in the continuation of the trial.
Follow-up
Apart from the three monthly routine surveillance visits to all children in the study area, all children enrolled in the trial were visited two and six months after inclusion to assess tuberculin reaction and BCG scarring. The children were followed for admissions to hospital and mortality to age 5 years through the demographic and hospital surveillance systems. A trained local physician implemented a standardised verbal autopsy when one of the study children had died. We intended to censor deaths from injury in the survival analyses as these are unrelated to any immune stimulatory effect of BCG. None of the participating children reportedly died from injuries.
Admissions to hospital—Two assistants from the Bandim Health Project work at the national hospital and register all children admitted to the only paediatric ward in the country. Using the vaccination cards they attempt to identify all children from the study area.
Tuberculosis surveillance—The project has maintained a tuberculosis surveillance system in the study area since 1997, and it has therefore been possible to conduct separate analyses for children who were or were not exposed to tuberculosis at home.30 33 34
Campaigns—The project documented individual participation in all vitamin A supplementation and vaccination campaigns in the study area during the conduct of the present trial. The annual campaigns conducted in 2002,35 2004, and 2005 provided oral polio vaccine and vitamin A supplementation. These campaigns were based on small mobile teams visiting all houses in the study area. Each team was accompanied by a project field worker, who noted participation on a registration list for all children in the subdistrict. In 2003 there was no oral polio vaccine campaign but a campaign was organised in November 2003 with vitamin A supplementation and missing vaccines being distributed from 15 fixed posts in the area.36 At each post, project field workers documented participation. Participation in this campaign was considerably lower than in the house to house campaigns. In May 2006, a campaign with measles vaccine administered to all children aged 6 months to 14 years was conducted in Guinea-Bissau. This campaign with fixed posts also included distribution of vitamin A supplementation to all children aged 6 months to 4 years and mebendazol to children aged 1-4 years. Project field workers at all fixed posts documented participation. The vaccination status and survival of all children less than 5 years was assessed in connection with these campaigns.
Conduct of the trial
As will be apparent from the accompanying commentaries and editorial, the present publication is controversial. We had no funding for a data monitoring and safety board and there were no predefined stopping rules. The senior author (PA), who is not a clinician and not involved in clinical care related to any child in the trial, monitored mortality registered by the routine registration system quarterly as a check of safety. As previous experience has shown large and unexpected effects of vaccinations on mortality3 15 37 we thought it essential to identify unexpected safety issues when a potential new vaccination strategy is tested in this environment with high childhood mortality.
Safety monitoring
When we checked data in the beginning of April 2004, we identified a sudden increase in mortality starting at the end of 2003. Eighteen children in the BCG vaccination and four in the control group had died between November 2003 and March 2004, whereas there had been nine and 14 deaths, respectively, in the preceding 14 months since the start of the trial. We decided to stop the trial temporarily to prevent a possible but unknown risk to more children; at that time only 130 of the planned 3000 children had not been enrolled. The increase in mortality apparently started in November, when several campaigns with vitamin A supplementation, missing vaccinations, and iron treatment had been implemented, and at the same time a major measles epidemic started in the area. In an attempt to explain the unexpected findings we used mid-November 2003 as the starting point of the analyses. Use of any month back to June 2003 as cut point between the two periods, however, produced a significant inversion in mortality trends.
When we discovered the increase in mortality we carried out a follow-up assessment of survival of all children in the study. Free access to clinical care and essential drugs, which initially had been planned for the first year after enrolment, was extended for the full duration of the study. With longer follow-up there was no difference between the groups. At the same time we noted that overall mortality in the trial was considerably lower (2.5% a year) than originally anticipated (4% a year); no further corrective measures were therefore introduced. As we could not explain these findings and because the addition of another 130 children would matter little to the outcome of the trial, we decided not to restart enrolment.
Explorative analyses
Because of the unexpected cluster of deaths, we initiated a series of explorative analyses to clarify possible environmental causes. The timing of the trial coincided with major health interventions, campaigns, and epidemics (see legend to fig 1). The explorative analyses were limited by the routine data available about these events; the detection of epidemics other than measles was restricted by the limited diagnostic data collected at the paediatric ward. The paediatric ward was totally overbooked by measles cases in precisely the months of the cluster.
It should be noted that the statistical analysis of the cluster was conducted with a method that does not depend on setting specific dates for defining the cluster (see below). The explorative analysis of possible causes, however, had to be restricted to the period in which the potential cause was operational. When the cluster was detected it looked like something had happened in November 2003; a vitamin A supplementation and missing vaccinations campaign had occurred in the third week of November,36 the measles epidemic had gained strength around this time, and iron supplements had been distributed in mid-November. We therefore focused our explorative analyses of increased mortality on the period mid-November 2003 to March 2004. Hence, July 2002 to mid-November 2003 was the period before the increase and April 2004 to May 2006 the period after the increase.
We planned to measure the effect of BCG revaccination at age 19 months, after DTP vaccination, which was usually administered at 18 months. After the protocol was originally written, we found that the sequence of vaccinations—for example, DTP vaccine given after measles vaccine—has a major impact on the mortality effect of a trial.3 12 16 38 We therefore also conducted explorative analyses of the sequence of vaccination and the effect of other vaccines administered after enrolment in the trial.
Statistical analyses
As defined by the protocol, we compared admissions to hospital and mortality rates in Cox proportional hazards analyses, controlling for age as underlying time and sex, to estimate incidence rate ratios for admission to hospital and hazard ratios for mortality for the two arms of the study. Results were presented as hazard ratios with 95% confidence intervals. The assumption of proportionality was tested in several models with age, time since enrolment, and calendar time as underlying scale. The Kaplan-Meier method was used to depict crude cumulative mortality curves.
Children were followed to death, migration, age 5 years, or the general measles vaccination campaign in May 2006, whichever came first. The analysis of the increase in mortality was censored 1 April 2004—that is, at the time we noted an unusual distribution of deaths. We used the non-parametric total-time-on-test plot and total-time-on-test statistics for censored data39 to test the hypothesis of an increase in mortality. The total-time-on-test determines whether the mortality intensity is constant versus the alternative (increasing or decreasing). The test does not need to split the follow-up into separate periods and thus avoids the problem of defining cut points for testing an increased or decreased mortality rate. For the test plot, boundaries corresponding to a Kolmogorov-Smirnov-type test can be drawn and if the total-time-on-test curve crosses any of the boundaries the hypothesis of a constant mortality rate can be rejected.39 For the total-time-on-test we used calendar time as the time scale with time unit equal to days and interpreted a significant result as an increase or decrease of mortality during the study period. A separate test was calculated for each randomisation group.
