Abstract
Background
Some malformations are clearly associated with older maternal age, but the effect of older age of the father is less certain. The aim of this study is to determine the degree to which maternal age and paternal age independently influence the risk of having a child with oral clefts.
Methods
Among the 1,489,014 live births in Denmark during 1973–1996, there were 1920 children with nonsyndromic cleft lip with or without cleft palate and 956 children with nonsyndromic cleft palate. We used logistic regression to assess the impact of parental age on the occurrence of cleft lip with or without cleft palate and cleft palate. Interaction between mother’s and father’s age was included in the analysis.
Results
Separate analyses of mother’s and father’s age showed that older age was associated with increased risk of both cleft lip with or without cleft palate and cleft palate only. In a joint analysis, both maternal and paternal ages were associated with the risk of cleft lip with or without cleft palate, but the contribution of each was dependent on the age of the other parent. In the analysis of cleft palate only, the effect of maternal age disappeared, leaving only paternal age as a risk factor.
Conclusion
Both high maternal age and high paternal age were associated with cleft lip with or without cleft palate. Higher paternal age but not maternal age increased the risk of cleft palate only.
Oral cleft is one of the most common congenital malformations with prevalence between 1 and 2 per 1000 live births.1 Family studies suggest that cleft lip with or without cleft palate and cleft palate only are etiologically distinct malformations.2 The etiology of both defects is thought to be multifactorial with genes playing the major role. Several potential risk factors have been studied, but no strong risk factor has yet been identified.3–5
Increased maternal age is a risk factor for both chromosomal6 and nonchromosomal abnormalities.7 There have been conflicting reports on whether older maternal or paternal ages are associated with risk of oral clefts. Only a few studies were conducted according to the recommendation of the International Consortium for Oral Clefts Genetics,8 population-based, distinguishing between cleft lip with or without cleft palate and cleft palate only, and excluding cases with associated anomalies. Furthermore, few controlled for the age of the other parent, which is known to be correlated. Ascertaining whether older parental age is associated with oral cleft is not only of interest for clarifying the etiology of oral clefts, but is also important from a biologic and a public health point of view. During the last 20 years, mean maternal and paternal ages have increased in Denmark by almost 3 years.9
Denmark provides nationwide population and health registers with all treatment free of charge and centralized. This ensures a high ascertainment of oral clefts and makes Denmark an ideal site for etiologic studies of clefts.
METHODS
The present study is based on linkage of 3 registers:
The Civil Registration System was established in 1968 and registers all individuals with residence in Denmark on and after April 1, 1968.10 Individuals are registered by personal identification numbers (PIN). The unique 10-digit PIN includes date of birth, sex, and a builtin check code disclosing most invalid numbers. The identification of individuals is therefore highly reliable. In addition to the PIN, the places of birth and the PINs of the legal parents are included.
The Danish Facial Cleft Register has been previously described in detail.11,12 It has recently been updated to include the 1988–2001 cohorts in addition to the 1936–1987 birth cohorts. Capture–recapture methods have indicated that nearly all (99%) live-born oral cleft cases without associated malformations in Denmark have been ascertained (except submucous cleft palate, which often remains asymptomatic).11 Owing to the incomplete ascertainment of defects of stillbirths, only live births are included in the register. The Danish Facial Cleft Register now includes 9483 cleft cases, of which 8623 (91%) are registered by a PIN. The PIN enables linkage to the Civil Registration System whereby data on parental ages of cleft cases were obtained. Clefts are grouped into cleft lip with or without cleft palate and cleft palate only. The cleft palate group comprises cases with clefts in the hard or soft palate and submucous cleft. Isolated bifid uvula is not considered a cleft palate.
The Danish Fertility Database comprised background data on maternal age and paternal age for every live birth in Denmark from 1973 through 1996.13 We therefore chose these years as the study period. During the period 1973–1989, records of 99.3% of the children were linked to their mother’s PIN, whereas 96.4% were linked to the father’s PIN.14 Data in the fertility database were retrieved from population-based registers with national coverage primarily from the Register of Population Statistics in Statistics Denmark,9 which is based on the Civil Registration System. In the Register of Population Statistics, each child has its own record, including a reference to the parents regardless of whether the parents are currently married. If the couple is married, the husband is automatically regarded as the father; for other cases, in this register, the “father” is the man who has acknowledged paternity or has been ordered (by the legal system) to accept paternity.
