Abstract
This study was performed to produce transgenic Korean native goat (Capra hircus) by laparoscopic embryo transfer (ET) to overcome the limitations of ET performed by laparotomy. Transgenic embryos were produced by DNA pronuclear microinjection of in vivo zygotes. The recipient goats were synchronized for estrus by using an introvaginal progesterone devices as a controlled internal drug-releasing insert (CIDR) for 13 days and injection of 400 IU PMSG 48 h before removal of the insert. Embryos were transferred on day 3 and 4 after removal of the insert. Recipient goats were deprived of feed for 48 h, then suspended in a laparotomy cradle at an angle of 45°. After obtaining a sufficient pneumoperitoneum, the laparoscope and forceps were inserted abdominally through 5 mm trocar sleeves. Examination of the ovaries and uterus was performed and then 213 embryos were transferred into the oviducts via the infundibula of 76 recipient goats. To compare pregnancy rates, ET was also performed by laparotomy in 82 recipient goats. The pregnancies in the recipient goats were diagnosed by ultrasound on day 30 after embryo transfer. The pregnancy rate with laparoscopic ET was significantly higher than with ET performed by laparotomy (46.1% vs. 28.6%, p < 0.05). In addition, the pregnancy rates were compared between ovulated and non-ovulated ovaries of the recipient goats in the laparoscopic ET group. No significant difference was observed between the pregnancy rates of ovulated and non-ovulated ovaries (41.3% vs. 33.3%, p < 0.05) suggesting that ET may also be possible in non-ovulated recipients through artificial rupture of Graafian follicles. These results suggest that laparoscopic ET is a highly efficient method for the transfer of goat embryos.
Keywords: embryo transfer, goat, laparoscopy, laparotomy, transgenic
Introduction
The mammary gland of transgenic farm animals has been proposed as the best available bioreactor for the production of human pharmaceutical proteins [4,5,13,21]. Dairy goats have been used as a bioreactor because of their relatively short gestation period and low maintenance costs compared to cattle. Many studies have reported on the production of transgenic goats [6-8,11]. The possibility of large-scale production for industrial application has been demonstrated [9,10]. In Korea, transgenic goats have been used to produce human granulocyte colony stimulating factor (G-CSF) in their milk [15,18].
To date, laparotomy methods have generally been used for goat embryo transfer (ET). However, this method can cause adhesions in the reproductive tract following repeated surgical ET and requires relatively long intervals before the re-use of a recipient female [15,18]. To overcome the limitations of laparotomy, laparoscopic ET has been performed in various species including sheep [19,20], cows [12] and pigs [3]. The laparoscopic method has also been performed in goats [1,2,16,17,24]. However, in the above mentioned studies, the laparoscopic method was used only for oocyte recovery by ovum pick-up and embryo recovery but not for embryo transfer.
Estrus synchronization is essential for successful ET and corpus luteum (CL) formation is necessary for pregnancy maintenance. However, it is difficult to know the exact status of the ovaries if they are not observed directly by exploratory surgery or ultrasonography. Therefore, if the CL is not formed during ET, artificial formation of the CL by follicle puncture is necessary for ovulation and progesterone support is required to maintain the pregnancy or the ET must be postponed until CL formation.
In the present study, we performed laparoscopic ET to overcome the limitations of laparotomy in the production of transgenic goats. The pregnancy rates resulting from the two methods were compared in the Korean native goat (Capra hircus). In addition, the pregnancy rates were compared between ovulated and non-ovulated animals in the laparoscopic ET group.
