Abstract
Idiopathic pulmonary fibrosis (IPF) is a destructive inflammatory disease with limited therapeutic options. To better understand the inflammatory responses that precede and concur with collagen deposition, we used three models of pulmonary fibrosis and identify a critical mechanistic role for IL-17A. After exposure to bleomycin (BLM), but not Schistosoma mansoni eggs, IL-17A produced by CD4+ and γδ+ T cells induced significant neutrophilia and pulmonary fibrosis. Studies conducted with C57BL/6 il17a−/− mice confirmed an essential role for IL-17A. Mechanistically, using ifnγ−/−, il10−/−, il10−/−il12p40−/−, and il10−/−il17a−/− mice and TGF-β blockade, we demonstrate that IL-17A–driven fibrosis is suppressed by IL-10 and facilitated by IFN-γ and IL-12/23p40. BLM-induced IL-17A production was also TGF-β dependent, and recombinant IL-17A–mediated fibrosis required TGF-β, suggesting cooperative roles for IL-17A and TGF-β in the development of fibrosis. Finally, we show that fibrosis induced by IL-1β, which mimics BLM-induced fibrosis, is also highly dependent on IL-17A. IL-17A and IL-1β were also increased in the bronchoalveolar lavage fluid of patients with IPF. Together, these studies identify a critical role for IL-17A in fibrosis, illustrating the potential utility of targeting IL-17A in the treatment of drug and inflammation-induced fibrosis.
Despite distinct etiological and clinical features, most chronic fibrotic disorders have in common a persistent irritant that sustains the production of growth factors, proteolytic enzymes, angiogenic factors, and fibrogenic cytokines (Wilson and Wynn, 2009). Together, these factors stimulate the deposition of connective tissue elements that progressively remodel normal tissue architecture. Although initially beneficial, tissue repair processes become pathogenic when they are not regulated, resulting in substantial deposition of extracellular matrix (ECM) components and development of scar tissue. In some diseases, like idiopathic pulmonary fibrosis (IPF), aberrant healing may lead to organ failure and death (Meltzer and Noble, 2008). Indeed, IPF and other chronic fibrotic lung diseases are associated with high morbidity and mortality and are generally refractory to existing pharmacological therapy (Shah et al., 2005). Therefore, better characterization of the molecular and immunological mechanisms of fibrosis is needed to identify new therapeutic modalities for these diseases.
Although a variety of cytokines, chemokines, and growth factors are important regulators of fibrosis, we identified a critical role for IL-13 in the development of fibrosis in schistosomiasis, a chronic liver disease caused by the parasitic helminth Schistosoma mansoni (Chiaramonte et al., 1999). Since then, IL-13 has been shown to exhibit fibrotic activity in a variety of diseases and tissues, including models of chronic asthma (Blease et al., 2001), skin fibrosis (Aliprantis et al., 2007), and bronchiolitis obliterans (Keane et al., 2007). A few recent studies have also suggested a role for IL-13 in bleomycin (BLM)-induced pulmonary fibrosis, a well-studied model of IPF (Jakubzick et al., 2003; Fichtner-Feigl et al., 2006). It has been suggested that IL-13 triggers fibrosis by inducing and activating TGF-β (Lee et al., 2001). Nevertheless, the mechanism of action of TGF-β in the development of pulmonary fibrosis remains controversial (Kaviratne et al., 2004; Varga and Pasche, 2008). Although it has been suggested that TGF-β contributes to BLM-induced inflammation and fibrosis by stimulating fibroblast proliferation and collagen-producing myofibroblasts (Cutroneo et al., 2007), recent studies also identified a critical role for TGF-β in the development of IL-17A–producing CD4+ T cells (Bettelli et al., 2006; Veldhoen et al., 2006), which regulate the pathogenesis of a variety of autoimmune and inflammatory diseases (Bettelli et al., 2008). Similarly, IL-1β can stimulate IL-17A production (Sutton et al., 2009), and IL-1β is a critical mediator of pulmonary fibrosis (Gasse et al., 2007). To date, however, a link between IL-17A–driven inflammation and pulmonary fibrosis has not been established.
The aim of the current study was to characterize the mechanisms of pulmonary fibrosis and to determine whether IL-17A in particular plays an important regulatory role. To do this, three distinct model systems were used, including S. mansoni egg-induced pulmonary fibrosis, BLM-induced pulmonary fibrosis, and the recently described IL-1β–driven fibrosis (Gasse et al., 2007). We report here that S. mansoni egg-mediated fibrosis is IL-13 dependent, as il13−/− mice developed minimal fibrosis compared with WT mice. In marked contrast, BLM-induced pulmonary fibrosis was independent of IL-13 at early time points. Instead, studies with il17a−/− mice revealed a critical role for IL-17A. Using IL-10gfp reporter mice and newly generated IL-10 and IL-17A double cytokine-deficient animals, we determined that CD4+ cell-derived IL-10 is required to limit the production and frequency of IL-17A+CD4+ and IL-17A+γδ+ T cells, thus preventing the development of severe IL-17A–driven fibrosis. We also show that IL-17A is essential for the development of fibrosis in response to IL-1β, thus extending recent studies that described an early innate role for IL-1β in pulmonary fibrosis (Gasse et al., 2007). Together, these studies demonstrate that fibrotic tissue remodeling is induced by distinct cytokine-dependent mechanisms, with the effector cytokines IL-13 and IL-17A playing central roles. Moreover, these findings suggest that TGF-β and proinflammatory mediators like IL-1β promote fibrosis by up-regulating the production of IL-17A, thus identifying IL-17A blockade as a potential treatment for fibrotic diseases like IPF.
RESULTS
Fibrosis is mediated by IL-13–dependent and IL-13–independent mechanisms
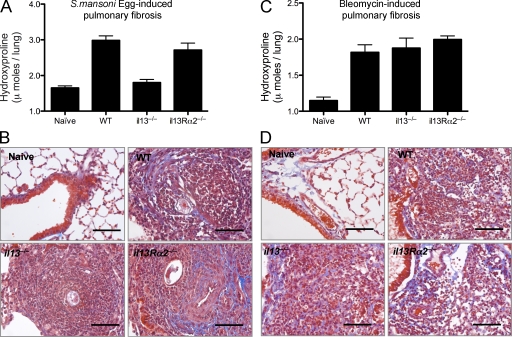
S. mansoni eggs delivered i.v. into S. mansoni egg–sensitized mice induce eosinophil-rich pulmonary granulomas around eggs trapped in the pulmonary microvasculature. The lesions are also associated with significant pulmonary fibrosis, as determined by hydroxyproline assay of lung tissue (Fig. 1 A). As observed in the liver during S. mansoni infection (Chiaramonte et al., 1999), studies conducted with il13−/− mice showed that pulmonary fibrosis is highly dependent on IL-13 (Fig. 1 A). However, unlike chronic liver fibrosis, hydroxyproline assays and Masson’s trichrome staining of lung tissue suggested that the IL-13 decoy receptor (IL-13Rα2) was not significantly involved in the regulation of collagen deposition in the lung (Fig. 1, A and B). In contrast to S. mansoni egg–induced pulmonary fibrosis, BLM-induced fibrosis appeared to be both IL-13 and IL-13Rα2 independent. Indeed, C57BL/6 WT, il13−/−, and il13Rα2−/− mice all displayed indistinguishable levels of interstitial fibrosis after intratracheal delivery of BLM (Fig. 1, C and D).
Figure 1.
IL-13–dependent and –independent pulmonary fibrosis. WT, il13−/−, or il13Rα2−/− mice were given either 5,000 S. mansoni eggs i.p. followed by 5,000 S. mansoni eggs i.v. 14 d later, with pulmonary fibrotic granulomas assessed 7 d later (d21; A and B), or an intratracheal delivery of 0.15 U BLM, with pulmonary fibrosis assessed on day 7 (C and D). One of two independent experiments is shown, with five animals per group. (A and C) Pulmonary collagen deposition, expressed as micromoles of hydroxyproline per lung. Data shown are mean ± SEM. (B and D) 5-µm sections of paraffin-embedded lung tissue were stained with Masson’s Trichrome. Images are shown at 10× magnification. Collagen, blue; nuclei, dark red; cytoplasm, red/pink. Bars, 60 µm.
BLM-induced pulmonary fibrosis is characterized by IFN-γ and IL-17A production
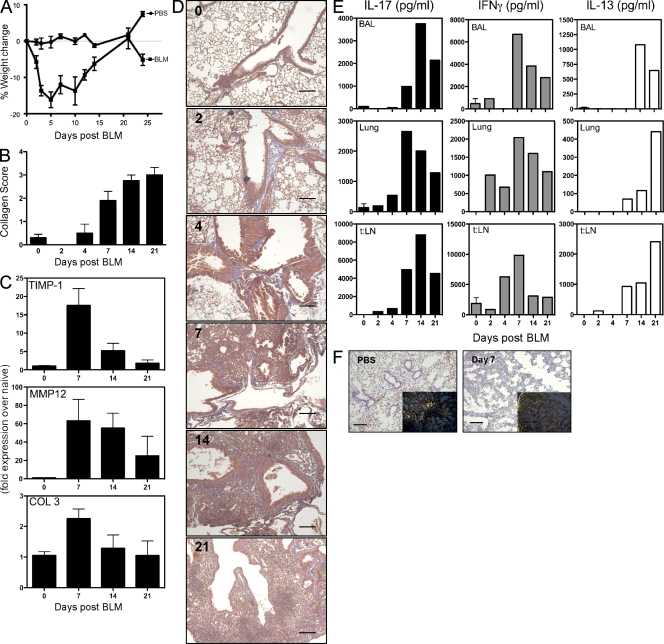
To elucidate the mechanisms involved in acute pulmonary inflammation and fibrosis after BLM administration, we characterized the immune and pathological responses in mice over a 21-d period. Significant weight loss occurred during the first week after intratracheal BLM (Fig. 2 A). Marked increases in collagen deposition were also seen by day 7, with peak pulmonary fibrosis developing between days 14 and 21 (Fig. 2, B, D, and F). Cachexia was associated with significant pneumonia and perivascular and peribronchial inflammation as early as day 4 after BLM and persisting through day 21 (Fig. 2 D). The peak in fibrosis was preceded by marked increases in matrix metalloproteinase (MMP) 12, tissue inhibitor of MMP (TIMP) 1 (Manoury et al., 2006), and COL-3 messenger RNA (mRNA) expression (Fig. 2 C), confirming activation of the collagen-producing machinery by BLM. Expression of IFN-γ, IL-13, and IL-17A was also monitored from restimulated bronchoalveolar lavage (BAL) cells, lung leukocytes, and local draining LN cells (thoracic LN) to determine whether BLM-induced fibrosis was associated with a Th1, Th2, and/or Th17-type response. Marked increases in IFN-γ, followed by IL-17A, were detected between days 2 and 7 after BLM, whereas significant increases in IL-13 were not observed before day 7. In fact, peak IL-13 production appeared to occur quite late when compared with IFN-γ and IL-17A (Fig. 2 E). The kinetics of these three mediators suggested that BLM-induced inflammatory and fibrotic responses are associated with a Th1/Th17 response at early time points and a mixed Th1/Th17/Th2-type pattern at later times. As such, they illustrate that BLM triggers significant collagen deposition before the development of the Th2 response (Fig. 2, B and E), thus contrasting with the S. mansoni egg–induced fibrosis, which is highly dependent on IL-4 and IL-13 (Chiaramonte et al., 1999).
