Abstract
We have recently demonstrated that peripheral CD8 T cells require two separate activation hits to accumulate to high numbers in the lungs after influenza virus infection: a primary interaction with mature, antigen-bearing dendritic cells (DCs) in the lymph node, and a second, previously unrecognized interaction with MHC I–viral antigen–bearing pulmonary DCs in the lungs. We demonstrate that in the absence of lung-resident DC subsets, virus-specific CD8 T cells undergo significantly increased levels of apoptosis in the lungs; however, reconstitution with pulmonary plasmacytoid DCs and CD8α+ DCs promotes increased T cell survival and accumulation in the lungs. Further, our results show that the absence of DCs after influenza virus infection results in significantly reduced levels of IL-15 in the lungs and that pulmonary DC–mediated rescue of virus-specific CD8 T cell responses in the lungs requires trans-presentation of IL-15 via DC-expressed IL-15Rα. This study demonstrates a key, novel requirement for DC trans-presented IL-15 in promoting effector CD8 T cell survival in the respiratory tract after virus infection, and suggests that this trans-presentation could be an important target for the development of unique antiviral therapies and more effective vaccine strategies.
Clearance of a primary influenza A virus (IAV) infection is known to require killing of virus-infected host cells by activated, antigen-specific CD8 T cells in the lungs (Topham et al., 1997). Until recently, antigen-specific CD8 T cells were thought to undergo programmed activation, whereby a single, brief interaction with a mature, antigen-bearing DC in the LN was sufficient to induce a full program of activation, division, and differentiation from naive to mature, cytotoxic CD8 T cells (Kaech and Ahmed, 2001; Wong and Pamer, 2001). Increasing evidence has suggested, however, that activation of antigen-specific CD8 T cells is not as simple as previously thought, and multiple factors, including cytokine signals such as IL-2 (Wong and Pamer, 2004), IFN-α (Marrack et al., 1999; Price et al., 2000; Kolumam et al., 2005), and IL-12 (Curtsinger et al., 2003a,b; Trinchieri, 2003), and late co-stimulatory signals such as CD70 (Dolfi and Katsikis, 2007) and 4-1BBL (Bertram et al., 2002; Lin et al., 2009), can regulate and fine tune the magnitude and duration of the effector response, as well as the nature of the ensuing memory T cell population.
We have recently demonstrated in a model of IAV infection that the absence of specific pulmonary DC subsets, including plasmacytoid DC (pDCs) and CD8α+ DCs, from the lungs leads to a significant decrease in the number of virus-specific CD8 T cells (McGill et al., 2008). Reconstitution of the lungs with physiological numbers of pDCs or CD8α+ DCs is able to restore the pulmonary IAV-specific CD8 T cell response to near normal levels via a mechanism that is dependent on direct DC–T cell interactions, DC-expressed MHC I, and the presence of viral antigen. Interestingly, however, this rescue is DC subset specific, as reconstitution with purified alveolar and airway DCs (aDCs) or alveolar macrophages (aMϕs) was unable to rescue the virus-specific CD8 T cell response (McGill et al., 2008). After IAV infection there is an abundance of IAV antigen– and MHC I–expressing cells present in the lungs, including infected epithelial cells. Given this fact and the inability of all DC subsets to rescue the virus-specific CD8 T cell response, it suggested that there were additional, undefined requirements for pDC- and CD8α+ DC–mediated rescue of the T cell response in the lungs. Further, it remained unclear what mechanism was contributing to decreased numbers of IAV-specific CD8 T cells in the lungs of aDC-depleted mice: impaired DC migration from the lungs to the LN, impaired CD8 T cell proliferation within the lungs, or impaired CD8 T cell survival within the lungs. It was also unclear what mechanism pulmonary DC subsets were using to rescue this defect.