Materials and Methods
Synchronization and embryo collection
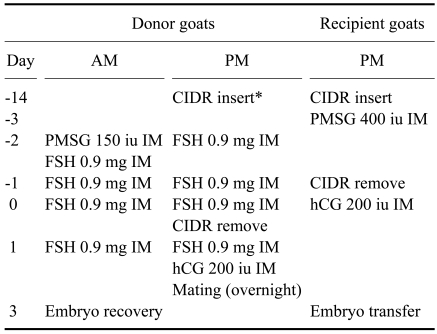
In this study, Korean native goats with a body weight ranging from 15 to 25 kg were used as donors and recipients from September to April 2001-2002. All goats were fed alfalfa/grass hay and a commercial diet with free access to water and trace-minerals. The estrous periods of the donors were synchronized using an intravaginal progesterone devices such as a controlled internal drug-releasing insert (CIDR; Pharmacia & Upjohn, New Zealand) for 13 to 14 days irrespective of the natural estrous cycles (Table 1). Superovulation was induced following combined treatment with FSH (ICPbio, New Zealand), PMSG (Horizon Technology, Australia) and hCG (Sigma, USA). FSH (0.9 mg/goat) was administered to the goats over a 4-day period, at 12-h intervals, starting 2.5 days before CIDR removal and continuing within 1 day of CIDR removal. PMSG (150 IU) was administrated at the time of the first FSH administration and hCG (200 IU) was administrated at the time of the last FSH injection to induce ovulation. The donors demonstrated estrus within 24 h following the CIDR removal and were mated with fertile bucks. At 66 h after the CIDR removal, embryos were surgically recovered by flushing both oviducts. All donors were fasted 24 h prior to surgery. A low dose of xylazine hydrochloride (0.12 mg/kg BW; Bayer Korea, Korea) was injected (im) as a pre-anesthetic agent. After a subcutaneous injection of lidocaine (0.1 g/ animal, Kwangmyung Pharm, Korea) for local anesthesia, a midventral incision was made and the reproductive tract exteriorized. The ovaries were examined for fresh ovulation sites to provide an estimate of the number of embryos. The oviducts were flushed with sterile phosphate-buffered saline. The recipient goats were also synchronized with the donor doses for 13 d using the CIDR with a single injection of 400 IU PMSG two days before CIDR removal. As no FSH was administered to the recipient goats, the injection of hCG and removal of the CIDR were both performed one day before they were performed in the donor goats.
Table 1.
The superovulation and estrous synchronization schedule for the donor and recipient Korean native goats
*Intravaginal progesterone insertion with controlled internal drug-releasing insert (CIDR).
Embryo manipulation and microinjection
Immediately after flushing, the number of oocytes/embryos was evaluated for each donor under a stereomicroscope. Zygotes were microcentrifuged at 10,000 g for 7 min to improve pronuclei visualization and the injection of DNA. A 3.7 kb BaaH II/Kpn I fragment of pGbc-hGCSF, in which the hG-CSF (human granulocyte-colony stimulating factor) gene was fused as the promoter sequence with the goat β-casein gene, was injected into one of the pronuclei of the 1-cell embryos. Following microinjection, the embryos were placed into modified synthetic oviduct fluid (mSOF) supplemented with 10% FBS and cultured for 1 or 2 h in a humidified (38.5℃) 5% CO2 incubator until transfer [22].
Embryo transfer
The equipment used for laparoscopic surgery included the following: a 5-mm laparoscope (MGB, Germany), a charge coupled device (IK-C43H 47; Toshiba, Japan), a flexible fiber-optic cable (Olympus, Japan), a camera control unit (IK-Cu43A; Toshiba, Japan), a light source (CLV-E; Olympus, Japan), a 5-mm trocar (MGB, Germany), a 5-mm laparoscopic assistant forceps for dissection, atraumatic grasping and allis forceps (MGB, Germany), and a 5-mm injection needle (MGB, Germany). Embryo transfer was performed 4 days after removal of the CIDR. The recipient goats were starved for 48 h prior to ET, and xylazine hydrochloride (0.7 mg/kg, IM) was administered as an anesthetic agent. The anesthetized goats were suspended head down on a laparotomy table at an angle of 45°. After disinfection of the surgical area, 2% lidocaine was infused for local anesthesia at the site of the proposed puncture. A Verres needle (Vomed, Germany) was inserted through the abdominal wall to create a pneumoperitoneum using a CO2 automatic insufflator. After obtaining a sufficient pneumoperitoneum, a 5-mm middle incision was made in the skin cranial to the mammary gland. The trocar was passed through the abdominal wall, the trocar sleeve was inserted and the laparoscope was inserted through the trocar sleeve. An injection needle was inserted cranial to the laparoscope and forceps were inserted lateral to the injection needle. After examination of the ovaries, oviducts and uterine horns, the embryos were transferred.
