Abstract
Many studies demonstrated that cancer sera contain antibodies which react with autologous cellular antigens generally known as tumor-associated antigens (TAAs). In our laboratories, the approach used in the identification of TAAs has involved initially examining the sera of cancer patients using extracts of tissue culture cells as source of antigens in Western blotting and by indirect immunofluorescence on whole cells. With these two techniques, we identify sera which have high-titer fluorescent staining or strong signals to cell extracts on Western blotting and subsequently use these sera as probes in immunoscreening cDNA expression libraries, and also in proteomic approach to isolate and identify targeted antigens which might potentially be involved in malignant transformation. In this manner, several novel TAAs including HCC1, p62, p90 and others have been identified. In extension of these studies, we evaluate the sensitivity and specificity of different antigen-antibody systems as markers in cancer in order to develop “tumor-associated antigen array” systems for cancer diagnosis, cancer prediction, and for following the response of patients to treatment.
Keywords: Autoantibodies, tumor-associated antigens (TAAs), cDNA expression library screening, two dimensional SDS- polyacrylamide gel electrophoresis (2D-SDS-PAGE), proteomic approach, cancer marker
1. Introduction
It is well established that cancer sera contain antibodies that react with a unique group of autologous cellular antigens called TAAs_(1–3). The types of cellular proteins that induce these autoantibody responses are quite varied and include the tumor suppressor p53 (4,5), oncogene products such as HER-2/neu and ras (6), proteins that protect mRNAs from degradation such as p62 (7) and CRD-BP (8), onconeural antigens (9), differentiation-antigens such as tyrosinase and the cancer/testis antigens (10), and anti-apoptotic proteins such as survivin (11) and LEDGF (12). Factors leading to the production of such autoantibodies are not completely understood but the available data show that many of the target antigens are cellular proteins whose aberrant regulation or overexpression could lead to tumorigenesis, such as p53 (4, 5). A highly informative study showed that lung tumors contained several types of p53 gene mutations including missense, stop codon and frameshift mutations, but it was the missense mutations with overexpression of protein which altered function and increased protein stability that correlated with antibody production (13). In the case of the mRNA binding protein p62, a fetal protein absent in adult tissues, immunogenicity appears to be related to abnormal expression of p62 in tumor cells (14). The immune system in certain cancer patients appears to have the capability of sensing these abnormalities in cellular proteins and responding by producing autoantibodies. Importantly, it has been shown that the patients' immune system is capable of sensing abnormalities in cellular proteins involved in malignant transformation earlier than clinical diagnostic procedures (5, 15). An emerging concept is that autoantibodies associated with a specific type of cancer are directed against aberrantly regulated or activated protein components of molecular pathways involved in the malignant transformation process in that particular type of cancer (1,16). Thus, cancer-associated autoantibodies might be regarded as reporters identifying aberrant cellular mechanisms in tumorigenesis. In recent years, research on humoral immunity to TAAs has received significant attention, and investigators are now beginning to address specific clinical questions such as the potential utility of TAA-autoantibody systems as early cancer biomarkers, tools to monitor therapeutic outcomes, or indicators of disease prognosis (17).
2. Materials
2.1. Cell culture and cell extracts
HepG2 (human hepatocellular carcinoma) cell lines were obtained from the American Type Culture Collection (ATCC) and cultured following the specific protocol.
Cells grown in monolayers were solubilized directly in Laemmli's sample buffer containing protease inhibitors (Boehringer Mannheim). Solubilized lysates were briefly sonicated and then electrophoresed on SDS-PAGE and transferred to nitrocellulose paper.
2.2. Materials for cDNA expression library cloning
Amplified premade hepatocellular carcinoma (HepG2) cDNA library constructed in the Uni-ZAP® XR vector (Stratagene, La Jolla, CA).
Host strains: Bacterial glycerol stock of XL1-Blue MRF' strain and SOLR™ strain (Stratagene, La Jolla, CA).
ExAssist® interference-resistant helper phage (Stratagene, La Jolla, CA).
Isopropyl-1-thio-β-D-galactopyranoside (IPTG) (Fisher Scientific, Houston, TX).
Nitrocellulose membrane (Osmonics Inc. MA).
Chemiluminescent reagent (Pierce Biotechnology, Rockford, IL).