Results
Trial children
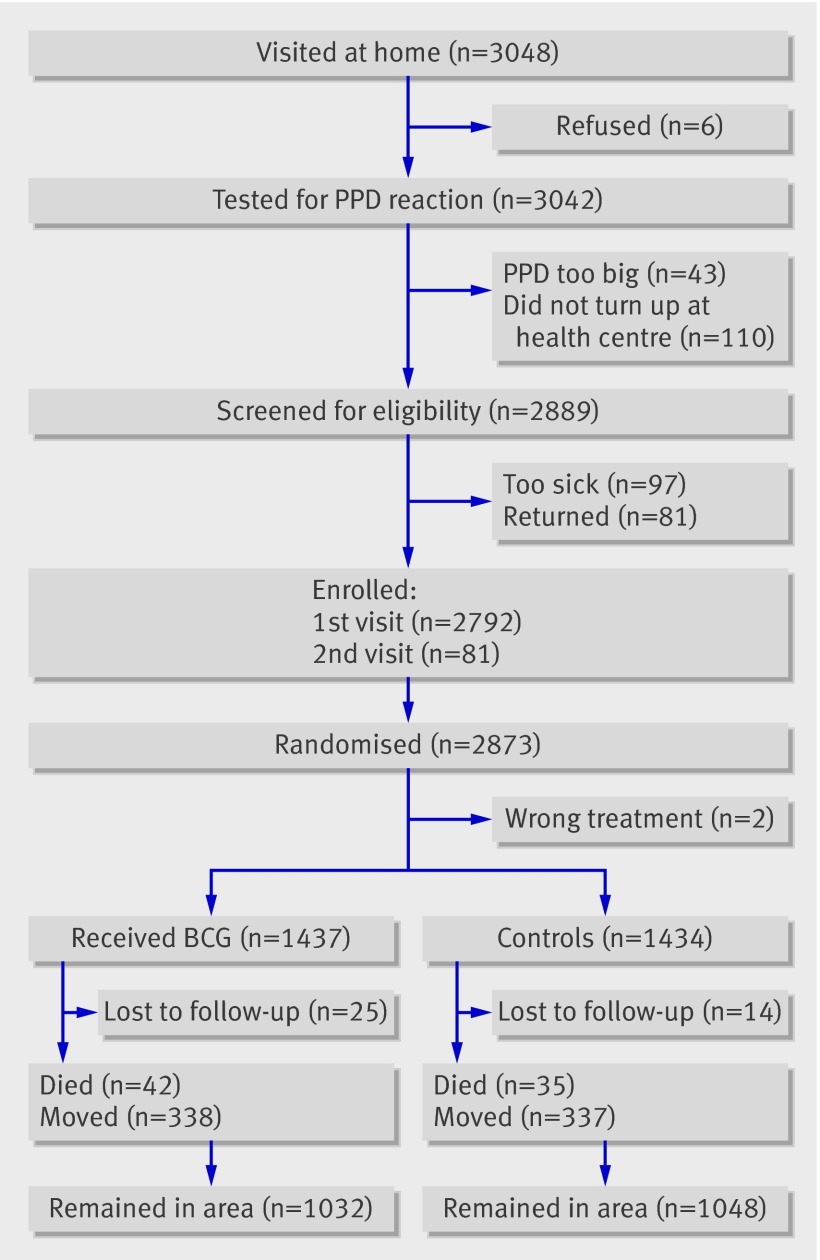
Between July 2002 and April 2004 we enrolled 2873 (96%) of the planned 3000 children (fig 1). Two children received the opposite treatment to the one indicated by their randomisation number and were not considered further. Thirty nine children (1%) had no follow-up beyond the day of enrolment, leaving 2832 children for analysis. Until censoring, enrolled children contributed 6827 of the planned 7500 person years of follow-up. There were no major differences in socioeconomic and biological background factors for the children enrolled in the two arms of the trial, except that revaccinated children had more scabies than control children (6% v 4%) (table 1).
Fig 1 BCG revaccination trial profile. PPD=tuberculin purified protein derivative
Table 1.
Distribution of background factors for two trial arms. Figures are numbers (percentage) unless stated otherwise
| Variables | BCG revaccination group (n=1437) | Control group (n=1434) |
|---|---|---|
| Boys | 732 (51) | 704 (49) |
| Twin | 49 (3) | 56 (4) |
| Ethnicity (% Pepel) | 482 (34) | 505 (35) |
| Living in Bandim-1 (district) | 557 (39) | 571 (40) |
| Family | ||
| Mother’s mean (SD) No of deliveries | 2.8 (1.9) | 2.9 (1.9) |
| Mean No (SD) of children still alive | 2.6 (1.6) | 2.6 (1.6) |
| Mother died | 3 (0.2) | 5 (0.3) |
| Father died | 10 (0.7) | 14 (1.0) |
| Mother had BCG scar | 524 (36) | 548 (38) |
| Mother had smallpox scar | 84 (6) | 89 (6) |
| Apartment had only 1 room | 586 (41) | 577 (40) |
| Median (IQR) No of people per family | 6 (4-8) | 6 (4-8) |
| Had chloroquine at home | 165 (11) | 172 (12) |
| Tuberculosis patient in house | 46 (3) | 40 (3) |
| Had pigs in house | 301 (21) | 317 (22) |
| Status at enrolment | ||
| Breast feeding (%) | 949 (66) | 983 (69) |
| Previous admission to hospital | 144 (10) | 165 (12) |
| Documented admission to paediatric ward before enrolment | 127 (9) | 130 (9) |
| Median (IQR) arm circumference (mm) | 148 (140-154) | 148 (140-154) |
| Median (IQR) height (cm) | 81 (78-83) | 81 (78-83) |
| Median (IQR) weight (kg) | 10.1 (9.3-11.1) | 10.2 (9.4-11.1) |
| Diarrhoea* | 119 (8) | 137 (10) |
| Fever* | 443 (31) | 442 (31) |
| Respiratory infection* | 265 (18) | 260 (18) |
| Scabies† | 93 (6) | 59 (4) |
| Eye infections† | 125 (9) | 133 (9) |
| Considered too sick to be enrolled at first visit† | 46 (3) | 34 (2) |
| Vaccination status | ||
| Showed vaccination card | 1298 (90) | 1298 (91) |
| Had BCG scar | 1117 (78) | 1089 (76) |
| Measles vaccinated | 1301 (91) | 1293 (90) |
| DTP3 | 1232 (86) | 1231 (86) |
| DTP4 (booster dose) | 571 (40) | 575 (40) |
IQR=interquartile range; DTP=diphtheria-tetanus-pertussis.
