Abstract
Systemic exacerbation of allergic responses, in which mast cells play a critical role, results in life-threatening anaphylactic shock. Sphingosine-1–phosphate (S1P), a ligand for a family of G protein–coupled receptors, is a new addition to the repertoire of bioactive lipids secreted by activated mast cells. Yet little is known of its role in human mast cell functions and in anaphylaxis. We show that S1P2 receptors play a critical role in regulating human mast cell functions, including degranulation and cytokine and chemokine release. Immunoglobulin E–triggered anaphylactic responses, including elevation of circulating histamine and associated pulmonary edema in mice, were significantly attenuated by the S1P2 antagonist JTE-013 and in S1P2-deficient mice, in contrast to anaphylaxis induced by administration of histamine or platelet-activating factor. Hence, S1P and S1P2 on mast cells are determinants of systemic anaphylaxis and associated pulmonary edema and might be beneficial targets for anaphylaxis attenuation and prophylaxis.
Allergic disease is endemic in developed nations, and none is more dramatic or deadly than systemic anaphylactic shock (Finkelman, 2007). Unfortunately, this collection of rapid changes to the cutaneous, vasculature, and pulmonary systems may also be the least understood of atopic diseases. A better understanding of the eliciting factors for systemic anaphylaxis is therefore critical for accurate diagnosis and therapeutic intervention. Mast cells, which reside in vascularized tissues and are strategically located at the interfaces of host and environment in skin and mucosal surfaces, are key effectors of IgE-mediated allergic disorders, including anaphylaxis, hay fever, eczema, and asthma. Mast cells express FcεRI (high-affinity receptors for IgE), which binds antigen (Ag)-specific IgE antibodies, and subsequent exposure to Ag initiates cross-linking and aggregation of FcεRI. This cascade of events triggers mast cell activation and secretion of a wide array of inflammatory mediators, such as histamine and other preformed mediators, bioactive lipids, including eicosanoids and platelet-activating factor (PAF), and numerous proinflammatory cytokines and chemokines (Kalesnikoff and Galli, 2008), which are essential to the pathogenesis of allergic diseases and anaphylaxis (Finkelman, 2007).
A recent addition to the recognized repertoire of lipid mediators secreted by mast cells is the sphingolipid metabolite sphingosine-1–phosphate (S1P; Jolly et al., 2004; Mitra et al., 2006; Olivera et al., 2006), which has been implicated in initiation and maintenance of diverse aspects of immune cell activation and function (Rosen et al., 2008; for review see Schwab and Cyster, 2007; Rivera et al., 2008). Focus on S1P in immune responses has increased after the elucidation of its critical role in lymphocyte trafficking (Rosen et al., 2008; for review see Schwab and Cyster, 2007), and observations of local increases of S1P in inflammatory disorders such as asthma (Ammit et al., 2001) and rheumatoid arthritis (Kitano et al., 2006). S1P is a ligand for five G protein–coupled receptors, designated S1P1-5, through which it exerts many of its actions (Spiegel and Milstien, 2003). Rodent mast cells express S1P1 and S1P2 receptors on their cell surface, which function in an autocrine manner to fine-tune mast cell functions (for review see Olivera, 2008; Price et al., 2008). Although S1P1 is important for migration of mast cells toward Ag, S1P2 contributes to the robustness of their degranulation (Jolly et al., 2004). FcεRI engagement on mast cells by allergens stimulates sphingosine kinase (SphK) 1 and 2, the two isoenzymes which produce S1P, and both contribute to mast cell functions (Jolly et al., 2004, 2005; Urtz et al., 2004; Olivera et al., 2006, 2007). SphK1, but not SphK2, was shown to be important for Ag-induced calcium mobilization, degranulation, and migration of human and rodent bone marrow–derived mast cells (Melendez and Khaw, 2002; Jolly et al., 2004, 2005; Oskeritzian et al., 2008). However, based on results with fetal liver–derived mast cells from SphK1 and SphK2 knockout mice, it was suggested that SphK2, but not SphK1, modulates calcium influx and downstream signaling, leading to degranulation and production of eicosanoids and cytokines (Olivera et al., 2007). Similarly, SphK2 was also necessary for secretion of both TNF and IL-6 by mature human mast cells (Oskeritzian et al., 2008). Remarkably, however, mice deficient in SphK1 displayed reduced levels of circulating S1P and were resistant to anaphylaxis, whereas anaphylactic responses in mice deficient in SphK2 were normal (Olivera et al., 2007). An intriguing finding of this study was the association between circulating concentrations of S1P and susceptibility to anaphylaxis, suggesting that S1P is a key factor that determines anaphylactic responses.
Although much has been learned about the roles of the SphKs that produce S1P in mast cell functions and anaphylactic responses, little is known about how S1P executes these important functions in vivo and which of the S1P receptors is involved. To this end, we examined the roles of S1P receptors in developing and mature human mast cells and their importance in IgE-mediated passive systemic anaphylaxis (PSA) in mice. Our data provide direct pharmacological, genetic, and biological evidence for the importance of S1P and the S1P2 receptor on mast cells in anaphylaxis and associated pulmonary edema. Hence, S1P and S1P2 may be therapeutic targets for clinical intervention in this poorly understood disease.
