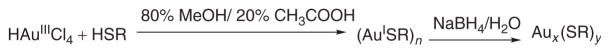Abstract
Centered on solid chemistry foundations, biology and materials science have reached a crossroad where bottom-up designs of new biologically important nanomaterials are a reality. The topics discussed here present the interdisciplinary field of creating biological mimics. Specifically, this discussion focuses on mimics that are developed using various types of metal nanoparticles (particularly gold) through facile synthetic methods. These methods conjugate biologically relevant molecules, e.g., small molecules, peptides, proteins, and carbohydrates, in conformationally favorable orientations on the particle surface. These new products provide stable, safe, and effective substitutes for working with potentially hazardous biologicals for applications such as drug targeting, immunological studies, biosensor development, and biocatalysis. Many standard bioanalytical techniques can be used to characterize and validate the efficacy of these new materials, including quartz crystal microbalance (QCM), surface plasmon resonance (SPR), and enzyme-linked immunosorbent assay (ELISA). Metal nanoparticle–based biomimetics continue to be developed as potential replacements for the native biomolecule in applications of immunoassays and catalysis.
THE IMPORTANCE OF BIOMIMICS
The accurate mimicking of biologically important materials in a benign form is critical for the development of drug carriers, sensors, and catalysts. The use of whole or modified pathogens presents many challenges to researchers in terms of personal safety, facility requirements, and overall time and cost. Additionally, while the inactivated or killed form of a given pathogen can be used, there are always risks such as conformational changes or losses during inactivation, or a specimen that remains partially active. These challenges require the development of a surrogate that circumvents the need for active biological systems. Biomimetic nanoparticles offer an easy way to present the active part of a biomolecule with better stability and without the harmful payload. Additionally, nanoparticles provide a way to modify a surface with multiple functional groups because of their high surface area. All these attributes have led to nanoparticles becoming a diverse platform for biomimicking.
Since the development of water-soluble, ligand-capped nanoparticles almost 15 years ago,1 the use of nanoparticles in biological systems has increased dramatically. This is due, in part, to the fact that they can be chemically modified to mimic an antigen or biological marker of interest. Unlike growing cell cultures or working with live animals, which is time consuming and expensive, nanoparticle synthesis is relatively straightforward and can be carried out on a larger scale. The chemistry to conjugate functional ligands and macromolecules to nanoparticles has been well developed (especially place exchange2 and amide linkage3) and can be adapted to fit a myriad of systems, for example, antigen/antibody interaction, via different synthetic routes. Nanoparticles offer a method whereby a surface can be multifunctionalized to create a broad spectrum of functionality, whether presenting multiple epitopes of the same antigen or two different reactive species from a catalyst. This review will discuss the creation, modification, characterization, and uses of nanoparticle-based biological mimics, and the tools that can be used to validate their biological activity.
NANOPARTICLE SYNTHESIS AND FUNCTIONALIZATION
The scientific study of colloidal metal particles dates back to Faraday in the mid-19th century.4 The synthesis and characterization, notably by electron microscope, of water ‘soluble’ gold colloids as small as 18 nm was completed by Turkevich and coworkers in 1951.5 Schiffrin and Brust, 43 years later, reported metal particles stabilized by alkanethiols. Murray and coworkers termed these ‘monolayer-protected clusters’ (MPCs) and defined them as differing from metal colloids because they can be repeatedly dried as well as isolated from and redissolved in common solvents without decomposing or aggregating.6 MPCs are synthesized using a bottom-up approach, suggesting that a wide variety of nanomaterials is possible from a small number of building blocks.7
Nanoparticles are created with a variety of core types and capping ligands to create water- or organic-soluble products with desired functions. Both metallic and nonmetallic starting materials are used in the creation of nanoparticles, such as MPCs,1,6,8–12 organic polymers,13–16 virus-like particles (VLPs),17–22 protein particles,23 colloidal particles,5,24,25 and semiconductor quantum dots.26 Thiol-capped MPCs have received more focus because of their ease of creation, water and air stability, electrochemical and optical properties, and their ability to be surface-functionalized by the addition of biologically relevant ligands, such as peptide sequences of epitopes. Gold MPCs can range in size from 1 to 10 nm, containing approximately 55–1000 gold atoms with molecular weights between 10 and 200 kDa.27
We acknowledge that a broad spectrum of nanometer-sized materials is present in the literature as previously mentioned. However, the focus of this review is stable, water-soluble gold-core MPCs and their targeted use in biological mimetics.
Synthetic Routes
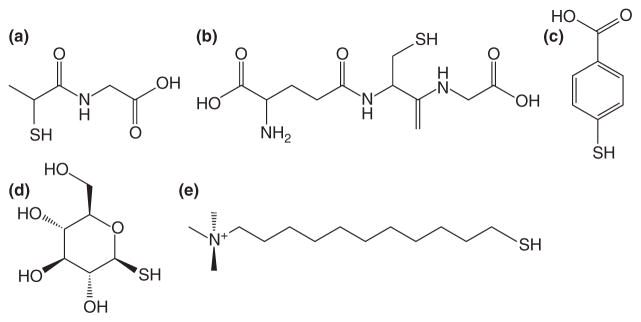
Water solubility of MPCs is best accomplished by using a thiolated, polar protecting ligand in a modified Brust reaction1,9 as seen in Figure 1. In the Brust reaction, tetrachloroauric acid is reduced from Au3+ to Au1+ in the presence of the thiol capping ligand, yielding a colorless gold-thiol solution. This is either composed of a gold-thiol polymer6 or tetramer.28 Following the initial reduction, the gold is further reduced to Au0 in the presence of sodium borohydride (NaBH4), yielding a black to dark brown solution. Other potent reducing agents, such as lithium aluminum hydride (LiAlH4) or lithium tri-ethylborohydride, have been used to reduce different metal cores such as palladium and platinum.11,12 Key examples of thiolate ligands that have been used to produce water-soluble and long-term (months) air- and water-stable clusters are tiopronin,9 glutathione,29 4-mercaptobenzoic acid,301-thio-β -D-glucose,31 and N, N, N-trimethyl(mercaptoundecyl)ammonium (TMA)32 as depicted in Figure 2.
FIGURE 1.
Modified Brust reaction scheme for polar ligands.
FIGURE 2.
Examples of thiolate ligands used in the synthesis of water-soluble Au monolayer-protected clusters (MPCs). (a) tiopronin, (b) glutathione, (c) 4-mercaptobenzoic acid, (d) 1-thio-β -D-glucose, (e) N, N, N-trimethyl (mercaptoundecyl)ammonium (TMA).
Functionalization of Monolayer-Protected Clusters
Transformation of water-soluble MPCs into biological mimics has been accomplished using a variety of synthetic functionalization strategies. However, the most widely used, straightforward method is the thiol place-exchange reaction seen in Figure 3.
FIGURE 3.
Scheme of the thiol place-exchange reaction. The stoichiometry of the incoming to exiting ligand is 1:1.