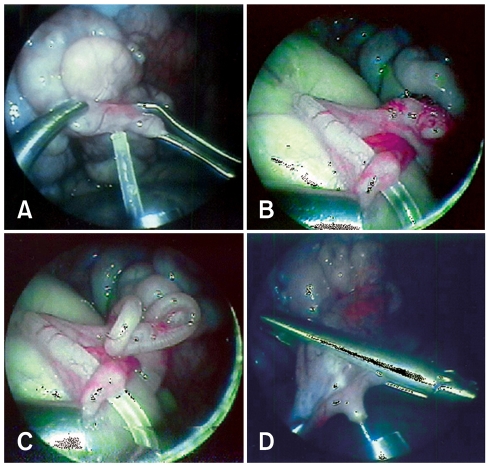
The stage and quality of the embryos were evaluated under a stereomicroscope, and the embryos were loaded into a polyethylene tube (SP65; Nastume, Japan) attached to the injection needle (Fig. 1). With the forceps, the infundibulum was grasped (Fig. 2A), the polyethylene tube was inserted into the oviduct via the infundibulum (Fig. 2B), and 2 to 3 embryos were then transferred (Fig. 2C). After transferring the embryos, the back flow of the medium, into the abdominal cavity, was prevented by grasping the infundibulum with the forceps (Fig. 2D). The polyethylene tube was washed with medium, then checked for any remaining embryos. Recipient goats were used up to three to four times if no pregnancy was established after the ET.
Fig. 1.
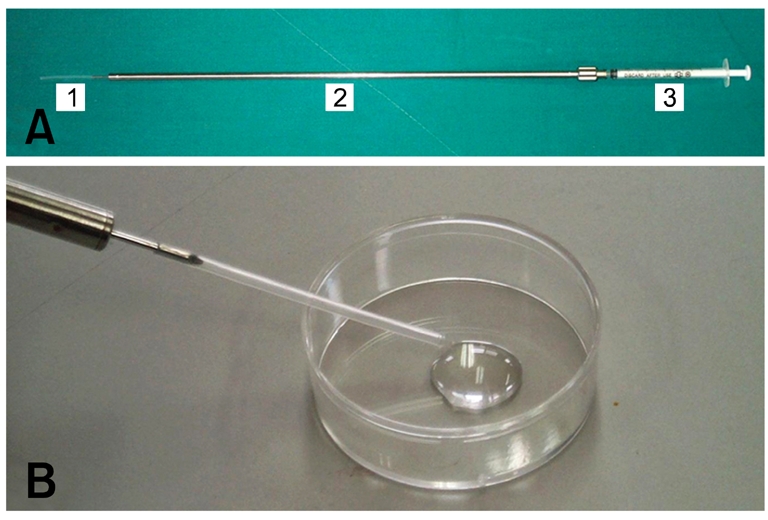
Injection set for laparoscopic embryo transfer and embryo loading. (A) 5-cm polyethylene tube (1), injection needle (2) and 1-ml syringe (3). (B) Embryo loading in the injection needle.
Fig. 2.
Laparoscopic embryo transfer. (A) Infundibulum was grasped with assist forceps, and then inserted into a polyethylene tube. (B) Insertion of polyethylene tube into the ampulla via the infundibulum. (C) Swollen oviduct after embryo transfer. (D) Preventing back flow of medium into the abdominal cavity by grasping the infundibulum with assist forceps.
To compare the pregnancy rates, ET by laparotomy was performed as described previously [18]. Briefly, 2-3 embryos were surgically transferred into 1 oviduct ipsilateral to the ovulated ovary, using a syringe connected to a sterile polyethylene tube, which was inserted into the oviduct lumen via the fimbria. The pregnancies were diagnosed by transrectal ultrasound scanning (SonoVet 600; Medison, Korea) using a transrectal 5-MHz linear array probe on day 30 and 40 following ET in both groups.