RESULTS AND DISCUSSION
S1P1 regulates migration of human mast cells toward Ag but is not involved in other mast cell functions
Cross-linking of FcεRI on mast cells activates SphK1 and SphK2, leading to generation and secretion of S1P (Melendez and Khaw, 2002; Urtz et al., 2004; Mitra et al., 2006; Olivera et al., 2006, 2007; Oskeritzian et al., 2008). Although these kinases that produce S1P have distinct and important roles in human mast cells, it is still not well understood how S1P executes these important functions in vivo and which of the S1P receptors is involved.
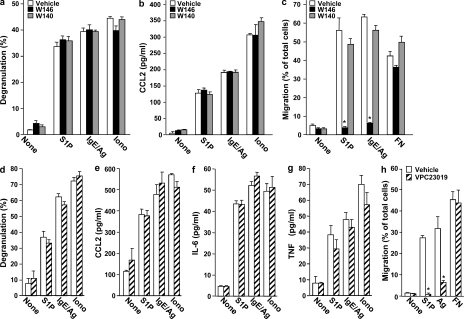
We first examined the role of S1P1, one of the two S1P receptors expressed by rodent and human mast cells (Jolly et al., 2004; Oskeritzian et al., 2008). In agreement with previous results (Oskeritzian et al., 2008), S1P potently induced degranulation of cord blood-derived mast cells (CB-MCs) to almost the same extent as Ag (Fig. 1 a) and also induced significant secretion of the chemokine CCL2 (Fig. 1 b), which is also known as monocyte chemoattractant protein (MCP) 1. However, pretreatment with W146, the preferred R enantiomer of a chiral S1P1 selective competitive antagonist (Sanna et al., 2006), did not affect either degranulation (Fig. 1 a) or secretion of CCL2 induced by S1P, Ag, or ionomycin (Fig. 1 b). In contrast, W146 ablated migration of CB-MC toward S1P and Ag without affecting migration toward fibronectin (Fig. 1 c). This was a specific effect of S1P1 antagonism, as the inactive S enantiomer (W140) had no significant effects (Fig. 1, a–c). S1P also significantly stimulated degranulation of skin-derived mast cells (Sk-MCs) nearly as effectively as Ag and triggered secretion of CCL2 and the cytokines IL-6 and TNF. Moreover, the S1P1 antagonist VPC23019 had no significant effects on S1P- or Ag-stimulated degranulation (Fig. 1 d) or CCL2 (Fig. 1 e) and cytokine release (Fig. 1, f and g), yet it eliminated migration of Sk-MC toward S1P and Ag (Fig. 1 h). Similarly, the S1P1 antagonist W146 also had no significant effects on secretory or excretory responses but prevented migratory responses of Sk-MC to S1P and Ag (Fig. S1).
Figure 1.
S1P1 is not involved in degranulation or cytokine and chemokine secretion from human CB-MCs and Sk-MCs but is essential for human mast cell motility. (a–c) Purified CB-MCs were stimulated with vehicle, 100 nM S1P, or 1 µM ionomycin, or sensitized overnight with IgE, and then stimulated with 30 ng/ml DNP-HSA (Ag), for 2 h. Where indicated, CB-MCs were pretreated for 30 min with vehicle, 1 µM W146, or 1 µM W140 before stimulation. Degranulation was assessed by the percentage of β-hexosaminidase release (a). Secretion of CCL2 (b) was measured by ELISA. (c) Duplicate cultures pretreated for 30 min with vehicle, 1 µM W146, or 1 µM W140 were allowed to migrate in transwell chambers toward vehicle, 100 nM S1P, 30 ng/ml Ag, or 20 µg/ml fibronectin (FN) for 24 h. Similar results were obtained in three independent experiments using CB-MC from three different donors. (d–h) Sk-MCs were stimulated with vehicle, 100 nM S1P, or 1 µM ionomycin, or sensitized overnight with IgE, and then stimulated with 30 ng/ml Ag for 2 h. Where indicated, Sk-MCs were pretreated for 30 min with vehicle or 1 µM VPC23019 before stimulation. Degranulation (d) and secretion of CCL2 (e), IL-6 (f), and TNF (g) was measured. (h) Migration of duplicate cultures toward vehicle, 100 nM S1P, 30 ng/ml Ag, or 20 µg/ml fibronectin was determined in transwell chambers. Data are expressed as the percentage of migrating cells and are the means ± SD of triplicate determinations. Similar results were obtained in three independent experiments using CB-MC and Sk-MC from three different donors. *, P < 0.01, compared with vehicle treatment.
As cross-linking of FcεRI on human mast cell induces production and secretion of S1P (Mitra et al., 2006), our finding that S1P alone potently induces degranulation and cytokine secretion from CB-MC and Sk-MC suggests that S1P acts in a positive-feedback loop to amplify and enhance mast cell–mediated inflammatory responses in a manner that could be destabilizing if not properly controlled. The observation that the S1P1 receptor regulates migration of human mast cell toward Ag, together with other studies demonstrating a role for S1P1 in trafficking of innate and adaptive immune cells (for review see Schwab and Cyster, 2007; Rivera et al., 2008), implies that this receptor may be involved in directing effector cells to sites of allergic inflammation.