*Information reported by mother/guardian.
†Observed by clinician.
Main outcome: overall mortality
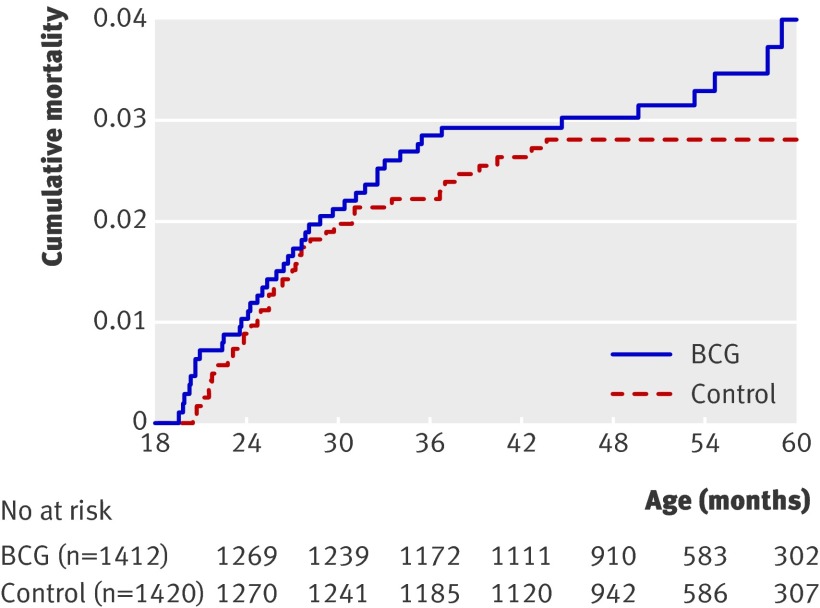
Figure 2 shows the distribution of deaths over time, and figure 3 shows cumulative mortality curves. BCG revaccination had no overall effect on mortality, the hazard ratio for BCG revaccinated children compared with non-BCG revaccinated children being 1.20 (95% confidence interval 0.77 to 1.89) (table 2). The pattern was the same for boys and girls (data not shown). A total of 98% (2811/2871) of the children had valid documentation of primary BCG vaccination. We had no such documentation for 60 children but 54 claimed to have received it. Exclusion of these 60 children had no effect on the overall results.
Fig 3 Cumulative mortality curves for BCG revaccinated children and controls
Table 2.
Mortality rates and hazard ratios for BCG revaccinated and controls overall* and by mortality periods and vaccination status at enrolment
| Mortality periods | Mortality rate/100 person years (deaths/days) | Hazard ratio (95% CI) for BCG v no BCG | |
|---|---|---|---|
| BCG revaccination | Controls | ||
| Total period | 1.2 (42/1 242 701) | 1.0 (35/1 251 051) | 1.20 (0.77 to 1.89) |
| Explorative analysis: mortality periods | |||
| Before increase | 1.5 (11/272 281) | 2.0 (15/269 691) | 0.73 (0.34 to 1.59) |
| During increase | 3.8 (16/154 401) | 1.4 (6/155 684) | 2.69 (1.05 to 6.88) |
| After increase | 0.7 (15/816 019) | 0.6 (14/825 676) | 1.08 (0.52 to 2.24) |
| Explorative analysis: vaccination status at enrolment | |||
| Booster DTP before enrolment | 0.4 (5/508 443) | 1.0 (14/513 158) | 0.36 (0.13 to 0.99) |
| No booster DTP before enrolment | 1.8 (37/734 258) | 1.0 (21/737 893) | 1.78 (1.04 to 3.04) |
DTP=diphtheria-tetanus-pertussis.
*Includes 2832 children who had at least some follow-up.
Exclusion of children who had been exposed to tuberculosis from six months before treatment was initiated had no effect on the results, the hazard ratio being 1.26 (0.80 to 1.99). Among children exposed to tuberculosis in their home some time during their life there were too few deaths to assess whether revaccination had any significant impact on survival; there was one death among revaccinated children (1/172 961 person days) and five in the control group (5/174 755 person days), the hazard ratio being 0.20 (0.02 to 1.69).
Secondary outcome: adverse events
Among the first 400 revaccinated and 400 control children included, nine (six and three, respectively) had a positive tuberculin reaction at inclusion (Mantoux test result diameter 1-14 mm). During the two months after inclusion adverse events were assessed in 787 of the 800 children. In the revaccinated children, 5.3% (21/394) had a large local reaction (≥10 mm). No reactions persisted six months after vaccination or led to surgical drainage, admission to hospital, or death. Among revaccinated children with a positive tuberculin reaction there was an over-representation of large local reactions (3/6) compared with revaccinated children with no tuberculin reaction (18/388) (risk ratio 10.78, 4.30 to 27.01), but two months after revaccination all had healed vaccination scars with no axillary lymph node enlargement, fever, suppurative lymphadenitis, or admission to hospital. As there were no serious or persistent adverse events among children with a positive tuberculin reaction, we decided to continue to include children with a tuberculin reaction <15 mm. There was a tendency for axillary lymph nodes to be enlarged (≥15 mm in diameter) in the two months after revaccination (24/394), compared with those who were not revaccinated (14/393) (1.28, 0.99 to 1.65), that was not present six months after revaccination.