Solution-Phase Place Exchange
In the place-exchange reaction, an incoming ligand, such as a thiol-containing biomolecule, replaces one of the original capping ligands in a 1:1 ratio. Place exchange on nanoparticles was first described by Murray and coworkers who used alkanethiolate clusters with ω-functionalized thiols in toluene.2 This reaction has since been expanded to aqueous solutions and can also be carried out in aqueous buffer solutions.10 Multiple research groups have studied the dynamics by which place exchange occurs in ligand solutions. According to Murray’s work, the rate of ligand exchange depends upon both the concentration of incoming and exiting ligands, implying an associative (Sn2-like) mechanism.33 Lennox and coworkers, on the other hand, report that the reaction is zero-order with respect to the incoming ligand.34 Zerbetto’s lab found that the associative mechanism is accurate, but that the newly introduced ligand interacts with multiple existing ligands on the cluster.35 These interactions cause the kinetics to change as the reaction proceeds. Nevertheless, while the exact mechanism for place-exchange reactions may be complicated, the utility of place-exchange for functionalizing MPCs results from its simplicity.
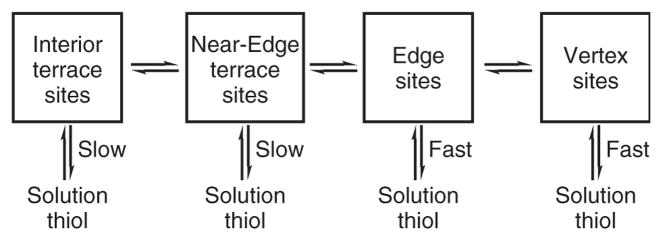
Reaction rates also play an important role in the place-exchange dynamics. The reaction rate increases with smaller-sized entering ligands and shorter chain length of the protecting ligand.33 Consequently, it is thermodynamically favorable to place-exchange a large biomolecule, such as a peptide or protein fragment, with a small protecting ligand such as tiopronin. Further, it is important to consider that subtle differences in the structure of the incoming ligand, such as branching, can have a significant effect on both the rate of place exchange and the stability of the monolayer.36 Additionally, it should be noted that the reaction proceeds more favorably at different sites on the core: vertex sites > edge sites > near-edge sites > terrace sites,33 as depicted in Figure 4.
FIGURE 4.
Chart of the different rates at which place exchange and (possibly) migration occur. (Reprinted, with permission, from Ref. 33. Copyright 1999 American Chemical Society.)
The variations in reactivity due to thermodynamics and kinetics originate from the differences in electron density37 and steric accessibility38 of these sites. This unique property of nanoparticles leads to some degree of predictability, and therefore control, in where the place-exchanged functional groups will anchor on the core. The rate of exchange is also increased by oxidative electronic charging of the core by electrochemical means39 or in the presence of dioxygen.40 The extent of reaction can be enhanced by increasing the incoming ligand concentration, but it should be noted that the extent of exchange rarely approaches 100%, owing to the difficulty of exchange at terrace sites.33
It is also important to realize that the rate of ligand place exchange on MPCs becomes slower as the particles age, probably because of a slow rearrangement of the ligands on the surface.41 Unfortunately, no kinetic or mechanistic study of place exchange has considered the new findings about the presence of gold-thiolate tetramer rings on the surface of MPCs as reported by the Cliffel group using mass spectrometry28 and Häkkinen and coworkers in a theoretical paper.42 Most recently, Kornberg et al. determined the specific crystal structure of a p-mercaptobenzoic acid MPC.43 The X-ray structure showed surface bridging interactions between gold atoms and the thiol groups of the protecting ligands. Also, the structure contains conformational features specific to the phenyl ligands, for example, phenyl stacking, T stacking, and sulfur–phenyl interactions. All the recent findings show nuances in surface structure that could help to better explain the complexities of the place-exchange mechanism.
Solid-Phase Place Exchange
As an alternative to the solution-phase place exchange discussed above, Huo and coworkers have studied solid-phase place-exchange reactions. In their original report of this reaction, they employed a polystyrene Wang resin with acetyl-protected 6-mercaptohexanoic acid attached via an ester bond.44 The thiol groups were deprotected and allowed to undergo place exchange with butanethiolate-protected gold nanoparticles, followed by washing away of unexchanged product and cleaving of the exchanged particles. Their results showed that they could place-exchange one ligand on to a particle surface. This was proven using coupling chemistry to make dimer nanoparticle complexes, rather than trimers or larger aggregates that would result from multiple exchanged ligands.
The same group has compared this solid-phase approach to the solution-phase approach and found the solid phase approach to be advantageous in terms of controlling the number of ligands attached per cluster and preserving the order of ligands on the surface.45 Recently, the same group has reported a solid-phase approach using a noncovalent interaction of the incoming ligand with silica gel,46 which is depicted in Figure 5. This strategy employs milder reaction conditions, thereby making it amenable to a wider class of molecules, such as large biologically relevant functional groups.
FIGURE 5.
The noncovalent interaction based place-exchange reaction. (Reprinted, with permission, Wiley Periodicals, Inc.)
Adapting Biological Functional Groups to Place Exchange
Macromolecules often contain thiols, for example, cysteine residues in proteins or adenosyl phosphothioate residues in DNA oligonucleotides.47 These groups make biomolecules readily amenable to the place-exchange reaction. Strategies to introduce thiol groups into macromolecules include, but are certainly not limited to, the use of Traut’s reagent (2-iminothiolane)48 in the case of proteins, the inclusion of terminal cysteine residues during the synthesis of peptides, and the conversion of phosphates to phosphorothioates using 3H-1,2-benzodithiole-3-one 1,1-dioxide.49 It is also possible to introduce ligands into the MPC monolayer which undergo electrostatic interactions with biomolecules, for example, the use of biotin–streptavidin interaction or biotin–anti-biotin interaction.50 All these routes provide straightforward methods to ready a group for place exchange and create functional nanoparticles.
Direct Monolayer Functionalization
There are other strategies to functionalize MPCs. An important class of these strategies, which is gaining popularity, is the use of simple organic reactions on ligands already bound to the MPC. Examples include triazole cycloaddition to a bromine functionality,51 direct functionalization of a hydroxyl group,52 amide coupling, and ester coupling.3 All these methods enable post-exchange reaction chemistry to occur, allowing a surface to be modified in a controlled fashion.