S1P2 is pivotal in immunological activation of human mast cells
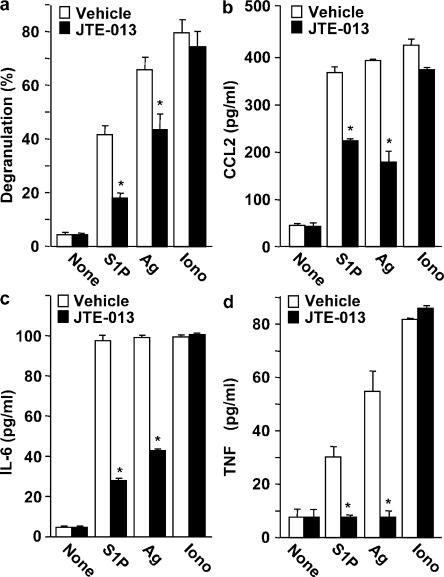
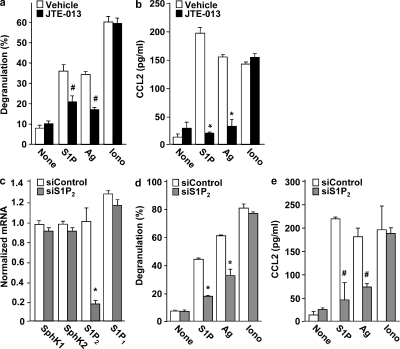
To examine the role of S1P2, the other S1P receptor present on mast cells, we used the selective S1P2 receptor antagonist JTE-013, which greatly suppressed degranulation of Sk-MC induced by S1P and Ag (Fig. 2 a) and also significantly reduced secretion of CCL2 (Fig. 2 b), IL-6 (Fig. 2 c), and TNF (Fig. 2 d). These effects of JTE-013 appeared to be specific, as it did not alter degranulation or chemokine/cytokine secretion induced by the calcium ionophore ionomycin (Fig. 2). Similarly, in CB-MC, JTE-013 also markedly suppressed degranulation induced by S1P and Ag, but not that induced by ionomycin (Fig. 3 a), and also reduced CCL2 secretion (Fig. 3 b).
Figure 2.
S1P2 antagonism impairs degranulation and cytokine and chemokine secretion from human Sk-MCs. Sk-MCs were stimulated with vehicle, 100 nM S1P, or 1 µM ionomycin, or sensitized overnight with IgE, and then stimulated with 30 ng/ml Ag for 2 h. Sk-MCs were pretreated for 30 min with vehicle or 1 µM JTE013 before stimulation, as indicated. Degranulation was assessed by β-hexosaminidase release (a). Secretion of CCL2 (b), IL-6 (c), and TNF (d) was measured by ELISA. Data are the means ± SD of triplicate determinations. Similar results were obtained in three independent experiments using Sk-MC from three different donors. *, P < 0.01, compared with vehicle treatment.
Figure 3.
S1P2 antagonism or down-regulation of S1P2 decreases S1P- and Ag-induced degranulation and chemokine secretion from CB-MCs. (a and b) Purified CB-MCs were stimulated with vehicle, 100 nM S1P, or 1 µM ionomycin, or sensitized overnight with IgE, and then stimulated with 30 ng/ml Ag for 2 h. CB-MCs were pretreated for 30 min with vehicle or 1 µM JTE013 before stimulation, as indicated. Degranulation (a) and secretion of CCL2 (b) were measured. *, P ≤ 0.01; #, P ≤ 0.05, compared with vehicle treatment. (c–e) CB-MCs transfected with control siRNA or S1P2 siRNA were stimulated with vehicle, 100 nM S1P, or 1 µM ionomycin, or sensitized overnight with IgE, and then stimulated with 30 ng/ml Ag for 2 h. (c) RNA was isolated and mRNA levels of S1P1, S1P2, SphK1, SphK2, and GAPDH were determined by quantitative real-time PCR. Degranulation (d) and secretion of CCL2 (e) were measured. *, P ≤ 0.01; #, P ≤ 0.05, compared with scrambled siRNA. Data are the means ± SD of triplicate determinations. Similar results were obtained in three independent experiments with CB-MC from three different donors.
A molecular approach was used to confirm and extend the involvement of S1P2 in the activation of human mast cell. To this end, S1P2 expression in CB-MC was down-regulated with a specific small interfering RNA (siRNA) that efficiently inhibited its expression without altering S1P1 (Fig. 3 c). Similar to the effects of S1P2 antagonism, depletion of the S1P2 receptor in CB-MC greatly reduced S1P- and Ag-mediated degranulation (Fig. 3 d) and CCL2 secretion (Fig. 3 e), whereas activation by ionomycin was not affected (Fig. 3, d and e). Moreover, transfection with scrambled siRNA had no significant effects. Hence, pharmacological and molecular approaches demonstrate that the S1P2 receptor, in contrast to S1P1, is essential for human mast cell functions, including degranulation and cytokine and chemokine release. Although the two types of human mast cells, CB-MC and Sk-MC, are not functionally identical (Oskeritzian et al., 2005), activation of both by cross-linking of FcεRI by Ag or by S1P involves the S1P2 receptor. Moreover, we previously reported that degranulation of bone marrow–derived mast cells derived from S1P2 knockout mice was dramatically impaired (Jolly et al., 2004). Altogether, these results suggest that the S1P–S1P2 receptor axis is an intrinsic regulator of mast cell responsiveness. Furthermore, the clear separation of responsibilities for S1P1 and S1P2 receptors in mast cell activation allows for independent control of migration and inflammatory mediator release.