Associated Anomalies
A case with an associated malformation or malformation syndrome is noted in the cleft register if the presence of such malformation has been documented in any of the sources for the register. All medical records of cases born between 1988 and 1996 were further scrutinized to enhance information about associated anomalies. Malformations such as neural tube defects, monogenic traits, syndromes, and sequences were designated as major anomalies. Defects such as congenital dislocation of the hip or polydactyly were considered minor malformations. As a result of the more thorough classification of associated anomalies in the 1988–1996 period, a higher percentage of cases was included in the group of clefts with associated anomalies (17.6% compared with 10.9% for the 1973–1987 period).
In the analyses, we considered first the group “nonsyndromic,” defined as oral cleft cases with fewer than 3 minor associated anomalies. Second, we repeated the analyses considering only the “strictly nonsyndromic” cases, ie, those with no associated anomalies. Finally, we repeated the analyses including only oral cleft cases with at least 1 associated minor or major anomaly.
Register Linkage
A total of 3451 infants with oral clefts were born during 1973–1996. Of these, 468 (13.5%) had at least 1 major or 3 minor associated anomalies. Maternal and paternal ages were obtained for 2876 (96%) of the nonsyndromic cases (46 cases had no PIN). Of these, 1920 cleft lip with or without cleft palate cases (97%) and 956 cleft palate only cases (96%) were born during 1973–1996, registered by a PIN and with parental age. During the same period, there were a total of 1,489,014 live births in the Danish population.
Analysis
We first analyzed association of clefts with maternal and paternal ages separately. The age data were stratified by 5-year intervals, except for the older age groups (paternal age >49 years and maternal age>39). We used simple logistic regression analyses with age as a continuous variable to test for any dependence of cleft lip with or without cleft palate and cleft palate only on maternal age or paternal age.
Second, we calculated the birth prevalence of cleft lip with or without cleft palate and cleft palate only per 1000 live births across paternal age in 3 overlapping maternal age intervals, and then the same across maternal age in 3 overlapping paternal age groups. Trends were characterized with regression lines fitted by weighted least squares using the reciprocal of the estimated variance of each prevalence as weight.
To visualize the dependence of cleft prevalence on mother’s and father’s age, we used 2-dimensional contour maps based on smoothed prevalences. For any combination of mother’s age (m) and father’s age (f), we considered all births from whom the sum of the absolute differences of father’s and mother’s ages was less than a certain threshold b(f,m). For example, the smoothed prevalence at f = 30 and m = 30 will be classified in a threshold b(30, 30) = 2 (ie, age difference between father and mother is less than 2 years); threshold b includes all births with the following combinations of father’s and mother’s ages: (30, 30), (29, 30), (30, 29), (30, 31), (31, 30), (31, 31), (29, 29), (31, 29), (29, 31), (30, 28), (28, 30), (30, 32), (32, 30). We chose values of b(f,m) sufficiently large to include at least 500,000 births. Within the neighboring population, we computed the prevalence as a weighted relative frequency, in which the weight of each birth decreased linearly with the sum of the distances of father’s and mother’s ages to f and m (ie, using a triangular kernel).
Multiple logistic regression models were used to investigate the joint dependence on maternal and paternal ages. Maternal and paternal ages were entered as continuous covariates. We adjusted for cohort effect by including year in the model and for the difference in thoroughness of the identification of associated anomalies in the 2 time periods (1973–1987 and 1988–1996) by including an indicator for year ≥1988. We computed odds ratios (ORs) and 95% confidence intervals (CIs). First, we fitted a model with interaction between maternal and paternal ages. If the interaction was not statistically significant, we fitted a second model without interaction. If the interaction was statistically significant, 2 further models with only the mother’s age and the father’s age were fitted and compared with the first model (the interaction model) using a likelihood ratio test.