Figure 2.
IL-17A and IFN-γ production during BLM-induced fibrosis. 0.15 U BLM was given to WT mice via intratracheal route, as in Fig. 1, with local immune profiling and assessment of tissue pathology analyzed from days 0 to 21, as indicated. One of three independent experiments is shown, with five animals per group. Data shown are mean ± SEM. (A) Weight loss, assessed at several time points after BLM, as indicated. (B) Pulmonary collagen score from histology sections. (C) RNA was extracted from lung tissue, with timp-1, mmp12, and pro–COL-3 mRNA quantified by quantitative RT-PCR. (D) 5-µm sections of paraffin-embedded lung tissue obtained from mice at the indicated day after BLM and stained with Masson’s Trichrome. Images are shown at a 5× magnification. Bars, 60 µm. (E) BAL, lung, and lung-draining thoracic LN (t:LN) cells were isolated and stimulated with anti-CD3ε for 4 d. IL-17A, IFN-γ, and IL-13 were measured in culture supernatants by ELISA. (F) 5-µm sections of paraffin-embedded lung tissue obtained from mice at day 7 after BLM stained with Giemsa or Picrosirius red (inset) shown under polarized light. Images are shown at 10× magnification. Bars, 60 µm.
IL-10 limits IL-17A/IFN-γ production and pulmonary fibrosis
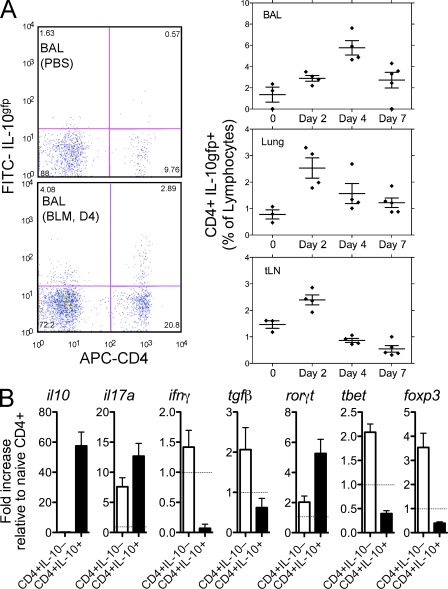
Exogenous IL-10 treatment suppresses BLM-induced fibrosis (Arai et al., 2000; Kitani et al., 2003; Kradin et al., 2004; Nakagome et al., 2006); however, the mechanism of action and role of endogenous IL-10 remains much less clear. Using IL-10gfp reporter mice, we identified the major source and pattern of expression of IL-10 after BLM challenge. IL-10 was detected in CD4+ and CD4− cells; however, the major increase in IL-10 was observed in the CD4+ T cell population with minor increases in CD8+, CD19+, and CD11c+ cells (unpublished data). The percentage of CD4+ lymphocytes producing IL-10 increased within 2 d after administration of BLM and remained elevated in the BAL and lung at all time points (Fig. 3 A). CD4+IL-10+ and CD4+IL-10− cells were FACS sorted from the lung of BLM-treated mice and analyzed for mRNA transcripts. IL-10 gene transcripts were highly up-regulated in CD4+IL-10+ sorted cells, confirming faithful reporter activity (Fig. 3 B). IL-17A mRNA transcripts were also up-regulated in CD4+IL-10+ cells, suggesting coexpression of IL-17A and IL-10, as previously reported (McGeachy et al., 2007; Stumhofer et al., 2007) and similar to IFN-γ and IL-10 coexpressing cells (Anderson et al., 2007; Jankovic et al., 2007; Saraiva et al., 2009). Neither IFN-γ and T-bet nor TGF-β and Foxp3 were coexpressed with IL-10, suggesting that IL-10–producing CD4 cells were not Th1 or Treg cells but, rather, were associated with IL-17A–producing cells.
Figure 3.
IL-10–producing CD4 cells accumulate in the lung. 0.15 U BLM was given to C57BL/6 IL-10gfp mice via intratracheal route, as in Fig. 1. One of two independent experiments shown with five animals per group. Data shown are mean ± SEM. (A) Lung, BAL, or thoracic LN cells were isolated from IL-10gfp reporter mice and stained with anti–mouse CD3 and CD4. Horizontal bars show the mean. (B) CD4+IL-10gfp− and CD4+IL-10gfp+ cells were FACS sorted (>98% pure) from the lung of BLM-treated mice at day 7. RNA was extracted and analyzed for mRNA transcripts. The dotted line refers to naive CD4+ cells.
Germ line deletion of the il10 gene significantly accelerated and increased pulmonary inflammation (Fig. S1, A and B), with the exacerbated response becoming most obvious by day 7. Histological scores of lung sections also revealed marked increases in perivascular and peribronchial inflammation in BLM-treated il10−/− mice, as well as marked increases in pulmonary fibrosis (Fig. S1 B). The increase in pulmonary fibrosis was also verified by quantitative measurements of lung hydroxyproline (Fig. 4 A). Marked increases in soluble collagen was also detected in the BAL of il10−/− mice (Fig. 4 B), which was consistent with the increased levels observed in patients with pulmonary fibrotic diseases (Uebelhoer et al., 1993). These observations are also consistent with severe degradation of the basement membrane, enhanced alveolar permeability, and extensive lung injury in IL-10–deficient animals.
Figure 4.
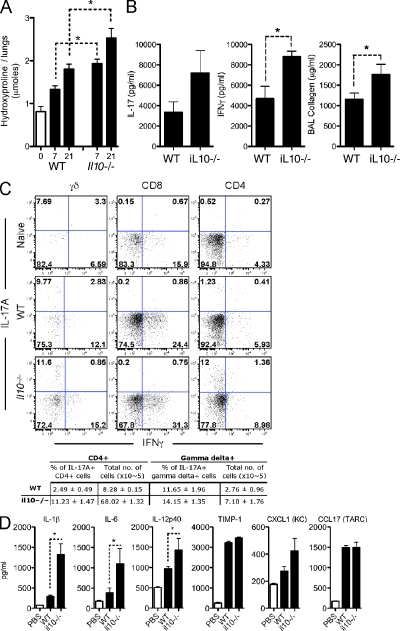
IL-10 restricts IL-17A and IFN-γ and the extent of pulmonary fibrosis. 0.15 U BLM was given to WT, IL-10gfp, or il10−/− mice, via the intratracheal route, as in Fig. 1. *, P < 0.05 using a Mann-Whitney test. One of three independent experiments is shown with five animals per group. Data shown are mean ± SEM. (A) Pulmonary collagen deposition, expressed as micromoles of hydroxyproline per lung. (B) Thoracic LN cells were stimulated with anti-CD3ε, with IL-17A or IFN-γ measured in culture supernatants. BAL collagen was quantified from BAL fluid using Sircol assay. (C) Lung cells, isolated at day 7 after BLM, were stained with anti–mouse, CD4, CD8, B220, γδTCR, NK1.1, IL-17A, and IFN-γ, after a brief stimulation with PMA and ionomycin in the presence of BFA. The percentage of CD4+ or γδ+ cells producing IL-17A, in addition to the total number of cells recovered from the lungs, is enumerated in the table. (D) Homogenized lung supernatant was assayed by ELISA for the indicated cytokines/chemokines.
The exaggerated pathologies observed in il10−/− mice were strongly correlated with increased production of IL-17A and IFN-γ (Fig. 4 B). FACS analysis identified IFN-γ–producing γδTCR+, CD8+, and CD4+ T cells in BLM-challenged mice (Fig. 4 C), as well as CD3+ γδTCR+ IL-17A+ and CD3+ CD4+ IL-17A+ cells in the lungs 7 d after BLM treatment. A 10-fold increase in the percentage of CD4+ IL-17A+ cells was observed in il10−/− mice (Fig. 4 C). In contrast, only a minor increase in the percentage of γδTCR+ IL-17A+ cells was noted. Nevertheless, a significant increase in the total number of both CD4+ and γδTCR+ populations in the lung (8.5-fold increase in CD4+ and 2.5-fold increase in γδTCR+) were observed in BLM-treatment of il10−/− mice, suggesting that IL-10 regulates the development and influx of both CD4+ and γδTCR+ populations.
Analyses of whole lung homogenates revealed significantly elevated levels of several IL-17A–promoting cytokines in BLM treated il10−/− mice. These mediators included IL-6, IL-12/23p40, the neutrophil chemokine KC (CXCL1), and, perhaps most strikingly, IL-1β, which was recently implicated in BLM-induced fibrosis (Gasse et al., 2007) and in amplifying Th17 responses (Sutton et al., 2006, 2009; Fig. 4 D). IL-10 may, therefore, operate to regulate the development, in addition to the recruitment, of CD4+ and γδ+IL-17A+ cells. In vitro Th17 cell differentiation was not directly influenced by rIL-10 or anti–IL-10R treatment (Fig. S2), indicating that IL-10 does not directly influence Th17 cells, which is consistent with a recent study examining the in vitro differentiation of human Th17 cells (Naundorf et al., 2009).
Neutrophilia is a common feature of pulmonary fibrosis (Hunninghake et al., 1981; Kinder et al., 2008), and IL-17A has been shown to be critically involved in the recruitment of neutrophils to sites of inflammation via induction of CXC chemokines (Laan et al., 1999; Witowski et al., 2000; Miyamoto et al., 2003). Consistent with the marked IL-17A response, we detected a significant number of neutrophils in the lungs in BLM-treated mice. Indeed, a substantial increase in neutrophils was observed in both the lung and BAL (not depicted), as well as in the peripheral circulation (Fig. S1 B). The neutrophil response was also exacerbated in il10−/− mice, which was consistent with enhanced IL-17A responses (Fig. 4 B). Collectively, these data indicate that IL-10 limits lung injury, neutrophilia, and pulmonary fibrosis by suppressing proinflammatory mediator release and the production of IL-17A from CD4αβ+ and γδTCR+ cells.