S1P2 antagonism decreases the severity of PSA
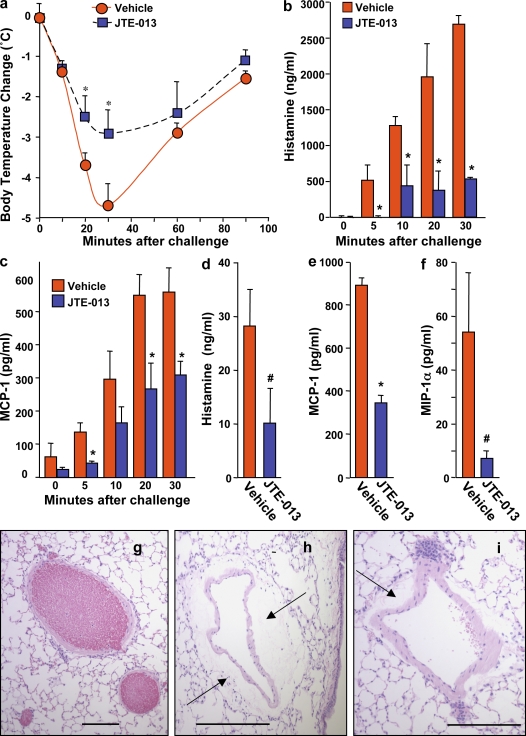
As it appears that S1P2 plays universal roles in activation of both rodent and human mast cell, it was of interest to evaluate the contribution of the S1P2 receptor to allergic reactions in an established mouse model in which mast cells play a prominent role. To this end, we used PSA (Finkelman, 2007), a mast cell–dependent model that circumvents the variables of Ag sensitization and IgE production. Passive immunization of mice with mouse IgE targeted against DNP, followed by i.p. injection of the Ag (DNP–human serum albumin [HSA]), caused profound systemic anaphylaxis, as judged by a drastic drop in core body temperature at 30 min that slowly recovered thereafter (Fig. 4 a). Pretreatment of mice with the S1P2 antagonist JTE-013 markedly attenuated the severe hypothermia induced during Ag challenge (Fig. 4 a). Elevated levels of serum histamine, which are responsible for hypothermia in IgE-mediated anaphylactic shock (Makabe-Kobayashi et al., 2002), were markedly increased within 5 min of Ag challenge, reached maximum levels by ∼30 min (Fig. 4 b), declined thereafter, and approached baseline as the animals recovered (Fig. 4 d). Administration of JTE-013 greatly diminished histamine levels at all time points (Fig. 4, b and d). In contrast to histamine, serum levels of the chemokine MCP-1/CCL2 remained elevated for a longer period of time after Ag challenge (Fig. 4 e). JTE-013 also reduced IgE-mediated production of both MCP-1/CCL2 (Fig. 4, c and e) and macrophage inhibitory protein (MIP) 1–α/CCL3 (Fig. 4 f), important modulators of monocyte and eosinophil recruitment which have previously been shown to increase after in vitro treatment of mouse mast cell with S1P (Jolly et al., 2004).
Figure 4.
S1P2 antagonist attenuates PSA. C57BL/6 mice were injected i.p. with IgE anti-DNP mAb. 12 h later, mice were injected i.p. with vehicle (DMSO; n = 6) or JTE013 (20 µg/mouse; n = 6) and, 30 min later, systemic anaphylaxis was induced by i.p. injection of DNP-HSA. Body temperature was monitored at the indicated times (a). Serum levels of histamine at the indicated times (b) or at 120 min (d), MCP-1/CCL2 at the indicated times (c) or at 120 min (e), and MIP-1α/CCL3 at 120 min (f) were determined by ELISA. Data are the means ± SD. (g–i) Representative micrographs of hematoxylin and eosin (H&E)–stained lung tissues from control mice (g), Ag-challenged mice (h), and mice treated with JTE013 before Ag challenge (i). Arrows indicate pulmonary vascular edema. Note the extensive vascular edema in vehicle-treated Ag-challenged lung (h) compared with JTE013-treated Ag-challenged lung (i). Bars, 100 µm. *, P ≤ 0.01; #, P ≤ 0.05, compared with vehicle treated mice. Similar results were obtained in three independent experiments.
Histamine, chiefly among other mediators secreted by activated mast cells, causes the signs of anaphylaxis predominantly by promoting vasodilatation and increasing vascular permeability (Finkelman, 2007). Accordingly, IgE-dependent anaphylaxis was associated with extensive lung perivascular edema (Fig. 4 h), which is indicative of increased vascular permeability, compared with the lungs of mice not challenged with Ag (Fig. 4 g). JTE-013 treatment dramatically reduced perivascular edema (Fig. 4 i), which is consistent with the attenuation of mast cell activation by this S1P2 antagonist (Figs. 2 and 3). Altogether, these results demonstrate that in vivo manifestations of Ag-induced anaphylaxis can be attenuated by preventing interaction of the mast cell S1P2 receptor with its ligand S1P.