Secondary outcome: admissions to hospital
Between enrolment and the end of the study in 2006, 250 children were admitted to hospital for the first time, the incidence rate ratio for BCG revaccination versus control group being 1.04 (0.81 to 1.33). There was an increase in the incidence of admissions to hospital at the same time as mortality increased (see figure on bmj.com). The annual incidence rate for the whole study period among the children who were revaccinated was 3.1% for girls and 4.8% for boys, giving an incidence ratio of 0.65 (0.46 to 0.93). Among the control group the incidence was 3.8% and 3.8%, giving an incidence rate ratio of 1.00 (0.70 to 1.43) (P=0.094 for interaction between revaccination and sex). Inclusion of subsequent admissions made no difference to the estimates (data not shown)
Explorative analyses of mortality
Increase in mortality
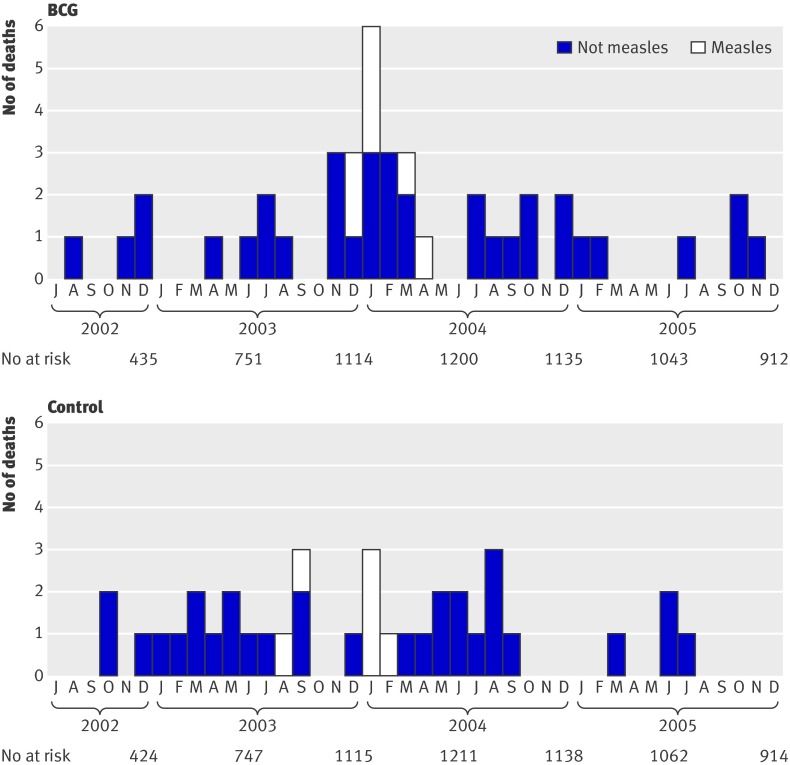
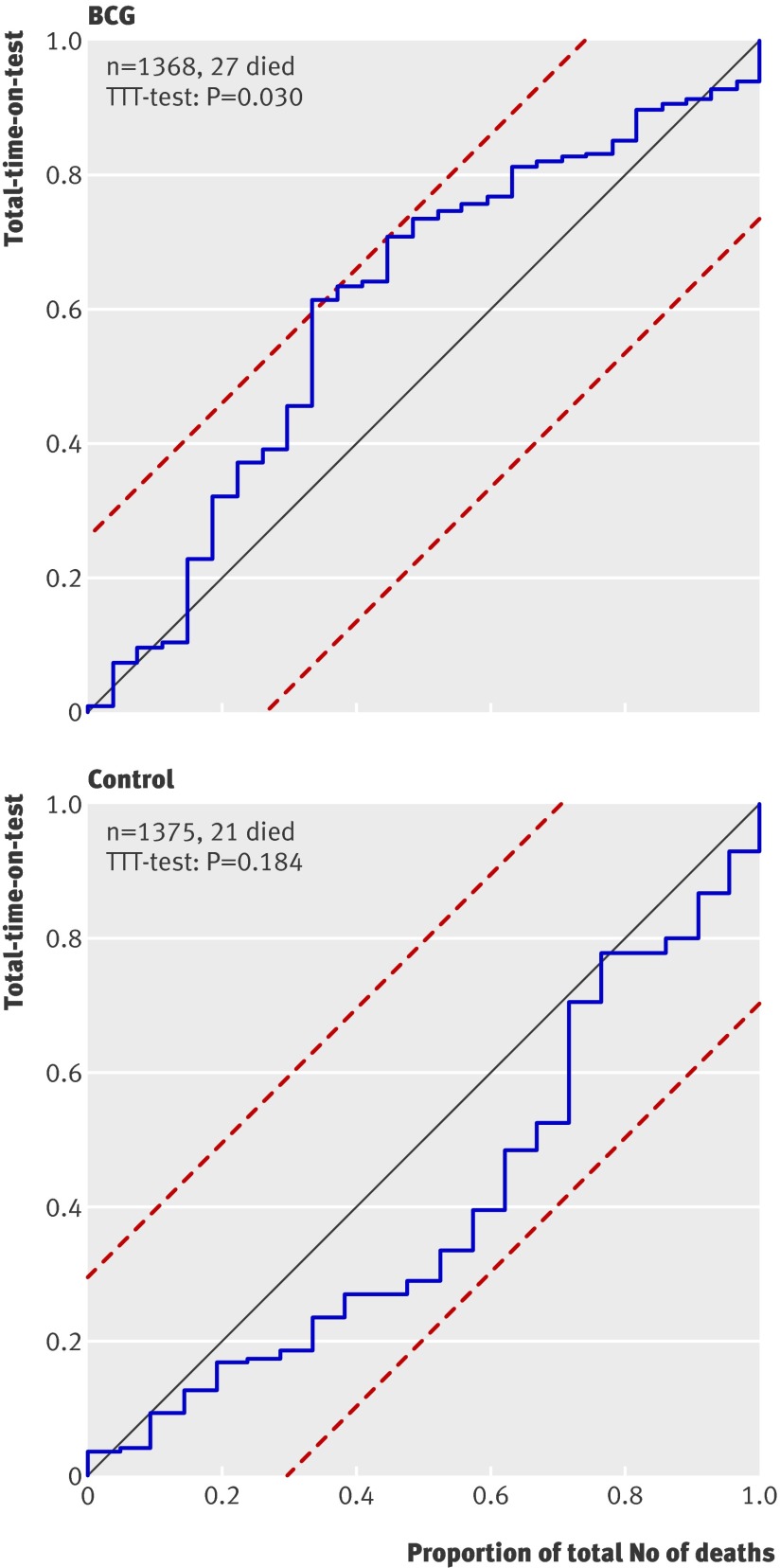
On 21 April 2004 the research team temporarily stopped enrolment as the data showed a sudden increase in mortality in the BCG revaccination arm (fig 2). During the autumn of 2003, the mortality rate in the revaccination group increased significantly with the total-time-on-test (P=0.030). The same increase was not observed in the control group (P=0.184) (fig 4).