IL-12/23p40 is required for IL-17A production and pulmonary fibrosis
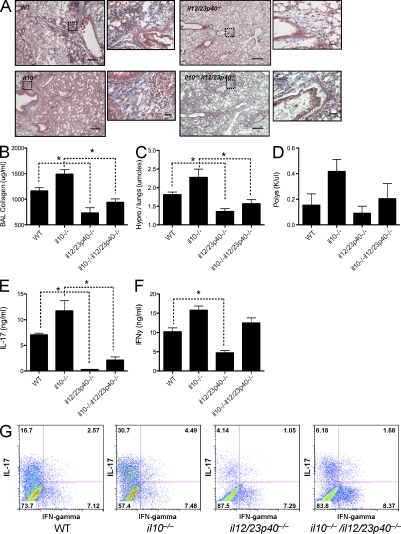
IL-12/23p40 promotes the differentiation and expansion of both IL-17A and IFN-γ–producing lymphocytes. Given that all of these mediators were elevated in WT mice after BLM treatment, and to a greater extent in il10−/− mice, we investigated whether IL-12/23p40 was regulating the development of IL-17A–producing cells and pulmonary fibrosis. Using mice deficient in the p40 subunit of IL-12/23, we determined that IL-12/23p40 is critically involved in orchestrating the lung inflammatory response after BLM administration (Fig. 5 A). The reduction in BLM-induced lung damage in p40−/− mice was characterized by significantly decreased collagen in the BAL (Fig. 5 B) and lung (Fig. 5 C), which is consistent with related studies (Maeyama et al., 2001; Huaux et al., 2002; Sakamoto et al., 2002). Circulating neutrophilia followed a similar pattern (Fig. 5 D). More importantly, however, we show that the exacerbated disease in il10−/− mice was highly dependent on IL-12/23p40 production because il10−/−il12/23p40−/− mice were almost completely protected from BLM-induced fibrosis (Fig. 5, B and C). We also extend previous studies by demonstrating mechanistically that deletion of IL-12/23p40 is associated with reduced IFN-γ production (Fig. 5 F) but markedly reduced expression of IL-17A (Fig. 5 E). Our data, however, suggested a particularly critical role for IL-17A because only IL-17A was significantly reduced in the double il10−/−il12/23p40−/− mice (Fig. 5 F), correlating with markedly reduced disease (Fig. 5, A and B). Similar results were obtained by ELISA (Fig. 5, E and F) and by intracellular cytokine staining (Fig. 5 G). Nevertheless, previous studies identified an important role for IFN-γ in BLM-induced fibrosis (Chen et al., 2001). Consequently, we revisited this work but investigated whether there was a possible mechanistic link between IFN-γ and IL-17A. Interestingly, we discovered that the reduced fibrotic response in ifnγ−/− mice (Fig. S3, C and D) was associated with decreased IL-17A expression (Fig. S3, B and E) and reduced circulating neutrophils (Fig. S3 A). Together, these data suggest that IFN-γ may facilitate an IL-17A response, as suggested in psoriatic lesions (Kryczek et al., 2008). This notion is also supported by an earlier induction of IFN-γ in the lung compared with IL-17A (Fig. 2 E).
Figure 5.
IL-12/23p40 deficiency significantly impacts IL-17A and IFN-γ and curtails pulmonary fibrosis. 0.15 U BLM was given to WT, il10−/−, il12/23p40−/−, or il10−/−il12/23p40−/− mice via the intratracheal route as in Fig. 1. *, P < 0.05 using a Mann-Whitney test. One of two independent experiments is shown, with five animals per group. Data shown are mean ± SEM. (A) 5-µm sections of paraffin-embedded lung tissue taken from WT or il10−/− mice at day 7 or 21 after BLM and stained with Masson’s Trichrome. Images are shown at 5× magnification with dotted squares magnified at 40× in the insets. Bars: 60 µm; (inset) 7 µm. (B) Lung injury, measured as BAL collagen, was quantified from BAL fluid using Sircol assay. (C) Pulmonary collagen deposition, expressed as micromoles of hydroxyproline per lung. (D) Absolute counts of circulating poly morphonuclear cells (PMNs) were obtained from CBC counts. (E and F) Thoracic LN cells were stimulated with anti-CD3ε, with IL-17A (E) and IFN-γ (F) measured in cultures supernatants. (G) Thoracic LN cells were stained with anti–mouse CD4, CD8, B220, γδTCR, NK1.1, IL-17A, and IFN-γ after a brief stimulation with PMA and ionomycin in the presence of BFA. Data shown is gated on CD4+ cells.
IL-17A is required for BLM-induced pulmonary fibrosis
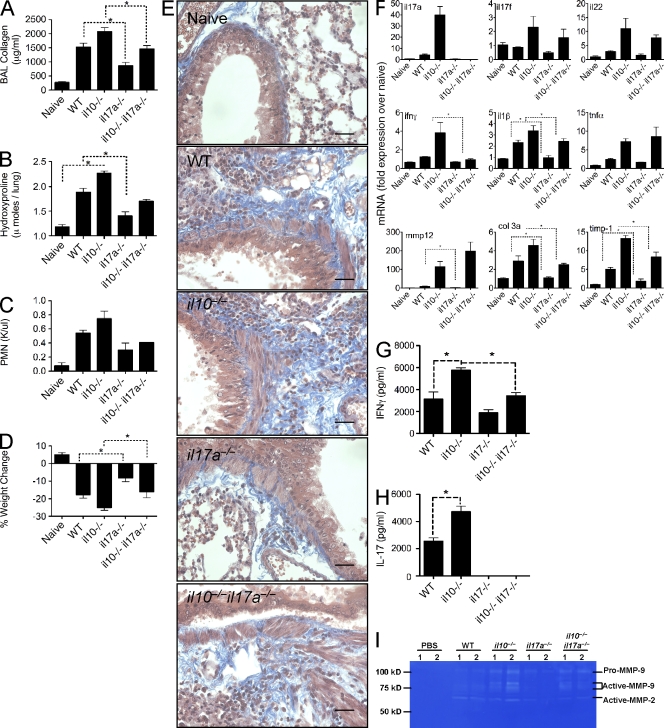
To formally investigate the role of IL-17A in BLM-induced pulmonary fibrosis, WT and il17a−/− mice were challenged with BLM. We also generated il10−/−il17a−/− mice (double KO [dKO]) mice to determine whether the exacerbated lung fibrosis in IL-10–deficient animals was attributed to the enhanced Th17 response. IL-17A deficiency significantly reduced BLM-induced fibrosis in WT mice, as shown by analysis of collagen expression in the BAL (Fig. 6 A) and collagen deposition in the lung (Fig. 6, B and E). The significant increase in IL-17A in il10−/− mice (Fig. 4, B and C; and Fig. 6 H), which correlated with increased fibrosis, was also reduced to WT levels by deleting IL-17A (Fig. 6, A, B, and E). The reduction in fibrosis observed in il17a−/− mice and il10−/−il17a−/− mice also correlated with a similar decrease in circulating neutrophils (Fig. 6 C) and degree of weight loss (Fig. 6 D), further illustrating an important role for IL-17A in BLM-induced pathology and morbidity. The findings with il10−/−il12/23p40−/− mice and il10−/−il17a−/− dKO mice also point to IL-17A, rather than IFN-γ, as the critical downstream mediator of fibrosis because the il10−/−il12/23p40−/− mice developed markedly reduced IL-17A and fibrotic responses yet maintained near normal IFN-γ production. These observations also suggest that IL-10 inhibits the development of BLM-induced fibrosis by targeting the proinflammatory IL-23–IL-17A pathway rather than the IL-12–IFN-γ axis.
Figure 6.
Attenuated pulmonary fibrosis in il17a−/− mice. 0.15 U BLM was given to WT, il10−/−, il17a−/−, or il10−/−il17a−/− mice, via the intratracheal route, as in Fig. 1. *, P < 0.05 using a Mann-Whitney test. One of two independent experiments is shown, with five animals per group. Data shown are mean ± SEM. (A) Lung injury, measured as BAL collagen, was quantified from BAL fluid using Sircol assay. (B) Pulmonary collagen deposition, expressed as micromoles of hydroxyproline per lung. (C) Absolute counts of circulating polymorphonuclear cells were obtained from CBC counts. (D) Percentage of weight change, 7 d after BLM. (E) 5-µm sections of paraffin-embedded lung tissue taken from WT or il10−/− mice at day 7 or 21 after BLM and stained with Masson’s Trichrome. Images are shown at a 40× magnification. Bar, 7 µm. (F) RNA was extracted from lung tissue, with indicated mRNA quantified by quantitative RT-PCR. (G and H) Thoracic LN cells were stimulated with anti-CD3ε, with IL-17A (G) and IFN-γ (H) measured in culture supernatants. (I) MMP2 and MMP9 bioactivity measured in BAL by zymography.
Il17f, il22, and tnfα were elevated in the lungs of BLM-treated il10−/− mice and remained elevated in il10−/−il17a−/− mice, even though the dKO animals developed much less fibrosis (Fig. 6 F). Consequently, it seems unlikely that these Th17-associated mediators are contributing to the exaggerated fibrotic phenotype in il10−/− mice. Interestingly, ifnγ mRNA (Fig. 6 F) and protein expression (Fig. 6 G) were significantly reduced in il10−/−il17a−/− mice compared with il10−/− mice, suggesting that IL-17A may facilitate the recruitment of IFN-γ–producing cells via lymphocyte-recruiting chemokines (Zrioual et al., 2008), cell survival (Sergejeva et al., 2005), or direct induction of IL-12 and IFN-γ by macrophages (Lin et al., 2009). It is interesting to note that IL-17A and IFN-γ synergistically promote chemokine responses in a variety of inflamed tissues (Albanesi et al., 1999; Andoh et al., 2001; Eid et al., 2009), suggesting a coordinated response between IL-17A and IFN-γ.
In contrast to il17f, il22, and tnfα, we observed a strong correlation between il1β expression and the degree of fibrosis in il10−/− and il10−/−il17a−/− mice. These observations were revealing because IL-1β was recently implicated in BLM-induced fibrosis in both humans and mice (Gasse et al., 2007; Ortiz et al., 2007; Hoshino et al., 2009). il1β expression was also significantly reduced in il17a−/− mice compared with WT animals, suggesting that either IL-17A or IL-17A–dependent responses promotes IL-1β production. IL-1β can indeed promote IL-17A responses (Sutton et al., 2009); however, whether IL-17A directly or indirectly feeds back to enhance IL-1β has not been reported. Expression of several ECM-associated genes, including col3, timp1, and mmp12, was also reduced in il17a−/− mice (Fig. 6 F). Furthermore, using zymography we observed reduced MMP-2 and MMP-9 activity in BAL fluid of il17a−/− mice compared with WT and in il10−/−il17a−/− mice compared with il10−/− mice (Fig. 6 I). Collectively, these data clearly identify IL-17A as a dominant and critical mediator in the pathogenesis of BLM-induced pulmonary fibrosis.