S1P2 deficiency attenuates PSA
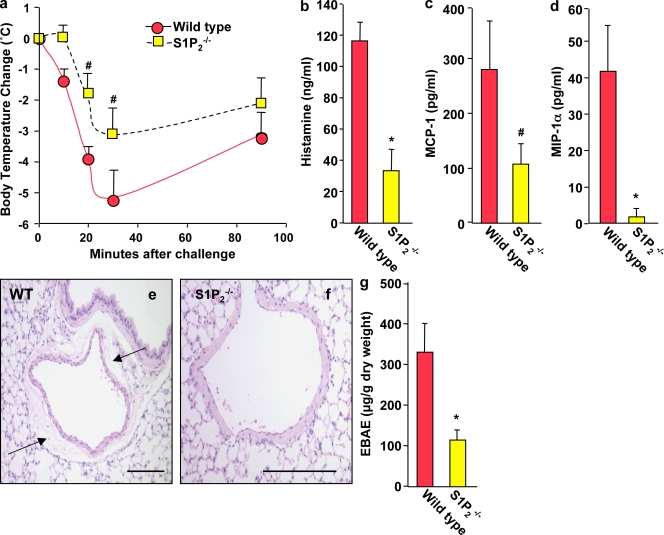
To confirm the importance of S1P2 through a genetic approach, we tested S1P2 knockout mice for their ability to undergo PSA. Indeed, the severity of anaphylaxis in S1P2 deficient mice was significantly decreased compared with their wild-type counterparts, as shown by an attenuation of the Ag-induced decrease of core body temperature (Fig. 5 a) and marked reductions in serum levels of histamine (Fig. 5 b), MCP-1/CCL2 (Fig. 5 c), and MIP1-α/CCL3 (Fig. 5 d). Lung histology also demonstrated increased vascular permeability and perivascular edema in wild-type animals after Ag challenge (Fig. 5 e), whereas S1P2-deficient mice showed much less edema around the blood vessels (Fig. 5 f).
Figure 5.
PSA is markedly reduced in S1P2 knockout mice. S1P2−/− mice (n = 6) and wild-type mice (n = 6) were injected i.p. with IgE anti-DNP mAb, and systemic anaphylaxis was elicited 12 h later by injection of DNP-HSA. Body temperature was monitored at the indicated times (a). Serum levels of histamine (b), MCP-1/CCL2 (c), and MIP-1α/CCL3 (d) at 120 min were determined by ELISA. Data are means ± SD. (e and f) Representative micrographs of H&E-stained lung tissues showing extensive pulmonary perivascular edema in Ag-challenged wild-type mice (e), which was nearly absent around lung blood vessels of Ag-challenged S1P2−/− mice (f). Arrows indicate pulmonary vascular edema. Lung vascular permeability was determined by quantifying Evans blue extravasation (EBAE; g). Similar results were obtained in three independent experiments. *, P ≤ 0.01; #, P ≤ 0.05, compared with wild-type mice. Bars, 100 µm.
To quantify pulmonary vascular permeability, mice were injected with Evan’s blue dye, as an increase in vascular permeability allows the dye to leak from the blood vessels into the surrounding tissue. After Ag challenge, wild-type mice had much greater extravasation of Evans blue into the lung than did S1P2−/− mice (Fig. 5 g). These data confirm the decreased vascular permeability and less severe anaphylaxis in mice devoid of S1P2.
Anaphylaxis triggered by histamine or PAF is unaltered by S1P2 blockade or deficiency
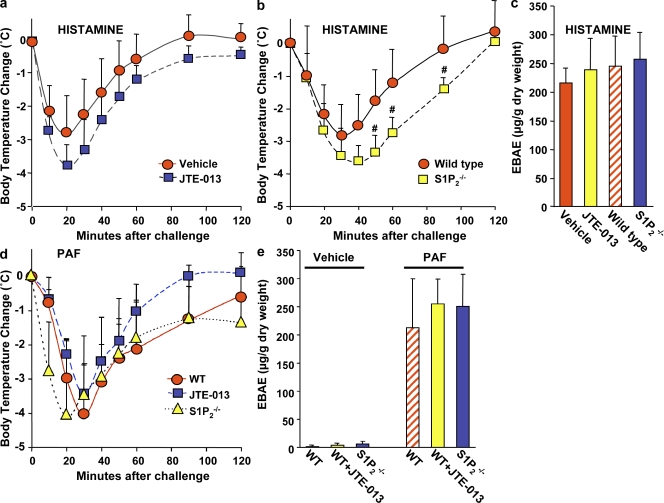
It was important to examine whether the S1P2 receptor on mast cells alone, and not on other responsive cell types, is critical for induction of mast cell–mediated anaphylaxis. We were unable to carry out adoptive transfer experiments using mast cell–deficient KitW-sh/W-sh mice on the C57BL/6 background reconstituted with S1P2-deficient mast cells derived from C57BL/6 × 129sv mixed background mice because minor histocompatibility Ags are not conserved between them, precluding transplantation experiments without rejection. Thus, to examine whether the reduced signs of anaphylaxis from blockade or deficiency of S1P2 are a result of their effects on mast cell functions, mast cell degranulation was bypassed by administration of exogenous histamine or PAF, which are major mediators of anaphylaxis in mice released by activated mast cells (Finkelman, 2007). Interestingly, neither JTE-013 (Fig. 6 a) nor S1P2 deficiency (Fig. 6 b) reduced, but rather slightly enhanced the drop in body temperature induced by histamine injection. Similarly, sensitivity to PAF-induced anaphylaxis was not significantly blunted by JTE-013 treatment or by deletion of S1P2 (Fig. 6 d). Moreover, extravasation of Evans blue in lung induced by histamine (Fig. 6 c) or PAF (Fig. 6 e) was not abrogated by S1P2 blockade or by S1P2 deficiency, which is in sharp contrast to IgE/Ag-induced anaphylaxis and edema in lung tissues (Figs. 4 and 5).
Figure 6.