Fig 2 Number of deaths by month, July 2002 to May 2006 (numbers should be interpreted with caution as number of enrolled children and their average age changed over time). Sequence of events: recruitment to trial started in July 2002; oral polio vaccine and vitamin A supplementation campaign (high coverage) occurred in October and November 2002; malaria survey and iron supplementation in August and November 2003; measles epidemic in November 2003-May 2004; missed vaccines and vitamin A supplementation campaign (low coverage) in November 2003; recruitment stopped in April 2004; oral polio vaccine and vitamin A supplementation campaign (high coverage) took place in October and November 2004, and October and November 2005; measles vaccine for all aged <15 and vitamin A supplementation campaign (high coverage) and end of follow-up was in May 2006
Fig 4 Mortality according to total-time-on-test analysis. Boundaries (dashed lines) corresponding to total-time-on-test (see text). Under assumption of constant mortality intensity, plot should approximate straight line with unit slope. If curve crosses any of boundaries hypothesis of constant mortality intensity can be rejected
The subsequent follow-up and increased clinical attention to study children after the increase in mortality had been detected could have affected the overall mortality results. If we censured follow-up on 1 April 2004, however, the hazard ratio of BCG revaccinated children compared with control children was 1.28 (0.72 to 2.26).
Mortality periods
In the period before the increased mortality, the hazard ratio had been slightly but not significantly lower in the BCG revaccinated group compared with the control group (table 2). During the period of increased mortality, mortality was higher in the BCG revaccination group, the hazard ratio being 2.69 (1.05 to 6.88). The hazard ratio for BCG revaccinated compared with control children changed significantly between the period before the increase and the mid-November 2003 to March 2004 period (P=0.036, test of interaction). After the increase there was no difference in mortality, the hazard ratio being 1.08 (0.52 to 2.24).
Causes of death
The increase in mortality occurred at the beginning of the dry season. During the period of increased mortality, deaths were caused by measles, diarrhoea, and respiratory infections and only one death was ascribed to malaria (table 3). The causes of deaths might have differed between the two groups (P=0.088, Fisher’s exact test). The incidence of measles was equally distributed between the two groups, while children who were revaccinated seemed more susceptible to diarrhoea and respiratory infections. In the periods with no increase in mortality, there were no differences in the causes of death between the children.
Table 3.
Causes of death by randomisation group, period, and vaccination status at enrolment
| Cause of death in verbal autopsy | Cluster period | Non-cluster periods | Total | |||||
|---|---|---|---|---|---|---|---|---|
| BCG+ | No BCG | BCG+ | No BCG | BCG+ | No BCG | |||
| Malaria | 0 | 1 | 8 | 6 | 9 | 7 | ||
| Diarrhoea | 6 | 0 | 5 | 7 | 11 | 7 | ||
| Measles | 6 | 4 | 1 | 2 | 7 | 6 | ||
| Respiratory infections | 3 | 0 | 1 | 0 | 4 | 0 | ||
| Other infections | 0 | 0 | 3 | 7 | 3 | 7 | ||
| No information/not done | 1 | 1 | 7 | 7 | 8 | 8 | ||
| Total | 16 | 6 | 26 | 29 | 42 | 35 | ||
There was no interaction between BCG revaccination and measles infection. The measles incidence ratio was not significantly higher (1.18, 0.88 to 1.58) in the BCG group than in the control group. There was no difference in case fatality between the groups. Missing vaccinations and vitamin A supplementation were linked to excess mortality.
Vitamin A supplementation
The hazard ratio for BCG vaccinated children versus controls was four times higher among the two thirds of the children who had received vitamin A supplementation, whereas there was no difference among the children who did not receive vitamin A in the campaign (table 4). Too few children had received iron to measure a separate effect, but supplementation with vitamin A or iron was associated with a hazard ratio of 4.97 (1.44 to 17.2) for BCG vaccinated children compared with controls, whereas it was 0.34 (0.04 to 3.29) for children who had received neither (P=0.042, test of homogeneity).
Table 4.
Mortality rate and hazard ratio for children revaccinated with BCG and controls during period with increased mortality according to receipt of vitamin A in November 2003
| Campaign status in November 2003 | Mortality rate/100 person years (deaths/days) | Hazard ratio (BCG v no BCG) | |
|---|---|---|---|
| BCG revaccination | Controls | ||
| Vitamin A supplementation | 4.4 (12/99 772) | 1.1 (3/102 383) | 4.14 (1.17 to 14.7) |
| No vitamin A | 2.7 (4/54 629) | 2.1 (3/53 301) | 1.29 (0.29 to 5.75) |
| Total | 3.8 (16/154 401) | 1.4 (6/155 684) | 2.69 (1.05 to 6.88) |
Vaccination status at enrolment
Contrary to our original assumption, only 40% of the children had received a booster DTP vaccine before enrolment in the trial (table 1). The BCG vaccinated children who had received a booster DTP vaccine before enrolment (table 2) had significantly lower mortality than controls, the hazard ratio being 0.36 (0.13 to 0.99). Children missing vaccinations at enrolment were told by the nurses to come back to the health centre for vaccination and many did so. The children who had not received a booster DTP vaccine before BCG vaccination had significantly higher mortality than controls, the hazard ratio being 1.78 (1.04 to 3.04). Hence, the effect of BCG revaccination differed significantly depending on whether they had received a booster dose of DTP vaccine or not (P=0.006, test of homogeneity). The negative effect of BCG revaccination in those who had not received a booster DTP vaccine before enrolment was marked during the period with increased mortality; the hazard ratio for BCG revaccination compared with controls was 3.06 (0.99 to 9.51) between mid-November 2003 and March 2004
Discussion
In this randomised trial in Africa, general BCG revaccination at 19 months of age had no beneficial effect on mortality. During a short period in the autumn of 2003, however, we observed an increase in mortality in children who were revaccinated, which significantly reversed the hazard ratio for revaccinated and control children. The unexplained increase in mortality led to the trial being stopped prematurely to prevent unnecessary risk for more children.
In subsequent explorative analyses, the increase in mortality seemed to be linked to a campaign with vitamin A supplementation and additional vaccinations in November 200336 and with iron treatment in a malaria survey conducted at the same time.29 Contrary to our expectations, BCG revaccination was associated with higher mortality in children who had not received DTP booster vaccine before enrolment and were likely to receive it after revaccination with BCG. This effect was particularly strong during the period when there was an increase in mortality. Taken together, these observations suggest that BCG revaccination—possibly in combination with subsequent immunostimulation with other vaccines and vitamin A—might affect the immune system, leading to increased mortality.
Strengths and weaknesses
Randomisation worked well, and there were apparently no major differences in baseline characteristics between the two randomisation groups.