Experimental design
The pregnancy rates following ET were compared between the laparoscopy and laparotomy groups in experiment 1. In experiment 2, the recipient goats were classified into two groups based on whether they had ovulated or non-ovulated ovaries (GF; ovary with Graafian follicle that was non-ovulated, CH; ovary with corpus hemorrhagicum after ovulation). The pregnancy rates were compared after the laparoscopic ET. In the GF group, the non-ovulated follicle was ruptured artificially by needle puncture prior to the ET. This experiment was performed to investigate the effects of artificial rupture, of non-ovulated Graafian follicles, on the efficiency of laparoscopic ET.
Statistical analysis
All values for each parameter were analyzed by ANOVA using a general linear model (PROC-GLM) in the SAS 8.1 program (p < 0.05).
Results
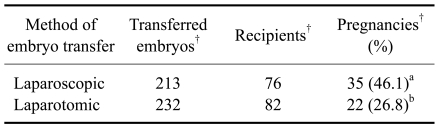
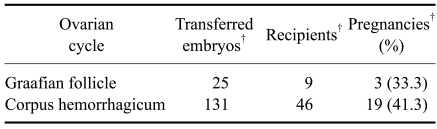
A comparison of the pregnancy rates between the laparoscopic ET and ET by laparotomy revealed that there was a significant difference between the two methods of ET (Table 2). Following the laparoscopic method, 213 transgenic embryos were transferred to 76 recipient goats and 35 recipients (46.1%) became pregnant. However, with the ET by laparotomy, only 22 out of the 82 recipients (26.8%) became pregnant. No significant difference was observed in the pregnancy rates between the ovulated (CH) and non-ovulated (GF) groups (Table 3).
Table 2.
Comparison of pregnancy rates between laparoscopic and laparotomic embryo transfer
a,bValues in the same column with different superscripts are different (p < 0.05). †: number.
Table 3.
Comparison of pregnancy rates according to ovarian findings of the recipient goat with laparoscopic embryo transfer
†: number.
Discussion
The results of this study showed a significantly higher pregnancy rate with laparoscopic ET compared to ET by laparotomy. Tittel et al. [23] noted that laparoscopic adhesiolysis resulted in a significantly reduced number of new adhesions compared to open surgery. The operation was performed only in transferable recipients after laparoscopic exploration of the ovary and uterus.
For efficient production of transgenic goats, by pronuclear microinjection, the pregnancy rate following ET is important. Relatively higher pregnancy rates have been recorded when transferring non-transgenic embryos by laparotomy ET [14], compared to the pregnancy rates after the transfer of transgenic embryos [18]. In previous studies using Korean native goats as recipients, the pregnancy rate following laparoscopic ET was lower than the rate observed in our study (25.7% and 36.8% vs. 46.1%).
The findings of our study suggest that by decreasing the disadvantages of ET by laparotomy, we achieved better results with laparoscopic ET. However, our pregnancy rate with ET by laparotomy was lower than reported in a previous study [11], suggesting that additional studies might lead to an improvement in pregnancy rates of ET by laparotomy.
We also compared the pregnancy rates between the GF and CH groups to evaluate the effects of artificial rupture on the non-ovulated Graafian follicles. When Graafian follicles were identified by laparoscopic ET, they were ruptured artificially for formation of the CL, essential for the maintenance of a pregnancy. We then investigated the effects of the artificially ruptured Graafian follicles by comparison of the pregnancy rates. The most appropriate period for transferring an embryo is within 24 h after ovulation. Although the recipient goats were synchronized with progesterone and PMSG for the ET, some of the recipient goats had not ovulated at the time of the ET. Out of 55 recipient goats, nine goats (16.4%) had not yet ovulated with the Graafian follicle and 46 goats (83.6%) were estimated to have passed beyond the 24 h after ovulation by observation of the corpus luteum (CL). In comparison of the pregnancy rates, there was no significant difference between the CH and GF groups (41.3% vs. 33.3%, respectively, p > 0.05). Although the pregnancy rates were lower than in the CH group, an acceptable pregnancy rate was achieved by artificial rupture in the GF group. Therefore, in cases with a non-ovulated Graafian follicle, artificial rupture was efficient for the formation of the CL, essential for pregnancy maintenance after embryo transfer. However, if artificial rupture was not performed in the Graafian follicle, medical induction of ovulation or additional embryo transfer after CL formation was needed.