Blockade of S1P2 or S1P2 deficiency does not mitigate anaphylaxis triggered by histamine or PAF. (a) C57BL/6 mice were injected i.v. with histamine (3 mg/mouse) together with vehicle (n = 6) or JTE-013 (20 µg/mouse; n = 6), and body temperature was monitored at the indicated times. (b) C57BL/6 × 129sv wild-type (n = 6) and S1P2 knockout mice (n = 9) were injected i.v. with histamine (3 mg/mouse), and body temperature was monitored. (c) Lung vascular permeability was determined by quantifying Evans blue extravasation. (d) C57BL/6 × 129sv wild-type mice were injected i.v. with PAF (500 ng/mouse) together with vehicle (n = 9) or JTE-013 (n = 9). S1P2 null mice were injected with PAF (500 ng/mouse; n = 9). Body temperature was monitored at the indicated times. (e) Lung vascular permeability was determined by quantifying Evans blue extravasation in C57BL/6 × 129sv wild-type mice and in S1P2 null mice injected i.v. with vehicle, PAF, or JTE-013, as indicated. Data are the means ± SD of triplicate determinations. Similar results were obtained in two independent experiments.
Concluding remarks
Collectively with the observations that S1P is released from mast cells after cross-linking of FcεRI and that their S1P2 receptors are critical for degranulation and chemokine and cytokine release from activated human (Figs. 2 and 3) and rodent mast cells (Jolly et al., 2004), our data provide evidence for the importance of S1P and its S1P2 receptor on mast cells in mast cell–mediated anaphylactic shock. Loss of S1P2 expression in mice or S1P2 blockade with a specific antagonist decreased the anaphylactic responses and lung perivascular edema triggered by Ag challenge. In contrast, direct induction of anaphylaxis and lung leakage by injection of the mast cell mediators histamine or PAF was independent of S1P2 receptor status. Hence, engagement of S1P2 receptors on mast cells by S1P upon cross-linking of FcεRI with IgE/Ag acts to enhance and amplify mast cells functions (Rivera and Gilfillan, 2006; for review see Price et al., 2008) that are important for anaphylaxis in mice. Indeed, interfering with the S1P–S1P2 receptor axis drastically reduced circulating levels of histamine, whose rapid secretion from mast cells results in systemic vasodilation associated with a sudden drop in body temperature, increased vascular permeability, and edema of bronchial mucosa (Makabe-Kobayashi et al., 2002; Finkelman, 2007; Galli et al., 2008).
Specifically targeting the S1P2 receptor may have important clinical and therapeutic implications for allergic diseases. Just as we found divergent roles for S1P1 and S1P2 in mast cell functions, there are other opposing physiological and pathological roles for these receptors, suggesting that complete S1P blockade may be complicated or even dangerous. Previous studies have conclusively demonstrated that S1P, via ligation of the S1P1 receptor, enhances endothelial cell barrier function in vivo and in vitro (Garcia et al., 2001; Paik et al., 2004; McVerry and Garcia, 2005). A recent elegant study provided convincing evidence that plasma S1P maintains vascular integrity and prevents lethal responses to leak-inducing agents in adult mice by a mechanism that involves activation of S1P1, most likely on endothelial cells (Camerer et al., 2009). Conversely, treatment with the S1P1 antagonist W146 caused pulmonary edema (Sanna et al., 2006). Thus, although S1P1 elicits inflammatory cell migration, it also has physiological protective effects on the vasculature that dampen endothelial permeability. In contrast, ligation of S1P2 on endothelial cells increases vascular permeability (Sanchez et al., 2007). However, it seems unlikely that the marked reduction in pulmonary edema after Ag challenge by the S1P2 antagonist JTE-013 or depletion of S1P2 is the result of an improvement of barrier integrity, as neither JTE-013 nor knockout of S1P2 reduced sensitivity to histamine or PAF. Thus, activation of mast cell S1P2 and endothelial cell S1P1 receptors (Camerer et al., 2009) may be critical in determining the extent of anaphylactic shock by regulating mast cell functions and vascular permeability, respectively. In sum, our data demonstrate the importance of S1P and its S1P2 receptor in mast cell activation and anaphylaxis and suggest that specific targeting of the S1P–S1P2 receptor axis could be a novel therapeutic strategy to control acute life threatening exacerbations of anaphylactic shock and other diseases where systemic mast cell activation has been identified as critical for the induction and promotion of initial inflammatory events.
MATERIALS AND METHODS
Reagents.
JTE-013 was obtained from Tocris Bioscience. VPC23019 and W-146 (and its inactive S isomer) were purchased from Avanti Polar Lipids, Inc.
Culture of human mast cells.
All protocols involving human tissues were approved by the human studies Internal Review Board at Virginia Commonwealth University (VCU). Surgical skin samples were obtained from the Cooperative Human Tissue Network of the National Cancer Institute or from the National Disease Research Interchange. Sk-MCs were prepared as previously described (Zhao et al., 2005) and were 100% mast cells, as determined by staining with toluidine blue. Purified stem cell factor (SCF)–dependent CB-MCs were prepared and cultured for 8–12 wk of culture in the presence of recombinant human SCF (Amgen) alone as previously described (Oskeritzian et al., 2008), and >95% stained positively with toluidine blue. Human umbilical cord blood samples were provided by the Labor and Delivery station at the VCU Health System (Richmond, VA).
siRNA transfection.