Additional reagents include 1M MgSO4, 20% maltose, tryptone, yeast extract, antibiotics (tetracycline, ampicillin), SM buffer (5.8g NaCl; 2.0g MgSO4; 50ml Tris, pH 7.4, 1M; 5ml 2% gelatin, add H2O to 1 liter and autoclave), PBS-Tween (PBST) solution, 3% non-fat dry milk in PBST, horseradish peroxidase(HRP)-conjugated goat anti-human IgG (Caltag Laboratories, San Francisco, CA).
2.3. Materials for proteomic approach
Protein assay Bradford dye reagent. (Bio-rad, Hercules, CA)
Immobilized pH gradient IPG gel strip. (Bio-rad, Hercules, CA)
Dithiothreitol (DTT) equilibration buffer 1 for sulfhydryl group reduction. (2% DTT, 6M Urea, 20% SDS, 1.5 M Tris/HCl, pH8.8, 50% glycerol, nanopure water)
Iodoacetamide equilibration buffer 2 for sulfhydryl group alkylation. (2.5% Iodoacetamide, 6M Urea, 20% SDS, 1.5 M Tris/HCl, pH8.8, 50% glycerol, nanopure water)
Nitrocellulose membrane. (Osmonics Inc. MA)
Chemiluminescent reagent (Pierce Biotechnology, Rockford, IL).
Sequencing grade modified trypsin.(Promega, Madison, WI )
C18 bead ziptips (POROS R2) from Applied Biosystems, CA.
Additional reagents include proteomics grade water, mineral oil, PBS-Tween (PBST) solution, 3% non-fat dry milk in PBST, horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Caltag Laboratories, San Francisco, CA), HPLC grade Acetonitrile, ammonium bicarbonate, formic acid (FA), Methonal, Trifluoroacetic acid (TFA).
3. Methods
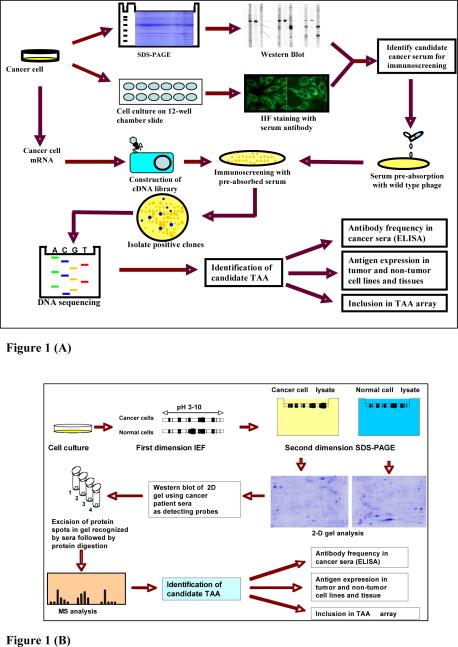
The methods which we have used in the identification of putative TAAs has involved initially examining the sera from cancer patients using extracts of tissue culture cells as source of antigens in Western blotting and by indirect immunofluorescence on whole cells. With these two techniques, we identify sera which have high-titer fluorescent staining or strong signals to cell extracts on Western blotting and subsequently use the antibodies in these sera to isolate cDNA clones from cDNA expression libraries. In this manner, several novel TAAs including HCC1 (18), SG2NA (19), CENP-F (20), p62 (7) and p90 (21) have been identified. Several novel as well as previously defined tumor antigens have been recently identified with autoantibodies from patients with different types of cancer (2) using a methodology called SEREX (serological analysis of recombination cDNA expression libraries) (22), which is essentially a modification of our previous approach (18, 19). Immunoscreening of cDNA libraries with serum antibodies for identifications of autoantigens is a well-established method and has been used not only to identify TAAs but also antigens in autoimmune diseases (23). This methodology was the basis of the methods described in SEREX (serological analysis of recombination cDNA expression libraries) with the difference that cDNA expression libraries constructed from autologous patient tumor were used as substrate in immunoscreening. Subsequent reports using the SEREX technique have shown that the TAAs identified are no different from standard methods using cDNA expression libraries from cell lines derived from different sources, so that there did not appear to be any advantage to using cDNA libraries from autologous patients. A proteome-based approach has been recently implemented for identifying tumor-associated antigens in cancer patients (24, 25). The proteome-based technology allows individual screening of a large number of sera, as well as potential identification of a large number of autoantigens. Proteome-based approach can also distinguish isoforms and the detection of autoantibodies directed against post-translational modifications of specific targets. The practical utility of this approach remains to be established with the proviso that efforts should be made to identify tumor-associated from tumor-irrelevant antigens. In this chapter, we will use the hepatocellular carcinoma (HCC)-associated antigens as examples to describe how to isolate and identify TAAs as markers in cancer. The schematic representation of TAA identification was shown in Figure 1.
