Abstract
Interferon (IFN) has therapeutic potential for a wide range of infectious and proliferative disorders. However, the half-life of IFN is too short to have a stable therapeutic effect. To overcome this problem, serum immunoglobulin has been fused to IFN. In this study, the efficacy of serum immunoglobulin fused INFs (si-IFN1 and si-IFN2) was evaluated on athymic mice bearing colon 26 adenocarcinoma cells. Seven days after the implantation of tumor cells, each group of mice was injected once a week with si-IFN1 and si-IFN2 at two different concentrations (10 × : 30 µg/kg and 50 × : 150 µg/kg). A slight anti-tumoral effect was observed in all 10 × groups compared to the control. In the 50 × groups, however, si-IFN1 and si-IFN2 showed significant anti- tumoral effects compared to the control. To gain more information on the mechanisms associated with the decrease of tumor size, a Western blot assay of apoptosis-related molecules was performed. The protein expression of cytochrome c, caspase 9, 6, and 3 were increased by si-IFN1 and si-IFN2. These 2 IFNs also increased the expressions of p53, p21, Bax and Bad. Interestingly, si-IFN1 and si-IFN2 decreased the expression of VEGF-β. Taken together, serum immunoglobulin fused IFNs increased therapeutic efficacy under current experimental condition.
Keywords: adenocarcinoma cell, interferon, serum immunoglobulin, tumor growth inhibition
Introduction
Interferon (IFN) is a cytokine produced and secreted by eukaryotic cells in response to stimulation by viruses, bacteria, and mitogens and mediates diverse biological activities by binding to a specific receptor on the cell surface [1,10]. IFNs have therapeutic potential for a wide range of infectious and proliferative disorders due to their pleitropic effects on multiple metabolic, immunologic and pathologic events [3,6,9]. However, the half-life of IFN is too short for a stable therapeutic effect. To overcome this problem, several methods are used for improving the stability of proteins. One of the methods is chemical modification of a polypeptide with highly soluble macromolecules such as polyethylene glycol (PEG) which prevents the polypeptides from making contact with proteases. However, such pegylated polypeptides have the disadvantage of lowering both the activity and production yield of an active substance as the molecular weight of PEG increases. Another approach for enhancing the in vivo stability of the IFN is to conjugate the IFN with a stable serum immunoglobulin. As an improved method for enhancing the stability of an active polypeptide and simultaneously maintaining the in vivo activity thereof, the two IFN conjugate si-IFN1 and si-IFN2 comprising an IFN, PEG and serum immunoglobulin, were interlinked to one another.
The present paper reports that IFN-α modified with serum immunoglobulin (si-IFN1 and si-IFN2) inhibits tumor growth in athymic mice bearing colon 26 adenocarcinoma cells.
Materials and Methods
Reagents and cell culture
Recombinant IFN-α, serum immunoglobulin fused IFN-α (si-IFN1 and si-IFN2), and peginterferon α-2a were supplied by Hanmi Pharmaceutical (Korea). The efficacy of several IFNs was compared to efficacy-proven interferon, peginterferon α-2a, which is a covalent conjugate of recombinant α-2a interferon with a single branched bis-monomethoxy PEG chain (Roche, USA). The si-IFN1, si-IFN2, and PEGASYS were administered at two different concentrations (10 × groups: 30 µg/kg and 50 × groups: 150 µg/kg). The mouse colon 26 adenocarcinoma cells (CT-26) were purchased from the Korean Cell Line Bank (KCLB, Korea). Colon 26 adenocarcinoma cells were cultured with RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) in an atmosphere of 5% CO2 in an incubator at 37℃.
Experimental animals
Specific pathogen-free male athymic BALB/c nude mice were purchased from the SLC (Shizuoka Institute for Laboratory Animals Center, Japan). Athymic nude mice were maintained at 23 ± 2℃ , with a relative humidity of 50 ± 20% and a 12 h light/dark cycle. After 7 days of tumor cell inoculation, the mice were grouped as follows: control, 10 × and 50 × IFN concentrations. All the procedures for handling and caring for the animals were followed by the guidelines given in the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1985, revised 1996, USA). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
Tumor cell inoculation and treatment procedure
Mice were inoculated subcutaneously with 5 × 105 colon 26 adenocarcinoma cells in RPMI 1640 with 10% FBS. Seven days after tumor cell inoculation, each mouse was treated with recombinant IFN-α, si-IFN1, si-IFN2 and peginterferon α-2a by intratumoral injection. IFN-α was injected every day for 21 days, the others were injected on days 7, 14 and 21 [5].