Characterization Methods for Monolayer-Protected Clusters
A thorough characterization of MPCs and functionalized MPCs is critical before applying them in biological uses. Determination of core size is easily accomplished via transmission electron microscopy (TEM).1 Core sizes have also been determined by mass spectrometric methods.53,54 Thermogravimetric analysis (TGA)55 provides an easy method to determine the ratio of organic ligand to inorganic core material. Combining the core size and organic : inorganic ratio data yields an approximate average molecular formula for homofunctionalized MPCs.54 Nuclear magnetic resonance (NMR) spectroscopy is useful for determining the structure and composition of the protecting monolayer. Protecting ligands have broadened peaks in both 1H and 13C spectra due to spin–spin relaxational (T2) broadening, dispersity in binding sites, and dipolar broadening due to packing density gradients.56,57
Once functionalized as a biomolecular mimic, it is critical to characterize MPCs for two attributes. The first is the quantity of biologically relevant functional groups attached per cluster, generally reported as an average of all the clusters in a sample. The second factor is the secondary structure of the biomimics post conjugation. 1H NMR is a simple way to semiquantitatively determine the number of antigen peptides per cluster via integration of known protecting ligand peaks versus new broadened biomolecule peaks. The accuracy of this method can be enhanced through the use of I2-induced MPC decomposition (termed the ‘death reaction’), which leads to sharper peaks with less overlap.58
Secondary structure determination has proven to be more challenging. Drobny and coworkers describe the use of novel solid-state NMR techniques to investigate the secondary structure of peptides immobilized on gold MPCs via amide coupling.59 For their experiments, they used cross-polarization magic angle spinning (CPMAS) and double-quantum dipolar recoupling with a windowless sequence (DQDRAWS). They showed that a peptide maintained a helical structure upon conjugation, but with a slight change in backbone torsion angle. Mandal and Kraatz recently described similar characterizations of peptides place-exchanged onto MPCs using Fourier transform infrared spectroscopy (FT-IR) and Fourier transform reflection absorption spectroscopy (FT-RAIRS).60 Using amide I bands, they observed that the secondary structure of a leucine-rich peptide bound to gold transitions from α-helical to β-sheet with greater surface curvature. Results showed that free peptides, 2-D self-assembled monolayers (SAMs) on gold, and peptides on 20-nm gold MPCs showed α-helical structure because of less surface curvature. However, 10-nm and particularly 5-nm gold MPCs showed increasing amounts of β-sheet conformation due to the increased surface curvature. Understanding the nature of primary and secondary structure becomes critical as functionalized nanoparticles are used for practical applications.
ANTIGENIC VALIDATION USING IMMUNOASSAYS
One of the most effective ways to validate functionalized nanoparticles’ ability to mimic a biological antigen is to look for recognition from a specific antibody. Since antibodies are generally targeted for one epitope of interest, they allow for specificity and serve as the keystone in many bioanalytical techniques. This section will quickly highlight some selected analytical tools used to detect antigen mimics in a sensitive and specific fashion.
Enzyme-Linked Immunosorbent Assay
ELISA describes a family of techniques used for the validation of antigen–antibody interaction through the detection of antigen or antibody. This technique was first described by Engvall and Perlmann in 1971.61 ELISA generally involves the adsorption of an antigen onto a plastic substrate, followed by recognition with a primary antibody. Then, detectable secondary antibody, specific to the primary, is incubated. Secondary antibodies use tags: for example, horseradish peroxidase or alkaline phosphatase that give a detectable signal upon activation with a specific substrate.
Quartz Crystal Microbalance
A powerful tool used by our lab and others to detect antigens against specific antibodies is the quartz crystal microbalance (QCM).47,62–68 This technique is based on a piezoelectric oscillator that changes frequency with the addition of a mass load, specifically, the antigen. This frequency shift is then converted to a mass load, so the instrument acts as a highly sensitive mass balance. Depicted in Figure 6 is a cartoon representation of a QCM biosensor, with a generic antibody–antigen system. The detection limit of QCM technology is continuously increasing as higher-frequency crystals are developed, reaching easily to the nanogram level and down to hundreds of picograms. A convenient reason to use QCM for measuring biomimic binding is the built-in amplification of using nanoparticles. Since QCM is essentially a mass detection method, the large molecular weight of the gold nanoparticle improves the sensitivity for biomimic studies.
FIGURE 6.
The quartz crystal microbalance (QCM) biosensor showing an antibody recognizing a functionalized nanoparticle (antigen mimic). (Reprinted, with permission, Wiley Periodicals, Inc.)
Surface Plasmon Resonance
Another popular tool for bioanalytical measurements is the optical technique, surface plasmon resonance (SPR).25,67–69 This technique detects the refractive change of an incident laser source, which then translates into the on and off rates of the antigens. SPR utilizes commercially available gold surfaces with prefabricated substrates. These prefabricated substrates allow easier surface functionalization to create the biosensor. With subnanogram detection limits, SPR is another powerful tool for bioassays.
NANOPARTICLE-BASED MIMETICS
Protein-Functionalized Nanoparticles
Protein A–Coated Nanoparticles
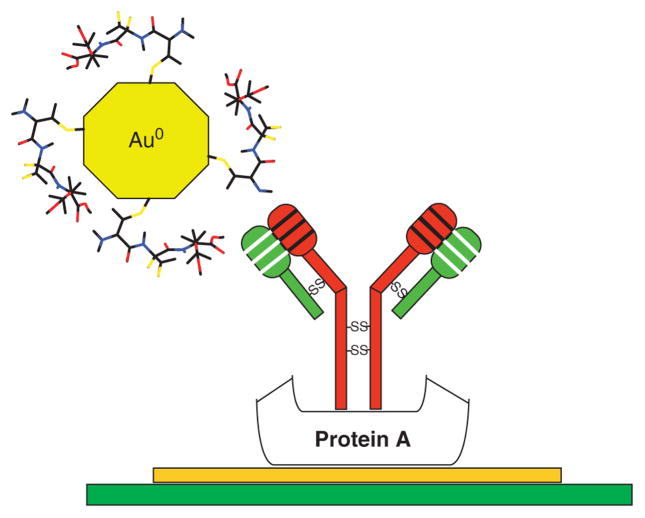
Nanoparticles are capable of being functionalized with whole proteins, while still undergoing the same biomolecular recognition events as the free proteins. Recently, Rosenzweig and Thanh demonstrated the viability of biomolecular recognition of whole protein–coated gold nanoparticles in the development of an aggregation-based assay.70 They were able to detect anti-protein A in serum by aggregating protein A-coated gold nanoparticles and observing an absorbance change at 620 nm.
Antibody Fragment–Conjugated Nanoparticles
Kornberg and coworkers described single-chain Fv (scFv) antibody fragments conjugated to glutathione gold MPCs.71 The scFv were rigidly coupled and exhibited specificity in binding to the antigen protein. By eliminating the flexible regions present in the whole antibody, rigidity was accomplished. Conjugation was accomplished by attaching a cysteine-terminated C-terminal affinity tag (FLAG) to the scFv. To assist the place exchange of the glutathione with scFv, they used oxidative charging of the metal core. Using cryo-electron microscopy, they were able to verify the antibody activity by observing the attachment of four Au71–scFv–glutathione units to single tetrameric influenza N9 neuraminidase units. In both these cases, the nanoparticle was used to aid in the detection of antibody–antigen binding, without actually using it as a biomimetic building block.