Fig. 1.
Schematic representation of TAA identification. (A) TAA identification using cDNA library immunoscreening. In brief, the sera from cancer patients were initially examined using extracts of tissue culture cells as source of antigens in Western blot and by indirect immunofluorescence (IIF) on whole cells. With these two techniques, we identify sera which have high-titer fluorescent staining or strong signals to cell extracts on Western blot and subsequently use the antibodies in these sera to isolate cDNA clones from cDNA expression libraries. (B) TAA identification using proteomic approach. In brief, cell extract of cultured cancer cells was applied onto the first dimension gel (isoelectrofocusing gel), and subsequently loaded onto the second-dimension gel (2DSDS-PAGE). The protein was transferred to the nitrocellulose membrane or visualized by silver staining. High-titer sera from cancer patients and sera from normal individuals (used as controls) were selected for the study. Reactivity with cancer cells for all selected serum samples were confirmed by Western blotting analysis and immunofluorescence assay. After immunoblotting with cancer sera and normal human sera, a number of protein spots of interest were excised from the 2-D gels, digested by trypsin, and subsequently analyzed by mass spectrometry (MS). In subsequent studies, we will characterize identified tumor-associated antibody-antigen systems that are potentially useful for the early detection of cancer, and then evaluate the sensitivity and specificity of different antigen-antibody systems as markers in cancer for further developing “TAA array” systems for cancer diagnosis, prediction, and for following the response of patients to treatment.
3.1. Methods for cDNA expression library immunoscreening
Immunoscreening HepG2 cDNA expression library: The HepG2 cDNA expression library constructed in the Uni-ZAP XR vector system was purchased from the company (Stratagene, La Jolla, CA). One selected HCC serum was diluted 1:200 and used as a probe for initial immunoscreening of the cDNA library. Before screening the cDNA library, the HCC serum was extensively adsorbed against wild-type Uni-ZAP XR phage infected E. coli mixture to reduce nonspecific antibody binding (See Notes 1–5). All screenings were performed on duplicate isopropyl β-D-thiogalactoside (IPTG) pre-impregnated nitrocellulose filters. Horseradish perosidase (HRP)-conjugated goat anti-human IgG (CALTAG, Burlingame, CA) at 1:3000 dilution was used as secondary antibody, immunoreactive clones were detected by chemiluminescence. Double positive phages in first screening were subsequently screened to 100% purity. At least, 400,000 phage recombinants need to be screened in each screening. Positive cDNA clones were isolated and converted to pBluescript phagemid by in vivo excision using the EXAssist helper phage (Stratagene Inc.) with SOLR strain. The phagemids were purified by Qiaprep spin columns (Qiagen, Valencia, CA) and the size of insert was determined by restriction enzyme digestion with EcoR1 and Xho1.
Sequencing of the positive cDNA clones derived from library screening: The cDNA inserts of the pBluescript phagemid were sequenced with T3 and T7 primers by the dideoxy chain termination method using SequiTherm EXCEL ™ II DNA Sequencing Kit (Epicentre Technologies, Madison, WI, USA). All sequence data were analyzed by BLAST search with known sequence databases.
3.2. Methods for proteomic approach
Two Dimensional SDS- Polyacrylamide Gel Electrophoresis (2D-SDS-PAGE) Analysis: Cells were briefly sonicated with lysis buffer (10mM Tris 5mM EDTA, 50mM Nacl, 30mM Na pyrophosphate, 50mM Na fluoride, 1mM Na orthovanadate, 1% Triton, pH 7.6). Protein concentration was measured with Bradford assay (Bio-Rad). For the first dimensional gel analysis, total protein 200 ug mixed with denatured buffer and applied on pH 3–10 11cm isoelectric focusing strip (IEF) from Bio-Rad. IEF was performed at a current of 50 mA per gel 300V 30 minute, followed by 8000V 2.5 hour and additional 5 hour (See Note 6). A total volthourproduct of 45 kvh. Strips were immediately stored at −80 °C for SDS-PAGE analysis. The second dimension was carried out using 15% polyacrylamide gels and 200V under an appropriate cooling system (See Note 7).