The cytokine IL-15 has been demonstrated to play a key role in promoting lymphoid homeostasis, particularly with respect to CD8 T cells (Budagian et al., 2006; Kim et al., 2008). IL-15 was initially thought to signal similar to IL-2, whereby IL-15Rα formed a heterotrimeric complex with IL-2/IL15Rβ and common γ for high affinity signaling. Although this model appears to hold true in certain situations, recent reports have demonstrated a unique, alternative signaling mechanism, termed trans-presentation. In this model, IL-15Rα is required for the processing and presentation of active IL-15 in trans to cells expressing the IL-2/IL15Rβ–common γ chain complex (Sandau et al., 2004; Schluns et al., 2004; Kobayashi et al., 2005). At this time, it is unclear which cell types serve as the primary trans-presenting cells during an immune response; however, several lines of evidence have indicated that DCs may play an important role (Burkett et al., 2003, 2004). It is known that DCs express protein for both IL-15 and IL-15Rα, and that stimulation by IFN-αβ (Mattei et al., 2001) or IFN-γ (Doherty et al., 1996; Musso et al., 1999), or exposure to viral infection leads to further up-regulation of these molecules (Liu et al., 2000; Dubois et al., 2005; Budagian et al., 2006; Mattei et al., 2009). Interestingly, DCs matured in the presence of IL-15 have been demonstrated to promote enhanced antigen-specific CD8 T cell proliferation (Jinushi et al., 2003; Mattei et al., 2009) and a robust Th1 skewing in vivo (Pulendran et al., 2004).
IL-15 has been best characterized for its role in maintaining memory CD8 T cell homeostasis, primarily through promoting enhanced basal proliferation (Becker et al., 2002; Goldrath et al., 2002; Schluns et al., 2002). More recently, however, there is accumulating evidence that IL-15 is also important for promoting primary effector CD8 T cell responses (Akbar et al., 1996; Bulfone-Paus et al., 1997; Vella et al., 1998; Schluns et al., 2002; Rausch et al., 2006; Yajima et al., 2006). Surface expression of both IL-15Rα and IL-2/IL15-Rβ is up-regulated after TCR activation (Vella et al., 1998), and IL-15 has been proposed to enhance activated CD8 T cell survival after challenge with staphylococcal enterotoxin A (Vella et al., 1998), Mycobacterium tuberculosis (Rausch et al., 2006), and vesicular stomatitis virus infection (Sandau et al., 2004). Collectively, these studies suggest a particularly important role for IL-15 in the generation and maintenance of an appropriate immune response; however, it remains unclear what role IL-15 plays during the effector phase of the immune response or in what context IL-15 contributes to activated CD8 T cell survival in vivo.
In this study, we demonstrate a previously unrecognized role for pulmonary DC–mediated IL-15 trans-presentation in regulating virus-specific CD8 T cell responses in the lungs after IAV infection. The reduction in T cell numbers observed in the lungs of aDC-depleted mice after IAV challenge results not from impaired proliferation within the lungs but is caused by significantly increased levels of apoptosis of virus-specific CD8 T cells compared with nondepleted controls. Further, reconstitution with purified pDCs or CD8α+ DCs rescues the IAV-specific CD8 T cell response by promoting increased CD8 T cell survival in the lungs of aDC-depleted mice. Additionally, our results show that IAV infection induces up-regulation of both IL-15 mRNA and protein in the lungs and that depletion of aDCs at 48 h post infection (p.i.) results in a significant reduction in pulmonary IL-15 expression. Finally, this study demonstrates that pulmonary DCs prevent virus-specific CD8 T cell apoptosis through trans-presentation of IL-15, as blockade of IL-15 or IL-15Rα on the surface of pulmonary DCs before adoptive transfer, or transfer of IL-15−/− pulmonary DC subsets ablates the rescue of the virus-specific CD8 T cell response in the lungs of aDC-depleted mice.