Peptide-Functionalized Gold Nanoparticles
Peptide-Functionalized Particles for Biological Recognition
Nanoparticles can be surface-functionalized by particle assembly and stabilization with a peptide or by place-exchange with the ligand after particle assembly. The first example of biologically relevant particles is the synthesis of nanoparticles with a protecting peptide from the histidine-rich protein II (HRP-II) of Plasmodium falciparum.72 Using standard fmoc procedures, Wright and coworkers recreated this peptide from HRP II and used it as a stabilizing ligand on different metal core particles: ZnS, Au0, Ag0, TiO2, and AgS. The biological significance comes from the recognition of the particle by a monoclonal antibody specific for P. falciparum. They were able to detect the peptide-encapsulated particles as they would the whole protein. This antibody–nanoparticle recognition shows that their particle mimics the native epitope.
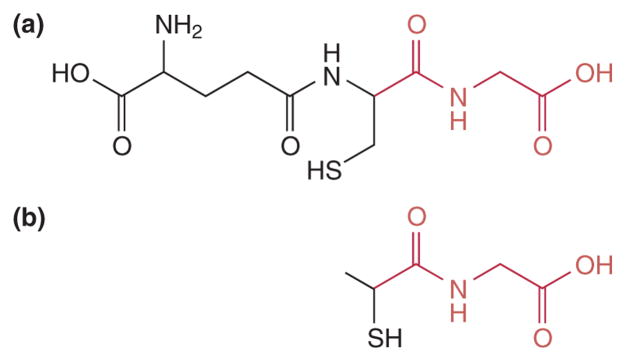
Recently, Cliffel and coworkers developed several MPCs that mimic antigens of interest. The first was a glutathione (GSH)-passivated gold cluster (GSH-MPC) that was then detected with a polyclonal anti-GSH antibody.63 The antibody very specifically recognized the GSH-MPC versus a standard tiopronin-passivated nanoparticle, even though both surface ligands only differ by about one amino acid, seen in Figure 7. While glutathione is not a traditional antigen, it serves as a proof of concept that an MPC can be functionalized with a surface peptide, and then specifically recognized via its antibody.
FIGURE 7.
Comparison of the glutathione (a) and tiopronin (b) ligands used to functionalize MPCs. Tiopronin is a truncated form of the 3-amino acid glutathione, with overlap shown in red. (Reprinted, with permission, from Ref. 62. Copyright 2005 American Chemical Society.)
Another MPC this group synthesized contains an epitope from the hemagglutinin (HA) protein of influenza,64 termed an HA-MPC. The 10-amino acid peptide was again synthesized with standard fmoc procedures with a terminating cysteine residue to promote place-exchange chemistry. This peptide was selected because it is a neutralizing site for influenza and there was a commercially available monoclonal antibody specific for this epitope on HA. Also, this experiment compared 2-D SAMs to 3-D nanoparticles as depicted in Figure 8. It was shown that the HA-MPC was more efficient in presenting the peptide to the antibody, resulting in a higher ratio of antibody to peptide binding when compared to the 2-D surface.
FIGURE 8.
Different nanostructures used: (a) the 3-D GSH-MPC, (b) the 3-D 10-amino acid hemagglutinin monolayer-protected clusters (HA-MPC), and (c) the same 10-amino acid sequence for HA as a 2-D self-assembled monolayer (SAM) with tiopronin spacers. (Reprinted, with permission, from Ref. 63. Copyright 2005 American Chemical Society.)
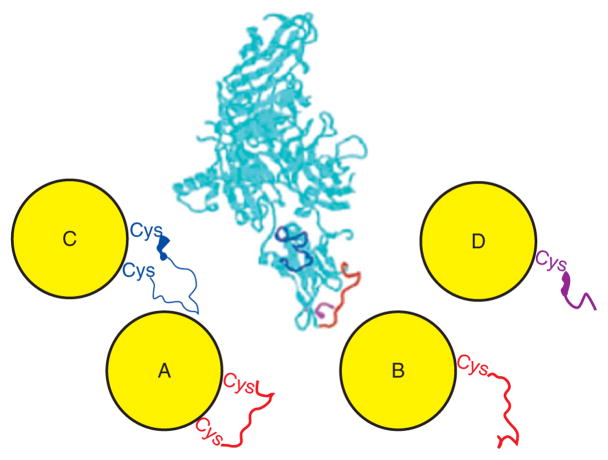
Another novel feature of epitope presenting MPCs is that they can be size-separated. Using a specific sized particle, the peptide is forced into adopting a conformation closer to the native structure. Previous work by Murray and coworkers had shown that ligands are dynamically attached to the surface and will therefore migrate across the MPC to find the most stable conformation possible.3,33,73 Cliffel’s research group applied this concept to their work on the protective antigen (PA) of Bacillus anthracis.66 The PA protein is one of three precursors of the anthrax toxin. PA was selected because it precedes the other two proteins (edema factor and lethal factor) in their transport for infection, which makes it an ideal target for neutralizing antibodies. Specifically, the C-terminus and two loops of the PA protein were identified as cell-receptor sites, making them the best candidates for their work.
Again, tiopronin MPCs were used and place-exchanged with the relevant peptide for the regions on PA. Since some of the PA epitopes selected were loop regions, the peptide was designed so that it could mimic its native conformation by putting cysteine residues on both the N- and C-termini. This allowed bidentate attachment across the nanoparticle surface to reconstruct the natural loop. Shown in Figure 9 is the stepwise process in the creation of the conformational mimic.
FIGURE 9.
Step-by-step creation of the conformational mimic nanoparticle using the synthetic protective antigen (PA) peptide.
For comparison, a second cluster was created that only had a cysteine on the C-terminus for monodentate attachment. This creates two types of clusters, both with the proper primary structure, but only one with the secondary structure closer to the native conformation, as illustrated in Figure 10.
FIGURE 10.
Loop presenting MPCs (left) shown compared to the native protein (middle) and the linear presenting monolayer-protected cluster (MPC) (right). (Reprinted, with permission, Wiley Periodicals, Inc.)
A QCM-based antibody–antigen binding study revealed that the loop-presenting cluster was more strongly recognized than the linear epitope cluster. More specifically, the loop epitope had a higher affinity constant (Ka) for this particular antibody than the linear epitope, especially at physiological saline concentrations. This data shows that the bidentate structure was better recognized and bound more tightly. This suggests that the commercial antibody may have a conformational paratope. The QCM was used to detect the antibody binding to the MPC, which was electrostatically held to the sensor.
Peptides are not only limited to use as the functional group. Naik and coworkers used peptides in a novel and sophisticated way. Multifunctional peptides were used as the reducing agent, gold-protecting ligand, and presenting epitope.74 Peptide A3 was selected from a phage peptide display library and found to both bind to gold and reduce it. Flg, a peptide commonly used in tagging proteins with a biomolecular recognition domain, was also found to reduce gold. They were able to produce Flg-A3 and A3-Flg gold nanoparticles in a one-pot synthesis with good monodispersity and were capable of binding to anti-Flg IgG on glass slides.
