Abstract
An herbal extract mixture and yogurt added to the herbal extract mixture were tested for their protective and therapeutic effects on ethanol-induced liver injury. The herbal extract mixture, yogurt and commercial drugs were used for treatment for two weeks prior to administering a single oral dose of ethanol (3 g/kg body weight). The herbal extract mixture and yogurt added to the herbal extract mixture were found to provide protection against ethanol-induced toxicity comparable to the commercial drug treatment, according to the serum and histopathological analysis. It was also shown that co-treatment with herbal extract mixture and yogurt against a triple oral dose of ethanol (2 g/kg body weight, over one week) provided protection against ethanol toxicity. After the initial set of experiments, the herbal extract mixture and yogurt treatments were extended for three more weeks. When compared to the positive control, further treatment with both the herbal extract and yogurt significantly reduced liver injury and resulted in a lower grade of lipid deposition.
Keywords: ethanol-induced toxicity, herbal extract mixture, protective effect, rat, yogurt
Introduction
Functional foods and food additives are substances that provide health benefits beyond basic nutrition due to certain physiologically active components. Some of these substances may help prevent disease, reduce the risk of developing disease, or enhance general health, especially in the liver. Consumer interest in functional foods has increased during the late twentieth century as people have become more focused on achieving and maintaining a healthy lifestyle [9].
Alcoholic liver disease (ALD) remains a serious health problem. ALD is a common consequence of prolonged and heavy alcohol intake. Changes fatal to the liver include fatty liver, hepatitis and hepatic cirrhosis [5,6,12]. Multiple mechanisms are likely to be involved in the pathogenesis of these problems; especially those associated with the toxic substances generated during alcohol metabolism. Accumulated evidence has demonstrated that both oxidative stress and abnormal cytokine production are important factors in the development of alcoholic liver damage [2,3,8].
Although much progress has been made in understanding the pathogenesis of alcoholic liver disease, there remains no effective therapy for this disease. In the absence of reliable drugs to protect the liver, herbs may play role in treating liver disorders. Many plants and herbal extracts demonstrate hepatoprotective activity; hence, many attempts have been made to formulate herbal preparations as functional foods [5,9].
This study was undertaken to investigate the protective and therapeutic effects of an herbal extract mixture and yogurt added to the herbal extract mixture on ethanol induced hepatic injury in a rat model.
Materials and Methods
Animals
Four week old male SD rats weighing 190-200 g were purchased from SLC (Japan) and maintained in a barrier room with a 12:12 h light and dark cycle. The room temperature (22 ± 1℃) and humidity (55 ± 5%) were controlled automatically. Laboratory pellet chow (Purina, Korea) and water were given ad libitum. All of the animals were acclimatized for one week prior to the experiment. The procedures involving the animals and their care were carried out according to the Guidelines for the Care and Use of Laboratory Animals of Seoul National University, Korea.
Materials
The herbal extract mixture was composed of two herbal extracts (alder tree extract: Alnus japonica Steud, labiate herb extract; Prunella vulgaris var. lilacina Nakai), two fermented ingredients (milk thistle extract, green bean-rice bran fermentation extract) and turnip concentrate. The herbal extract mixture preparation included the following: 100 g of the xylem and bark from a dried alder tree was added to 1,000 g of water, then boiled at 100℃ for 1-5 h. The solution was filtered through a 5 µm filter to acquire 500 g of an alder tree extract. Fifty grams of the dried labiate herb was boiled at 100℃ in 1,000 g of water for 1-5 h, followed by filtration through a 400 mesh filter to produce 600 g of labiate herb extract. Milk thistle extract was purchased in powder form (TGS, Japan) and had, by weight, a total flavonoid content of 80-95% and 28-32% sylibin content. In addition, a liquid green bean-rice bran fermentation extract was purchased (Toyo Hakko, Japan) along with a liquid turnip concentrate (34-36%) (KangHwa Product, Korea).
In this study, two different doses (1 × and 2 ×) of herbal extract mixture and yogurt were used. The herbal extract mixture 1 × with the dose of the yogurt additive, was expected to be put on the market. The herbal extract mixture 2 × had a two-fold concentration. The commercial drug (Legalon; Bukwang Pharm, Korea) consisted of sylibin (Carduus marianus extract) was used as a control. The ethanol purchased from the Sigma-Aldrich (USA).
