Abstract
Canine heart worm disease is often life-threatening due to its various complications, including right side heart failure, caval syndrome and pulmonary eosinophilic granulomatosis. Several preventive medications and melarsomine have been developed and they are very effective to control heartworm infestation. However, in a case of severe infestation, melarsomine therapy often results in an unfavorable outcome because of the severe immune reaction caused by rapid killing of the adult worm. Surgical removal and an interventional method using flexible alligator forceps have been well described in the literature. Despite the usefulness of mechanical removal using flexible alligator forceps, the methodology still needs to be upgraded for increasing the applicability for treating dogs with severe infestation. We describe herein a newly developed percutaneous removal method for heartworms and this was successfully applied to 4 dogs with severe heartworm infestation. The follow-up studies also showed favorable outcomes with no complications.
Keywords: caval syndrome, dirofilariasis, dogs, heartworm, percutaneous removal
Introduction
The mainstays of heartworm treatment for small animals are monthly macrolide preventives, adulticidal therapeutics (i.e. melarsomine and thiacetarsamide) and mechanical heartworm removal with using retrieval devices (known as worm embolectomy), depending on the severity of the heartworm infestation [1,4,5,9]. The efficacy of melarsomine for adulticidal therapy has already been well described in the literature [6], although the adverse effects related to adulticidal treatment (i.e. pulmonary thromboembolism due to rapid worm killing) are often frustrating for practitioners [7]. Despite the excellent efficacy of melarsomine, the survival of residual heart worms after 2 to 3 melarsomine injections has also been well described in literature [1]. However, adulticidal therapy is generally not accepted in cases of severe infestation because of the severe immune response from the rapid killing of worms. Therefore, either mechanical or surgical heartworm removal is indicated in severe cases of heartworm infestation. Mechanical removal using retrieval devices (i.e. alligator forceps, a basket retrieval device or a loop snare device) has been successfully applied in the veterinary field [2,3,8]. The main advantages of this method are the reduced invasiveness of the procedure, less damage to the vascular endothelium and a shorter duration of general anesthesia [1]. A recent study found the rate of worm removal by using alligator forceps was 91.4% during 30.0 ± 7.6 min of procedure time [10]. Despite these advantages of mechanical removal (worm embolectomy) over surgical removal, these methods still need further improvement to obtain better accessibility to the pulmonary arteries, to minimize the bleeding that occurs during catheterization and for better heartworm removal. Therefore in this study, we developed a new modified percutaneous heartworm removal method and we successfully applied it to 4 dogs with severe heartworm infestation. The subsequent follow-up studies found that this method is a good alternative treatment for severe heartworm infestation.
Materials and Methods
Procedure for percutaneous heartworm removal
To minimize the side effects of the procedure, all the dogs were premedicated with aspirin (5 mg/kg, BID; Shinpoong, Korea), clopidogrel hydrogen sulfate (1 mg/kg, PO, SID; Sinil Pharmaceutical, Korea) and prednisolone (0.5 mg/kg, BID, PO; Daewoo Pharmaceutical, Korea) from the week prior to the heartworm removal procedure. Heparin (100 U/kg, SC, SID; Greencross, Korea) was also administered at the day prior to the procedure. The dogs were premedicated with atropine (0.05 mg/kg, SC; Daewoo Pharmaceutical, Korea) and diazepam (0.5 mg/kg; Daewon Pharmaceutical, Korea); this was followed by induction of anesthesia with propofol (4 mg/kg; Jeil Pharmaceutical, Korea). After tracheal intubation, the anesthesia was maintained by isoflurane with a 2-5% concentration depending on the dog's size. The animal test subject was mechanically ventilated at a rate of 30 times per minute with using a volume-cycled respirator (MDS Matrix 3000; Hallowell, USA). After achieving surgical anesthesia, venipuncture was performed at the right jugular vein with an 18G needle. A guidewire (COOK, USA) was inserted into the needle and this was located at the pulmonary artery. Under fluoroscopy, the guidewire was pushed to the cranial vena cava and right atrium, and then the tip of the guidewire was nosed down and advanced to the right ventricle and pulmonary artery (Fig. 1). An introducer sheath (Flexer Tuohy-Borst Side-Arm Introducer; COOK, USA) was inserted to the right external jugular vein with guidance of the pre-placed guidewire and this was located at the pulmonary artery (Fig. 2). The guide wire was then removed from the jugular vein. Either an endoscopic grasping forceps (FG-53SX-1; Olympus, Japan) or a flexible three wires nail tipped forceps (Rosot, USA) was inserted into the introducer and this was used to grasp the heartworm under fluoroscopic guidance (Fig. 3). Initially, the heartworm was removed from the pulmonary artery. After the heartworm was no longer being retrieved from the pulmonary artery, the introducer was pulled back to the right ventricle. The heartworm was then removed from the right ventricle. Lastly, the introducer was pulled back to the right atrium and the worm was removed. After the procedure, the venipunctured jugular vein was tightly tied with a surgical nylon.