RESULTS
aDC depletion at 48 h p.i. does not alter proliferation of IAV-specific CD8 T cells in the lungs
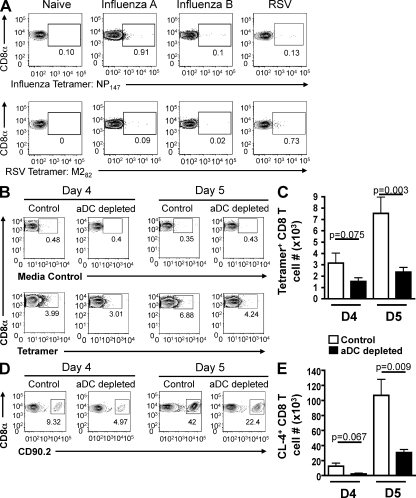
We have previously demonstrated that aDC depletion at 48 h after IAV infection results in increased virus-associated mortality, increased pulmonary viral titers, and significantly reduced numbers of virus-specific CD8 T cells in the lungs on day 6 p.i. (McGill et al., 2008). To determine the mechanism contributing to reduced numbers of CD8 T cells observed in the lungs of aDC-depleted mice, we first extended our previous studies to determine the extent of this T cell defect in the lungs at an earlier time p.i. (i.e., day 4 p.i., when CD8 T cells are first beginning to arrive in the lungs; Lawrence and Braciale, 2004; Lawrence et al., 2005). Given that there are only small numbers of virus-specific CD8 T cells in the lungs at early time points p.i., we first wanted to confirm that tetramer staining was specific and sensitive enough to detect the small frequencies of cells in the lungs at day 4 p.i. Therefore, BALB/c mice were infected with a sublethal dose of IAV, a sublethal dose of influenza type B (which expresses distinct CD8 T cell epitopes that do not cross react with those of IAV), or a sublethal dose of respiratory syncytial virus (RSV). On day 4 p.i., the mice were sacrificed and their lungs were examined for the frequency of CD3+CD8+ CD8 T cells specific for the HA204, HA529 (unpublished data), and NP147 epitopes of IAV as well as the M282 epitope of RSV. A group of naive mice was included as an additional staining control. As seen in Fig. 1 A (top), we observe specific staining of tetramer+ CD8 T cells after IAV infection, with ∼1% of CD8 T cells being specific to the NP147 epitope, and only ∼0.1% of staining in samples from naive, influenza type B– or RSV-infected mice. Further, we observe only background levels of staining in IAV-infected samples using a tetramer containing the RSV M282 epitope, whereas 0.7% of CD8 T cells are specific to this epitope after RSV infection (Fig. 1 A, bottom). Collectively, these results indicate that IAV tetramer staining is both sensitive and specific enough to detect very small numbers of antigen-specific CD8 T cells found in the lungs early after IAV infection. Therefore, we next chose to use this method to examine the defect in virus-specific CD8 T cell numbers in the lungs of aDC-depleted mice at early time points p.i. To this end, BALB/c mice were infected with a sublethal dose of IAV with or without aDC depletion at 48 h p.i., and the total number of pulmonary CD3+CD8+tetramer+ IAV-specific CD8 T cells was examined on days 4 and 5 p.i. As seen in Fig. 1 (B and C), aDC depletion at 48 h p.i. results in reduced numbers of virus-specific CD8 T cells in the lungs on day 4 p.i. and significantly reduced numbers of T cells by day 5 p.i. Similarly, in experiments that used the adoptive transfer of transgenic clone-4 (CL-4) CD8 T cells, which are specific for the HA protein of IAV (McGill et al., 2008), we observed a reduction in the numbers of CL-4 CD8 T cells in the lungs of aDC-depleted host mice relative to nondepleted controls on days 4 and 5 p.i. (Fig. 1, D and E).
Figure 1.
aDC depletion at 48 h p.i. results in reduced numbers of IAV-specific CD8 T cells in the lungs on days 4 and 5 p.i. (A) Groups of mice were infected with either a sublethal dose of IAV, influenza type B, or the A2 strain of RSV. One group of mice remained uninfected. On day 4 p.i., the mice were sacrificed and their lungs were analyzed by flow cytometry for the frequencies of influenza NP147-specific and RSV M282-specific tetramer+ CD8 T cells. Representative FACS plots are gated on CD3+CD8+ T cells. Data are representative of one experiment (n = 2–3 mice/group). (B and C) Groups of BALB/c mice were infected with a sublethal dose of IAV. Half of the mice were aDC depleted at 48 h p.i. (aDC depleted), whereas the other half remained nondepleted (control). On days 4 and 5 p.i., the frequency (B) and number (C) of antigen-specific tetramer+ CD8 T cells in the lungs were enumerated. Representative FACS plots are gated on CD3+CD8+ T cells. Numbers of tetramer+ CD8 T cells in the lungs were determined by subtracting background staining using the media control (B, top). Data are pooled from two separate experiments and represent means ± SEM (n = 5–9 mice/group). (D and E) Influenza-specific, CD90.2+ CL-4 T cells were adoptively transferred to groups of CD90.1+ BALB/c mice as described in Materials and methods. 24 h later, mice were infected with a sublethal dose of IAV. Half of the mice were aDC depleted at 48 h p.i. (aDC depleted), whereas the other half remained nondepleted (control). On days 4 and 5 p.i., the frequency (D) and number (E) of CD90.2+, adoptively transferred CL-4 T cells were determined by flow cytometry. Representative FACS plots are gated on CD3+CD8+ T cells. Data are representative of three separate experiments and represent means ± SEM (n = 3–4 mice/group).