Immunoblotting analysis: Proteins separated by first and second dimension were transferred to pure nitrocellulose membranes (Osmonics Inc.). After preblocking with phosphate-buffered saline containing 0.05% Tween-20 (PBST) and 5% nonfat milk for 30 min at room temperature, the nitrocellulose membranes were incubated for 2 hour at room temperature with 1:300 dilution of serum. Horseradish peroxidase-conjugated goat anti-human IgG (Caltag Laboratories, San Francisco, CA) was applied as secondary antibody using 1:3000 dilution. Immunoreactive spots were visualized by the ECL kit (Amersham, Arlington Heights, IL) according to the manufacturer's instructions.
In-gel protein Digestion: The proteins of interest were excised from a Coomassie stained preparative gel (See Note 8) and then washed with HPLC grade water, followed by destaining with acetonitrile (ACN) for 15 min to remove coomassie blue staining, and dried in a SpeedVac concentrator as described previously (26). After the reduction (with 5mM DTT) and alkylation (with 10mM iodoacetoamide) of cysteine residues, digestion was performed by addition of 12.5 ng/μL of typsin (Promega, Madison, WI) in 50 mM ammonium bicarbonate containing 5 mM CaCl2 (See Note 9). Following the enzymatic digestion overnight at 37 °C, the peptides were extracted with 25 mM ammonium bicarbonate/50% ACN, and with 5% formic acid (FA) in 50% ACN solution. After removal of acetonitrile by SpeedVac concentrator, the sample was desalted by C18 bead ziptips (POROS R2, Applied Biosystems). The samples were dried out by SpeedVac concentrator before mass spectrometry analysis.
LC-MS/MS Analysis: Samples were resuspended in 0.1% FA before running the LC-MS/MS analysis. The liquid chromatography mass spectrum (LC-MS/MS) analysis was carried using a nano HPLC (LC packings system, Ultimate) coupled to the ESI-QUAD-TOF-MS (Micromass). Peptides were separated with the following gradient: 100% solvent A for 5 min, 0–50% solvent B in 25 min, 50–90% solvent B in 1 min and 90% solvent B for 5 min (solvent A = 5% ACN/0.1% FA; solvent B = 80% ACN/0.1% FA). The mass spectrometry (MS) spectra were collected for 2 sec in a range between 400 and 1800 a.m.u. Each peptide with intensity higher than 10 counts was submitted only once to fragmentation, using ramp collision energy (22–60 eV). The MS/MS spectra were collected for 3 sec in a range between 50 and 2050 a.m.u.
Protein Identification: Protein identification was performed using MASCOT software (www.matrixscience.com), and all tandem mass spectra were searched against the IPI Human database. The following search parameters were used: trypsin is used as the cutting enzyme, carbamidomethyl (C) and Oxidation (M) are chosen as fixed and variable modifications, mass tolerance for monoisotopic peptide is set to 500 ppm, fragment mass tolerance is set to 0.8 Da, protein mass is unrestricted, one missed cleavage is allowed. Briefly, the algorithm searches candidate sequences from the database by intact peptide masses. After this step, all candidates were submitted to a in silico fragmentation, and the resulting theoretical fragments were compared with the fragments from experimental spectrum. Finally a statistical analysis was used to validate the sequences. Mascot validates its results based on the probability of the identification being a random event.
Acknowledgments
This work was supported by grants from National Institutes of Health (2S06GM008012-37, 5G12RR08124, CA56956).
Supported by grants from National Institutes of Health (2S06GM008012-37, 5G12RR08124 and CA56956).
Footnotes
All sera, even “normals”, may have anti-bacterial and anti-phage antibodies. Sera used for screening must therefore be absorbed against bacteria and phages.
Use wild type λ phage, specific clone(s), or a mixture of clones from the same library being screened.
Plating as described above, using ~20,000 pfu per small plate or 200,000 pfu per large square plate. After 4–6 hours when the plagues are visible, overlay plate with corresponding filter presoaked in IPTG. After several hours or overnight, flip nitrocellulose filter upside down for several more hours of incubation at 37°C so that both side of the filter will be coated with phage / E. Coli. The number and size of nitrocellulose filter required for absorption depends on the background signal for each screening serum.
Proceed as for immunoblotting above, block filters with 3% milk/PBST, use 15 ml of 1:50 diluted serum in 3% milk in PBST against 8filters. Incubate primary antibody with filters for 1–2 hours with occasional mixing.
Remove absorbed sera and add equal volum of 3% milk PBST to generate 30 ml of 1:100 pre-absorbed antibody. Use immediately or store at −20°C. Save filters for stripping and reuse.
The current of IEF strips should be maintained below 100 mA. All proteins should be run through the desalt procedures if the voltage of the gel cannot go up to the desired setting.