IL-1β–driven fibrosis is dependent on IL-17A
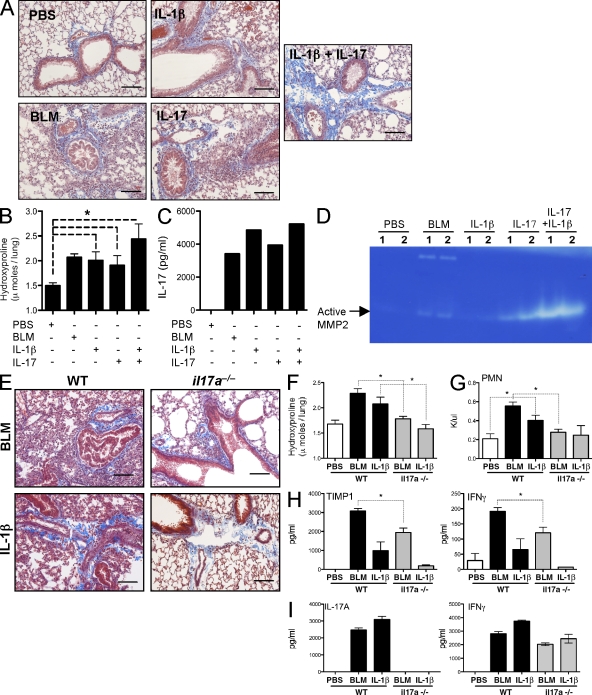
IL-1β mRNA expression (Fig. 6 F) and cytokine production (Fig. 4 D) correlated with the Th17 response and degree of fibrosis after BLM administration. Several recent studies have also identified an important role for IL-1β in the development of BLM-induced fibrosis. Indeed, administration of IL-1β alone mimics much of the pulmonary pathology caused by BLM (Wang et al., 2000; Gasse et al., 2007; Ortiz et al., 2007; Hoshino et al., 2009). We confirmed and extended these observations by showing that intratracheal administration of IL-1β induces a marked IL-17A response (Fig. 7 C), as well as significant pulmonary fibrosis (Fig. 7, A and B). The administration of 0.5 µg each of both IL-1β and IL-17A had a mild additive effect with increased collagen deposition and, most strikingly, increased MMP2 bioactivity (Fig. 7 D) compared with IL-1β, IL-17A, or BLM treatment alone, suggesting increased turnover of the ECM by IL-1β and IL-17A.
Figure 7.
IL-17A–dependent IL-1β–induced collagen deposition. Mice were given intratracheal 0.15 U BLM, 1 µg IL-1β, 1 µg IL-17A, or 0.5 µg each of both IL-1β and IL-17A with pulmonary collagen deposition assessed on day 7. *, P < 0.05 using a Mann-Whitney test. One of two independent experiments is shown, with five animals per group. Data shown are mean ± SEM. (A and E) 5-µm sections of paraffin-embedded lung tissue were stained with Masson’s Trichrome. Images are shown at a 20× magnification. Bars, 20 µm. (B and F) Pulmonary collagen deposition, expressed as micromoles of hydroxyproline per lung. (C and I) Thoracic LN cells were stimulated with anti-CD3ε, with IL-17A and IFN-γ measured in culture supernatants. (D) MMP2 bioactivity in BAL fluid measured by zymography. (G) Absolute counts of circulating polymorphonuclear cells were obtained from CBC counts. (H) BAL fluid TIMP-1 and IFN-γ was measured by ELISA.
To test the requirement of IL-17A on IL-1β–induced fibrosis, we administered IL-1β to either WT or il17a−/− mice. As observed earlier, BLM-induced fibrosis was significantly reduced in il17a−/− mice (Fig. 6), and, furthermore, IL-1β–mediated inflammation and fibrosis was also significantly reduced, almost to background levels, in the absence of IL-17A (Fig. 7, E and F). As observed with BLM, intratracheal IL-1β induced significant neutrophilia (Fig. 7 G), TIMP-1 in the BAL (Fig. 7 H), and IFN-γ and IL-17A production in the local LNs (Fig. 7 I). IFN-γ was only slightly elevated in the BAL after IL-1β treatment (Fig. 7 I). These mediators were all reduced in IL-1β–treated il17a−/− mice, indicating that IL-1β–induced pulmonary disease, like BLM, is dependent on IL-17A for airway inflammation and pulmonary fibrosis.
IL-17A–dependent pulmonary fibrosis requires TGF-β
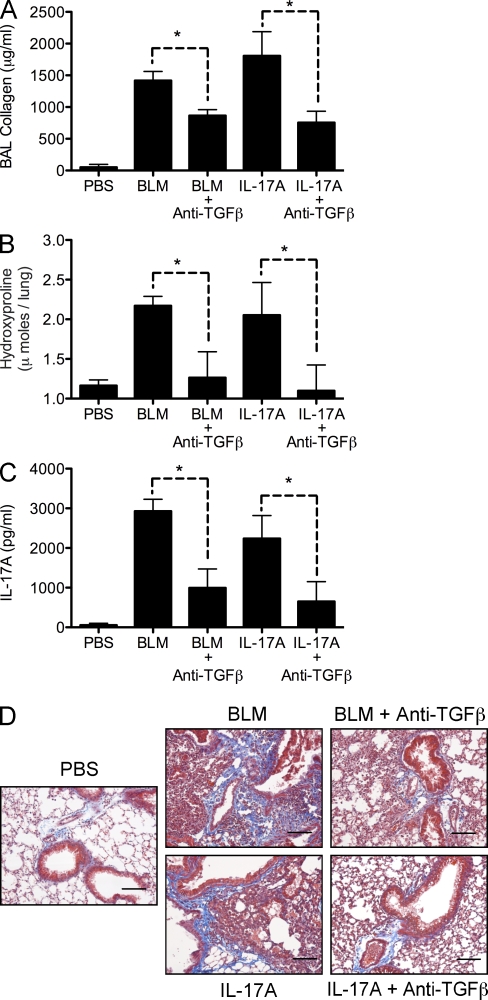
Many studies have identified a profibrotic role for TGF-β, particularly in the pathogenesis of pulmonary fibrosis (Cutroneo et al., 2007). More recently, studies have also suggested important inflammatory activities for TGF-β, with TGF-β providing an integral differentiation signal for the development of proinflammatory IL-17A–secreting cells (Bettelli et al., 2006; Veldhoen et al., 2006). We therefore tested whether the profibrotic properties of TGF-β were linked to IL-17A. Intratracheal delivery of BLM or rIL-17A induced significant collagen release in the BAL (Fig. 8 A) and deposition in the lung (Fig. 8, B and D). TGF-β blockade significantly reduced BLM and IL-17A–induced fibrosis (Fig. 8, B and D), identifying a critical requirement for TGF-β in both BLM and IL-17A–induced fibrosis. rIL-17A and BLM both increased IL-17A responses in the local LNs (Fig. 8 C), and TGF-β blockade curtailed endogenous IL-17A production, indicating that TGF-β is required for the genesis of endogenous IL-17A. Together, these findings suggest cooperative and amplifying roles TGF-β and IL-17A in the development of fibrosis.
Figure 8.
IL-17A–dependent fibrosis requires TGF-β. Mice were given 0.15 U of intratracheal BLM or 1 µg IL-17A with or without 500 µg of anti–TGF-β treatment on days −1, 3, and 5. Pulmonary collagen deposition was assessed on day 7. *, P < 0.05 using a Mann-Whitney test. One of two independent experiments is shown, with five animals per group. Data shown are mean ± SEM. (A) Lung injury, measured as BAL collagen, was quantified from BAL fluid using Sircol assay. (B) Pulmonary collagen deposition, expressed as micromoles of hydroxyproline per lung. (C) Thoracic LN cells were stimulated with anti-CD3ε, with IL-17A and IFN-γ measured in culture supernatants. (D) 5-µm sections of paraffin-embedded lung tissue were stained with Masson’s Trichrome. Images are shown at a 20× magnification. Bars, 20 µm.
IL-13–dependent pulmonary fibrosis is IL-17A independent
To determine whether IL-17A was functioning as a master regulator of fibrosis, we also exposed il17a−/−and il10−/−il17a−/− mice to another model of pulmonary fibrosis. Previous studies have shown that S. mansoni egg–induced pulmonary fibrosis is highly dependent on IL-13 signaling (Ramalingam et al., 2008). However, IL-17A has been implicated in IL-13–associated airway hyperreactivity (Nakae et al., 2002). The possible connection between IL-17A expression and Th2-dependent fibrosis, however, has not been previously investigated (Wynn, 2008). We therefore assessed the impact of IL-17A on IL-13–dependant pulmonary fibrosis using the S. mansoni egg–induced pulmonary granuloma model. CD4+IL-17A+ (1.1%), CD4+IFN-γ+ (13.1%), and CD4+IL-13+ (22.5%) lymphocytes were observed in WT mice 7 d after i.v. delivery of S.mansoni eggs to the lungs (Fig. 4 A), suggesting a potential role for IL-17A in the development of fibrosis in this model. Deletion of il10 resulted in an approximate doubling of IL-17A–producing cells (1.1–2.5%) and a modest increase in IFN-γ–producing cells (13.1–16.3%). Similarly, when whole lung tissue was analyzed by quantitative RT-PCR, significant increases in il17a and ifnγ mRNAs were observed, with only modest changes in il13 (Fig. S4, A and E). To test the importance of IL-17A in this model, WT, il17a−/−, il10−/−, and il10−/−il17a−/− mice were challenged i.v. with S. mansoni eggs. In marked contrast to the BLM model (Fig. 6), BAL collagen (Fig. S4 B), hydroxyproline measurements (Fig. S4 C), and microscopic assessments of lung fibrosis (Fig. S4, D and G) revealed little to no role for IL-17A. Therefore, although IL-17A was significantly up-regulated and increased further in the absence of IL-10 (Fig. S4, A and E), IL-13 appears to function as the dominant profibrotic mediator in this model. Production of IL-13 was not significantly affected by the presence or absence of IL-17A, suggesting no obvious regulatory role for IL-17A (Fig. S4, E and F). In fact little modulation of the ECM-related genes (mmp12, timp1, and col6a) was observed in the absence of IL-17A (Fig. S4 E). Moreover, although there was a modest increase in IFN-γ in the IL-10–deficient groups, it had no significant impact on the development of IL-13–dependent fibrosis, perhaps because IL-13 was up-regulated ∼20-fold more than IFN-γ. Thus, in marked contrast to the BLM model, where IL-17A and, to a lesser extent, IFN-γ appeared to play critical roles in the development of fibrosis, pulmonary fibrosis induced by schistosome eggs appeared to be dependent on Th2 cytokines. Thus, unique cytokine-dependent mechanisms can be exploited to induce pulmonary fibrosis, with IL-17A playing a critical role in BLM-induced fibrosis.