The decrease in virus-specific CD8 T cell numbers observed in the lungs of aDC-depleted mice could be attributed to two potential possibilities: (1) decreased proliferation of antigen-specific CD8 T cells after arriving in the lungs or (2) decreased survival/increased apoptosis of antigen-specific CD8 T cells after arriving in the lungs. Although a potential third mechanism exists whereby virus-specific CD8 T cells are impaired in their ability to home to the lungs in the absence of pulmonary DC subsets, our previous work (McGill et al., 2008) suggests that this is not likely to be the primary mechanism contributing to the decreased pulmonary CD8 T cell numbers. First, we have observed similar numbers of IAV-specific CD8 T cells in the blood, spleens, and nondraining LN of control and aDC-depleted mice. Further, the adoptive transfer of β2M−/− or influenza B antigen–bearing pulmonary DC subsets was not sufficient to rescue the virus-specific T cell response, whereas influenza B antigen–bearing DCs pulsed with IAV antigens were able to rescue the response (McGill et al., 2008). These findings suggest the requirement for a direct interaction between pulmonary DCs and CD8 T cells already present in the lungs rather than a defect in DC-mediated recruitment of CD8 T cells into the lungs from the blood or secondary lymphoid organs.
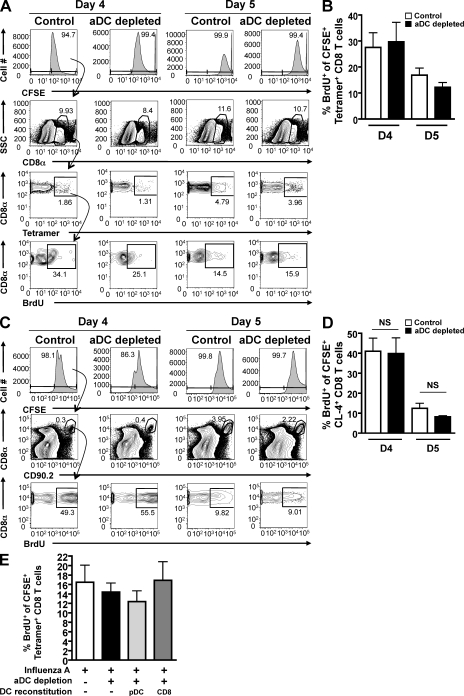
Given the cognate MHC I–viral peptide–dependent nature of pulmonary DC–T cell interactions in the lungs of aDC-depleted mice (McGill et al., 2008), we expected that increased T cell proliferation was the primary mechanism contributing to the rescue of the CD8 T cell response after pulmonary DC reconstitution. Therefore, we first assessed proliferation by virus-specific CD8 T cells in the lungs of aDC-depleted mice compared with nondepleted controls. Our laboratory has previously developed a novel intranasal (i.n.) CFSE/BrdU dual-labeling technique that allows one to specifically assess cell division by T cells in the lungs (McGill and Legge, 2008, 2009). In brief, CFSE was administered i.n. to day 4 and 5 infected control and aDC-depleted mice, followed 2 h later by i.n. BrdU. Using the gating strategy outlined in Fig. 2 A, lung-resident proliferation was assessed 4 h after BrdU administration by measuring BrdU incorporation by CFSE+tetramer+ CD8 T cells present in the lungs at the time of assay. As seen in Fig. 2 B, the frequency of virus-specific CFSE+ CD8 T cells that incorporated BrdU in the lungs during the time of assay was the same between aDC-depleted mice and nondepleted control mice on days 4 and 5 p.i. Further, we observed no significant difference in BrdU incorporation by transgenic CL-4 CD8 T cells in the lungs of aDC-depleted mice compared with control mice on days 4 or 5 p.i. (Fig. 2, C and D). Similarly, no difference in Ki67 staining was observed between the polyclonal virus-specific T cell response from aDC-depleted and nondepleted mice (Fig. S1 C) or between the transgenic CL-4 T cell response from aDC-depleted and nondepleted mice (Fig. S1, A and B). Furthermore, when pDCs and CD8α+ DCs were adoptively transferred into aDC-depleted lungs, a reconstitution that rescues pulmonary virus-specific CD8 T cell numbers in aDC-depleted mice, we still observed no change in the frequency of pulmonary antigen-specific CD8 T cells incorporating BrdU (Fig. 2 E) or expressing Ki67 (Fig. S1, D and E) on day 5 p.i. Collectively, these results suggest that although total numbers of antigen-specific CD8 T cells in the lungs are reduced in aDC-depleted mice compared with nondepleted controls, the proliferative capacity of IAV-specific CD8 T cells is the same between these two groups, and therefore the reduction in CD8 T cell numbers in the lungs is not likely a result of differences in lung-resident T cell expansion.