Experiment design
To examine protective and therapeutic efficacy of the herbal extract mixture and yogurt, we carried out two animal experiments as follows:
Protective effects of herbal extract mixture with a single ethanol challenge
For the herbal extract mixture, the rats were divided into four groups (herbal extract mixture 1 ×, herbal extract mixture 2 ×, negative, and positive control groups) containing five animals each. Before inducing ethanol toxicity, the negative and positive control groups received PBS 5 ml/kg body weight (BW) orally once per day for two weeks. The herbal extract mixture, 1 × and 2 × treatment groups, was administered at a dose of 5 ml/kg body weight of herbal extract mixture, 1 × and 2 ×, orally once per day for two weeks. After the two-week treatment period, a single dose of ethanol (50%, 3 g/kg BW) was administered orally to all groups except for the negative control group. The negative control animals were administered PBS in equivalent volumes instead of ethanol.
For the yogurt preparation, the rats were divided into four groups (yogurt, commercial drug, negative, and positive control groups) containing five animals each. Before inducing ethanol toxicity, the negative and positive control groups were treated the same as in the herbal extract mixture experiment. The commercial drug group was administered 70 mg/kg BW (recommended consumption dose for adults) in PBS once a day orally during the same period. The yogurt treatment groups received 5 ml/kg BW of yogurt orally once a day for two weeks. After the two-week treatment, ethanol administration was carried out as mentioned above.
Protective and therapeutic effects of the herbal extract mixture and yogurt to triple ethanol challenge
For the herbal extract mixture, the rats were divided into four groups (herbal extract mixture 1 ×, herbal extract mixture 2 ×, negative, and positive control groups) containing ten animals each. The negative and positive control groups were given PBS 5 ml/kg BW orally once per day for one week. The herbal extract mixture, 1 × and 2 ×, treatment groups were given 5 ml/kg BW of herbal extract mixture, 1 × and 2 ×, orally once a day for one week. Ethanol (50%, 2 g/kg BW) was administered orally to all groups, except for the negative control group, once a day on the first, fourth and seventh day. The negative control animals were given PBS in an equivalent volume instead of the ethanol. Ethanol administration was given 30 min after the herbal extract mixture and the PBS treatment.
For the yogurt preparation, the experimental groups were the same as the single ethanol challenge. The negative and positive control groups were treated the same herbal extract mixture triple ethanol challenge. The commercial drug group was given 70 mg/kg BW of the drug for the liver (recommended consumption dose for adults) in PBS once a day orally during the same period. The yogurt treatment groups were given 5 ml/kg BW of yogurt once a day orally for one week. Ethanol (50%, 2 g/kg BW) administration was carried out as mentioned above. Six hours later after the last ethanol dose, one-half of all animals in the experimental group (five animals) were sacrificed and necropsied; liver and blood samples were collected on the seventh day.
To examine the therapeutic properties, the remaining experimental groups of animals were continuously treated with herbal extract mixture, yogurt and PBS for three weeks, in the same way, without ethanol administration.
All experimental animals were denied food and water for 5 h prior to treatment. After each treatment, the animals were given feed pellets and water ad libitum except on the day of necropsy. Clinical evaluation and general appearances were noted once daily, and body weights and food consumption levels were measured twice per week. Six hours after the final administration and treatment, all animals were subsequently sacrificed under anesthesia. Liver and blood samples were collected for further examination.
Serum analysis
From the collected blood samples, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin levels were measured using a standard clinical automatic analyzer (Hitachi 7200; Hitachi High-Tech, Japan).
Necropsy and histopathology
Livers from all experimental groups were examined immediately and fixed in 10% buffered formalin for 24 h, processed in an alcohol-xylene series, embedded in paraffin wax, and sectioned at 2 µm. The sections were stained with hematoxylin and eosin. The sections were graded for the degree of fatty change, inflammation and pericentral fibrosis. Steatosis was scored as follows: 1 when less than 25% of the cells contained fat droplets, 2 when 25-50%, 3 when 50-75%, and 4 when > 75% contained fat droplets. Inflammation was graded 0 to 3, as previously described [11]: 1 indicated the presence of scattered inflammatory cells, 2 indicated the presence of foci of inflammatory cells and 3 corresponded to diffuse inflammation. The sum of steatosis and inflammation scores was used in the results. Persons unaware of our experimental treatments evaluated the liver sections.