The results of this study demonstrated that laparoscopic ET was a reliable and effective technique for efficient production of transgenic goats after pronuclear DNA microinjection. In addition, we found that artificial rupture of the Graafian follicle was an efficient method for the formation of the CL for pregnancy maintenance. More work is needed to better understand the factors involved in this process for further improvement of the pregnancy rate in caprine laparoscopic ET.
Acknowledgments
This study was supported by the High-Technology Development Project (No. 2003-6628) and Research Project on the Production of Bio-organs (No. 200606031401) from Ministry of Agriculture and Forestry, and the BioGreen 21 Program from Rural Development Administration, Korea.
References
- 1.Baldassarre H, Wang B, Kafidi N, Keefer C, Lazaris A, Karatzas CN. Advances in the production and propagation of transgenic goats using laparoscopic ovum pick-up and in vitro embryo production technologies. Theriogenology. 2002;57:275–284. doi: 10.1016/s0093-691x(01)00671-9. [DOI] [PubMed] [Google Scholar]
- 2.Baldassarre H, Wang B, Kafidi N, Gauthier M, Neveu N, Lapointe J, Sneek L, Leduc M, Duguay F, Zhou JF, Lazaris A, Karatzas CN. Production of transgenic goats by pronuclear microinjection of in vitro produced zygotes derived from oocytes recovered by laparoscopy. Theriogenology. 2003;59:831–839. doi: 10.1016/s0093-691x(02)01128-7. [DOI] [PubMed] [Google Scholar]
- 3.Besenfelder U, Mödl J, Müller M, Brem G. Endoscopic embryo collection and embryo transfer into the oviduct and the uterus of pigs. Theriogenology. 1997;47:1051–1060. doi: 10.1016/s0093-691x(97)00062-9. [DOI] [PubMed] [Google Scholar]
- 4.Clark AJ. The mammary gland as a bioreactor: expression, processing, and production of recombinant proteins. J Mammary Gland Biol Neoplasia. 1998;3:337–350. doi: 10.1023/a:1018723712996. [DOI] [PubMed] [Google Scholar]
- 5.Colman A. Production of proteins in the milk of transgenic livestock: problems, solutions, and successes. Am J Clin Nutr. 1996;63:639S–645S. doi: 10.1093/ajcn/63.4.639. [DOI] [PubMed] [Google Scholar]
- 6.Denman J, Hayes M, O'Day C, Edmunds T, Bartlett C, Hirani S, Ebert KM, Gordon K, McPherson JM. Transgenic expression of a variant of human tissue-type plasminogen activator in goat milk: purification and characterization of the recombinant enzyme. Biotechnology. 1991;9:839–843. doi: 10.1038/nbt0991-839. [DOI] [PubMed] [Google Scholar]
- 7.Ebert KM, Selgrath JP, DiTullio P, Denman J, Smith TE, Memon MA, Schindler JE, Monastersky GM, Vitale JA, Gordon K. Transgenic production of a variant of human tissue-type plasminogen activator in goat milk: generation of transgenic goats and analysis of expression. Biotechnology. 1991;9:835–838. doi: 10.1038/nbt0991-835. [DOI] [PubMed] [Google Scholar]
- 8.Ebert KM, Schindler JES. Transgenic farm animals: progress report. Theriogenology. 1993;39:121–135. [Google Scholar]
- 9.Ebert KM, DiTullio P, Barry CA, Schindler JE, Ayres SL, Smith TE, Pellerin LJ, Meade HM, Denman J, Roberts B. Induction of human tissue plasminogen activator in the mammary gland of transgenic goats. Biotechnology. 1994;12:699–702. doi: 10.1038/nbt0794-699. [DOI] [PubMed] [Google Scholar]
- 10.Edmunds T, Van Patten SM, Pollock J, Hanson E, Bernasconi R, Higgins E, Manavalan P, Ziomek C, Meade H, McPherson JM, Cole ES. Transgenically produced human antithrombin: structural and functional comparison to human plasma-derived antithrombin. Blood. 1998;91:4561–4571. [PubMed] [Google Scholar]