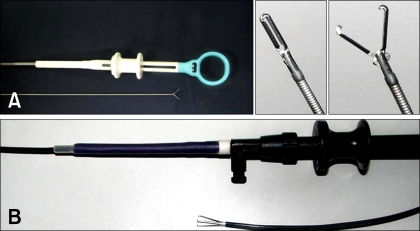
Fig. 1.
The heartworm removal devices used in this study. (A) Endoscopic grasping forceps. (B) Flexible three wires nail-tipped forceps.
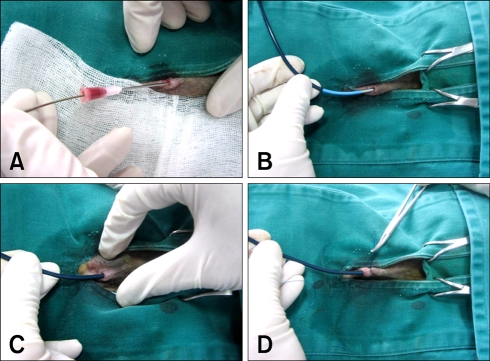
Fig. 2.
The procedure for mechanical heartworm removal. (A) After achieving surgical anesthesia, venipuncture was performed at the right jugular vein with an 18G needle. Then a guidewire was inserted into the needle and this was located at the pulmonary artery. (B) An introducer sheath was inserted to the right external jugular vein with guidance of a pre-placed guidewire, and the sheath was located at the pulmonary artery. (C) The guide catheter was then removed from the sheath. (D) The sheath was temporarily tied with simple interrupted suture.
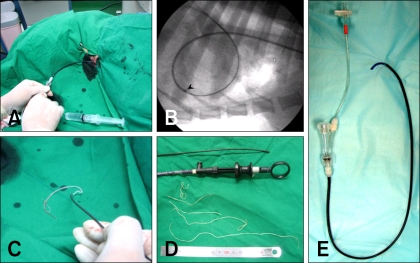
Fig. 3.
The procedure for mechanical heartworm removal (continued). (A) After the sheath was inserted into the right cardiac chamber or pulmonary artery, the removal device was inserted into the sheath. (B) Heartworm removal was performed at the pulmonary artery with fluoroscopic guidance. (C) The heartworms were removed from the sheath. (D) The mechanically removed heartworms. E: An introducer sheath used in this study.