Before starting the second dimension the strips are equilibrated in DTT equilibration buffer 1 for 15 min. Afterward, they were briefly rinsed in double distilled water and equilibrated in Iodoacetamide equilibration buffer 2 for an additional 15 min. The IEF strips were overlayered with a solution containing agarose (0.5% w/v) on the top of second dimension gels. The second dimension was carried out using 10% or 15% polyacrylamide gels and current is non-limited per gel in a cooling system.
Spot patterns on each membrane are matched to an equivalent spot on original 2D-PAGE gel. This gel analysis can be done by using PDQuest 2-D Analysis Software (Bio-Rad, Hercules, CA).
If the gel pieces still have Coomassie blue staining, the gel pieces need to be rehydrated again in an equal volume of ammonium bicarbonate and acetonitrile.
References
- 1.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J. Clin. Invest. 2001;108:1411–1415. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Old LJ, Chen YT. New paths in human cancer serology. J. Exp. Med. 1998;187:1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EKL, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol. Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- 4.Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int. J. Cancer. 1982;30:403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- 5.Soussi T. p53 antibodies in the sera of patients with various types of cancer. Cancer Res. 2000;60:1777–1788. A review. [PubMed] [Google Scholar]
- 6.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–7. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JY, Chan EKL, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J. Exp. Med. 1999;189:1101–1110. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle GA, Bourdeaux-Heller JM, Coulthard S, Meisner LF, Ross J. Amplification in human breast cancer of a gene encoding a c-myc mRNA binding protein. Cancer Res. 2000;60:2756–2759. [PubMed] [Google Scholar]
- 9.Keene JD. Why is Hu where? Shuttling of early response gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of humoral immune response of cancer patients to a panel of human tumor antigens. J. Exp. Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3:917–992. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 12.Daniels T, Zhang J, Gutierrez I, Elliot ML, Yamada B, Heeb MJ, Sheets SM, Wu X, Casiano CA. Antinuclear autoantibodies in PCa: Immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. 2005;62:14–26. doi: 10.1002/pros.20112. [DOI] [PubMed] [Google Scholar]
- 13.Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–4174. [PubMed] [Google Scholar]
- 14.Lu M, Nakamura RM, Dent ED, Zhang JY, Nielsen FC, Christiansen J, Chan EK, Tan EM. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am. J. Pathol. 2001;159:945–953. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer. 1993;71:26–35. doi: 10.1002/1097-0142(19930101)71:1<26::aid-cncr2820710106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disis ML, Montgomery RB, Goodell V, dela Rosa C, Salazar LG. Antibody immunity to cancer-associated proteins. The Education Book of the 96th Annual AACR Meeting; Anaheim, CA. 2005. pp. 166–169. [Google Scholar]
- 18.Imai H, Chan EKL, Kiyosawa K, Fu XD, Tan EM. Novel nuclear antoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J Clin Invest. 1993;92:2419–26. doi: 10.1172/JCI116848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landberg G, Tan EM. Characterization of a DNA-binding nuclear autoantigen mainly associated with S phase and G2 cells. Exp Cell Res. 1994;212:255–61. doi: 10.1006/excr.1994.1141. [DOI] [PubMed] [Google Scholar]
- 20.Casiano CA, Landberg G, Ochs R, Tan EM. Autoantibodies to a novel cell cycle-regulated protein that accumulates in the nuclear matrix during S phase and is localized in the kinetochores and spindle midzone during mitosis. J Cell Sci. 1993;106:1045–56. doi: 10.1242/jcs.106.4.1045. [DOI] [PubMed] [Google Scholar]
- 21.Soo Hoo L, Zhang JY, Chan EKL. Cloning and characterization of a novel 90kDa `companion' auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21:5006–15. doi: 10.1038/sj.onc.1205625. [DOI] [PubMed] [Google Scholar]
- 22.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers JC, Keene JD. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci USA. 1985;82:2115–9. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Naour F, Brichory F, Beretta L, Hanash SM. Identification of tumor-associated antigens using proteomics. Technology in Cancer Research & Treatment. 2002;1:257–262. doi: 10.1177/153303460200100406. [DOI] [PubMed] [Google Scholar]
- 25.Le Naour F, Brichory F, Misek DE, Brechot C, Hanash SM, Beretta L. A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Mol Cell Proteomics. 2002;1:197–203. doi: 10.1074/mcp.m100029-mcp200. [DOI] [PubMed] [Google Scholar]
- 26.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyarcylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]