- 11.Gootwine E, Barash I, Bor A, Dekel I, Friedler A, Heller M, Zaharoni U, Zenue A, Shani M. Factors affecting success of embryo collection and transfer in a transgenic goat program. Theriogenology. 1997;48:485–499. doi: 10.1016/s0093-691x(97)00257-4. [DOI] [PubMed] [Google Scholar]
- 12.Fayrer-Hosken RA, Younis AI, Brackett BG, McBride CE, Harper KM, Keefer CL, Cabaniss DC. Laparoscopic oviductal transfer of in vitro matured and in vitro fertilized bovine oocytes. Theriogenology. 1989;32:413–420. doi: 10.1016/0093-691x(89)90007-1. [DOI] [PubMed] [Google Scholar]
- 13.Houdebine LM. Production of pharmaceutical proteins from transgenic animals. J Biotechnol. 1994;34:269–287. doi: 10.1016/0168-1656(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 14.Kiessling AA, Hughes WH, Blankevoort MR. Superovulation and embryo transfer in the dairy goat. J Am Vet Med Assoc. 1986;188:829–832. [PubMed] [Google Scholar]
- 15.Ko JH, Lee CS, Kim KH, Pang MG, Koo JS, Fang N, Koo DB, Oh KB, Youn WS, Zheng GD, Park JS, Kim SJ, Han YM, Choi IY, Lim J, Shin ST, Jin SW, Lee KK, Yoo OJ. Production of biologically active human granulocyte colony stimulating factor in the milk of transgenic goat. Transgenic Res. 2000;9:215–222. doi: 10.1023/a:1008972010351. [DOI] [PubMed] [Google Scholar]
- 16.Koeman J, Keefer CL, Baldassarre H, Downey BR. Developmental competence of prepubertal and adult goat oocytes cultured in semi-defined media following laparoscopic recovery. Theriogenology. 2003;60:879–889. doi: 10.1016/s0093-691x(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 17.Kühholzer B, Müller S, Besenfelder U, Prokofiev MI, Ernst LK, Brem G. Laparoscopic recovery of pronuclear-stage goat embryos. Vet Rec. 1998;142:40–42. doi: 10.1136/vr.142.2.40. [DOI] [PubMed] [Google Scholar]
- 18.Lee CS, Fang NZ, Koo DB, Lee YS, Zheng GD, Oh KB, Youn WS, Han YM, Kim SJ, Lim JH, Shin ST, Jin SW, Lee KS, Ko JH, Koo JS, Park CS, Yoo OJ, Lee KK. Embryo recovery and transfer for the production of transgenic goats from Korean native strain, Capra hircus aegagrus. Small Rumin Res. 2000;37:57–63. doi: 10.1016/s0921-4488(99)00139-x. [DOI] [PubMed] [Google Scholar]
- 19.McKelvey WA, Robinson JJ, Aitken RP. A simplified technique for the transfer of ovine embryos by laparoscopy. Vet Rec. 1985;117:492–494. doi: 10.1136/vr.117.19.492. [DOI] [PubMed] [Google Scholar]
- 20.Mutiga ER, Baker AA. Transfer of sheep embryos through a laparoscope. Vet Rec. 1984;114:401–402. doi: 10.1136/vr.114.16.401. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph NS. Biopharmaceutical production in transgenic livestock. Trends Biotechnol. 1999;17:367–374. doi: 10.1016/s0167-7799(99)01341-4. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, First N. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology. 1992;37:963–978. doi: 10.1016/0093-691x(92)90096-a. [DOI] [PubMed] [Google Scholar]
- 23.Tittel A, Treutner KH, Titkova S, Ottinger A, Schumpelick V. New adhesion formation after laparoscopic and conventional adhesiolysis: a comparative study in the rabbit. Surg Endosc. 2001;15:44–46. doi: 10.1007/s004640000256. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Baldassarre H, Tao T, Gauthier M, Neveu N, Zhou JF, Leduc M, Duguay F, Bilodeau AS, Lazaris A, Keefer C, Karatzas CN. Transgenic goats produced by DNA pronuclear microinjection of in vitro derived zygotes. Mol Reprod Dev. 2002;63:437–443. doi: 10.1002/mrd.10199. [DOI] [PubMed] [Google Scholar]