RESULTS
Figure 1 presents birth prevalences of nonsyndromic cleft lip with or without cleft palate and cleft palate only per 1000 live births in each parental age group. Birth prevalence of both cleft lip with or without cleft palate and cleft palate increases with increasing maternal age from 20 to 40 years and with increasing paternal age from 20 to 50 years. The extreme age groups seem only partially to follow this general tendency, although small numbers in these groups reduce precision. Within the age range of 20–40 years, the OR with maternal age was 1.20 per 10-year increase in age (95% CI = 1.08–1.33) for cleft lip with or without cleft palate and 1.16 per 10-year increase (1.00–1.35) for cleft palate only. For fathers in the range of 20 to 50 years, ORs per 10-year increase in age were 1.12 (1.02–1.22) for cleft lip with or without cleft palate and 1.24 (1.10–1.40) for cleft palate only.
FIGURE 1.
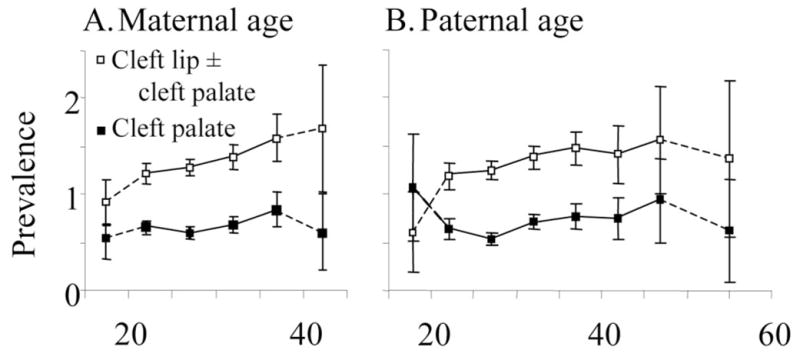
Birth prevalence of cleft lip with or without cleft palate and cleft palate only per 1000 live births in relation to mean (A) maternal age and (B) paternal age in 5-year intervals. The data points are placed at the mean age in each interval. The extremes (maternal age <20 and >40, paternal age <20 and >50) are grouped as a result of relatively small numbers.
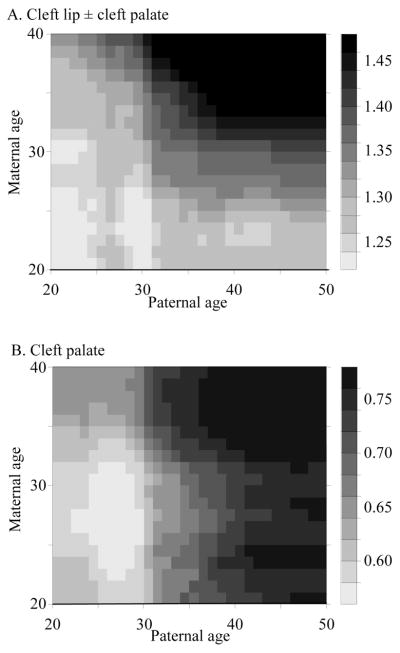
Figure 2 shows the prevalence of nonsyndromic cleft lip with or without cleft palate as a function of father’s age stratified by the age of the mother and vice versa. Prevalence increases with the age of the father if the mother is old, but not if the mother is young. Similarly, the birth prevalence increases with the age of the mother, especially if the father is old. This interaction between father’s and mother’s ages can also be seen in the contour map in Figure 3A. There was interaction between father’s and mother’s ages in the multiple regression analysis (P = 0.04). Leaving out paternal age and the interaction from the model did not result in a significantly worse fit (P = 0.11), whereas the fit was worse when leaving out maternal age and the interaction (P = 0.00).
FIGURE 2.