As an extension of peptide-epitope-protected gold MPCs, a collaboration of the Cliffel and Wright research groups synthesized tiopronin MPCs functionalized with either the flag epitope (flag-MPC), HA epitope (HA-MPC), flag and HA epitope (flag/HA-MPC), or no epitope.75 The peptide epitopes were attached to the cluster via a cysteine-terminated polyethylene glycol (PEG) hexamer using place exchange. The PEG linker provides enhanced accessibility by moving the epitope away from the particle’s surface. QCM immunosensors, as previously described, using either anti-flag or anti-HA IgG were used to evaluate the immunological activity of the mimics synthesized. They were able to detect the HA-MPC and HA/flag-MPC using the anti-HA immunosensor, and the flag-MPC and HA/flag-MPC using the anti-flag immunosensor. Neither one detected the tiopronin MPCs without peptide epitopes. In all these trials, biological recognition serves as a quick means to validate peptide nanoparticles and determine binding constants.
Peptide-Functionalized Gold Nanoparticles for Biological Targeting
Biomimetic nanoparticles have shown promise as a tool for targeted cell entry. Targeted entry is complex, but the small size of gold nanoparticles and the functionality available from synthetic peptides make this delicate task a possibility. Inspired by viruses, Tkachenko and coworkers conjugated peptides to bovine serum albumin (BSA) via an ester linker, and then conjugated the BSA to gold nanoparticles.76 The four peptides they used were from viral cell entry/targeting proteins, and they were able to achieve targeted entry of the gold nanoparticles into the nucleus of HepG2 cells. Furthermore, it should be noted that the cells were still viable after entry of the gold nanoparticles.76
Cell-Binding Studies of Functionalized Nanoparticles
Gold nanoparticles, as previously mentioned, can be functionalized with many different ligands. Results from Rotello’s group show that the charge of the capping ligand can affect how the particle binds to cell surfaces.77 Positively charged ligands, such as TMA, cause an attraction between the particle and the negatively charged cell wall. The increased binding leads to higher toxicity and cell lysis. Conversely, the same negatively charged cell wall has little attraction to a carboxylate nanoparticle, leading to less cell lysis. Cell walls with no overall charge, however, lysed slightly more with negatively charged particles. These findings present interesting considerations when conducting studies at the cellular level.
Further work by Schmid and coworkers has shown that very small gold nanoparticles (Au55 cores, 1.4 nm) can actually bind to DNA in the cell. This is due to gold’s preference for the negatively charged backbone of DNA, which partially removes the protecting ligand group.78 Au55 clusters entrenched in the DNA grooves are depicted in Figure 11. Cell entry and specific targeting can serve as tools to either mark cells for imaging or cause controlled cell death.
FIGURE 11.
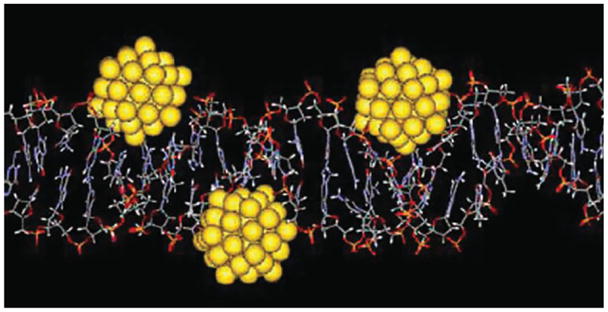
Model of Au55 clusters irreversibly bound to the grooves of DNA. (Reprinted, with permission, Wiley Periodicals, Inc.)
Peptide-Functionalized Gold Nanoparticles for Biological Catalysis
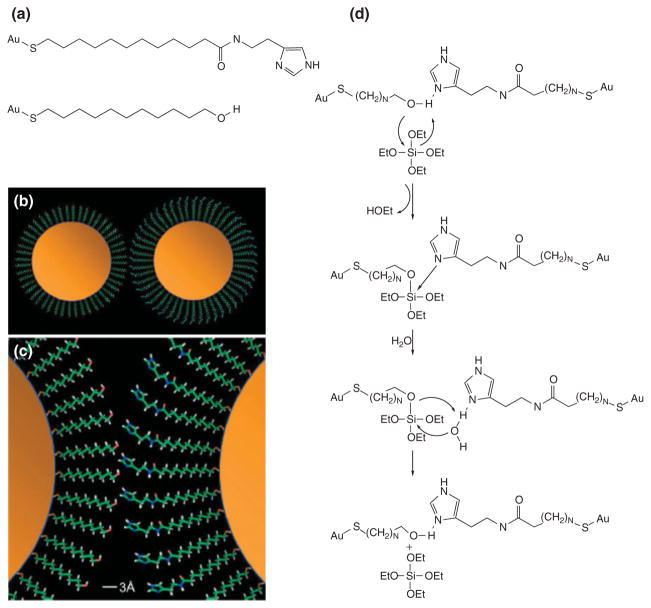
The first report of using multifunctionalized gold MPCs for catalysis was by Frigeri and coworkers earlier this decade.79 Using N-methylimidazole-functionalized gold nanoparticles, they were able to catalyze the hydrolysis of an activated ester. Scrimin and coworkers have created water-soluble gold MPCs place-exchanged with histidine-phenylalanine dipeptides that are capable of mimicking hydrolytic enzymes.80 These two examples represent steps toward gold nanoparticle–based enzyme mimics that inspired Scrimin and coworkers to term them ‘nanozymes’. More recently, Morse and coworkers were able to use gold nanoparticles to mimic the catalytic activity of an enzyme in the sponge Tethya aurantia responsible for producing silica needles by simply conjugating organic molecules to the protecting monolayer of gold nanoparticles.81 The catalytic site of the aforementioned enzyme in T. aurantia uses a nucleophilic -OH group interacting with a hydrogen-bonding imidazole group to accomplish hydrolysis of a silicon alkoxide precursor and subsequent polycondensation to silica. Hydroxy-terminated nanoparticles were afforded simply by using 11-mercaptoundecanol as the protecting ligand in a Brust synthesis. The imidazole-terminated nanoparticles were obtained by using amide coupling of an imidazole functionality to 11-mercaptoundecanoic-protected gold nanoparticles. This idea is illustrated in Figure 12.
FIGURE 12.
Hydroxy- and imidazole-functionalized nanoparticles working together to catalyze silica formation. The ligands used to functionalize the particles are shown in (a), while the interaction between particles in (b) and (c). Part (d) shows the stepwise synthetic route of the ligands. (Reprinted, with permission, Wiley Periodicals, Inc.)
Carbohydrate-Functionalized Nanoparticles
Many important processes in biology rely on carbohydrate–protein interactions, and it may become convenient to functionalize gold nanoparticles with carbohydrates instead of proteins or peptides. This was first accomplished by Penades and coworkers when they used carbohydrate-functionalized gold nanoparticles to mimic glycocalyx, the sticky film found on the outside of many different cells.82
As a further example of non-protein-related gold nanoparticle biomimetics, Chen and coworkers observed high affinity and specificity for binding of carbohydrate-encapsulated nanoparticles to concanavalin A.83 Carbohydrates were attached to the gold core using a thiol linker in a place-exchange reaction. Interaction with concanavalin A was monitored using SPR.