Serum and hematological analysis
Blood samples for hematology were obtained from 5 mice in each group every week. Every week clinical biochemistry determinations were also made on the serum harvested from blood samples obtained from the mice. The following parameters were assayed: total protein (TPROT), albumin (ALB), total bilirubin (TBILI), aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose (GLU), blood urea nitrogen (BUN), creatinine (CREAT).
Measurement of tumor volume
Tumor volume was measured on days 7, 14 and 21 with the aid of vernier calipers. At necropsy, the tumor volume was estimated for the largest (a) and the smallest (b) diameter, and the tumor volume was calculated as V = ab2/2.
Western blot
Protein was extracted from tumor tissues in recombinant IFN-α, si-IFN1, si-IFN2, and peginterferon α-2a in 50 ×. The extracts were added to the sample buffer. Samples were boiled for 10 min, and proteins were separated on 15% SDS-PAGE for 18 h. The nitrocellulose membrane was rinsed twice in Tween 20-TBS (T-TBS) with 5% skim milk. Subsequently, the membranes were incubated with a 1:2,500 dilution of primary antibody (cytochrom c, caspase 3, 6, and 9, and p53, p21, Bax, Bad, VEGF-β, and FGF-2) in T-TBS buffer for 3 h. They were then washed twice for 10 min in T-TBS buffer, incubated with a 1:5,000 dilution of secondary antibody conjugated to HRP (Santa Cruz, USA) in T-TBS buffer for 1 h. After washing, the bands-of-interest were pictured by luminescent image analyzer LAS-3000 (Fujifilm, Japan).
Statistical analysis
The results were expressed as mean ± SD values for independent experiments. Statistical analysis was performed on all groups using the t test.
Results
Clinical hematology and biochemistry
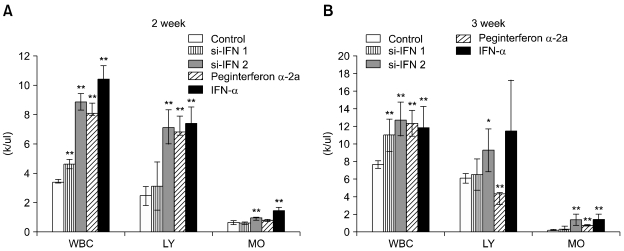
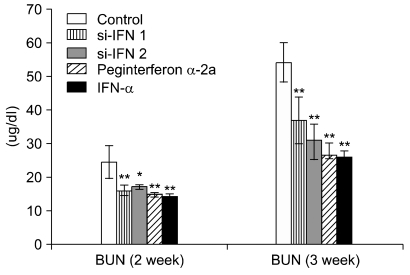
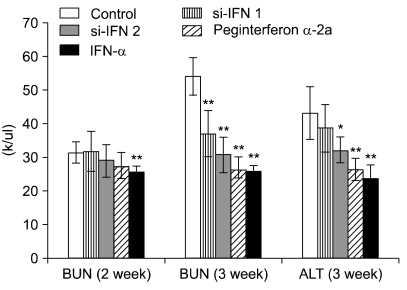
In the hematological test of the 10 × group, there was no significant change after 1, 2 and 3 weeks (data not shown), however, in the 50 × group, the WBC value was increased in si-IFN2, peginterferon α-2a and IFN-α compared to the control after 2 weeks. This pattern was also observed in the lymphocyte value (Fig. 1A). After 3 weeks, the increase of WBC's and lymphocytes was observed in the si-IFN2, peginterferon α-2a and IFN-α groups compared to the control (Fig. 1B). Interestingly, the significant change in the monocyte level observed at 2 weeks was not maintained until 3 weeks (Fig. 1). In the biochemical test of 10 × group, a decrease of BUN was observed at 2 weeks in si-IFN2, peginterferon α-2a and IFN-α group compared to the control and this decrease was maintained at 3 weeks (Fig. 2). In the biochemical test of the 50 × group, a decrease of BUN was observed only at 3 weeks in the si-IFN2, peginterferon α-2a and IFN-α group comparing to the control (Fig. 3). In particular, the level of ALT was decreased in peginterferon α-2a and IFN-α group comparing to the control at 3 weeks (Fig. 3).
Fig. 1.
Hematological assay of differential leukocytes in the 50 × interferon group. Blood samples for hematological determinations were obtained from 5 mice. The differential leukocyte count was measured at 1 week (data not shown), 2 weeks (A) and 3 weeks (B) after tumor cells inoculation (n = 5). 50 × interferon group: concentration of si-IFN1 and si-IFN2 (150 µg/kg), WBC: white blood cell, LY: lymphocyte, MO: monocyte. Each point represents the mean ± SD. *Significantly different from control (p < 0.05). **Significantly different from control (p < 0.01).