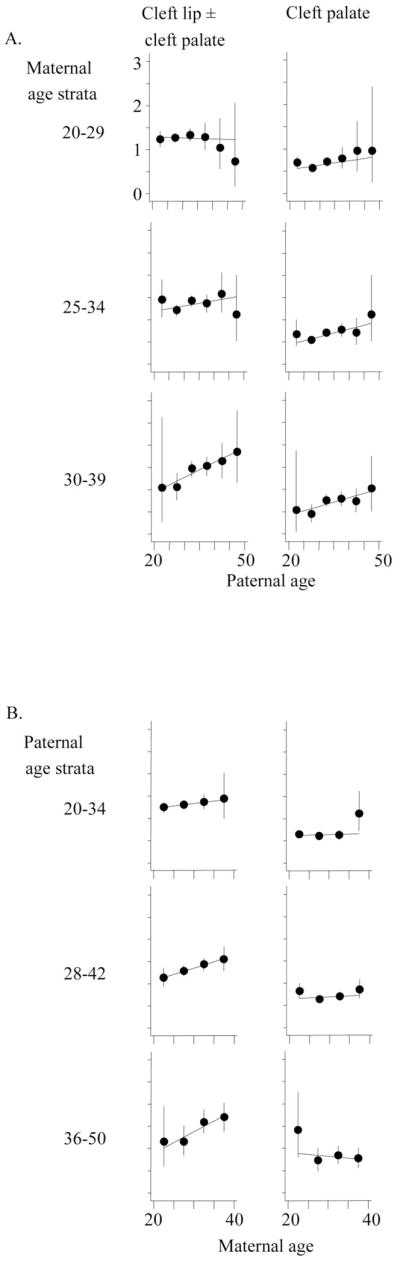
Birth prevalence of cleft lip with or without cleft palate and cleft palate only per 1000 live births by strata of maternal and paternal age (years) (Age strata are overlapping.) (A) in 3 maternal age strata, by paternal age (5-year categories) and (B) in 3 paternal age strata by maternal age (5-year categories).
FIGURE 3.
Contour maps of smoothed birth prevalence of oral clefts per 1000 live births in relation to maternal and paternal ages (years). (A) Cleft lip with or without cleft palate. (B) Cleft palate only.
The stratification of maternal age for cleft palate only in Figure 2 revealed different results. In each maternal age stratum, there was an increase of cleft risk with increasing paternal age. When stratifying for paternal age, there was no effect of maternal age. This is corroborated by the contour map in Figure 3B. Results from the multiple logistic regression analysis confirm that, for fixed paternal age, maternal age has no influence (OR per 10 years = 0.99; 95% CI = 0.81–1.20). There was, however, a clear effect of father’s age (OR per 10-year age increase = 1.27; CI = 1.09–1.49).
In the multiple logistic regression analysis, we found a slightly decreasing prevalence of cleft lip with or without cleft palate with more recent birth year (OR per 10-year increase = 0.91; CI = 0.80–1.03). For cleft palate only, no cohort effect was found (1.00; 0.84–1.19).
When restricting the outcome to the “strictly nonsyndromic,” the results remained virtually the same both in the univariate and the multivariate analyses (Table 1). Table 1 furthermore includes results from univariate and multivariate analyses of cleft cases accompanied by at least 1 minor or major anomaly. The univariate analyses show in general the same pattern as the analyses of the nonsyndromic clefts, although the trends generally are stronger. The multivariate analyses for cleft palate suggests again that mother’s age has no influence if one takes father’s age into account, but the confidence intervals are wide.
TABLE 1.
Cleft Lip With or Without Cleft Palate and Cleft Palate Only in Relation to Maternal and Paternal Age
| Nonsyndromic* OR (95% CI) | Strictly Nonsyndromic† OR (95% CI) | Syndromic‡ OR (95% CI) | |
|---|---|---|---|
| Cleft lip with or without cleft palate | |||
| (n = 1825) | (n = 1791) | (n = 169) | |
| Univariate analysis | |||
| Maternal age | 1.20 (1.08–1.33) | 1.21 (1.09–1.34) | 1.19 (0.84–1.67) |
| Paternal age | 1.12 (1.02–1.22) | 1.12 (1.03–1.22) | 1.32 (1.00–1.75) |
| Multivariate analysis | |||
| Maternal age | — | — | 0.79 (0.50–1.24) |
| Paternal age | — | — | 1.34 (0.93–1.92) |
| Cleft palate only | |||
| (n = 907) | (n = 879) | (n = 276) | |
| Univariate analysis | |||
| Maternal age | 1.16 (1.00–1.35) | 1.15 (0.99–1.34) | 1.39 (1.07–1.81) |
| Paternal age | 1.24 (1.10–1.40) | 1.23 (1.09–1.39) | 1.57 (1.27–1.94) |
| Multivariate analysis | |||
| Maternal age | 0.99 (0.81–1.20) | 1.00 (0.81–1.22) | 0.88 (0.62–1.25) |
| Paternal age | 1.27 (1.09–1.49) | 1.26 (1.07–1.48) | 1.58 (1.20–2.08) |
Odds ratios per 10-yr increase in age.