As seen in the accompanying commentaries and editorial, our publication is controversial for at least two reasons. Firstly, we did not follow best practice rules for conducting randomised controlled trials, which include having a stopping rule and an external data monitoring and safety board. Secondly, we stopped the trial because of the excess mortality detected in April 2004, which could have been an accidental finding from a statistical perspective. Unfortunately, we did not have sufficient funding for a data monitoring and safety board. Thus we proceeded with the trial and did our own quarterly safety monitoring as this was not a blinded trial, and unexpectedly we found a safety concern when the mortality ratio between the two arms of the study changed completely within a short period. Thus we decided to stop enrolment temporarily to complete the data with follow-up of all children in the trial—as there is always a delay in the mortality data available in a surveillance system—and to examine possible causes of this change. A data monitoring and safety board would probably not have stopped the trial because the P value for change was not below 0.001, but, as a result of past experience of unfavourable events in such trials and studies of routine vaccinations,3 12 13 14 15 37 38 we thought it would be better to secure safety.
The initial assessment suggested that something had happened in November 2003 when there were several campaigns in the community. It should be noted, however, that the increase in mortality in the BCG arm did not depend on whether we used a particular definition of the period before and during the increase. The total-time-on-test measured a change in mortality over the whole period of the trial until the decision was made to stop. Hence, an unexpected cluster of deaths was confirmed, and we had to conduct explorative analyses to find possible causes.
Mortality during the trial was considerably lower than originally anticipated (table 2). There might have been an improvement in child survival in recent years because of campaigns and better malaria control,40 but better access to clinical examinations and treatment because of the trial might also have been a factor. Though the study did not have the power originally planned, it was large enough to show a strong differential effect on overall mortality of BCG revaccination in different periods of the study and according to vaccination status.
Consistency with previous studies: potential non-specific effects of BCG revaccination
Possible beneficial effects
From animal studies and cancer treatment we know that BCG is an immunomodulator, and observational studies of primary BCG vaccination have suggested major non-specific beneficial effects on survival of children in low income countries.4 41 Only one previous study has assessed the impact of revaccination on child survival. In a major study in Algeria using alternate allocation to vaccination and no vaccination with oral BCG, mortality in the intervention group was reduced by 27% after BCG revaccination at age 1 and 3 years.25 26 We therefore tested whether BCG revaccination reduced child mortality by 30%.
As expected BCG revaccination was beneficial among children who had received booster DTP before enrolment and hence did not receive DTP after enrolment. Whatever beneficial effects exist might be stronger for girls as girls revaccinated with BCG had significantly lower rates of admission to hospital than boys revaccinated with BCG, in concordance with previous findings.4
Possible negative effects
Our findings show that under certain circumstances BCG revaccination might be problematic. BCG revaccination was associated with an increase in mortality during the autumn of 2003, which might have started in November when vitamin A and missing vaccines were administered in a campaign.36 Furthermore, mortality was increased throughout the study for BCG vaccinated children who had not received booster DTP before enrolment. These children were likely to receive booster DTP during follow-up (data not shown). Hence, BCG revaccination followed by booster DTP or vitamin A supplementation, or both, might have contributed to an inadequate response to infections as the excess deaths in the BCG group in the period with increased mortality were caused mainly by diarrhoea and respiratory infections (table 3). We have previously reported from this campaign that younger children who received DTP and vitamin A supplementation simultaneously had increased mortality compared with children who received only vitamin A supplementation.36 Studies have also found that children receiving additional doses of DTP after measles vaccine have increased mortality.12 38
Our study produced several unexpected results. In an observational study these could have been attributed to uncontrolled confounding, the selection of subgroups, and time periods. We did not randomise children to receive a booster dose of DTP vaccine before enrolment in the trial nor did we randomise the children to vitamin A supplementation in the campaign in 2003. Receipt, or not, of these, however, was equally distributed between the BCG revaccination and control groups. As these interactions were found within a randomised study it seems likely that BCG revaccination contributes to non-specific negative immunological effects in some contexts.
There has been concern that BCG might have negative health consequences in children with HIV.31 This is unlikely to have been an explanation in our study because then the effect should also have been visible among the BCG revaccinated children before the increase in mortality and among the BCG revaccinated children who had received booster DTP vaccination before enrolment. Furthermore, the level of HIV-1 infection is low in the study area, and few children would have been infected and survived to inclusion at 19 months of age.42
Implications
There are general lessons from the present randomised trial. Firstly, non-specific effects of vaccines are important for child survival in low income countries. As the non-specific effects happened within a randomised trial, it seems unlikely that uncontrolled confounding or selection bias can explain the observed contrasting trends.
Secondly, BCG revaccination might have had a negative effect when given with vitamin A supplementation and when booster DTP was given after BCG revaccination. We have recently reported from another randomised trial that vitamin A supplementation at birth43 interacted negatively with DTP, particularly for girls.44 45 A negative interaction between DTP vaccine and vitamin A supplementation was also observed in a randomised trial in Ghana.38 The present trial supports the theory that administration of vitamin A might amplify the non-specific effects of vaccines, a hypothesis we proposed several years ago.46 The possible interactions with different vaccinations or micronutrients are virtually never reported when a new intervention is examined, with dire effects as shown with the high titre measles vaccine.3
Thirdly, the trial suggests that interventions that are usually considered beneficial might also have negative consequences under certain circumstances. We and others have advocated BCG at birth as having beneficial non-specific effects.41 In the present study BCG revaccination also had negative effects. In a study from Bangladesh, children who were vaccinated with BCG after 9 months of age tended to have increased mortality and BCG in general was associated with twofold increased mortality between 9 months and 5 years of age, the reported hazard ratio being 2.12 (1.28 to 3.51).47 As the Bangladeshi children are likely to have received vitamin A supplementation after 6 months of age, this study might represent a similar negative interaction between BCG and vitamin A.
Fourthly, the conduct of the trial was not ideal and would have benefited from stopping rules and a data monitoring and safety board because we might have been unduly cautious in stopping the trial too early. Randomised trials are doubly difficult to conduct in low income countries, however, where unexpected events and unplanned outside interventions during the trial commonly occur. Thus a flexible and interactive monitoring system to support investigators in the field is necessary but expensive.
Fifthly, international public health assumes that our common interventions have the expected beneficial effects irrespective of other conditions. There are, however, an increasing number of studies documenting that this is not the case. Most interventions have not been tested for their impact on child survival even though reduction in child mortality is the justification for the intervention. But even when they have been found to reduce mortality in randomised clinical trials the conditions under which they were tested might change and negate the beneficial effect. Given the lack of studies and general surveillance of mortality and interventions in low income countries we might simply not know that the beneficial impact has disappeared.