Results
Case 1: The patient was an 8-year-old intact female Jindo dog (18.5 kg BW). The dog showed clinical signs of anorexia, depression, severe dyspnea and severe ascites. Heart auscultation revealed a grade IV/VI systolic regurgitant murmur on the right apex. The main ECG findings were the presence of an S wave in leads I, II and III, suggesting right ventricular enlargement. The main laboratory findings were anemia, leukocytosis with eosinophilia, hypoproteinemia, prerenal azotemia (blood urea nitrogen: 50 mg/dl, creatinine: 2.5 mg/dl) and mildly increased hepatic enzymes (alanine transaminase: 80 IU/l, aspartate transaminase: 140 IU/l). Abdominal fluid analysis revealed a blood tinged modified transudate. The immunological tests for adult worms and microfilaria were positive. On the thoracic radiography, the right atrium and caudal vena cava were severely enlarged, although the lung fields were relatively clear. An echocardiographic study found a severe heartworm infestation in the right atrium and ventricle, and tricuspid regurgitation was also noted. In this dog, 13 heartworms (8 females, 5 males) were removed from the heart. After the heartworm removal, the dog was treated with prednisolone (0.5 mg/kg, BID, PO), amoxicillin (20 mg/kg, BID, PO; Chong Kun Dang, Korea) and heparin (100 U/kg, SC, SID) for 3 days. The dog's abdominal fluid was removed (300ml). This dog was also medicated with diuretics (furosemide, 2.5 mg/kg, BID; Handok, Korea) and nitroglycerine (transdermal patch, 1/8 of 25 mg/h, every other 12 h; Daewoong Pharmaceutical, Korea) to prevent further abdominal fluid accumulation. On the echocardiography taken 2 weeks after heartworm removal, a heartworm still existed in the right ventricle and pulmonary artery and the dog's fluid accumulation still persisted. Adulticidal therapy (melarsomine, 2.5 mg/kg, IM; Merial, USA) was done for 2 consecutive days with cage rest at a month after the heartworm removal. For 2 months, the dog was treated with furosemide (3 mg/kg, PO, BID) and enalapril (0.5 mg/kg, Cellart Pharm, Korea) to reduce the right atrial dilation and the abdominal fluid accumulation; during these 2 months, the dog was treated with prednisolone (0.5 mg/kg, PO, BID) and clopidogrel hydrogen sulfate (18 mg/kg, PO, SID). No worm was detected on the echocardiography that performed a month after the adulticidal treatment. No further fluid accumulation was observed after 2 months of the diuretic therapy. The dog showed a negative reaction on the antigen test for adult worm and Knott's test for microfilaria; these tests were done 4 months after the heartworm removal.
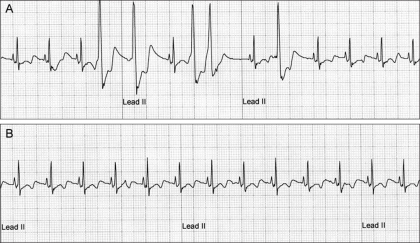
Case 2: The patient was a 3-year-old intact male Boston terrier (4.9 kg of body weight). The dog showed clinical signs of severe dyspnea, occasional cough, hemoglobinuria (Fig. 4B) and exercise intolerance. On thoracic auscultation, a grade IV/VI systolic murmur with a split S2 sound was heard at the right cardiac apex. Electrocardiographic (ECG) studies showed sinus tachycardia (145 per min) with occasional ventricular premature contractions (Fig. 5A). The hemogram revealed hemolytic anemia and leukocytosis with eosinophilia. The blood biochemistry revealed increased hepatic enzymes (alanine transaminase, 120 IU/l; aspartate transaminase 110 IU/l), azotemia (blood urea nitrogen, 41 mg/dl; creatinine, 1.5 mg/dl) and bilirubinemia (1.5 mg/dl). On the heartworm antigen test (SNAP kit; IDEXX Laboratories, USA), the dog showed a positive reaction with microfilaria being observed on the direct smear. On the thoracic radiography, a remarkable enlargement of the pulmonary artery with pruning and moderate right atrial and ventricular enlargement, and the lung fields showed an interstitial pattern. An echocardiographic study showed the enlargement of the pulmonary artery and right atrium, the movement of heartworm, and especially in the right outflow tract, and the turbulent flow at the tricuspid and pulmonary valve areas. In this dog, 15 heartworms (8 females, 7 males) were removed from the right atrium at a single retrieval (Fig. 4A). Seven more worms were removed with successive retrievals from the right ventricle and pulmonary artery. Since the cardiac contractibility suddenly dropped, we stopped the procedure. After the removal of the introducer and retrieval device, the cardiac contractibility returned to normal. The dog was then treated with prednisolone (0.5 mg/kg, BID, PO), amoxicillin (20 mg/kg, BID, PO) and heparin (100 U/kg, SC, SID) for 3 days and then the dog was changed to prednisolone, amoxicillin, aspirin (5 mg/kg, PO, BID) and clopidogrel hydrogen sulfate (18 mg/kg, PO, SID) for another 7 days. On the clinical examination immediately after the heartworm removal, the systolic murmur disappeared on the thoracic auscultation. More interestingly, the ventricular premature contractions also disappeared after the heartworm removal (Fig. 5B). The hemoglobinuria no longer persisted from the next day after the heartworm removal (Fig. 4B). For the first week of the follow-up study, the dog showed dramatic improvement of its physical condition without any complications. The dog was then medicated with a preventive dose of ivermectin (PO, monthly; Merial, USA). Subsequent melarsomine therapy (2 consecutive injections) was performed at a month after heartworm removal. The dog showed negative reaction on the antigen test for adult worm and on Knott's test for microfilaria; these tests were performed 4 months after the heartworm removal.