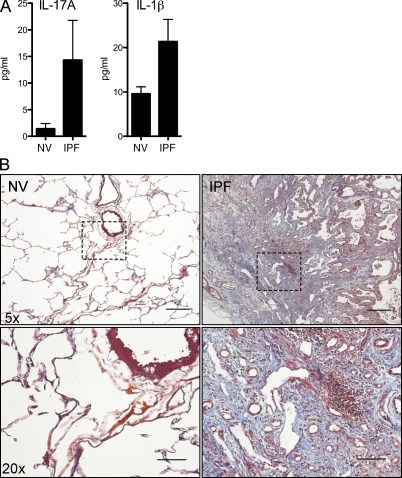
Increased IL-1β and IL-17A in BAL fluid of IPF patients
To determine whether IL-1β and IL-17A are involved in human IPF, we obtained lung biopsies and BAL fluid from normal volunteers (NVs) and IPF patients. Masson’s trichrome–stained lung sections revealed inflammatory foci within the parenchyma, surrounded by dense collagen deposits (Fig. 9 B). Similar to the mouse model, IL-17A (NV, 1.48 ± 0.98 pg/ml; IPF, 14.31 ± 3.76 pg/ml) and IL-1β (NV, 9.58 ± 1.58 pg/ml; IPF, 21.36 ± 3.97 pg/ml), but not IL-22 (NV, 56.1 ± 2.00 pg/ml; IPF, 50.7 ± 1.47 pg/ml; Whittington et al., 2004), in BAL fluid of IPF patients were increased (Fig. 9 A), indicting that an IL-1β–IL-17A pathway may be involved in the development of human IPF.
Figure 9.
Elevated IL-17A and IL-1β in human IPF patient BAL fluid. BAL fluid and lung biopsies were collected from NVs and IPF patients. (A) BAL fluid was assayed for IL-17A and IL-1β by ELISA. (B) 5-µm lung sections were cut from paraffin-embedded lung biopsies and stained with Masson’s trichrome. Data shown are mean ± SEM. Images are shown at 5× (top) and dashed rectangles are magnified at 20× (bottom). Bars: (top) 60 µm; (bottom) 20 µm.
DISCUSSION
We have demonstrated that BLM-induced, but not S. mansoni egg–induced, pulmonary fibrosis is dependent on IL-17A produced by γδ+ and CD4+αβ+ T cells. In addition, we show that IL-17A is required for the development of inflammation, neutrophilia, and pulmonary fibrosis after exposure to IL-1β, a recently described initiator of fibrosis (Wang et al., 2000; Ortiz et al., 2007; Gasse et al., 2007; Cassel et al., 2008; Hoshino et al., 2009). Mechanistically, using il10−/−, il10−/−il17a−/−, and il10−/−il12p40−/− dKO mice and TGF-β blockade, we also show that fibrosis is tightly controlled by IL-10–producing CD4+ lymphocytes, which regulate IL-17A production. Together, these studies identify IL-17A as a critical mediator of pulmonary fibrosis after BLM administration, illustrating the potential utility of targeting IL-17A in the treatment of IPF and other fibrotic diseases.
Previous studies have identified important roles for TGF-β1 and IL-13 in the development of fibrosis in a variety of tissues (Chiaramonte et al., 1999; Lee et al., 2001; Varga and Pasche, 2008), whereas a role for IL-17A has remained unclear. Some studies found that IL-13 induces and activates TGF-β1 (Lee et al., 2001), which then serves as the primary mediator of fibrosis by stimulating collagen synthesis in fibroblasts (Czaja et al., 1989). Nevertheless, other studies demonstrated that IL-13 could function independently of TGF-β1 (Kaviratne et al., 2004). To better characterize both the unique and convergent pathways of fibrosis, we dissected the mechanisms of pulmonary fibrosis in two distinct models in which TGF-β1 and IL-13 have been shown to function as critical mediators. Unexpectedly, although small interfering RNA gene silencing studies implicated IL-13 in the mechanism of BLM-induced pulmonary fibrosis (Fichtner-Feigl et al., 2006), our studies conducted with C57BL/6 il13−/− and il13Rα2−/− mice failed to reveal a significant role for IL-13 signaling at this early stage of fibrosis in this model. In contrast, S. mansoni egg–induced pulmonary fibrosis was completely IL-13 dependent. Together, these studies indicate that pulmonary remodeling and fibrosis can be induced by distinct nonoverlapping mechanisms, with IL-13 functioning as the key driver of S. mansoni egg–driven fibrosis (Kaviratne et al., 2004) and IL-17A serving as a key mediator of BLM-induced fibrosis. The discovery that distinct immunological mechanisms can regulate fibrosis suggests that the etiology and/or route of the initial insult, as well as the character of the subsequent inflammatory response, may have a significant bearing on the nature of the lung wound healing response. The distinct route of tissue damage observed in both models may contribute to the unique fibrotic mechanisms used. Indeed, S. mansoni eggs delivered i.v. primarily disrupt endothelial cells lining the pulmonary vasculature, whereas BLM administered intratracheally is directly toxic to epithelial cells lining the airway. S. mansoni eggs are also highly immunogenic, inducing robust antigen-specific CD4+ Th2 responses (Everts et al., 2009; Steinfelder et al., 2009), whereas BLM induces rapid epithelial cell death and destruction of DNA (Hay et al., 1991), leading to acute proinflammatory Th1/Th17-type cytokine production.
Most studies of BLM-induced fibrosis have focused on the hypothesis that TGF-β regulates the development of pulmonary fibrosis by directly promoting the differentiation and activation of collagen-producing myofibroblasts (Varga and Pasche, 2008). However, recent studies demonstrated that TGF-β is also critically involved in the development of IL-17A–producing lymphocytes (Bettelli et al., 2006; Veldhoen et al., 2006), which regulate the development of a variety of inflammatory and autoimmune diseases (Tesmer et al., 2008). Using il17a−/− mice, we show that IL-17A is essential for the development of BLM-induced fibrosis. Similarly, blockade of TGF-β also significantly reduces BLM and IL-17A–induced fibrosis and correlates with reduced IL-17A production. These findings suggest that the profibrotic activity of TGF-β may, at least in part, be attributed to the induction of IL-17A. Therefore, by stimulating myofibroblast activation and the production of IL-17A by T cells, TGF-β likely promotes BLM-induced fibrosis through both direct and indirect mechanisms. In contrast, development of S. mansoni egg–induced pulmonary fibrosis was completely IL-17A independent. Therefore, IL-17A appears to induce fibrosis in models where TGF-β, but not IL-13, has been shown to play critical roles. IL-17A can directly induce the collagenase MMP-1 (Cortez et al., 2007) and progelatinase, MMP-3 (Beklen et al., 2007) from various fibroblasts, suggesting that IL-17A may facilitate tissue disruption, in addition to its ability to promote granulopoiesis (Schwarzenberger et al., 1998) and inflammation. To this end, many studies have identified the proinflammatory properties of IL-17A. In the context of inflammation and pulmonary fibrosis, IL-17A can stimulate IL-6, IL-8, and MCP-1 from bronchial epithelial cells (Laan et al., 2001) or fibroblasts (Hata et al., 2002; Mahanonda et al., 2008) and, as mentioned in the previous paragraph, is inducible by IL-1β and IL-23, placing IL-17A in a central position, magnifying the inflammatory cascade. One previous study suggested that IL-17A producing γδ+ T cells regulate cellular recruitment with γδ+ T cell–deficient mice developing increased inflammation and collagen deposition (Braun et al., 2008). These data suggest that γδ+ T cells may be heterogeneous and responsible for more than IL-17A, with populations of γδ+ T cells providing a brake on the inflammatory response in addition to the early IL-17A production. A similar scenario could be envisioned for CD4+ T cells, providing both proinflammatory and fibrotic IL-17A in addition to antiinflammatory IL-10.
Several studies have identified an important regulatory role for IL-10 in BLM-induced pulmonary fibrosis (Kradin et al., 2004; Nakagome et al., 2006). These studies suggested that IL-10 controls BLM-induced inflammatory and fibrotic response by regulating chemokine and TGF-β1 production (Kradin et al., 2004; Nakagome et al., 2006). However, we also found a marked increase in IL-17A in il10−/− mice, which develop markedly exacerbated inflammation, neutrophil mobilization, and pulmonary fibrosis after BLM administration. These observations are consistent with studies that suggested IL-10 could regulate the magnitude and effector function of Th17 responses (Fitzgerald et al., 2007; McGeachy et al., 2007; Chang et al., 2008). Similar to previous studies (McGeachy et al., 2007; Stumhofer et al., 2007), IL-10 was often coexpressed with IL-17A and RoRγt, suggesting that Th17 cells may develop into IL-10–secreting cells to limit Th17 effector function.
To determine whether there was a functional connection between IL-10 and IL-17A, we generated il10−/−il17a−/− dKO mice and then challenged the animals with BLM. Strikingly, the increase in BLM-induced pulmonary fibrosis observed in il10−/− mice was completely reversed in the absence of IL-17A. As such, these studies explain the severe inflammatory response observed in il10−/− mice (Kradin et al., 2004). They also identify IL-17A, which is produced predominantly by CD4+ T cells, as a critical target of IL-10. Gene transcripts for the IL-17A–related molecules IL-17F and IL-22 were also increased in the absence of IL-10, correlating with exaggerated inflammation and fibrosis (Fig. 6). However, these molecules were not largely affected in il17a−/−il10−/− mice despite a significant reduction in pulmonary fibrosis, suggesting that IL-17A, but not IL-17F or IL-22, functions as critical mediators of pulmonary inflammation and fibrosis at this stage. Furthermore, IL-22 levels were not significantly different between control and pulmonary fibrosis patients (Whittington et al., 2004). A comprehensive study by Ishigame et al. (2009) highlighted the overlapping but also distinct roles of IL-17A and IL-17F. For example, IL-17A, but not IL-17F, was required for DTH, autoimmune encephalomyelitis, and collagen-induced arthritis. However, IL-17A and IL-17F were both required for host defense against Staphylococcus aureus and Citrobacter rodentium. Functionally, IL-17F and IL-17A also appear to have opposing roles in the allergic lung, with IL-17F negatively regulating and IL-17A positively regulating Th2-mediated inflammation (Yang et al., 2008). From our data, it appears that IL-17A, and not IL-17F or IL-22, is the dominant molecule involved in BLM and IL-1β–mediated pulmonary inflammation and fibrosis.