Figure 2.
aDC depletion at 48 h p.i. does not alter IAV-specific CD8 T cell proliferation in the lungs. (A and B) Groups of BALB/c mice were infected with a sublethal dose of IAV. Half of the mice were subsequently aDC depleted at 48 h p.i. (aDC depleted), whereas the other half remained nondepleted (control). On days 4 and 5 p.i., mice were administered CFSE i.n, followed 2 h later by BrdU i.n. 4 h after BrdU administration, the proliferation, as measured by BrdU incorporation, of lung-resident CFSE+tetramer+ CD8 T cells was assessed by flow cytometry using the gating strategy outlined in A. Data in A and B are representative of two to three separate experiments (n = 5–8 mice/group). (C and D) Influenza-specific, CD90.2+ CL-4 T cells were adoptively transferred to groups of CD90.1+ BALB/c mice as in Fig. 1 (D and E). 24 h later, mice were infected with a sublethal dose of IAV with or without aDC depletion. On days 4 and 5 p.i., mice were treated with i.n. CFSE and BrdU. 4 h after BrdU administration, mice were sacrificed and their lungs were analyzed by flow cytometry for the frequency of BrdU+ cells among CFSE+CD8+CD90.2+ T cells using the representative gating strategy shown in C. Data in C and D are representative of one experiment (n = 3–5 mice/group). (E) Mice were infected and aDC depleted as in A and B; however, groups of aDC-depleted mice were subsequently reconstituted with purified pulmonary pDCs (light gray bars) or CD8α+ DCs (dark gray bars) on day 3 p.i. (i.e., 24 h after depletion). On day 5 p.i., virus-specific CD8 T cell proliferation was determined as in A and B. Data in E are representative of two to three separate experiments (n = 5–8 mice/group). Means ± SEM are shown. No statistical difference was observed between the analyzed groups. SSC, side scatter.
aDC depletion at 48 h p.i. results in increased virus-specific CD8 T cell apoptosis
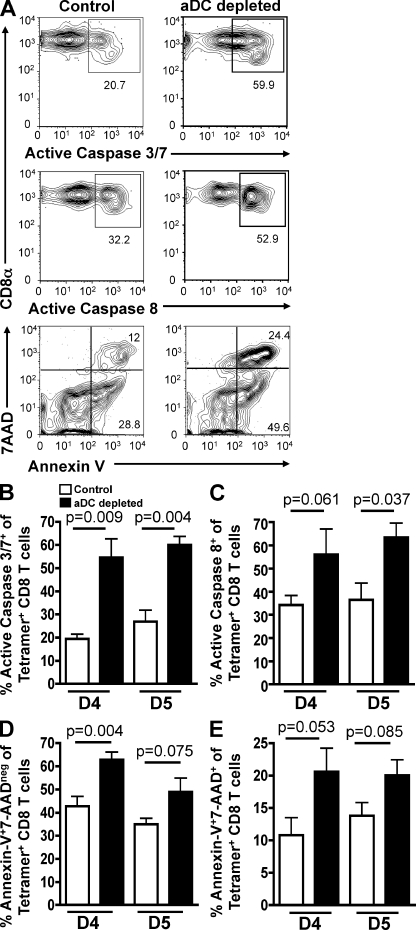
Given that aDC depletion and pulmonary DC reconstitution did not alter the levels of lung-resident, IAV-specific CD8 T cell proliferation during IAV infection, we next wanted to determine if the decrease in virus-specific CD8 T cell numbers in the lungs of aDC-depleted mice was a result of increased CD8 T cell apoptosis after their arrival in the lungs. To this end, BALB/c mice were infected with or without aDC depletion as in Fig. 1 B and assessed for virus-specific CD8 T cell apoptosis using an FAM caspase 3/7 kit on days 4 and 5 p.i. As seen in Fig. 3 (A and B), aDC depletion at 48 h p.i. results in significantly increased frequencies of active caspase 3/7+ pulmonary virus-specific CD8 T cells on both days 4 and 5 p.i., suggesting that aDC-depleted IAV-specific CD8 T cells are undergoing