CONCLUSION
The exciting field of nanoparticle-based biological mimetics is rapidly developing. The results will continue to grow as new discoveries are made in the secondary structure of proteins, opening the door for even more material to incorporate into an expanding toolbox. Also, new techniques are being created to better characterize the interface between biological recognition and nanoscale structures.
The field has brought out many interesting results concerning the synthesis and conjugation of nanoparticle-based mimetics that can compete with their native analogs in functional complexity. The future of this field will be focused on creating more sophisticated nanoparticle-based biomimetics, centered on biological recognition, catalysis, and targeting. Metal nanoparticle–based drug targets may work well under ideal conditions for analytical merit and in vitro studies, but their effect in complex biological systems is only beginning to unravel. The field of biological mimetics will continue to thrive, with nanoparticles playing a critical role in research, diagnostics, and therapeutics.
Footnotes
RELATED ONLINE ARTICLES
Metal nanoparticles for DNA and antigen detection. Using nanoparticles to push the limits of detection.
References
- 1.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a two-phase liquid-liquid system. Chem Commun. 1994;7:801–902. [Google Scholar]
- 2.Hostetler MJ, Green SJ, Stokes JJ, Murray RW. Monolayers in three dimensions: synthesis and electrochemistry of omega-functionalized alkanethiolate-stabilized gold cluster compounds. J Am Chem Soc. 1996;118(17):4212–4213. [Google Scholar]
- 3.Templeton AC, Hostetler MJ, Warmoth EK, Chen S, Hartshorn CM, et al. Gateway reactions to diverse, polyfunctional monolayer-protected gold clusters. J Am Chem Soc. 1998;120:4845–4849. [Google Scholar]
- 4.Faraday M. Experimental relations of gold (and other metals) to light. Philos Trans R Soc Lond. 1857;147:145–181. [Google Scholar]
- 5.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. [Google Scholar]
- 6.Templeton AC, Wuelfing WP, Murray RW. Monolayer-protected cluster molecules. Acc Chem Res. 2000;33:27–36. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 7.Niemeyer CM. Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angew Chem Int Ed. 2001;40:4128–4158. doi: 10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Rowe RP, Plass KE, Kim K, Kurdak C, Zellers ET, et al. Single-phase synthesis of functionalized gold nanoparticles. Chem Mater. 2004;16:3513–3517. [Google Scholar]
- 9.Templeton AC, Chen S, Gross SM, Murray RW. Water-soluble, isolable gold clusters protected by tiopronin and coenzyme a monolayers. Langmuir. 1999;15:66–76. [Google Scholar]
- 10.Templeton AC, Cliffel DE, Murray RW. Redox and fluorophore functionalization of water-soluble, tiopronin-protected gold clusters. J Am Chem Soc. 1999;121:7081–7089. [Google Scholar]
- 11.Yee C, Scotti M, Ulman A, White H, Rafailovich M, et al. One-phase synthesis of thiol-functionalized platinum nanoparticles. Langmuir. 1999;15:4314–4316. [Google Scholar]
- 12.Yee CK, Jordan R, Ulman A, White H, King A, et al. Novel one-phase synthesis of thiol-functionalized gold palladium and iridium nanoparticles using superhydride. Langmuir. 1999;15:3486–3491. [Google Scholar]
- 13.Croce TA, Hamilton SK, Chen ML, Muchalski H, Harth E. Alternative o-chinodimethane crosslinking precursors for intramolecular chain collapse nanoparticles. Macromolecules. 2007;40:6028–6031. [Google Scholar]
- 14.Ahmed F, Photos PJ, Discher DE. Polymersomes as viral capsid mimics. Drug Dev Res. 2006;67:4–14. [Google Scholar]
- 15.Joralemon MJ, Smith NL, Holowka D, Baird B, Wooley KL. Antigen-decorated shell cross-linked nanoparticles: synthesis, characterization, and antibody interactions. Bioconjug Chem. 2005;16:1246–1256. doi: 10.1021/bc0501505. [DOI] [PubMed] [Google Scholar]
- 16.Taubert A, Napoli A, Meier W. Self-assembly of reactive amphiphlic block copolymers as mimetics for biological membranes. Curr Opin Chem Biol. 2004;8:598–603. doi: 10.1016/j.cbpa.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, et al. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology. 2004;326:280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11(9):438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara Y, Amexis G, Yamaguchi H, Kajigaya S, Leppla SH, et al. Recombinant viral-like particles of parvovirus B19 as antigen carriers of anthrax protective antigen. In vivo. 2006;20:319–324. [PubMed] [Google Scholar]
- 20.Raja KS, Wang Q, Finn MG. Icosahedral virus particles as polyvalent carbohydrates display platforms. Chembiochem. 2003;4:1348–1351. doi: 10.1002/cbic.200300759. [DOI] [PubMed] [Google Scholar]
- 21.Szécsi J, Boson B, Johnsson P, Dupeyrot-Lacs P, Matrosovich M, et al. Induction of neutralising antibodies by virus-like particles harbouring surface proteins from highly pathogenic H5N1 and H7N1 influenza viruses. Virol J. 2006;3:1–7. doi: 10.1186/1743-422X-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Lin T, Tang L, Johnson JE, Finn MG. Icosahedral virus particles as addressable nanoscale building blocks. Angew Chem Int Ed. 2002;41:459–462. doi: 10.1002/1521-3773(20020201)41:3<459::aid-anie459>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Jahanshahi M, Williams S, Lyddiatt A, Shojaosadati SA. Preparation and purification of synthetic protein nanoparticles. IEE Proc Nanobiotechnol. 2004;151(5):176–182. doi: 10.1049/ip-nbt:20041085. [DOI] [PubMed] [Google Scholar]
- 24.Gole A, Vyas S, Phadtare S, Lachke A, Sastry M. Studies on the formation of bioconjugates of endoglucanase with colloidal gold. Colloids Surf B. 2002;25:129–138. [Google Scholar]
- 25.Lyon LA, Peña DJ, Natan MJ. Surface plasmon resonance of au colloid-modified au films: particle size dependence. J Phys Chem B. 1999;103:5826–5831. [Google Scholar]