Oil red O staining
Some of the liver tissues were removed and placed in 4% paraformaldehyde in PBS for 4 h. Briefly, the tissues were rinsed with PBS and cryoprotected by soaking in 40% sucrose solution at 4℃ overnight. The tissues were then snap-frozen in OCT and stored at -70℃. Eight micrometer frozen sections were prepared on glass slides (Super FrostPlus; Fisher, USA) and stored at -70℃. Frozen sections were incubated with oil red O, washed with 60% isopropanol and then counterstained with hematoxylin. The hepatic lipid deposit area (red color), in the stained liver section, was analyzed by an image analyzer (TDI Scope Eye Version 3.0 for Windows; Olympus, Japan).
Statistical analysis
All data (BW, food intake, blood chemistry, histological grade, lipid area) are reported as a mean ± SD (n = 5). The data was assessed using one-way analysis of variance coupled with Duncan's test using the SAS system. A p < 0.05 was considered significant.
Results
Change of body weight and food consumption
There were no clinical abnormalities in any of the experimental animals during the experimental period. The BW and food consumption levels were similar and showed no significant differences (data not shown). However, when the ethanol was administered, the food consumption levels in the ethanol-treated experimental animals decreased slightly when compared to the negative control animals (data not shown).
Protective effects of herbal extract mixture and yogurt with a single ethanol challenge
Ethanol-induced liver damage was evaluated by measuring liver injury markers (AST, ALT and total bilirubin) and assessing histopathological changes.
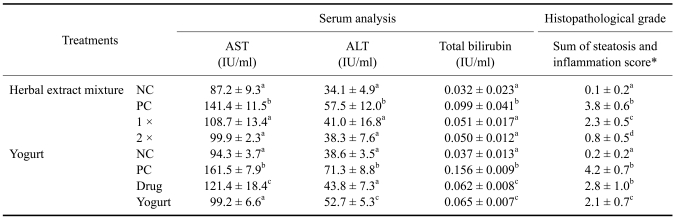
For the serum analysis, the herbal extract mixture, 1 × and 2 ×, treatment group animals showed significantly decreased levels of all injury makers compared to the positive control animals (Table 1). However, a dose dependent difference between the herbal extract mixture, 1 × and 2 ×, was not observed. When compared with the commercial drug treatment, the AST levels of the animals in the herbal extract mixture 2 × treatment group were decreased compared to the negative control animals, but by contrast, the levels of ALT and total bilirubin were not. However, all liver injury makers in the blood were significantly decreased with the yogurt treatment (Table 1).
Table 1.
Results from serum analysis and histopathological grade of the protective effect of herbal extract mixture and yogurt to a single ethanol challenge (n = 5)
Superscript letters (a, b, c, d) indicate groups that are significantly different (p < 0.05) from each other. *Scores of steatosis and inflammation were analyzed through the description of Materials and Methods. NC: negative control, PC: positive control, 1 ×: herbal extract mixture 1 × pretreatment, 2 ×: herbal extract mixture 2 × pretreatment, Drug: commercial liver drug pretreatment, Yogurt: yogurt pretreatment.
The livers from the ethanol treated animals showed hepatic microvesicular steatosis; the fat accumulated in vesicles that displaced the cytoplasm. Hepatocyte necrosis, degeneration and lymphocyte infiltration were also observed. For the histopathological grading, the herbal extract mixture 1 × and 2 × and the yogurt treatment groups showed a significantly lower grade when compared with the positive control animals; interestingly, the commercial drug treatment group did not show a lower histopathological grade (Table 1).
Protective and therapeutic effects of herbal extract mixture and yogurt with the triple ethanol challenge
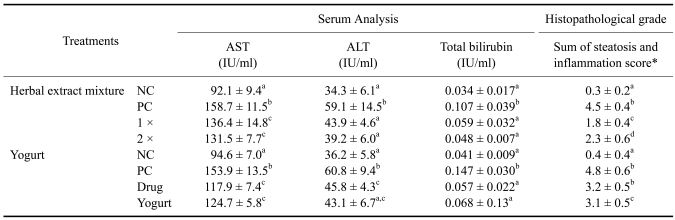
As shown in Table 2, the triple ethanol administration caused a higher increase in all serum injury marker levels and a more severe histopathological grade than did the single administration. However, one week of treatment with the herbal extract mixture, 1 × and 2 × and the yogurt preparation reduced the hepatic injury.