Cleft with fewer than 3 associated minor anomalies.
Cleft with no other associated anomalies.
Cleft with at least a minor or a major associated anomaly.
Dash indicates OR not calculated because of interaction between mother’s and father’s age (P < 0.05).
DISCUSSION
Using the population-based Danish Facial Cleft Database, we found that the influence of maternal and paternal ages on the risk of cleft lip with or without cleft palate increases with the advancing age of the other parent, and that the influence vanishes if the other parent is young. In contrast, the risk of having a child with cleft palate is influenced only by father’s age, not mother’s age.
There were 1920 nonsyndromic cleft lip with or without cleft palate and 956 nonsyndromic cleft palate only cases eligible for studying maternal and paternal ages. All cases were born in Denmark during a 23-year time period. No important cohort effect was found when adjusting for various exclusion rates (of cases with associated anomalies) as a result of different thoroughness of the classifications of associated anomalies in the 2 periods 1973–1987 and 1988–1996.
A possible weakness of the study is that we could not control for potential confounders such as nutrition, alcohol consumption, maternal smoking, epilepsy, birth order, income, and social status. However, of these risk factors, smoking is the only consistent (although weak) risk factor for oral clefts.15,16
Among the strengths of the present study was the high ascertainment and the accurate data on parental age of the background population. Few cases were excluded (3.6%) and these cases occurred throughout the whole study period, thus minimizing the risk of selection bias.
The percentage of cleft lip with or without cleft palate and cleft palate only with at least 1 major or 3 minor associated anomaly for the 1973–1996 cohorts were 7.9% and 22.9%, respectively. Compared with some studies, these percents are small, indicating that associated anomalies in our data are probably underreported. We cannot dismiss the possibility that we have a mixture of cases with and without associated anomalies in our population. However, we can assume that the majority of the nonsyndromic cases are correctly classified and those identified as syndromic are probably the most severe cases, leaving only the milder forms unidentified and mixed in the nonsyndromic case group.
We assumed that there is no difference in risk factors for isolated oral clefts and oral clefts with 1 or 2 minor associated malformations, which is the reason we included them in the analyses. Excluding cases with minor associated anomalies from the analyses did not change the results and therefore support this assumption. Excluding cases with minor anomalies might further bias the study, because the isolated cases would represent a healthier group.
The analysis of cases with associated anomalies generally showed a similar pattern with a tendency to stronger effects of paternal and maternal age. However, the confidence intervals were wide.
Maternal age has been widely studied as a risk factor for oral clefts, but results are conflicting. Several studies17–19 have found a positive association with increasing maternal age for cleft lip with or without cleft palate, cleft palate, or both. Others20–23 find no association with maternal age. None of the larger studies by Vieira et al20 and Robert et al24 adjusted for paternal age. An increasing risk of oral clefts with increasing maternal age may, however, reflect an increasing paternal age.
Although less studied, paternal age as a risk factor for oral cleft is likewise inconsistent.25,26 One of the major studies27 did adjust for maternal age but included cleft cases with associated anomalies, and results were inconclusive. Older studies often did not analyze cleft lip with or without cleft palate and cleft palate only as 2 groups, or simply compared mean paternal age of cleft cases and controls.
Only 1 larger study by Hay28 controlled for paternal age and excluded cases with associated anomalies.28 They studied the birth prevalence of cleft lip with or without cleft palate and cleft palate only among mothers younger or older than 35 years and fathers younger or older than 40 years. High maternal age, and especially high paternal age, were found to increase the risk of cleft palate. This is consistent with our results. For cleft lip with or without cleft palate, there was, however, no clear trend.