Fig. 2.
Concentrations of blood urea nitrogen (BUN) in the 10 × interferon group. Clinical biochemistry determinations were made on serum harvested from the blood of mice. BUN was measured at 1 week (data not shown), 2 weeks and 3 weeks after tumor cells inoculation (n = 5). 10 × interferon group: concentration of si-IFN1 and si-IFN2 (30 µg/kg), Each point represents the mean ± SD. *Significantly different from control (p < 0.05). **Significantly different from control (p < 0.01).
Fig. 3.
Concentrations of blood urea nitrogen (BUN) and alanine aminotransferase (ALT) in the 50 × interferon group. BUN and ALT were measured after tumor cells inoculation (n = 5). 50 × interferon group: concentration of si-IFN1 and si-IFN2 (150 µg/kg), Each point represents the mean ± SD. *Significantly different from control (p < 0.05). **Significantly different from control (p < 0.01).
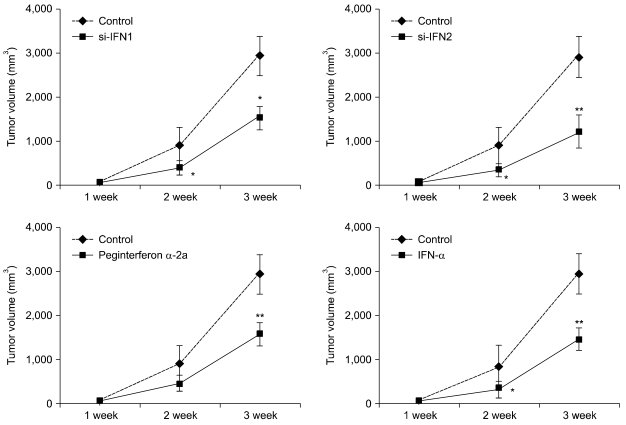
Tumor growth was inhibited by si-IFN1 and si-IFN2 treatment
In the 10 × group, the tumor volume decreased slightly in the si-IFN1, si-IFN2, peginterferon α-2a and IFN-α group compared to control (data not shown). In the 50 × group, however, the tumor volume decreased significantly in mice treated with si-IFN1, si-IFN2, peginterferon α-2a and IFN-α compared to the control (Fig. 4).
Fig. 4.
Effects of interferons on tumor growth inhibition. Mice were inoculated with 5 × 105 colon 26 adenocarcinoma cells in RPMI 1640 with 10% FBS subcutaneously. Seven days after tumor cell inoculation, the tumor size was measured. The mice were then treated with different interferons by intratumoral injection. IFN-α was injected every day for 3 weeks, the others were injected at 1 week, 2 weeks and 3 weeks. Tumor volume was estimated for the largest (a) and smallest (b) diameter, and the tumor volume was calculated using V= ab2/2. Each point represents the mean ± SD. *Significantly different from control (p < 0.05). **Significantly different from control (p < 0.01).
Level of pro-apoptotic molecules was increased in tumor tissue by modified IFNs
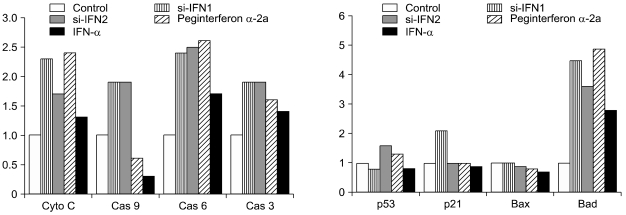
The expression levels of cytochrome c, caspase 6, and caspase 3 were increased in the si-IFN1, si-IFN2, peginterferon α-2a and IFN-α treated groups of 50 × concentration compared to the control. The expression of caspase 9 was observed clearly in si-IFN1 and si-IFN2 only. The expression level of p53 was slightly increased, however, a distinct increase of p21 was observed in si-IFN1 only. The expression of BAD was significantly increased in si-IFN1 and si-IFN2; however, no change of Bax was observed (Fig. 5).
Fig. 5.
Change of pro-apoptotic molecules in tumor tissue treated with 50 × groups. Protein samples were extracted from the tumor tissues of control, si-IFN1, si-IFN2, peginterferon α-2a and IFN-α treated groups at 50 × concentrations. Protein sample were prepared for Western blot using antibodies to mouse cytochrome c, caspase 9, caspase 6, and caspase 3, p53, p21, Bax and Bad. Each band was further analyzed by densitometer. Each number on the figure represents the density compared control.