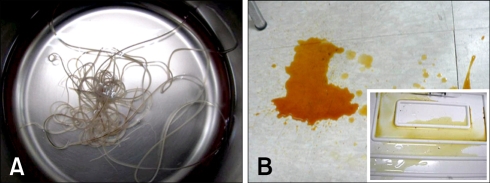
Fig. 4.
Images from Case No. 2. (A) The heartworms (8 females, 7 males) removed from the right atrium with a single retrieval. (B) The urine before and after (inset) the heartworm removal. The dog's hemoglobinuria disappeared after the heartworm removal.
Fig. 5.
Electrocardiograms (ECG) from Case No. 2. (A) The ECG recorded before the heartworm removal revealed normal sinus rhythm with occasional ventricular premature complexes (the 4th, 5th, 7th 8th and 10th QRS complexes). (B) The heart rhythm returned to sinus rhythm. No ventricular premature complexes were recorded on the 1 h ECG recordings.
Case 3: The patient was a 4-year-old intact female Yorkshire terrier (5.5 kg of body weight). The dog showed clinical signs of tachypnea, tachycardia, depression, nocturnal cough and severe exercise intolerance. On the thoracic auscultation, a grade V/VI systolic murmur with precordial thrills was heard at the right cardiac apex. No particular abnormalities were observed on the ECG except for tachycardia (190 per min). No significant abnormalities were observed on the routine CBC and biochemistry tests. On the heartworm antigen test, the dog showed a positive reaction with the observation of microfilaria on the direct smear. Thoracic radiography revealed right sided cardiac enlargement (reverse-D shape) with a severe enlargement of the right atrium (the central venous pressure was 22 cm H2O at presentation), tortuous and pruned right and left pulmonary arteries with increased density of the lung fields. Echocardiography revealed a heavy heartworm infestation in the right atrium, right ventricle and pulmonary artery. With performing our procedure, 22 heartworms (15 females, 7 males) were removed from the right atrium, right ventricle and pulmonary artery. After the heartworm removal, the dog was treated as was described in Case 1. In addition, the dog was medicated with diuretics (2 mg/kg PO, BID) and nitroglycerine (transdermal patch, 1/8 of 25 mg/h, every other 12 h) to reduce the preload of the right atrium for 2 weeks after the heartworm removal. On the clinical examination performed 2 weeks after the procedure, the condition of dog was clinically improved, but the dog still showed mild respiratory signs. Prednisolone (0.5 mg/kg, PO, BID) and cough suppressant (codeine, 0.2 mg/kg, PO, TID; Guju Pharmaceutical, Korea) were prescribed for another 2 weeks with administration of heartworm preventive medication (ivermectin, PO, monthly). Subsequent melarsomine therapy was performed after the dog's condition was stabilized. The owner reported the dog physically returned to normal after a short time.