Table 2.
Results from serum analysis and histopathological grade of the protective effect of herbal extract mixture and yogurt to a triple ethanol challenge (day 7, n = 5)
Superscript letters (a, b, c, d) indicate groups that are significantly different (p < 0.05) from each other. *Score of steatosis and inflammation were analyzed through the description of Materials and Methods. NC: negative control, PC: positive control, 1 ×: herbal extract mixture 1 × treatment, 2 ×: herbal extract mixture 2 × treatment, Drug: commercial liver drug treatment, Yogurt: yogurt treatment.
Serum and histopathological analysis
The serum analysis showed that the herbal extract mixture, 1 × and 2 ×, treatment did not reach dose-dependent significance, but did indicate a trend. The yogurt treatment did not reduce the levels of AST or total bilirubin similar to the results of the commercial drug. On the other hand, the ALT levels in the herbal extract mixture and yogurt treatment group showed a further decrease when compared to the commercial drug treatment (Table 2).
The histopathological grade also showed a significant reduction in steatosis and inflammation in accordance with the yogurt treatments, but the commercial drug administration did not reduce the histopathological lesions as did the herbal extract mixture and yogurt preparations (Table 2).
To investigate the therapeutic effects of the herbal extract mixture and yogurt, the animals were treated for an additional three weeks with the triple administration of ethanol, the herbal extract mixture and the yogurt preparation.
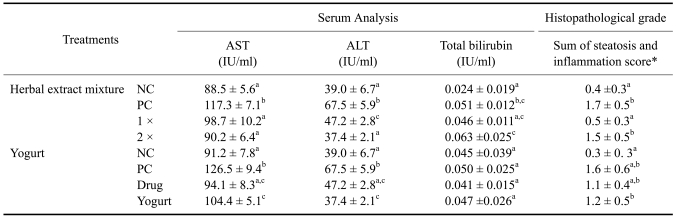
Two serum markers in the herbal extract mixture and yogurt treatment groups (AST and ALT) had lower levels on the day seven analysis and these differences were statistically significant. However, the total bilirubin showed no significant changes. The total bilirubin levels did not show any trend at the end of experiment (Table 3).
Table 3.
Results from serum analysis and histopathological grade of the protective effect of herbal extract mixture and yogurt to triple ethanol challenge (day 28, n = 5)
Superscript letters (a, b, c, d) indicate groups that are significantly different (p < 0.05) from each other. *Score of steatosis and inflammation were analyzed through the description of Materials and Methods. NC: negative control, PC: positive control, 1 ×: herbal extract mixture 1 × treatment, 2 ×: herbal extract mixture 2 × treatment, Drug: commercial liver drug treatment, Yogurt: yogurt treatment.
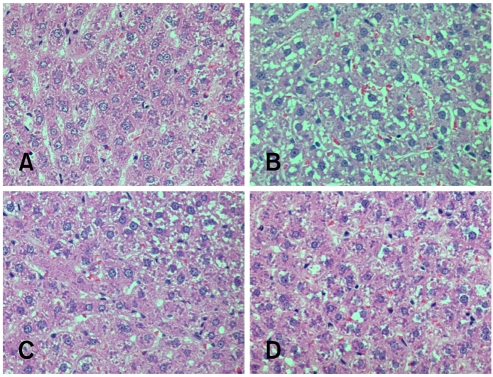
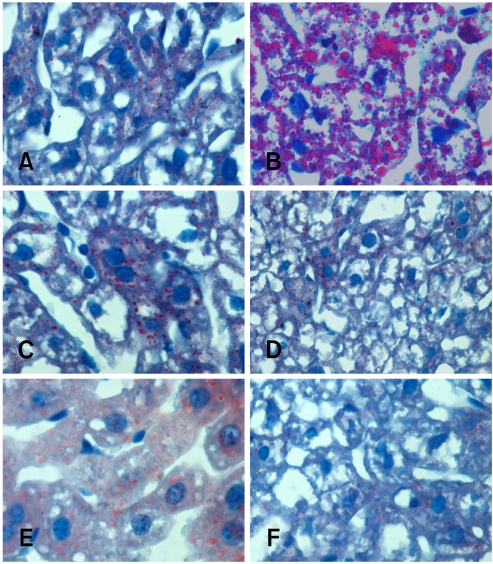
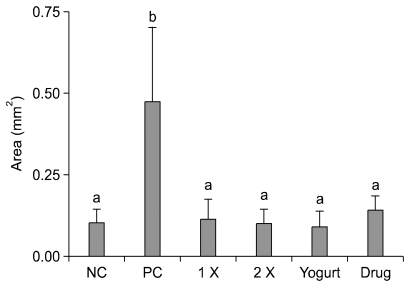
For the histopathological grading, only the herbal extract mixture 1× and yogurt treatment groups showed significantly decreased scores. By contrast, the herbal extract mixture 2× and commercial drug treatment showed no significant changes (Table 3). It was difficult to estimate the level of steatosis because of glycogen deposits in the liver (Fig. 1). To determine the lipid distribution, oil red O staining was carried out and the lipid areas were analyzed (Fig. 2 and 3). The lipid accumulation showed only a high level in positive control (Fig. 3); however, the herbal extract mixture, yogurt and commercial drug treatment groups had significantly lower lipid accumulation similar to negative control.