Previous studies identified a role for IFN-γ in BLM-induced fibrosis (Chen et al., 2001), which was consistent with our findings. Surprisingly, we found that IFN-γ deficiency was associated with reduced IL-17A production, suggesting a link between IFN-γ and IL-17A. BLM studies performed with il12p40−/− and il10−/−il12p40−/− dKO mice confirmed this association; however, they indicated that IL-17A was functioning as the dominant inducer of fibrosis. Indeed, reduced BLM-induced fibrosis in il12/23p40−/− mice was associated with a much greater decrease in IL-17A than IFN-γ. A similar pattern was observed in BLM-treated il10−/−il12p40−/− dKO mice. il17a−/− and il10−/−il17a−/− dKO mice also developed relatively normal IFN-γ responses, despite significantly reduced fibrosis. IFN-γ responses in the lungs precede IL-17A responses (Fig. 2 E), suggesting that the IL-12–IFN-γ axis may be involved in the recruitment of IL-23–dependent IL-17A– producing cells into the lung, as previously reported (Kryczek et al., 2008). Nevertheless, these findings strongly suggest that the IL-23–IL-17A axis, rather than the IL-12–IFN-γ pathway, plays the dominant role in BLM-induced fibrosis, although both mechanisms are clearly involved and connected.
In addition to TGF-β1 and IL-23 (Langrish et al., 2005), recent studies identified an important role for IL-1β in Th17 responses (Sutton et al., 2006). Interestingly, a recent study showed that IL-1R signaling is involved in BLM-induced pulmonary fibrosis, with exogenous IL-1β treatment recapitulating much of the lung pathology seen with BLM (Gasse et al., 2007). We confirmed and extended these observations by showing that IL-1β–mediated inflammation and fibrosis is dependent on IL-17A. These findings suggest that IL-17A functions as a critical downstream mediator of fibrosis. Interestingly, the reduced inflammation and fibrosis observed in BLM-treated il17a−/− animals was associated with reduced IL-1β expression, suggesting that IL-17A promotes IL-1β production, as has been observed in vitro with cultured fibroblasts (Beklen et al., 2007). These data suggest that IL-17A and IL-1β cross-regulate each other, thus providing an explanation for their additive roles in BLM-induced fibrosis.
BLM-induced fibrosis is associated with significant neutrophil recruitment to the lung. Indeed neutrophilia is a common feature of fibrosis and pulmonary alveolitis in IPF patients (Hunninghake et al., 1981; Kinder et al., 2008), with airway neutrophilia identified as a predictor of early mortality in IPF (Kinder et al., 2008). Interestingly, it is now well appreciated that IL-17A is involved in the recruitment of neutrophils to sites of inflammation via induction of IL-8 and CXC chemokines, including MIP-2 (Laan et al., 1999; Witowski et al., 2000; Miyamoto et al., 2003; Lindén et al., 2005). Nevertheless, a direct link between IL-17A, neutrophilia, and IPF has not been previously investigated. In our study, we observed a strong correlation between IL-17A and neutrophil recruitment, with il17a−/− and il10−/−il17a−/− mice displaying significantly fewer neutrophils in the lung than their respective control groups. Finally, IL-17A and IL-1β were also detected in the BAL fluid of IPF patients. When viewed together, these observations make a compelling case for IL-17A in the pathogenesis of pulmonary inflammation and fibrosis.
In conclusion, genetic deletion of IL-17A significantly attenuated lung inflammation, neutrophilia, and fibrosis induced by BLM treatment in both WT and IL-10–deficient mice. In contrast, IL-17A appeared to play little to no role in the development of IL-13–dependent fibrosis. As such, these data identify distinct nonoverlapping immunological roles for IL-13 and IL-17A in the development of fibrosis. They also suggest that the IL-17A pathway might provide a novel therapeutic target for the treatment of pulmonary fibrosis, for which few therapeutic options currently exist.
MATERIALS AND METHODS
Animals.
Female 6–10-wk-old C57BL/6 (WT), il10−/−, il12/23p40−/−, ifnγ−/−, il13−/−, il13Rα2−/−, il10−/−il12/23p40−/−, and OVA-specific OT-II [C57BL/6-Tg(TCR-α TCR-β)] (all C57BL/6 background for ≥10 generations) were obtained from National Institute of Allergy and Infectious Disease (NIAID) animal facilities at Taconic. IL-10gfp reporter mice were provided by R. Flavell (Yale University, New Haven, CT; Kamanaka et al., 2006). il17a−/− animals were generated and provided by Regeneron (Leppkes et al., 2009). il10−/−il17a−/− animals were generated by crossing il10−/− with il17a−/−, with gene expression and protein production confirmed by PCR and ELISA. All animals were housed under specific pathogen-free conditions at the National Institutes of Health in an American Association for the Accreditation of Laboratory Animal Care–approved facility. The NIAID animal care and use committee approved all experimental procedures. A minimum of five mice per group were used in each experiment, unless indicated.
Human tissues.
Paraffin-embedded lung sections and BAL fluid were obtained from 14 NVs and 7 patients diagnosed with IPF. 5-µm lung sections were stained with Masson’s trichrome as described in Histopathology and fibrosis. Subjects were enrolled in protocols 99-HG-0056 and/or 04-HG-0211, which were approved by the National Human Genome Research Institute Institutional Review Board. Written informed consent was obtained from all subjects. Bronchoscopy with BAL was performed as previously described (Ren et al., 2007).
Reagents and cell culture.
For in vitro cell culture, LN cells were isolated, washed, and plated at 5 × 105 cells per well of a 96-well plate and stimulated with 1 µg/ml of anti-CD3ε antibody (clone 2C11; eBioscience). For in vitro Th17 differentiation, OVA peptide323-328 (New England Peptide) was used at the indicated concentration to differentiate FACS-purified naive CD4+CD62LhiCD44lo T cells from OT2 OVA Tg mice into Th17 cells with 20 ng/ml rIL-6 (R&D Systems), 5 ng/ml recombinant human TGF-β (R&D Systems), 10 µg/ml of anti–IL-4 (11D11), and 10 µg/ml of anti–IFN-γ (XMG1.1), in combination with irradiated splenocytes or 1 µg/ml of anti-CD3ε (eBioscience) and 10 µg/ml of anti-CD28 (Invitrogen). rIL-10 (R&D Systems) or anti–IL-10RAb (BioXell; clone 1B1.3a) were added to cultures at the indicated concentrations. Anti–TGF-β (BioXell; clone 1D11) was used at 0.5 mg/mouse on days −1, 3, and 5.
Complete blood count (CBC) analysis.
EDTA-treated blood was processed for automated counting using a Vista Analyzer (Siemens).
Pulmonary fibrosis models.
For Schistosoma mansoni egg–induced pulmonary fibrosis, mice were immunized with 5,000 S. mansoni eggs by i.p. injection. Tail vein injection of 5,000 eggs was given on day 14, with analysis of fibrotic granuloma development 7 d later on day 21. For BLM-induced pulmonary fibrosis, mice were anaesthetized with a xylazine and ketamine cocktail and given 0.15 U BLM sulfate (EMD) in saline via the intratracheal route. Weight change, pulmonary inflammation, and fibrosis were assessed daily or 7 d later, as designated in figure legends. 1 µg IL-1β (R&D Systems) or 1 µg IL-17A (R&D Systems) was administered by the intratracheal route to anesthetized mice in the same way as BLM.
BAL fluid and differential cell counts.
Mice were terminally anaesthetized with sodium pentobarbital. The trachea was cannulated and pulmonary airspaces lavaged with an initial 500 µl of sterile PBS, followed by two 500-µl PBS washes. Fluids were centrifuged at 1,200 g, and pellets recovered from all three lavage washes were pooled into 1 ml PBS for total BAL cell counts and cellular analysis. The supernatants of the initial 500 µl BAL fluid were stored at −80°C for biochemical analyses. BAL collagen was measured using a Sircol assay, according to the manufacturer’s recommendations.
ELISA.
Cytokines, chemokines, and TIMP-1 were measured by ELISA using Immulon 2HB plates (Thermo Fisher Scientific) and the manufacturer’s guidelines. Paired capture and detection antibodies (R&D Systems) for IL-17A (human and mouse), IFN-γ, IL-13, IL-10, TIMP-1, CXCL1 (KC), IL-6, IL-12/23p40, and IL-1β (human and mouse), and CCL17 (TARC) and IL-22 (human) were used. Plates were washed with 0.05% Tween 20 in PBS (PBST) and blocked with 5% milk in PBST. Recombinant cytokine standards (R&D Systems) were used to assess quantity using a standard curve, with OD acquired at 405 nm in an ELISA reader.
Flow cytometry.
After a 3-h incubation with 10 µg/ml PMA,1 µg/ml ionomycin, and 10 µg/ml brefeldin A (BFA), cells were stained with antibodies diluted in PBS with 0.5% BSA (Sigma-Aldrich) and 0.05% sodium azide (Sigma-Aldrich) for 20 min at 4°C. Surface molecule staining (CD4 [BD], CD8 [eBioscience], CD44 [BioLegend], B220 [BD], CD19 [BD], and γδ [BD]), followed by fixation and permeabilization (Cytofix/Cytoperm; BD) and intracellular cytokine staining (IL-17A [BD], IFN-γ [BD], IL-10 [BD], IL-13 [Contocor], and IL-22 [provided by Wyeth; Ma et al., 2008]), was performed on freshly isolated cells. IL-10gfp+ cells were detected ex vivo, without stimulation, and were only stained for surface molecules. The expression of surface molecules and intracellular cytokines was analyzed on a flow cytometer (LSR II; BD) using FlowJo software (v.8; Tree Star, Inc.).
Histopathology and fibrosis.
Pulmonary collagen was measured as hydroxyproline after hydrolysis of a known weight of lung tissue in 5 ml of 6 N HCl at 110°C for 18 h. The increase in pulmonary hydroxyproline per mouse was calculated based upon total lung weight and expressed as micromoles in the lungs. For histopathological analyses, lungs were inflated with 4% phosphate-buffered formalin and embedded in paraffin for sectioning (Histo-Path of America, Inc.). Wright’s Giemsa or Masson’s trichrome (collagen, blue; nuclei, dark red; cytoplasm, red/pink) stains were used for analysis of airway inflammation and pathological changes. Perivascular and peribronchial inflammation and collagen evaluations were scored by a blinded observer on an arbitrary 1–4+ basis. The same individual scored all histological features and had no knowledge of the experimental design.