Case 4: The patient was a 4-year-old intact male Siberian Husky (48 kg of body weight). The dog showed clinical signs of tachypnea (90 respiratory rate/m), persistent cough and exercise intolerance. No abnormal sound was detected on the cardiac auscultation. The ECG showed occasional bundle branch blocks. For the hematology, the dog showed leukocytosis with eosinophilia. The blood chemistry tests showed increased hepatic enzyme (alanine transaminase, 320 IU/l; aspartate transaminase 150 IU/l) and an increased phosphorus concentration (7.2 mg/dl). Although the dog showed a positive reaction on the antigen test, negative findings were observed on the concentration testing method and by direct observation. Strangely, on the thoracic radiography, no enlargement of the cardiac silhouette was observed, although the dog showed severe vascular and pulmonary changes, including pruned and tortuous pulmonary arteries and nodular infiltration on both lung fields. On the echocardiography, only a few worms were detected in the pulmonary arteries. No other abnormalities were observed on further echocardiographic studies. However, in this dog, 18 heartworms (15 females, 3 males) were removed from the pulmonic artery. The dog was treated with the prescription described earlier. On the physical examination performed a day after the heartworm removal, the respiration rate had returned to the normal range (25-35/min) and the dog did not cough. After a week of care in our clinic, the dog was then released with the prescription described earlier. Monthly heartworm preventive medication with ivermectin was also prescribed. Subsequent melarsomine therapy was performed after the dog's condition was stabilized. No further deterioration was reported by the owner.
Discussion
Mechanical heartworm removal using a flexible alligator forceps has previously been successfully applied [2,8,10]. A subsequent study has also found this method is safer and more efficient than melarsomine administration, if it is applied under fluoroscopic guidance by a skilled practitioner [1,10]. However, subsequent melarsomine administration for adequate worm destruction is still required after worm removal [1]. The main advantages to this technique are i) the minimal risk of potential arsenic toxicity by the adulticidal therapy to asymptomatic patients, and ii) the minimal risk of thromboembolism, while main drawbacks are i) the need for general anesthesia and fluoroscopy, ii) the requirement that the personnel performing the procedure be highly skilled, and iii) the subsequent melarsomine administration.
Despite these advantages over conventional arsenic therapy, mechanical worm removal often produces frustrating outcomes. However, this new method of mechanical removal is advantageous over the method used by Sasaki et al. [8].
Firstly, this procedure does not require venotomy to access to the jugular vein, so the bleeding associated with the insertion and retraction of the heartworm removal device was remarkably reduced. Furthermore, surgical closure is not necessary since this procedure doesn't required venotomy.
Secondly, this procedure has better accessibility and it is safer. For this procedure, because the introducer catheter was pre-placed to the area of the pulmonary artery or right ventricle with using a very flexible guidewire, the removal device can easily access to the pulmonary artery without effort. This can minimize the vascular and intra-cardiac damage that is caused by the insertion of the removal device and this can save time when locating the removal device to the pulmonary artery.
Thirdly, this procedure can be done for a dog with marked right atrial enlargement. Direct insertion of removal devices is often frustrating in giant breed dogs and dogs that have an enlarged right atrium since the device can not be easily located into the right ventricle and pulmonary artery, despite the flexibility of the device that's used. However, because this procedure uses various types of guidewires that are easier to handle and locate to the target area, the removal device can be located at any place of the heart, even though the device is more rigid than the flexible alligator forceps.
Lastly, this procedure has better flexibility. Although we only used two removal devices, with this procedure we can use various types of commercially available retrieval devices. The large size (7-8 Fr) of the introducer can accommodate most types of retrieval devices that are usually used for endoscopic foreign body removal. For instance, removing heartworm from the smaller pulmonary arteries with Ishihara's flexible alligator forceps is problematic since this device needs enough space to open the jaws. However, this can be overcome with other types of retrieval devices (e.g. an extraction catheter with a wire guide), which can be applicable without the guidance of a pre-placed introducer. Furthermore, the introducer used in this study has a side-arm connector that enables us to monitor the central venous pressure and to perform angiocardiography. This features helped us to adjust the fluid speed and to delineate the vascular structures while this procedure is carried out.