Fig. 1.
Histopathology of the liver in the experimental groups (A) negative control, (B) positive control, (C) commercial liver drug treatment, (D) yogurt treatment, H&E stain, ×200.
Fig. 2.
Oil red O stain of the liver in the experimental groups. (A) negative control, (B) positive control, (C) herbal extract mixture 1 × treatment, (D) herbal extract mixture 2 × treatment, (E) commercial liver drug treatment, (F) yogurt treatment, Oil red O stain, ×200.
Fig. 3.
Lipid areas in the experimental groups. NC: negative control, PC: positive control, 1 ×: herbal extract mixture 1 × treatment, 2 ×: herbal extract mixture 2 × treatment, Drug: commercial liver drug treatment, Yogurt: yogurt treatment. The lipid areas are presented as the mean ± SD (n = 5). Superscript letters (a, b) indicate groups that are significantly different (p < 0.05) from each other.
Discussion
Ethanol causes toxicity in mice and rats [4,6,14]. The factors responsible for ethanol-induced liver injury are the levels of unmetabolized acetaldehydes and reactive oxygen species (ROS). Acetaldehyde, which is toxic itself, is a very unstable ethanol metabolite in living orangims, and the release of ROS from hepatocytes induces apoptosis [2,10,15]. An overdose of ethanol or chronic alcohol intake increases these toxic compounds and this results in acute and/or chronic liver disease manifested by steatosis, inflammation and hepatic failure [4,7,11,13].
In this study, a mixture of an herbal extract and yogurt additive were tested for their protective and therapeutic efficacy against ethanol-induced toxicity in rats. The two-week treatment with the herbal extract mixture and yogurt subsequent to a single ethanol dose challenge showed decreased liver injury in both the serum and histopathological analysis when compared with the positive control animals. The effects were similar to the commercial drug treatment. The results also showed that a triple ethanol challenge, in the one-week herbal extract mixture and yogurt co-treatment as well as the three-week continuous treatment, reduced lipid deposition caused by the ethanol-induced toxicity.
We previously reported that the herbal extract mixture had a protective effect against ethanol-induced toxicity in a mouse model [1]. In this study, the herbal extract mixture and yogurt also showed protective and therapeutic effects for the ethanol-induced toxicity in a rat model. The results suggest that treatment with an herbal extract mixture and yogurt effectively protected and treated the ethanol associated liver injury in the rats studied. To determine the precise mechanism underlying the efficacy of the herbal extract mixture for ethanol toxicity, studies focusing on the antioxidant effects of the herbal extract mixture and its neutralizing capabilities at the molecular level are being carried out.
Acknowledgments
This work was supported by a grant from the Research Institute for Veterinary Science, College of Veterinary Medicine Seoul National University and Korea Research Foundation Grant (KRF-005-F00077).