Unanswered questions and future research
In the analysis and presentation of this trial, the unexpected cluster of deaths took precedence. The main research question was whether BCG revaccination after the age at which children receive booster DTP vaccination had a beneficial effect on child survival. Fewer children than expected had received booster DTP and many therefore received it after BCG revaccination. BCG revaccination had a stronger than expected beneficial effect among the children who had received BCG after a DTP booster vaccination, but the effect was opposite among those who had not received a DTP booster vaccination, and there was therefore no overall beneficial effect. In countries where booster DTP vaccination is still used, it might be worth testing whether BCG revaccination given to children who have received the booster dose of DTP reduces child mortality as suggested by our results.
What is already known on this topic
BCG vaccination has stimulatory effects on the immune system
Old studies have reported that oral BCG revaccination is associated with improved survival
What this study adds
There was no overall beneficial effect of BCG revaccination on child survival
The mortality in BCG revaccinated children increased significantly during a short period
The impact of BCG revaccination on survival might depend on other health interventions such as vaccines and vitamin A supplementation
Contributors: AER and PA were the chief investigators and are guarantors. AER, CSB, MY, HW, IML, and PA designed the study. AER, CSB, and PA initiated the study. AER, AR, and IML were responsible for the recruitment and follow-up of participants. HR was responsible for statistical analysis. PA wrote the first draft of the paper. All authors contributed to and approved the final version of the paper.
Funding: The study was funded by the EU (ICA4-CT-2002-10053) and the Danish National Research Foundation. The Bandim Health Project received support from DANIDA. PA holds a research professorship grant from the Novo Nordisk Foundation. The funding agencies had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report.
Competing interests: None declared.
Ethical approval: The protocol was approved by the Danish Central Ethical Committee and the Guinean Ministry of Health’s Research Coordination Committee and all participants gave informed consent. Participants had access to free consultations at local health centres and to essential drugs free of charge.
Data sharing: Data on number of children receiving vaccinations after enrolment available from corresponding author.
Cite this as: BMJ 2010;340:c671
Web Extra. Extra material supplied by the author
Number of first admissions by month from July 2002 to May 2006
References
- 1.Aaby P, Samb B, Simondon F, Coll Seck AM, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 1995;311:481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen KM, Aaby P, Whittle H, Rowe M, Samb B, Simondon F, et al. Child mortality following standard, medium or high titre measles immunization in West Africa. Int J Epidemiol 1996;25;665-73. [DOI] [PubMed]
- 3.Aaby P, Jensen H, Samb B, Cisse B, Sodeman M, Jakobsen M, et al. Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: reanalysis of West African studies. Lancet 2003;361:2183-8. [DOI] [PubMed] [Google Scholar]
- 4.Roth AE, Garly ML, Jensen H, Nielsen J, Aaby P. Bacille Calmette Guerin vaccination and infant mortality. Expert Rev Vaccines 2006;5:277-93. [DOI] [PubMed] [Google Scholar]
- 5.Naeslund C. Resultats des experiences de vaccination par le BCG poursuivies dans le Norrbotten (Suede). In: Institut Pasteur. Vaccination preventive de la tuberculose de l’homme et des animaux per le BCG. Masson et Cie, 1932.
- 6.Medical Research Council Tuberculosis Vaccines Clinical Trials Committee. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescents. BMJ 1959;ii:379-96. [PMC free article] [PubMed]
- 7.Aronson JD. Protective vaccination against tuberculosis with special reference to BCG vaccination. Am Rev Tuberc 1948;58:255-81. [DOI] [PubMed] [Google Scholar]
- 8.Levin MI, Sackett MF. Results of BCG immunization in New York City. Am Rev Tuberc 1946;53:517-32. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal SR, Loewinsohn E, Graham ML, Liveright D, Thorne MG, Johnson V. BCG vaccination in tuberculous households. Am Rev Respir Dis 1961;84:690-704. [DOI] [PubMed] [Google Scholar]
- 10.Garly ML, Martins CL, Balé C, Baldé MA, Hedegaard KL, Gustafson P, et al. BCG scar and positive tuberculin reaction associated with reduced child mortality: a non-specific beneficial effect of BCG? Vaccine 2003;21:2782-90. [DOI] [PubMed] [Google Scholar]
- 11.Roth A, Sodemann M, Jensen H, Poulsen A, Gustafson P, Gomes J, et al. Tuberculin reaction, BCG scar and lower female mortality. Epidemiology 2006;17:562-8. [DOI] [PubMed] [Google Scholar]
- 12.Aaby P, Garly ML, Jensen H, Martins C, Balé C, Benn CS, et al. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: observations from vaccination trials in Guinea-Bissau. Pediatr Infect Dis J 2007;26:247-52. [DOI] [PubMed] [Google Scholar]
- 13.Veirum JE, Sodemann M, Biai S, Jakobsen M, Hedegaard K, Jensen H, et al. Routine vaccinations associated with divergent effects on female and male mortality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine 2005;23:1197-204. [DOI] [PubMed] [Google Scholar]
- 14.Aaby P, Jensen H, Garly ML, Balé C, Martins C, Lisse I. Routine vaccinations and child survival in war situation with high mortality: effect of gender. Vaccine 2002;21:15-20. [DOI] [PubMed] [Google Scholar]
- 15.Aaby P, Jensen H, Gomes J, Fernandes M, Lisse IM. The introduction of diphtheria-tetanus-pertussis vaccine and child mortality in rural Guinea-Bissau: an observational study. Int J Epidemiol 2004,33:374-80. [DOI] [PubMed]
- 16.Martins CL, Garly ML, Balé C, Rodrigues A, Ravn H, Whittle HC, et al. Protective efficacy of standard Edmonston-Zagreb measles vaccination in infants aged 4.5 months: interim analysis of a randomised clinical trial. BMJ 2008;337:a661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet 2005;366:1290-5. [DOI] [PubMed] [Google Scholar]
- 18.Fourie PB. BCG vaccination and the EPI. S Afr Med J 1987;72:323-6. [PubMed] [Google Scholar]