A decrease of proangiogenic molecule was observed in tumor tissue treated with modified IFNs
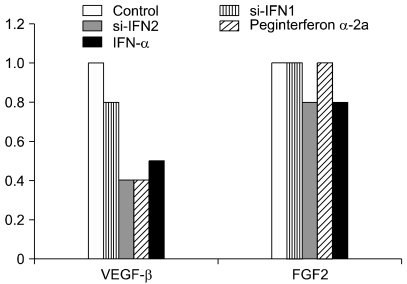
In the Western blot of pro-angiogenic molecule, the expression level of VEGF-β was decreased in the si-IFN1, si-IFN2, peginterferon α-2a and IFN-α treated groups. The decrease of the expression level was observed particularly in si-IFN1, si-IFN2, and peginterferon α-2a compared to the control (Fig. 6).
Fig. 6.
Change of angiogenesis-related molecules in tumor tissue of mice. Protein samples were extracted from the tumor tissues of control, si-IFN1, si-IFN2, peginterferon α-2a and IFN-α treated groups in 50 ×. Protein samples were prepared for Western blot using antibodies to mouse VEGF-β and FGF-2. Each band was further analyzed by densitometer program. Each number on the figure represents the density compared to the control.
Discussion
IFN-α, the first cytokine to be produced by recombinant DNA technology, has been identified as a pivotal regulator of cellp growth, differentiation, cell to cell communication and signal pathway [4]. Although there are many advantages in the use of IFN-α in various diseases, clinical trials have been limited due to their disadvantages such as their short half-life in in vivo systems. To overcome this disadvantage, various trials have been carried out using them in combination with other agents and making modifications to recombinant IFN-α. The present paper reports that a once-a-week injection of serum immunoglobulin fused IFN-α into athymic nude mice bearing tumor cells can inhibit tumor growth effectively. IFN-α is a multifunctional regulatory cytokine regulating cell function and proliferation. In the present experiments, serum immunoglobulin fused IFN-α named si-IFN1 and si-IFN2 was used. The results showed that at 21 days after tumor cells inoculation, the mean tumor volumes of si-IFN1, si-IFN2, peginterferon α-2a and IFN-α treated with 50 × were significantly decreased compared to the control. In the biochemical tests of the 50 × group, a decrease of BUN was observed in the si-IFN2, peginterferon α-2a and IFN- α group comparing to the damage but also dehydration. ALT is a predominantly hepatocyte enzyme and increase of ALT activity is known to be highly associated with liver damage. However, the current study is an initial report on the screening tests for the potential effects of various IFN types as antitumor drugs. These tests are based on various blood or serum measurements: (a) total white blood cell counts; (b) BUN for kidney toxicity and (c) ALT for liver toxicity. On the basis of the limited parameters tested, although varying in sensitivity, our data seem to correlate with the reduced toxicity and antitumor effects of the IFNs tested. However, the final validation of these tests, especially the BUN and ALT, will require both further study of the histopathologic effects and correlation with the results from further efficacy trials over an extended period. Further studies of this type are essential.
The pivotal events of tumor growth inhibition are apoptosis and anti-angiogenesis. Apoptosis is orchestrated by various molecules including extrinsic and intrinsic pathways. Also, a recent study reported that the IFN-family increased apoptosis in vitro [2]. To confirm the effect of serum immunoglobulin fused IFN-α to the pro-apoptotic function, we performed a Western Blot test. In the extrinsic pathway related to the death receptor, an increase of cytochrome c, caspase 3, 6, and 9 was observed in si-IFN1 and si-IFN2. Cytochrome c is located in the mitochondria of all aerobic cells and is involved in the electron transport system that functions in oxidative phosphorylation. In addition to its role in oxidative phosphorylation, the release of cytochrome c from the mitochondrial intermembrane space results in nuclear apoptosis. Binding of Apaf-1 to cytochrome c allows Apaf-1 to form a ternary complex with, and activate, the initiator procaspase-9 [8]. In the intrinsic pathway associated with mitochondria, an increase of p53, p21 and Bad was also detected. Takaoka et al. [7] demonstrated that transcription of the p53 gene was induced by IFN-α, accompanied by an increase in p53 protein levels and they provided examples in which p53 gene induction by IFN-α indeed contributed to tumor suppression. Our study also confirmed that si-IFN1 and si-IFN2 showed strong anti-tumor activity similar to that of peginterferon α-2a.