Recently, Vieira et al29 found an increasing risk with increasing birth order for cleft lip with or without cleft palate and cleft palate only. According to the authors, their results could partly be explained by longer periods of exposure to infections or environmental toxins among older mothers. The data were not adjusted for either maternal or paternal age and therefore birth order could be a proxy for parental age.
For cleft lip with or without cleft palate, we found an interaction between maternal and paternal age. This interaction might be the result of social confounding. The constellation of a young mother and an old father, who in our analysis had a relatively low risk of cleft lip with or without cleft palate, may be associated with preventive environmental factors such as vitamin intake or high intake of dietary folic acid. (Folic acid supplementation was not officially recommended to pregnant women in Denmark until 1997.)
Several single-gene disorders are now described in which the expression range is broad enough for affected cases to masquerade as nonsyndromic clefts. These disorders include the van der Woude syndrome (IRF6), clefting and hypodontia (MSX1), clefting and ankyloglossia (TBX22). and Kallmann syndrome (FGFR1).30–33 Because single-gene disorders may pose special risks for paternal mutations, some of the increase might relate to single-gene defects in which the syndromic features are not apparent or not expressed until later in life and thus are excluded from analysis here.
Our analyses suggest that parental age is a risk factor for oral clefts and emphasize the importance of taking paternal age into account when analyzing the effect of maternal age.
Acknowledgments
Funding for this study provided by the Egmont Foundation U.S. National Institute of Dental Research (R01 DE 11948) Faculty of Health, University of Southern Denmark.
We appreciate the collaboration of the 2 National Institutes of Defects of Speech, the University Hospital of Copenhagen, and Statistics Denmark.
References
- 1.Christensen K. The 20th century Danish facial cleft population—epidemiological and genetic–epidemiological studies. Cleft Palate Craniofac J. 1999;36:96–104. doi: 10.1597/1545-1569_1999_036_0096_tcdfcp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 2.Fogh-Andersen P. Genetic and non-genetic factors in the etiology of facial clefts. Scand J Plast Reconstr Surg Hand Surg. 1967;1:22–29. [Google Scholar]
- 3.Leite IC, Paumgartten FJ, Koifman S. Chemical exposure during pregnancy and oral clefts in newborns. Cad Saude Publica. 2002;18:17–31. doi: 10.1590/s0102-311x2002000100003. [DOI] [PubMed] [Google Scholar]
- 4.Schutte BC, Murray JC. The many faces and factors of orofacial clefts. Hum Mol Genet. 1999;8:1853–1859. doi: 10.1093/hmg/8.10.1853. [DOI] [PubMed] [Google Scholar]
- 5.Wyszynski DF, Beaty TH, Maestri NE. Genetics of nonsyndromic oral clefts revisited. Cleft Palate Craniofac J. 1996;33:406–417. doi: 10.1597/1545-1569_1996_033_0406_gonocr_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 6.Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58:282–285. [PubMed] [Google Scholar]
- 7.Hollier LM, Leveno KJ, Kelly MA, et al. Maternal age and malformations in singleton births. Obstet Gynecol. 2000;96:701–706. doi: 10.1016/s0029-7844(00)01019-x. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell LE, Beaty TH, Lidral AC, et al. Guidelines for the design and analysis of studies on nonsyndromic cleft lip and cleft palate in humans: summary report from a Workshop of the International Consortium for Oral Clefts Genetics. Cleft Palate Craniofac J. 2002;39:93–100. doi: 10.1597/1545-1569_2002_039_0093_gftdaa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 9.Eurostat Statistics Denmark. Statistics on Persons; A Register-Based Statistical System. Copenhagen: Statistics Denmark; 1995. [Google Scholar]
- 10.The Civil Registration System in Denmark. 2003 Available at: http://www.cpr.dk/