- 26.Matsuya T, Tashiro S, Hoshino N, Shibata N, Nagasaki Y, et al. A core-shell type fluorescent nanosphere possessing reactive poly(ethylene glycol) tethered chains on the surface of zeptomole detection of protein in time-resolved fluorometric immunoassay. Anal Chem. 2003;75:6124–6132. doi: 10.1021/ac034346e. [DOI] [PubMed] [Google Scholar]
- 27.Whetten RL, Shafigullin MN, Khoury JT, Schaaff TG, Vezmar I, et al. Crystal structures of molecular gold nanocrystal arrays. Acc Chem Res. 1999;32:397–406. [Google Scholar]
- 28.Gies AP, Hercules DM, Gerdon AE, Cliffel DE. Electrospray mass spectrometry study of tiopronin monolayer-protected gold nanoclusters. J Am Chem Soc. 2007;129(5):1095–1104. doi: 10.1021/ja0639057. [DOI] [PubMed] [Google Scholar]
- 29.Schaaff TG, Knight G, Shafigullin MN, Borkman RF, Whetten RL. Isolation and selected properties of a 10.4 kDa gold: glutathione cluster compound. J Phys Chem B. 1998;102(52):10643–10646. [Google Scholar]
- 30.Johnson SR, Evans SD, Brydson R. Influence of a terminal functionality on the physical properties of surfactant-stabilized gold nanoparticles. Langmuir. 1998;14(23):6639–6647. [Google Scholar]
- 31.Ackerson CJ, Jadzinsky PD, Kornberg RD. Thiolate ligands for synthesis of water-soluble gold clusters. J Am Chem Soc. 2005;127(18):6550–6551. doi: 10.1021/ja046114i. [DOI] [PubMed] [Google Scholar]
- 32.Cliffel DE, Zamborini FP, Gross SM, Murray RW. Mercaptoammonium-monolayer-protected, water-soluble gold, silver, and palladium clusters. Langmuir. 2000;16(25):9699–9702. [Google Scholar]
- 33.Hostetler MJ, Templeton AC, Murray RW. Dynamics of place-exhange reactions on monolayer-protected gold cluster molecules. Langmuir. 1999;15:3782–3789. [Google Scholar]
- 34.Kassam A, Bremner G, Clark B, Ulibarri G, Lennox RB. Place exchange reactions of alkyl thiols on gold nanoparticles. J Am Chem Soc. 2006;128(11):3476–3477. doi: 10.1021/ja057091q. [DOI] [PubMed] [Google Scholar]
- 35.Montalti M, Prodi L, Zaccheroni N, Baxter R, Teobaldi G, et al. Kinetics of place-exchange reactions of thiols on gold nanoparticles. Langmuir. 2003;19(12):5172–5174. [Google Scholar]
- 36.Hong R, Fernandez JM, Nakade H, Arvizo R, Emrick T, et al. In situ observation of place exchange reactions of gold nanoparticles. Correlation of monolayer structure and stability. Chem Commun. 2006;22:2347–2349. doi: 10.1039/b603988j. [DOI] [PubMed] [Google Scholar]
- 37.Häkkinen H, Barnett RN, Landman U. Electronic structure of passivated Au-38(SCH3)(24) nanocrystal. Phys Rev Lett. 1999;82(16):3264–3267. [Google Scholar]
- 38.Donkers RL, Song Y, Murray RW. Substituent effects on the exchange dynamics of ligands on 1.6 nm diameter gold nanoparticles. Langmuir. 2004;20(11):4703–4707. doi: 10.1021/la0497494. [DOI] [PubMed] [Google Scholar]
- 39.Song Y, Murray RW. Dynamics and extent of ligand exchange depend on electronic charge of metal nanoparticles. J Am Chem Soc. 2002;124(24):7096–7102. doi: 10.1021/ja0174985. [DOI] [PubMed] [Google Scholar]
- 40.Myung N, Bae Y, Bard AJ. Effect of surface passivation on the electrogenerated chemiluminescence of CdSe/ZnSe nanocrystals. Nano Lett. 2003;3(8):1053–1055. [Google Scholar]
- 41.Chechik V. Reduced reactivity of aged Au nanoparticies in ligand exchange reactions. J Am Chem Soc. 2004;126(25):7780–7781. doi: 10.1021/ja048879w. [DOI] [PubMed] [Google Scholar]
- 42.Häkkinen H, Walter M, Grönbeck H. Divide and protect: capping gold nanoclusters with molecular gold-thiolate rings. J Phys Chem B. 2006;110:9927–9931. doi: 10.1021/jp0619787. [DOI] [PubMed] [Google Scholar]
- 43.Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. Science. 2007;318:430–433. doi: 10.1126/science.1148624. [DOI] [PubMed] [Google Scholar]
- 44.Worden JG, Shaffer AW, Huo Q. Controlled functionalization of gold nanoparticles through a solid phase synthesis approach. Chem Commun. 2004;5:518–519. doi: 10.1039/b312819a. [DOI] [PubMed] [Google Scholar]
- 45.Shaffer AW, Worden JG, Huo Q. Comparison study of the solution phase versus solid phase place exchange reactions in the controlled functionalization of gold nanoparticles. Langmuir. 2004;20(19):8343–8351. doi: 10.1021/la049308k. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Worden JG, Dai Q, Zou JH, Wang JH, et al. Monofunctional gold nanopartictes prepared via a noncovalent-interaction-based solid-phase modification approach. Small. 2006;2(10):1126–1129. doi: 10.1002/smll.200600162. [DOI] [PubMed] [Google Scholar]
- 47.Bardea A, Dagan A, Ben-Dov I, Amit B, Willner I. Amplified microgravimetric quartz-crystal-microbalance analyses of olignucleotide complexes: a route to a Tay-Sachs biosensor device. Chem Commun. 1998:839–840. [Google Scholar]
- 48.Ghosh SS, Kao PM, McCue AW, Chappelle HL. Use of maleimidethiol coupling chemistry for efficient syntheses of oligonucleotide-enzyme conjugate hybridization probes. Bioconjug Chem. 1990;1:71–76. doi: 10.1021/bc00001a009. [DOI] [PubMed] [Google Scholar]
- 49.Iyer RP, Egan W, Regan JB, Beaucage SL. 3H-1,2-benzodithiole-3-one 1,1-dioxide as an improved sulfurizing reagent in the solid-phase synthesis of oligodeoxyribonucleoside phosphorothioates. J Am Chem Soc. 1990;112:1253–1254. [Google Scholar]
- 50.Connolly S, Fitzmaurice D. Programmed assembly of gold nanocrystals in aqueous solution. Adv Mater. 1999;11(14):1202–1205. [Google Scholar]
- 51.Fleming DA, Thode CJ, Williams ME. Triazole cycloaddition as a general route for functionalization of Au nanoparticles. Chem Mater. 2006;18(9):2327–2334. [Google Scholar]
- 52.Tan H, Zhan T, Fan WY. Direct functionalization of the hydroxyl group of the 6-mercapto-1-hexanol (MCH) ligand attached to gold nanoclusters. J Phys Chem B. 2006;110(43):21690–21693. doi: 10.1021/jp065652+. [DOI] [PubMed] [Google Scholar]
- 53.Tai Y, Murakami J, Saito K, Ikeyama M, Tajiri K, et al. Plasma desorption mass spectroscopy of thiol-passivated gold nanoparticles. Eur Phys J D. 2003;24(1–3):261–263. [Google Scholar]
- 54.Whetten RL, Khoury JT, Alvarez MM, Murthy S, Vezmar I, et al. Nanocrystal gold molecules. Adv Mater. 1996;8(5):428. [Google Scholar]