- 19.Rahman M, Sekimoto M, Hira K, Koyama H, Imanaka Y, Fukui T. Is Bacillus Calmette-Guerin revaccination necessary for Japanese children? Prev Med 2002;35:70-7. [DOI] [PubMed] [Google Scholar]
- 20.Aydinlioglu H, Caglayan S, Kansoy S, Yaprak I, Seckin E, Bakiler AR, et al. The decline of BCG immunity after neonatal vaccination: what about revaccination at one year? Paediatr Perinat Epidemiol 1993;7:334-8. [DOI] [PubMed] [Google Scholar]
- 21.Tala-Heikkila M, Nurmela T, Tala E, Tuominen J. Evaluation of the BCG revaccination programme of schoolchildren in Finland. Bull Int Union Tuberc Lung Dis 1991;66:57-9. [PubMed] [Google Scholar]
- 22.Shaaban MA, Abdul AM, Bahr GM, Standford JL, Lockwood DN, McManus IC. Revaccination with BCG: its effects on skin tests in Kuwaiti senior school children. Eur Respir J 1990;3:187-91. [PubMed] [Google Scholar]
- 23.Ildirim I, Hacimustafaoglu M, Ediz B. Correlation of tuberculin induration with the number of Bacillus Calmette-Guerin vaccines. Pediatr Infect Dis J 1995;14:1060-3. [DOI] [PubMed] [Google Scholar]
- 24.Karonga Prevention Trial Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 1996;348:17-24. [PubMed] [Google Scholar]
- 25.Sergent E, Catanei A, Ducros-Rougebief H. Premunition antituberculeuse par le BCG. Campagne poursuivie depuis 1935 sur 21,244 nouveau-nés vaccines et 20,063 non vaccines. Arch L’Institut Pasteur d’Algerie 1954;32:1-8. [PubMed] [Google Scholar]
- 26.Sergent E, Catanei A, Ducros-Rougebief H. Premunition antituberculeuse par le BCG. Campagne controlé poursuivie a Alger depuis 1935. Arch L’Institut Pasteur d’Algerie 1960;38:131-7. [Google Scholar]
- 27.Jensen H, Benn CS, Nielsen J, Lisse IM, Rodrigues A, Andersen PK, et al. Survival bias in observational studies of the impact of routine vaccinations on childhood survival. Trop Med Int Health 2007;12:5-14. [DOI] [PubMed] [Google Scholar]
- 28.Aaby P, Jensen H, Walraven G. Age-specific changes in female-male mortality ratio related to the pattern of vaccinations: an observational study from rural Gambia. Vaccine 2006;24:4701-8. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues A, Schellenberg JA, Roth A, Benn CS, Aaby P, Greenwood B. Revaccination with Bacillus Calmette-Guerin (BCG) vaccine does not reduce morbidity from malaria in African children. Trop Med Int Health 2007;12:224-9. [DOI] [PubMed] [Google Scholar]
- 30.Gustafson P, Gomes VF, Vieira CS, Rabna P, Seng R, Johansson P, et al. Tuberculosis in Bissau: incidence and risk factors in an urban community in sub-Saharan Africa. Int J Epidemiol 2004;33:163-72. [DOI] [PubMed] [Google Scholar]
- 31.Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, et al. Bacille Calmette-Guerin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis 2006;42:548-58. [DOI] [PubMed] [Google Scholar]
- 32.Kohl KS, Ball L, Gidudu J, Hammer SJ, Halperin S, Heath P, et al. Abscess at injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007;25:5821-38. [DOI] [PubMed] [Google Scholar]
- 33.Gustafson P, Gomes VF, Vieira CS, Seng R, Samb B, Nauclér A, et al. Tuberculosis mortality during a civil war in Guinea-Bissau. JAMA 2001;286:599-603. [DOI] [PubMed] [Google Scholar]
- 34.Seng R, Gustafson P, Gomes VF, Vieira C, Rabna P, Larsen O, et al. Community study of the relative impact of HIV1 and HIV2 on intrathoracic tuberculosis. AIDS 2002;16:1059-66. [DOI] [PubMed] [Google Scholar]
- 35.Benn CS, Martins C, Rodrigues A, Lisse IM, Aaby P. Randomised study of the impact of different doses of vitamin A on childhood morbidity and mortality. BMJ 2005;331:1428-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benn CB, Martins C, Rodrigues A, Ravn H, Fisker AB, Christoffersen D, et al. The effect of vitamin A supplementation administered with missing vaccines during national immunisation days in Guinea-Bissau. Int J Epidemiol 2009;38:304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garly ML, Jensen H, Martins CL, Balé C, Balde MA, Lisse IM, et al. Hepatitis B vaccination associated with higher female than male mortality in Guinea-Bissau: an observational study. Pediatr Infect Dis J 2004;23:1086-92. [PubMed] [Google Scholar]
- 38.Benn CS, Aaby P, Nielsen J, Binka FN, Ross DA. Does vitamin A supplementation interact with routine vaccinations? An analysis of the Ghana vitamin A supplementation trial. Am J Clin Nut 2009;90:629-39. [DOI] [PubMed] [Google Scholar]
- 39.Andersen PK, Borgan Ø, Gill R, Keiding N. Statistical models based on counting processes. Springer-Verlag, 1993.
- 40.Rodrigues A, Schellenberg JA, Kofoed PE, Aaby P, Greenwood B. Changing pattern of malaria in Bissau, Guinea-Bissau. Trop Med Int Health 2008;13:410-7. [DOI] [PubMed] [Google Scholar]
- 41.Roth A, Gustafson P, Nhaga A, Djana Q, Poulsen A, Garly ML, et al. BCG-vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol 2005;34:540-7. [DOI] [PubMed] [Google Scholar]
- 42.Da Silva ZJ, Oliveira I, Andersen A, Dias F, Rodrigues A, Holmgren B, et al. Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau. Is HIV-2 disappearing? AIDS 2008;22:1195-202. [DOI] [PubMed] [Google Scholar]
- 43.Benn CS, Diness BR, Roth A, Nante E, Fisker AB, Lisse IM, et al. Effect of 50,000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ 2008;336:1416-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benn CS, Rodrigues A, Yazdanbakhsh M, Fisker AB, Ravn H, Whittle H, et al. The effect of high-dose vitamin A supplementation administered with BCG vaccine at birth may be modified by subsequent DTP vaccination. Vaccine 2009;27:2891-8. [DOI] [PubMed] [Google Scholar]
- 45.Fisker AB, Lisse IM, Aaby P, Erhardt JG, Rodrigues A, Bibby BM, et al. Impact of neonatal vitamin A supplementation with BCG vaccine on vitamin A status at 6 weeks and 4 months of age. Am J Clin Nut 2007;86:1032-9. [DOI] [PubMed] [Google Scholar]
- 46.Benn CS, Bale C, Sommerfelt H, Friis H, Aaby P. Vitamin A supplementation and childhood mortality: amplification of the non-specific effects of vaccines? Int J Epidemiol 2003;32:822-8. [DOI] [PubMed] [Google Scholar]
- 47.Breiman RF, Streatfield PK, Phelan M, Shifa N, Rashi M, Yunus M. Effect of infant immunization on childhood mortality in rural Bangladesh: analysis of health and demographic surveillance data. Lancet 2004;364:2204-11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of first admissions by month from July 2002 to May 2006