Despite these advantages, there is a potential risk of cardiac arrest, and this is due to cardiac contractile dysfunction that caused by the insertion of the introducer in the right ventricle, and especially in dogs under 5 kg of body weight. The dog in case 2 in this study showed marked contractile dysfunction when the introducer and retrieval device were placed in the right ventricle. The rigidity of the catheter and retrieval device may induce cardiac muscular fatigue and this may cause contractile dysfunction and subsequent cardiac arrest in dogs with a small chamber (a small sized dog). Another disadvantage is that this method still requires subsequent melarsomine administration for adequate worm destruction [1,7,9]. In addition, the total cost for this procedure is more expensive than the other method because it uses a disposable intracardiac catheter. However a fair outcome and better accessibility may compensate for these disadvantages. This method may be a good therapeutic option for the cases with a severe heartworm burden and the cases with complications such as caval syndrome or pulmonary thromboembolism.
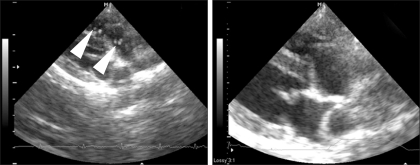
Fig. 6.
Echocardiographic evaluation of Case 2 before and after the heartworm removal (right parasternal short axis view, right outflow tract level) Left: before the procedure, many heartworms (arrowheads) are visible in the right ventricular outflow tract and pulmonary arteries. Right: after the procedure, no heartworms are visible in the right ventricular outflow tract and pulmonary arteries.
Acknowledgments
This study was supported by the research fund from Kangwon National University (3005055-1-1).
References
- 1.Atkins C. Canine heartworm disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Philadelphia: Saunders; 2004. pp. 1118–1136. [Google Scholar]
- 2.Atwell RB, Litster AL. Surgical extraction of transplanted adult Dirofilaria immitis in cats. Vet Res Commun. 2002;26:301–308. doi: 10.1023/a:1016042709194. [DOI] [PubMed] [Google Scholar]
- 3.Glaus TM, Jacobs GJ, Rawlings CA, Watson ED, Calvert CA. Surgical removal of heartworms from a cat with caval syndrome. J Am Vet Med Assoc. 1995;206:663–666. [PubMed] [Google Scholar]
- 4.Grieve RB, Frank GR, Stewart VA, Parsons JC, Belasco DL, Hepler DI. Chemoprophylactic effects of milbemycin oxime against larvae of Dirofilaria immitis during prepatent development. Am J Vet Res. 1991;52:2040–2042. [PubMed] [Google Scholar]
- 5.Paul AJ, Todd KS, Jr, Sundberg JP, DiPietro JA, McCall JW. Efficacy of ivermectin against Dirofilaria immitis larvae in dogs 30 and 45 days after induced infection. Am J Vet Res. 1986;47:883–884. [PubMed] [Google Scholar]
- 6.Polizopoulou ZS, Koutinas AF, Saridomichelakis MN, Patsikas MN, Leontidis LS, Roubies NA, Desiris AK. Clinical and laboratory observations in 91 dogs infected with Dirofilaria immitis in northern Greece. Vet Rec. 2000;146:466–469. doi: 10.1136/vr.146.16.466. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings CA, Raynaud JP, Lewis RE, Duncan JR. Pulmonary thromboembolism and hypertension after thiacetarsamide vs melarsomine dihydrochloride treatment of Dirofilaria immitis infection in dogs. Am J Vet Res. 1993;54:920–925. [PubMed] [Google Scholar]
- 8.Sasaki Y, Kitagawa H, Ishihara K, Masegi T. Improvement in pulmonary arterial lesions after heartworm removal using flexible alligator forceps. Nippon Juigaku Zasshi. 1990;52:743–752. doi: 10.1292/jvms1939.52.743. [DOI] [PubMed] [Google Scholar]
- 9.Strickland KN, Atkins CE. Heartworm disease, dog. In: Cote E, editor. Clinical Veterinary Advisor: Dogs and Cats. 6th ed. Philadelphia: Saunders; 2007. pp. 465–467. [Google Scholar]
- 10.Yoon HY, Jeong SW, Kim JY, Han HJ, Jang HY, Lee B, Namkang HS. The efficacy of surgical treatment with flexible alligator forceps in dogs with heartworm infection. J Vet Clin. 2005;22:309–313. [Google Scholar]