The expansion of the tumor masses depends on neovascularization and the formation of new vasculature involves multiple, interdependent steps [3]. In addition, the onset of angiogenesis involves a change in the local equilibrium between pro-angiogenic and anti-angiogenic molecules [3,9]. The key molecules associated with pro-angiogenesis include fibroblast growth factor 2 (FGF2) and vascular endothelial cell growth factor beta (VEGF-β). FGF2 is a wide-spectrum mitogenic, angiogenic, and neurotrophic factor that is expressed at low levels in many tissues and cell types and reaches high concentrations in the brain and pituitary. FGF2 has been implicated in a multitude of physiologic and pathologic processes, including limb development, angiogenesis, wound healing, and tumor growth. In our study, to evaluate the efficiency of serum immunoglobulin fused IFN-α to anti-angiogenesis, the expression level of VEGF-β and FGF2 was measured by Western Blot. Our results showed that the expression level of VEGF-β was decreased in the si-IFN1, si-IFN2, peginterferon α-2a and IFN-α treated groups. The decrease of the expression level was observed prominently in the si-IFN2 and peginterferon α-2a treated groups compared to the control. These data suggest that inhibition of tumor growth may be also due to the anti-angiogenic effect of si-IFN1 and si-IFN2.
In conclusion, serum immunoglobulin fused IFN-α, si-IFN1 and si-IFN2 are able to inhibit the tumor growth of athymic mice bearing colon 26 adenocarcinoma cells and the inhibitory effects may be associated with facilitating apoptosis and suppressing angiogenesis.
References
- 1.Chen SA, Sawchuk RJ, Brundage RC, Horvath C, Mendenhall HV, Gunther RA, Braeckman RA. Plasma and lymph pharmacokinetics of recombinant human interleukin-2 and polyethylene glycol-modified interleukin-2 in pigs. J Pharmacol Exp Ther. 2000;293:248–259. [PubMed] [Google Scholar]
- 2.de Luján Alvarez M, Ronco MT, Ochoa JE, Monti JA, Carnovale CE, Pisani GB, Lugano MC, Carrillo MC. Interferon α-induced apoptosis on rat preneoplastic liver is mediated by hepatocytic transforming growth factor β1. Hepatology. 2004;40:394–402. doi: 10.1002/hep.20307. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. Regulation of neoplastic angiogenesis. J Natl Cancer Inst Monogr. 2000;28:10–14. doi: 10.1093/oxfordjournals.jncimonographs.a024251. [DOI] [PubMed] [Google Scholar]
- 4.Gutterman JU. Cyt okine therapeutics: lessons from interferon α. Proc Natl Acad Sci USA. 1994;91:1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CW, Hong DH, Han SB, Jung SH, Kim HC, Fine RL, Lee SH, Kim HM. A novel stereo-selective sulfonylurea, 1-[1-(4-aminobenzoyl)-2,3-dihydro-1H-indol-6-sulfonyl]-4-phenyl-imidazolidin-2-one, has antitumor efficacy in in vitro and in vivo tumor models. Biochem Pharmacol. 2002;64:473–480. doi: 10.1016/s0006-2952(02)01105-x. [DOI] [PubMed] [Google Scholar]
- 6.Morinaga Y, Suga Y, Ehara S, Harada K, Nihei Y, Suzuki M. Combination effect of AC-7700, a novel combretastatin A-4 derivative, and cisplatin against murine and human tumors in vivo. Cancer Sci. 2003;94:200–204. doi: 10.1111/j.1349-7006.2003.tb01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, Taniguchi T. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 8.Tsunoda S, Ishikawa T, Yamamoto Y, Kamada H, Koizumi K, Matsui J, Tsutsumi Y, Hirano T, Mayumi T. Enhanced antitumor potency of polyethylene glycolylated tumor necrosis factor-α: a novel polymer-conjugation technique with a reversible amino-protective reagent. J Pharmacol Exp Ther. 1999;290:368–372. [PubMed] [Google Scholar]
- 9.von Marschall Z, Scholz A, Cramer T, Schäfer G, Schirner M, Oberg K, Wiedenmann B, Höcker M, Rosewicz S. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J Natl Cancer Inst. 2003;95:437–448. doi: 10.1093/jnci/95.6.437. [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Lu W, Dong Z. Tumor-infiltrating macrophages are involved in suppressing growth and metastasis of human prostate cancer cells by INF-beta gene therapy in nude mice. Clin Cancer Res. 2002;8:2942–2951. [PubMed] [Google Scholar]