- 11.Christensen K, Holm NV, Olsen J, et al. Selection bias in genetic–epidemiological studies of cleft lip and palate. Am J Hum Genet. 1992;51:654–659. [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen K, Fogh-Andersen P. Etiological subgroups in non-syndromic isolated cleft palate. A genetic–epidemiological study of 52 Danish birth cohorts. Clin Genet. 1994;46:329–335. doi: 10.1111/j.1399-0004.1994.tb04173.x. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen LB. The Danish Fertility Database. Dan Med Bull. 1998;45:221–225. [PubMed] [Google Scholar]
- 14.Knudsen LB. Fertility Trends in Denmark in the 1980s. Copenhagen: Statistics Denmark; 1993. [Google Scholar]
- 15.Chung KC, Kowalski CP, Kim HM, et al. Maternal cigarette smoking during pregnancy and the risk of having a child with cleft lip/palate. Plast Reconstr Surg. 2000;105:485–491. doi: 10.1097/00006534-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Zeiger JS, Beaty TH. Is there a relationship between risk factors for oral clefts? Teratology. 2002;66:205–208. doi: 10.1002/tera.10104. [DOI] [PubMed] [Google Scholar]
- 17.Womersley J, Stone DH. Epidemiology of facial clefts. Arch Dis Child. 1987;62:717–720. doi: 10.1136/adc.62.7.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxen I. Cleft lip and palate in Finland: parental histories, course of pregnancy and selected environmental factors. Int J Epidemiol. 1974;3:263–270. doi: 10.1093/ije/3.3.263. [DOI] [PubMed] [Google Scholar]
- 19.Shaw GM, Croen LA, Curry CJ. Isolated oral cleft malformations: associations with maternal and infant characteristics in a California population. Teratology. 1991;43:225–228. doi: 10.1002/tera.1420430306. [DOI] [PubMed] [Google Scholar]
- 20.Vieira AR, Orioli IM, Murray JC. Maternal age and oral clefts: a reappraisal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:530–535. doi: 10.1067/moe.2002.128875. [DOI] [PubMed] [Google Scholar]
- 21.Baird PA, Sadovnick AD, Yee IM. Maternal age and birth defects: a population study. Lancet. 1991;337:527–530. doi: 10.1016/0140-6736(91)91306-f. [DOI] [PubMed] [Google Scholar]
- 22.Baird PA, Sadovnick AD, Yee IM. Maternal age and oral cleft malformations: data from a population-based series of 576,815 consecutive livebirths. Teratology. 1994;49:448–451. doi: 10.1002/tera.1420490604. [DOI] [PubMed] [Google Scholar]
- 23.Perry TB, Fraser FC. Paternal age and congenital cleft lip and cleft palate. Teratology. 1972;6:241–246. doi: 10.1002/tera.1420060218. [DOI] [PubMed] [Google Scholar]
- 24.Robert E, Kallen B, Harris J. The epidemiology of orofacial clefts. 1. Some general epidemiological characteristics. J Craniofac Genet Dev Biol. 1996;16:234–241. [PubMed] [Google Scholar]
- 25.Savitz DA, Schwingl PJ, Keels MA. Influence of paternal age, smoking, and alcohol consumption on congenital anomalies. Teratology. 1991;44:429–440. doi: 10.1002/tera.1420440409. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury AR, Mukherjee M, Sharma A, et al. Study of 126,266 consecutive births for major congenital defects. Indian J Pediatr. 1989;56:493–499. doi: 10.1007/BF02722422. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh GC, Olshan AF, Baird PA. Paternal age and the risk of birth defects in offspring. Epidemiology. 1995;6:282–288. doi: 10.1097/00001648-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Hay S. Incidence of clefts and parental age. Cleft Palate J. 1967;4:205–213. [PubMed] [Google Scholar]
- 29.Vieira AR, Orioli IM. Birth order and oral clefts: a meta analysis. Teratology. 2002;66:209–216. doi: 10.1002/tera.10088. [DOI] [PubMed] [Google Scholar]
- 30.Kondo S, Schutte BC, Richardson RJ, et al. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jezewski PA, Vieira AR, Nishimura C, et al. Complete sequencing shows a role for MSX1 in non-syndromic cleft lip and palate. J Med Genet. 2003;40:399–407. doi: 10.1136/jmg.40.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braybrook C, Doudney K, Marcano AC, et al. The T-box transcription factor gene TBX22 is mutated in X-linked cleft palate and ankyloglossia. Nat Genet. 2001;29:179–183. doi: 10.1038/ng730. [DOI] [PubMed] [Google Scholar]
- 33.Dode C, Levilliers J, Dupont JM, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]