Expression of S1P2 was down-regulated by transfection with 100 nM of sequence-specific siRNAs (ON-TARGETplus SMARTpool siRNA; Thermo Fisher Scientific) in RPMI 1640 using Oligofectamine (Invitrogen).
Quantitative RT-PCR.
Total RNA was isolated with TRIzol reagent (Invitrogen) and was reverse transcribed with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative PCR was performed with premixed primer-probe sets using the ABI 7800 (Applied Biosystems).
Degranulation.
Degranulation of 106 human mast cells was measured by assaying β-hexosaminidase release as described previously (Oskeritzian et al., 2008). Degranulation was expressed as a percentage of the total cellular β-hexosaminidase released into the medium.
Chemotaxis.
Chemotaxis was measured in transwells (Costar; Corning) with 8-µm pores, as previously described (Oskeritzian et al., 2008).
Measurements of cytokines and chemokines.
Human IL-6, TNF, and CCL2/MCP-1 were measured by ELISA (Oskeritzian et al., 2008).
Animals.
Protocols and studies involving animals were performed in accordance with the VCU Institutional Animal Care and Use Committee guidelines. Mice were maintained in a pathogen-free facility at VCU. C57BL/6J and C57BL/6J × 129SV mice were obtained from The Jackson Laboratory, and S1P2 knockout mice (R. Proia, National Institutes of Health, Bethesda, MD) were maintained on mixed C57BL/6, 129/Sv backgrounds.
PSA.
All injections were performed i.p. in a final volume of 100 µl per injection. Mice were injected with 75 µg DNP-specific mouse IgE (provided by D. Conrad, VCU, Richmond, VA) and, 12 h later, with 100 µg DNP-albumin (Sigma-Aldrich) in PBS. Immediately before the Ag challenge, body temperature of each animal was measured using a rectal microprobe (Physitemp Instruments) and monitored up to 90 min after Ag challenge. Mice were then euthanized, blood was collected by cardiac puncture for serum analysis, and lungs were removed for histological examination. Control mice injected with PBS showed no significant change in body temperature (±1°C) and had blood histamine levels near the limit of detection.
Histology.
Lung samples were inflated at the time of dissection with 0.5% low melting point agarose solution in 10% formalin. Samples were embedded in paraffin, serial sections stained with H&E, and perivascular areas were evaluated in 8–12 randomly selected sections for each sample. Investigators had no knowledge of the treatment group assignments at the time of analysis. For quantification of lung capillary leakage, mice were injected i.v. with a solution of Evans blue dye in albumin (20 mg/kg). The dye was allowed to circulate for 30 min, mice were killed, and their lungs were flushed with PBS containing 5 mM EDTA. Lungs were then weighed and Evans blue was extracted overnight at 60°C with formamide and measured by absorbance at 650 nm (Tauseef et al., 2008).
Histamine determination.
Mouse serum histamine levels were determined using a histamine competitive direct ELISA kit (Neogen).
Statistical analysis.
Results were analyzed for statistical significance with the Student’s t test for unpaired samples in Excel (Microsoft). Experiments were repeated at least three times in triplicate with consistent results. In vivo experiments were repeated three times and each experimental group consisted of six mice. All data are presented as means ± SD.
Online supplemental material.
Fig. S1 shows the effects of treatment of Sk-MC with a S1P1 antagonist on migration, degranulation, and cytokine and chemokine secretion. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091513/DC1.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants KO1AR053186 to C.A. Oskeritzian and RO1AI50094 and U19AI077435 to S. Spiegel.
We are very grateful to Drs. Lawrence Schwartz, Donatas Kraskauskas, Irina Petrache, and Norbert Voelkel for advice and helpful discussion. We extend our gratitude to the Labor and Delivery team at the Virginia Commonwealth University Health System for kindly providing us with human umbilical cord blood samples, Amgen for SCF, and Dr. Daniel Conrad for providing IgE.
The authors declare that they have no competing financial interests.