RNA isolation, purification, and quantitative real-time PCR.
Total RNA was extracted from lung tissue samples in 1 ml TRIZOL reagent (Invitrogen). The sample was homogenized using a tissue polytron (Omni International), and total RNA was extracted according to the recommendations of the manufacturer and further purified using the RNeasy Mini kit (QIAGEN). RNA was reverse transcribed using Superscript II Reverse transcription (Invitrogen). Real-time RT-PCR was performed on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Relative quantities of mRNA for several genes was determined using SYBR Green PCR Master Mix (Applied Biosystems) and by the comparative threshold cycle method, as described by the manufacturer, for the ABI Prism 7700/7900HT Sequence Detection System. mRNA levels for each sample were normalized to hypoxanthine guanine phosphoribosyl transferase. Primers were either adopted from previously published primer sequences (col6a, mmp12, col3, il13 [Wilson et al., 2007], ifnγ [Pesce et al., 2006], and tnfα [Ramalingam et al., 2008]) or designed using Primer Express software (version 2.0; Applied Biosystems; timp1 sense, 5′-GCAAAGAGCTTTCTCAAAGACC-3′, and anti-sense, 5′-AGGGATAGATAAACAGGGAAACACT -3′; il17a sense, 5′-TGTGAAGGTCAACCTCAAAGTC-3′, and anti-sense, 5′-AGGGATATCTATCAGGGTCTTCATT-3′; il17f sense, 5′-TGCTACTGTTGATGTTGGGAC-3′, and anti-sense, 5′-AATGCCCTGGTTTTGGTTGAA-3′; il22 sense, 5′-GTGAGAAGCTAACGTCCATC-3′, and anti-sense, 5′-GTCTACCTCTGGTCTCATGG-3′; and il1β sense, 5′-AAGGAGAACCAAGCAACGACAAAA-3′, and anti-sense, 5′-TGGGGAACTCTGCAGACTCAAACT-3′).
Zymography.
15 µl BAL fluid was mixed with an equal volume of Tris-Glycine SDS sample buffer (Invitrogen) and subjected to electrophoresis with 10% gelatin zymogram gels (Invitrogen). According to manufacturer’s instructions, the gels were renatured, incubated in a developing buffer for 24 h at 37°C, and stained with Simply Blue Safe Stain (Invitrogen). Pro and active forms of MMP2 and MMP9 were detected using protein molecular weight standards (Bio-Rad Laboratories) and MMP standards (EMD).
Statistical analysis.
Datasets were compared by a Mann Whitney test or one-way analysis of variance, as specified in the figure legends, using Prism software v5. Differences were considered significant (*) at P < 0.05.
Online supplemental material.
Fig. S1 demonstrates increased pulmonary inflammation and circulating neutrophilia in BLM-treated il10−/− mice, compared with BLM-treated WT mice. Fig. S2 shows that neither recombinant IL-10 nor anti–IL-10R blockade has a direct impact on Th17 cell generation in vitro. Fig. S3 corroborates other studies and demonstrates, using ifnγ−/− mice, that IFN-γ contributes to BLM-induced pulmonary inflammation and fibrosis. Fig. S4 shows that IL-17A is not required for S. mansoni egg–induced pulmonary inflammation and fibrosis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092121/DC1.
Acknowledgments
We are very grateful to the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Disease, and National Human Genome Research Institute, who supported this research.
The authors acknowledge the meticulous care of animals used in this study by Nicole Relerford, Joy McFarlane, Jose Encarnacion, Lauren Donato, and SoBran staff. We thank Sandra D. White, Robert W. Thompson, and clinical center staff for additional animal care, technical assistance, and CBC processing. We also thank Drs. Margaret Karow, Andrew J. Murphy, David M. Valenzuela, and George D. Yancopoulos (Regeneron Pharmaceuticals, Inc.) for providing breeding pairs of il17–/– mice. We also thank Amy Klion, Luke Barron, Katrin Mayer, Margaret Mentink-Kane, and Kevin Shenderov for helpful and constructive discussion.
The authors have no competing financial interests.
Footnotes
Abbreviations used:
- BAL
- bronchoalveolar lavage
- BFA
- brefeldin A
- BLM
- bleomycin
- CBC
- complete blood count
- dKO
- double KO
- ECM
- extracellular matrix
- IPF
- idiopathic pulmonary fibrosis
- MMP
- matrix metalloproteinase
- mRNA
- messenger RNA
- NV
- normal volunteer
- TIMP
- tissue inhibitor of MMP
References
- Albanesi C., Cavani A., Girolomoni G. 1999. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J. Immunol. 162:494–502 [PubMed] [Google Scholar]
- Aliprantis A.O., Wang J., Fathman J.W., Lemaire R., Dorfman D.M., Lafyatis R., Glimcher L.H. 2007. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc. Natl. Acad. Sci. USA. 104:2827–2830 10.1073/pnas.0700021104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C.F., Oukka M., Kuchroo V.J., Sacks D. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:285–297 10.1084/jem.20061886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A., Takaya H., Makino J., Sato H., Bamba S., Araki Y., Hata K., Shimada M., Okuno T., Fujiyama Y., Bamba T. 2001. Cooperation of interleukin-17 and interferon-gamma on chemokine secretion in human fetal intestinal epithelial cells. Clin. Exp. Immunol. 125:56–63 10.1046/j.1365-2249.2001.01588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T., Abe K., Matsuoka H., Yoshida M., Mori M., Goya S., Kida H., Nishino K., Osaki T., Tachibana I., et al. 2000. Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L914–L922 [DOI] [PubMed] [Google Scholar]
- Beklen A., Ainola M., Hukkanen M., Gürgan C., Sorsa T., Konttinen Y.T. 2007. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J. Dent. Res. 86:347–351 10.1177/154405910708600409 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Oukka M., Kuchroo V.K. 2008. Induction and effector functions of T(H)17 cells. Nature. 453:1051–1057 10.1038/nature07036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blease K., Jakubzick C., Westwick J., Lukacs N., Kunkel S.L., Hogaboam C.M. 2001. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 166:5219–5224 [DOI] [PubMed] [Google Scholar]
- Braun R.K., Ferrick C., Neubauer P., Sjoding M., Sterner-Kock A., Kock M., Putney L., Ferrick D.A., Hyde D.M., Love R.B. 2008. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 31:167–179 10.1007/s10753-008-9062-6 [DOI] [PubMed] [Google Scholar]
- Cassel S.L., Eisenbarth S.C., Iyer S.S., Sadler J.J., Colegio O.R., Tephly L.A., Carter A.B., Rothman P.B., Flavell R.A., Sutterwala F.S. 2008. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA. 105:9035–9040 10.1073/pnas.0803933105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H., Hanawa H., Yoshida T., Hayashi M., Liu H., Ding L., Otaki K., Hao K., Yoshida K., Kato K., et al. 2008. Alteration of IL-17 related protein expressions in experimental autoimmune myocarditis and inhibition of IL-17 by IL-10-Ig fusion gene transfer. Circ. J. 72:813–819 10.1253/circj.72.813 [DOI] [PubMed] [Google Scholar]
- Chen E.S., Greenlee B.M., Wills-Karp M., Moller D.R. 2001. Attenuation of lung inflammation and fibrosis in interferon-gamma-deficient mice after intratracheal bleomycin. Am. J. Respir. Cell Mol. Biol. 24:545–555 [DOI] [PubMed] [Google Scholar]
- Chiaramonte M.G., Donaldson D.D., Cheever A.W., Wynn T.A. 1999. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Invest. 104:777–785 10.1172/JCI7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D.M., Feldman M.D., Mummidi S., Valente A.J., Steffensen B., Vincenti M., Barnes J.L., Chandrasekar B. 2007. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 293:H3356–H3365 10.1152/ajpheart.00928.2007 [DOI] [PubMed] [Google Scholar]
- Cutroneo K.R., White S.L., Phan S.H., Ehrlich H.P. 2007. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J. Cell. Physiol. 211:585–589 10.1002/jcp.20972 [DOI] [PubMed] [Google Scholar]
- Czaja M.J., Weiner F.R., Flanders K.C., Giambrone M.A., Wind R., Biempica L., Zern M.A. 1989. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J. Cell Biol. 108:2477–2482 10.1083/jcb.108.6.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid R.E., Rao D.A., Zhou J., Lo S.F., Ranjbaran H., Gallo A., Sokol S.I., Pfau S., Pober J.S., Tellides G. 2009. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 119:1424–1432 10.1161/CIRCULATIONAHA.108.827618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B., Perona-Wright G., Smits H.H., Hokke C.H., van der Ham A.J., Fitzsimmons C.M., Doenhoff M.J., van der Bosch J., Mohrs K., Haas H., et al. 2009. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 206:1673–1680 10.1084/jem.20082460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner-Feigl S., Strober W., Kawakami K., Puri R.K., Kitani A. 2006. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 12:99–106 10.1038/nm1332 [DOI] [PubMed] [Google Scholar]
- Fitzgerald D.C., Zhang G.X., El-Behi M., Fonseca-Kelly Z., Li H., Yu S., Saris C.J., Gran B., Ciric B., Rostami A. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 8:1372–1379 10.1038/ni1540 [DOI] [PubMed] [Google Scholar]
- Gasse P., Mary C., Guenon I., Noulin N., Charron S., Schnyder-Candrian S., Schnyder B., Akira S., Quesniaux V.F., Lagente V., et al. 2007. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest. 117:3786–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K., Andoh A., Shimada M., Fujino S., Bamba S., Araki Y., Okuno T., Fujiyama Y., Bamba T. 2002. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G1035–G1044 [DOI] [PubMed] [Google Scholar]
- Hay J., Shahzeidi S., Laurent G. 1991. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 65:81–94 10.1007/BF02034932 [DOI] [PubMed] [Google Scholar]
- Hoshino T., Okamoto M., Sakazaki Y., Kato S., Young H.A., Aizawa H. 2009. Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice. Am. J. Respir. Cell Mol. Biol. 41:661–670 10.1165/rcmb.2008-0182OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F., Arras M., Tomasi D., Barbarin V., Delos M., Coutelier J.P., Vink A., Phan S.H., Renauld J.C., Lison D. 2002. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J. Immunol. 169:2653–2661 [DOI] [PubMed] [Google Scholar]
- Hunninghake G.W., Gadek J.E., Lawley T.J., Crystal R.G. 1981. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J. Clin. Invest. 68:259–269 10.1172/JCI110242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 30:108–119 10.1016/j.immuni.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Jakubzick C., Choi E.S., Joshi B.H., Keane M.P., Kunkel S.L., Puri R.K., Hogaboam C.M. 2003. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J. Immunol. 171:2684–2693 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., Sher A. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283 10.1084/jem.20062175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M., Kim S.T., Wan Y.Y., Sutterwala F.S., Lara-Tejero M., Galán J.E., Harhaj E., Flavell R.A. 2006. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 25:941–952 10.1016/j.immuni.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Kaviratne M., Hesse M., Leusink M., Cheever A.W., Davies S.J., McKerrow J.H., Wakefield L.M., Letterio J.J., Wynn T.A. 2004. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J. Immunol. 173:4020–4029 [DOI] [PubMed] [Google Scholar]