References
- 1.Baek MW, Seok SH, Lee HY, Kim DJ, Lee BH, Ahn YT, Lim KS, Huh CS, Park JH. Protective Effect of Y-mix in an Ethanol-induced Toxicity Model. Lab Anim Res. 2005;21:413–417. [Google Scholar]
- 2.Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–1326. doi: 10.1002/hep.510280521. [DOI] [PubMed] [Google Scholar]
- 3.Bautista AP. Acute alcohol intoxication and endotoxemia desensitize HIV-1 gp120-induced CC-chemokine production by Kupffer cells. Life Sci. 2001;68:1939–1949. doi: 10.1016/s0024-3205(01)00986-9. [DOI] [PubMed] [Google Scholar]
- 4.Chawla RK, Jones DP. Abnormal metabolism of S-adenosyl-L-methionine in hypoxic rat liver. Similarities to its abnormal metabolism in alcoholic cirrhosis. Biochim Biophys Acta. 1994;1199:45–51. doi: 10.1016/0304-4165(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 5.Choi JS, Yoon TJ, Kang KR, Lee KH, Kim WH, Suh YH, Song J, Jung MH. Glycoprotein isolated from Acanthopanax senticosus protects against hepatotoxicity induced by acute and chronic alcohol treatment. Biol Pharm Bull. 2006;29:306–314. doi: 10.1248/bpb.29.306. [DOI] [PubMed] [Google Scholar]
- 6.Feo F, Pascale R, Garcea R, Daino L, Pirisi L, Frassetto S, Ruggiu ME, Di Padova C, Stramentinoli G. Effect of the variations of S-adenosyl-L-methionine liver content on fat accumulation and ethanol metabolism in ethanol-intoxicated rats. Toxicol Appl Pharmacol. 1986;83:331–341. doi: 10.1016/0041-008x(86)90310-8. [DOI] [PubMed] [Google Scholar]
- 7.Horie Y, Kato S, Ohki E, Tamai H, Yamagishi Y, Ishii H. Hepatic microvascular dysfunction in endotoxemic rats after acute ethanol administration. Alcohol Clin Exp Res. 2000;24:691–698. [PubMed] [Google Scholar]
- 8.Kannan M, Wang L, Kang YJ. Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med. 2004;229:553–559. doi: 10.1177/153537020422900614. [DOI] [PubMed] [Google Scholar]
- 9.Katan MB, De Roos NM. Promises and problems of functional foods. Crit Rev Food Sci Nutr. 2005;44:369–377. doi: 10.1080/10408690490509609. [DOI] [PubMed] [Google Scholar]
- 10.Kitazawa T, Nakatani Y, Fujimoto M, Tamura N, Uemura M, Fukui H. The production of tumor necrosis factor-alpha by macrophages in rats with acute alcohol loading. Alcohol Clin Exp Res. 2003;27:72S–75S. doi: 10.1097/01.ALC.0000078611.55696.F0. [DOI] [PubMed] [Google Scholar]
- 11.Leo MA, Lieber CS. Hepatic fibrosis after long-term administration of ethanol and moderate vitamin A supplementation in the rat. Hepatology. 1983;3:1–11. doi: 10.1002/hep.1840030101. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda Y, Takada A, Takase S, Yasuhara M. Effects of ethanol on the secretion of hepatic secretory protein in rat alcoholic liver injury. Alcohol. 1991;8:433–437. doi: 10.1016/s0741-8329(91)90051-w. [DOI] [PubMed] [Google Scholar]
- 13.Marotta F, Barreto R, Wu CC, Naito Y, Gelosa F, Lorenzetti A, Yoshioka M, Fesce E. Experimental acute alcohol pancreatitis-related liver damage and endotoxemia: synbiotics but not metronidazole have a protective effect. Chin J Dig Dis. 2005;6:193–197. doi: 10.1111/j.1443-9573.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 14.Thurman RG, Gao W, Connor HD, Adachi Y, Stachlewitz RF, Zhong Z, Knecht KT, Bradford BU, Mason RP, Lemasters JJ. Role of Kupffer cells in failure of fatty livers following liver transplantation and alcoholic liver injury. J Gastroenterol Hepatol. 1995;10:S24–S30. doi: 10.1111/j.1440-1746.1995.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama H, Fukuda M, Okamura Y, Mizukami T, Ohgo H, Kamegaya Y, Kato S, Ishii H. Superoxide anion release into the hepatic sinusoid after an acute ethanol challenge and its attenuation by Kupffer cell depletion. Alcohol Clin Exp Res. 1999;23:71S–75S. doi: 10.1111/j.1530-0277.1999.tb04538.x. [DOI] [PubMed] [Google Scholar]