- 55.Terrill RH, Postlethwaite TA, Chen CH, Poon CD, Terzis A, et al. Monolayers in three dimensions: NMR, SAXS, thermal, and electron hopping studies of alkanethiol stabilized gold clusters. J Am Chem Soc. 1995;117(50):12537–12548. [Google Scholar]
- 56.Badia A, Cuccia L, Demers L, Morin F, Lennox RB. Structure and dynamics in alkanethiolate monolayers self-assembled on gold nanoparticles: A DSC, FT-IR, and deuterium NMR study. J Am Chem Soc. 1997;119(11):2682–2692. [Google Scholar]
- 57.Hostetler MJ, Wingate JE, Zhong CJ, Harris JE, Vachet RW, et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir. 1998;14(1):17–30. [Google Scholar]
- 58.Templeton AC, Hostetler MJ, Kraft CT, Murray RW. Reactivity of monolayer-protected gold cluster molecules: steric effects. J Am Chem Soc. 1998;120:1906–1911. [Google Scholar]
- 59.Bower PV, Louie EA, Long JR, Stayton PS, Drobny GP. Solid-state NMR structural studies of peptides immobilized on gold nanoparticles. Langmuir. 2005;21(7):3002–3007. doi: 10.1021/la040092w. [DOI] [PubMed] [Google Scholar]
- 60.Mandal HS, Kraatz H-B. Effect of the surface curvature on the secondary structure of peptides adsorbed on nanoparticles. J Am Chem Soc. 2007;129:6356–6357. doi: 10.1021/ja0703372. [DOI] [PubMed] [Google Scholar]
- 61.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (Elisa) quantitative assay of Immunoglobulin-G. Immunochemistry. 1971;8(9):871. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 62.Patolsky F, Ranjit K, Lichtenstein A, Willner I. Dendritic amplification of DNA analysis by oligonucleotide-functionalized Au-nanoparticles. Chem Commun. 2000:1025–1026. [Google Scholar]
- 63.Gerdon AE, Wright DW, Cliffel DE. Quartz crystal microbalance detection of glutathione-protected nanoclusters using antibody recognition. Anal Chem. 2005;77:304–310. doi: 10.1021/ac048953t. [DOI] [PubMed] [Google Scholar]
- 64.Gerdon AE, Wright DW, Cliffel DE. Hemagglutinin linear epitope presentation on monolayer-protected clusters elicits strong antibody binding. Biomacromolecules. 2005;6:3419–3424. doi: 10.1021/bm050475o. [DOI] [PubMed] [Google Scholar]
- 65.Gerdon AE, Wright DW, Cliffel DE. Quartz crystal microbalance characterization of nanostructures assemblies in biosensing. In: Kumar CSSR, editor. Nanosystem Characterization Tools in the Life Sciences. Vol. 3. Wiley-VCH; 2006. pp. 109–144. [Google Scholar]
- 66.Gerdon AE, Wright DW, Cliffel DE. Epitope mapping of the protective antigen of B. Anthracis by using nanoclusters presenting conformational peptide epitopes. Angew Chem Int Ed. 2006;45:594–598. doi: 10.1002/anie.200503328. [DOI] [PubMed] [Google Scholar]
- 67.Janshoff A, Galla H-J, Steinem C. Piezoelectric mass-sensing devices as biosensors - an alternative to optical biosensors? Angew Chem Int Ed. 2000;39:4004–4032. doi: 10.1002/1521-3773(20001117)39:22<4004::aid-anie4004>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 68.Kößlinger C, Uttenthaler E, Drost S, Aberl F, Wolf H, et al. Comparison of the QCM and the SPR method for surface studies and immunological applications. Sens Actuators B. 1995;24–25:107–112. [Google Scholar]
- 69.Hutter E, Fendler JH, Roy D. Surface plasmon resonance studies of gold and silver nanoparticles linked to gold and silver substrates by 2-aminoethanethiol and 1,6-hexanedithiol. J Phys Chem B. 2001;105:11159–11168. [Google Scholar]
- 70.Thanh NTK, Rosenzweig Z. Development of an aggregation-based immunoassay for anti-protein A using gold nanoparticles. Anal Chem. 2002;74(7):1624–1628. doi: 10.1021/ac011127p. [DOI] [PubMed] [Google Scholar]
- 71.Ackerson CJ, Jadzinsky PD, Jensen GJ, Kornberg RD. Rigid, specific, and discrete gold nanoparticle/antibody conjugates. J Am Chem Soc. 2006;128(8):2635–2640. doi: 10.1021/ja0555668. [DOI] [PubMed] [Google Scholar]
- 72.Slocik JM, Moore JT, Wright DW. Monoclonal antibody recognition of histidine-rich peptide encapsulated nanoclusters. Nano Lett. 2002;2(3):169–173. [Google Scholar]
- 73.Ingram RS, Hostetler MJ, Murray RW. Poly-hetero-ω -fuctionalized alkanethiolate-stabilized gold cluster compounds. J Am Chem Soc. 1997;119:9175–9178. [Google Scholar]
- 74.Slocik JM, Stone MO, Naik RR. Synthesis of gold nanoparticles using multifunctional peptides. Small. 2005;1(11):1048–1052. doi: 10.1002/smll.200500172. [DOI] [PubMed] [Google Scholar]
- 75.Miller SA, Keil RG, Hiatt LA, Cliffel DE, Wright DW. Nanoparticle simulant for influenza virus. Nano Lett. 2007 In Submission. [Google Scholar]
- 76.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, et al. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125(16):4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 77.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 78.Tsoli M, Kuhn H, Brandau W, Esche H, Schmid G. Cellular uptake and toxicity of Au55 clusters. Small. 2005;1(8–9):841–844. doi: 10.1002/smll.200500104. [DOI] [PubMed] [Google Scholar]
- 79.Pasquato L, Rancan F, Scrimin P, Mancin F, Frigeri C. N-methylimidazole-functionalized gold nanoparticles as catalysts for cleavage of a carboxylic acid ester. Chem Commun. 2000;(22):2253–2254. [Google Scholar]
- 80.Pasquato L, Pengo P, Scrimin P. Functional gold nanoparticles for recognition and catalysis. J Mater Chem. 2004;14(24):3481–3487. [Google Scholar]
- 81.Kisailus D, Najarian M, Weaver JC, Morse DE. Functionalized gold nanoparticles mimic catalytic activity of a polysiloxane-synthesizing enzyme. Adv Mater. 2005;17(10):1234. [Google Scholar]
- 82.Barrientos AG, de la Fuente JM, Rojas TC, Fernandez A, Penades S. Gold glyconanoparticles: synthetic polyvalent ligands mimicking glycocalyx-like surfaces as tools for glycobiological studies. Chem Eur J. 2003;9(9):1909–1921. doi: 10.1002/chem.200204544. [DOI] [PubMed] [Google Scholar]
- 83.Lin CC, Yeh YC, Yang CY, Chen GF, Chen YC, et al. Quantitative analysis of multivalent interactions of carbohydrate-encapsulated gold nanoparticles with concanavalin A. Chem Commun. 2003;(23):2920–2921. doi: 10.1039/b308995a. [DOI] [PubMed] [Google Scholar]