Footnotes
Abbreviations used:
- Ag
- antigen
- CB-MC
- cord blood–derived mast cell
- HSA
- human serum albumin
- MCP
- monocyte chemoattractant protein
- MIP
- macrophage inhibitory protein
- PAF
- platelet-activating factor
- PSA
- passive systemic anaphylaxis
- S1P
- sphingosine-1–phosphate
- SCF
- stem cell factor
- siRNA
- small interfering RNA
- Sk-MC
- skin-derived mast cell
- SphK
- sphingosine kinase
References
- Ammit A.J., Hastie A.T., Edsall L.C., Hoffman R.K., Amrani Y., Krymskaya V.P., Kane S.A., Peters S.P., Penn R.B., Spiegel S., Panettieri R.A., Jr 2001. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 15:1212–1214 [DOI] [PubMed] [Google Scholar]
- Camerer E., Regard J.B., Cornelissen I., Srinivasan Y., Duong D.N., Palmer D., Pham T.H., Wong J.S., Pappu R., Coughlin S.R. 2009. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 119:1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F.D. 2007. Anaphylaxis: lessons from mouse models. J. Allergy Clin. Immunol. 120:506–515 10.1016/j.jaci.2007.07.033 [DOI] [PubMed] [Google Scholar]
- Galli S.J., Grimbaldeston M., Tsai M. 2008. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 8:478–486 10.1038/nri2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.G., Liu F., Verin A.D., Birukova A., Dechert M.A., Gerthoffer W.T., Bamberg J.R., English D. 2001. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108:689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly P.S., Bektas M., Olivera A., Gonzalez-Espinosa C., Proia R.L., Rivera J., Milstien S., Spiegel S. 2004. Transactivation of sphingosine-1–phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 199:959–970 10.1084/jem.20030680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly P.S., Bektas M., Watterson K.R., Sankala H., Payne S.G., Milstien S., Spiegel S. 2005. Expression of SphK1 impairs degranulation and motility of RBL-2H3 mast cells by desensitizing S1P receptors. Blood. 105:4736–4742 10.1182/blood-2004-12-4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnikoff J., Galli S.J. 2008. New developments in mast cell biology. Nat. Immunol. 9:1215–1223 10.1038/ni.f.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano M., Hla T., Sekiguchi M., Kawahito Y., Yoshimura R., Miyazawa K., Iwasaki T., Sano H., Saba J.D., Tam Y.Y. 2006. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 54:742–753 10.1002/art.21668 [DOI] [PubMed] [Google Scholar]
- Makabe-Kobayashi Y., Hori Y., Adachi T., Ishigaki-Suzuki S., Kikuchi Y., Kagaya Y., Shirato K., Nagy A., Ujike A., Takai T., et al. 2002. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J. Allergy Clin. Immunol. 110:298–303 10.1067/mai.2002.125977 [DOI] [PubMed] [Google Scholar]
- McVerry B.J., Garcia J.G. 2005. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell. Signal. 17:131–139 10.1016/j.cellsig.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Melendez A.J., Khaw A.K. 2002. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J. Biol. Chem. 277:17255–17262 10.1074/jbc.M110944200 [DOI] [PubMed] [Google Scholar]
- Mitra P., Oskeritzian C.A., Payne S.G., Beaven M.A., Milstien S., Spiegel S. 2006. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. USA. 103:16394–16399 10.1073/pnas.0603734103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A. 2008. Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model. Prostaglandins Other Lipid Mediat. 86:1–11 10.1016/j.prostaglandins.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A., Urtz N., Mizugishi K., Yamashita Y., Gilfillan A.M., Furumoto Y., Gu H., Proia R.L., Baumruker T., Rivera J. 2006. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J. Biol. Chem. 281:2515–2525 10.1074/jbc.M508931200 [DOI] [PubMed] [Google Scholar]
- Olivera A., Mizugishi K., Tikhonova A., Ciaccia L., Odom S., Proia R.L., Rivera J. 2007. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 26:287–297 10.1016/j.immuni.2007.02.008 [DOI] [PubMed] [Google Scholar]
- Oskeritzian C.A., Zhao W., Min H.K., Xia H.Z., Pozez A., Kiev J., Schwartz L.B. 2005. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 115:1162–1168 10.1016/j.jaci.2005.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskeritzian C.A., Alvarez S.E., Hait N.C., Price M.M., Milstien S., Spiegel S. 2008. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 111:4193–4200 10.1182/blood-2007-09-115451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J.H., Skoura A., Chae S.S., Cowan A.E., Han D.K., Proia R.L., Hla T. 2004. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 18:2392–2403 10.1101/gad.1227804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M.M., Oskeritzian C.A., Milstien S., Spiegel S. 2008. Sphingosine-1-phosphate synthesis and functions in mast cells. Future Lipidol. 3:665–674 10.2217/17460875.3.6.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., Gilfillan A.M. 2006. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 117:1214–1225, quiz :1226 10.1016/j.jaci.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Rivera J., Proia R.L., Olivera A. 2008. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8:753–763 10.1038/nri2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gonzalez-Cabrera P., Marsolais D., Cahalan S., Don A.S., Sanna M.G. 2008. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol. Rev. 223:221–235 10.1111/j.1600-065X.2008.00645.x [DOI] [PubMed] [Google Scholar]
- Sanchez T., Skoura A., Wu M.T., Casserly B., Harrington E.O., Hla T. 2007. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 27:1312–1318 10.1161/ATVBAHA.107.143735 [DOI] [PubMed] [Google Scholar]
- Sanna M.G., Wang S.K., Gonzalez-Cabrera P.J., Don A., Marsolais D., Matheu M.P., Wei S.H., Parker I., Jo E., Cheng W.C., et al. 2006. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat. Chem. Biol. 2:434–441 10.1038/nchembio804 [DOI] [PubMed] [Google Scholar]
- Schwab S.R., Cyster J.G. 2007. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8:1295–1301 10.1038/ni1545 [DOI] [PubMed] [Google Scholar]
- Spiegel S., Milstien S. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4:397–407 10.1038/nrm1103 [DOI] [PubMed] [Google Scholar]
- Tauseef M., Kini V., Knezevic N., Brannan M., Ramchandaran R., Fyrst H., Saba J., Vogel S.M., Malik A.B., Mehta D. 2008. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 103:1164–1172 10.1161/01.RES.0000338501.84810.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtz N., Olivera A., Bofill-Cardona E., Csonga R., Billich A., Mechtcheriakova D., Bornancin F., Woisetschläger M., Rivera J., Baumruker T. 2004. Early activation of sphingosine kinase in mast cells and recruitment to FcepsilonRI are mediated by its interaction with Lyn kinase. Mol. Cell. Biol. 24:8765–8777 10.1128/MCB.24.19.8765-8777.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Oskeritzian C.A., Pozez A.L., Schwartz L.B. 2005. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J. Immunol. 175:2635–2642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.