- Keane M.P., Gomperts B.N., Weigt S., Xue Y.Y., Burdick M.D., Nakamura H., Zisman D.A., Ardehali A., Saggar R., Lynch J.P., III, et al. 2007. IL-13 is pivotal in the fibro-obliterative process of bronchiolitis obliterans syndrome. J. Immunol. 178:511–519 [DOI] [PubMed] [Google Scholar]
- Kinder B.W., Brown K.K., Schwarz M.I., Ix J.H., Kervitsky A., King T.E., Jr 2008. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest. 133:226–232 10.1378/chest.07-1948 [DOI] [PubMed] [Google Scholar]
- Kitani A., Fuss I., Nakamura K., Kumaki F., Usui T., Strober W. 2003. Transforming growth factor (TGF)-β1–producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-β1–mediated fibrosis. J. Exp. Med. 198:1179–1188 10.1084/jem.20030917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kradin R.L., Sakamoto H., Jain F., Zhao L.H., Hymowitz G., Preffer F. 2004. IL-10 inhibits inflammation but does not affect fibrosis in the pulmonary response to bleomycin. Exp. Mol. Pathol. 76:205–211 10.1016/j.yexmp.2003.12.010 [DOI] [PubMed] [Google Scholar]
- Kryczek I., Bruce A.T., Gudjonsson J.E., Johnston A., Aphale A., Vatan L., Szeliga W., Wang Y., Liu Y., Welling T.H., et al. 2008. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J. Immunol. 181:4733–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M., Cui Z.H., Hoshino H., Lötvall J., Sjöstrand M., Gruenert D.C., Skoogh B.E., Lindén A. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162:2347–2352 [PubMed] [Google Scholar]
- Laan M., Lötvall J., Chung K.F., Lindén A. 2001. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br. J. Pharmacol. 133:200–206 10.1038/sj.bjp.0704063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.G., Homer R.J., Zhu Z., Lanone S., Wang X., Koteliansky V., Shipley J.M., Gotwals P., Noble P., Chen Q., et al. 2001. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 194:809–821 10.1084/jem.194.6.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes M., Becker C., Ivanov I.I., Hirth S., Wirtz S., Neufert C., Pouly S., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., et al. 2009. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 136:257–267 10.1053/j.gastro.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Lin Y., Ritchea S., Logar A., Slight S., Messmer M., Rangel-Moreno J., Guglani L., Alcorn J.F., Strawbridge H., Park S.M., et al. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 31:799–810 10.1016/j.immuni.2009.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén A., Laan M., Anderson G.P. 2005. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 25:159–172 10.1183/09031936.04.00032904 [DOI] [PubMed] [Google Scholar]
- Ma H.L., Liang S., Li J., Napierata L., Brown T., Benoit S., Senices M., Gill D., Dunussi-Joannopoulos K., Collins M., et al. 2008. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeyama T., Kuwano K., Kawasaki M., Kunitake R., Hagimoto N., Hara N. 2001. Attenuation of bleomycin-induced pneumopathy in mice by monoclonal antibody to interleukin-12. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L1128–L1137 [DOI] [PubMed] [Google Scholar]
- Mahanonda R., Jitprasertwong P., Sa-Ard-Iam N., Rerkyen P., Charatkulangkun O., Jansisyanont P., Nisapakultorn K., Yongvanichit K., Pichyangkul S. 2008. Effects of IL-17 on human gingival fibroblasts. J. Dent. Res. 87:267–272 10.1177/154405910808700314 [DOI] [PubMed] [Google Scholar]
- Manoury B., Caulet-Maugendre S., Guénon I., Lagente V., Boichot E. 2006. TIMP-1 is a key factor of fibrogenic response to bleomycin in mouse lung. Int. J. Immunopathol. Pharmacol. 19:471–487 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8:1390–1397 10.1038/ni1539 [DOI] [PubMed] [Google Scholar]
- Meltzer E.B., Noble P.W. 2008. Idiopathic pulmonary fibrosis. Orphanet J. Rare Dis. 3:8 10.1186/1750-1172-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Prause O., Sjöstrand M., Laan M., Lötvall J., Lindén A. 2003. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 170:4665–4672 [DOI] [PubMed] [Google Scholar]
- Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17:375–387 10.1016/S1074-7613(02)00391-6 [DOI] [PubMed] [Google Scholar]
- Nakagome K., Dohi M., Okunishi K., Tanaka R., Miyazaki J., Yamamoto K. 2006. In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-beta in the lung. Thorax. 61:886–894 10.1136/thx.2005.056317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naundorf S., Schröder M., Höflich C., Suman N., Volk H.D., Grütz G. 2009. IL-10 interferes directly with TCR-induced IFN-gamma but not IL-17 production in memory T cells. Eur. J. Immunol. 39:1066–1077 10.1002/eji.200838773 [DOI] [PubMed] [Google Scholar]
- Ortiz L.A., Dutreil M., Fattman C., Pandey A.C., Torres G., Go K., Phinney D.G. 2007. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA. 104:11002–11007 10.1073/pnas.0704421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce J., Kaviratne M., Ramalingam T.R., Thompson R.W., Urban J.F., Jr., Cheever A.W., Young D.A., Collins M., Grusby M.J., Wynn T.A. 2006. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J. Clin. Invest. 116:2044–2055 10.1172/JCI27727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam T.R., Pesce J.T., Sheikh F., Cheever A.W., Mentink-Kane M.M., Wilson M.S., Stevens S., Valenzuela D.M., Murphy A.J., Yancopoulos G.D., et al. 2008. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat. Immunol. 9:25–33 10.1038/ni1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P., Rosas I.O., Macdonald S.D., Wu H.P., Billings E.M., Gochuico B.R. 2007. Impairment of alveolar macrophage transcription in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 175:1151–1157 10.1164/rccm.200607-958OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Zhao L.H., Jain F., Kradin R. 2002. IL-12p40(-/-) mice treated with intratracheal bleomycin exhibit decreased pulmonary inflammation and increased fibrosis. Exp. Mol. Pathol. 72:1–9 10.1006/exmp.2001.2409 [DOI] [PubMed] [Google Scholar]
- Saraiva M., Christensen J.R., Veldhoen M., Murphy T.L., Murphy K.M., O’Garra A. 2009. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 31:209–219 10.1016/j.immuni.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberger P., La Russa V., Miller A., Ye P., Huang W., Zieske A., Nelson S., Bagby G.J., Stoltz D., Mynatt R.L., et al. 1998. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 161:6383–6389 [PubMed] [Google Scholar]
- Sergejeva S., Ivanov S., Lötvall J., Lindén A. 2005. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 33:248–253 10.1165/rcmb.2004-0213OC [DOI] [PubMed] [Google Scholar]
- Shah N.R., Noble P., Jackson R.M., King T.E., Jr., Nathan S.D., Padilla M., Raghu G., Rhodes M.B., Schwarz M., Tino G., Dubois R.W. 2005. A critical assessment of treatment options for idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffuse Lung Dis. 22:167–174 [PMC free article] [PubMed] [Google Scholar]
- Steinfelder S., Andersen J.F., Cannons J.L., Feng C.G., Joshi M., Dwyer D., Caspar P., Schwartzberg P.L., Sher A., Jankovic D. 2009. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J. Exp. Med. 206:1681–1690 10.1084/jem.20082462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer J.S., Silver J.S., Laurence A., Porrett P.M., Harris T.H., Turka L.A., Ernst M., Saris C.J., O’Shea J.J., Hunter C.A. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8:1363–1371 10.1038/ni1537 [DOI] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., Mills K.H. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 31:331–341 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Tesmer L.A., Lundy S.K., Sarkar S., Fox D.A. 2008. Th17 cells in human disease. Immunol. Rev. 223:87–113 10.1111/j.1600-065X.2008.00628.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelhoer M., Bewig B., Oldigs M., Nowak D., Magnussen H., Petermann W., Barth J. 1993. Protein profile in bronchoalveolar lavage fluid from patients with sarcoidosis and idiopathic pulmonary fibrosis as revealed by SDS-PAGE electrophoresis and Western blot analysis. Scand. J. Clin. Lab. Invest. 53:617–623 [PubMed] [Google Scholar]
- Varga J., Pasche B. 2008. Antitransforming growth factor-beta therapy in fibrosis: recent progress and implications for systemic sclerosis. Curr. Opin. Rheumatol. 20:720–728 10.1097/BOR.0b013e32830e48e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Wang R., Ibarra-Sunga O., Verlinski L., Pick R., Uhal B.D. 2000. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L143–L151 [DOI] [PubMed] [Google Scholar]
- Whittington H.A., Armstrong L., Uppington K.M., Millar A.B. 2004. Interleukin-22: a potential immunomodulatory molecule in the lung. Am. J. Respir. Cell Mol. Biol. 31:220–226 10.1165/rcmb.2003-0285OC [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Wynn T.A. 2009. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121 10.1038/mi.2008.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S., Elnekave E., Mentink-Kane M.M., Hodges M.G., Pesce J.T., Ramalingam T.R., Thompson R.W., Kamanaka M., Flavell R.A., Keane-Myers A., et al. 2007. IL-13Ralpha2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J. Clin. Invest. 117:2941–2951 10.1172/JCI31546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witowski J., Pawlaczyk K., Breborowicz A., Scheuren A., Kuzlan-Pawlaczyk M., Wisniewska J., Polubinska A., Friess H., Gahl G.M., Frei U., Jörres A. 2000. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J. Immunol. 165:5814–5821 [DOI] [PubMed] [Google Scholar]
- Wynn T.A. 2008. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214:199–210 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.O., Chang S.H., Park H., Nurieva R., Shah B., Acero L., Wang Y.H., Schluns K.S., Broaddus R.R., Zhu Z., Dong C. 2008. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205:1063–1075 10.1084/jem.20071978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrioual S., Toh M.L., Tournadre A., Zhou Y., Cazalis M.A., Pachot A., Miossec V., Miossec P. 2008. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J. Immunol. 180:655–663 [DOI] [PubMed] [Google Scholar]