increased levels of apoptosis in the lungs relative to nondepleted control CD8 T cells. Because active caspase 3/7 can also be expressed by activated nonapoptotic CD8 T cells (Alam et al., 1999), we wanted to confirm that the aDC-depleted CD8 T cells were indeed undergoing increased levels of apoptosis and were not simply more activated than their control counterparts. We therefore confirmed our results using the same experimental approach as in Fig. 3 (A [top] and B), but instead assessed apoptosis using an FAM active caspase 8 kit (Fig. 3, A [middle] and C), as well as annexin V/7-AAD staining (Fig. 3 A, bottom) as a measurement of early (Fig. 3 D) and late (Fig. 3 E) stage apoptosis. Similar to the results examining active caspase 3/7 (Fig. 3 B), we observed an increased frequency of caspase 8+ virus-specific CD8 T cells on days 4 and 5 p.i. in aDC-depleted mice. Further, using annexin V/7-AAD staining as a measure of apoptosis, we again observed increased levels of virus-specific CD8 T cell death in the lungs of aDC-depleted mice relative to nondepleted control lungs. In further support of our results examining apoptosis by the polyclonal IAV-specific T cells, we observed a similar increase in apoptotic, transgenic CL-4 CD8 T cells in the lungs of aDC-depleted mice compared with control mice (Fig. S2). Collectively, these results demonstrate that aDC depletion at 48 h p.i. results in increased apoptosis of virus-specific CD8 T cells in the lungs on days 4 and 5 p.i. and suggest that virus-specific CD8 T cells require interactions with pulmonary DCs in the lungs to survive and accumulate to sufficient numbers to control viral infection.
Figure 3.
aDC depletion at 48 h p.i. results in increased apoptosis of IAV-specific CD8 T cells in the lungs. Groups of BALB/c mice were infected with a sublethal dose of IAV with or without aDC depletion as in Fig. 1. On days 4 and 5 p.i., mice were sacrificed and their lungs were analyzed by flow cytometry for apoptosis of tetramer+ CD8 T cells as measured by the frequency of active caspase 3/7+ (A, top; and B), active caspase 8+ (A, middle; and C), and annexin V+7-AADneg and annexin V+7-AAD+tetramer+ CD8 T cells (A, bottom; and D and E). Representative FACS plots from day 4 p.i. are gated on CD3+CD8+tetramer+ CD8 T cells. Data are representative of three to four separate experiments (n = 3–6 mice/group). Means ± SEM are shown.
Pulmonary DC reconstitution promotes increased lung-resident, virus-specific CD8 T cell survival after aDC depletion
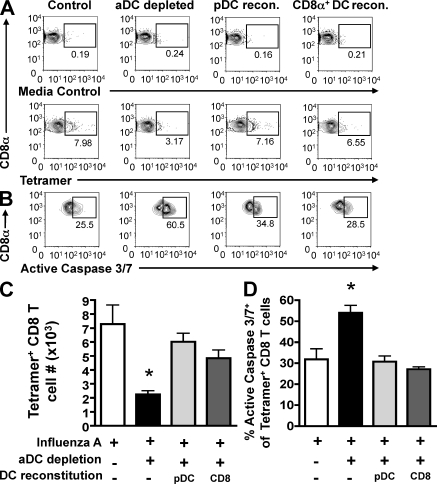
Given our results demonstrating that aDC depletion induced increased apoptosis of virus-specific CD8 T cells in the lungs after IAV infection, we next determined if pulmonary DC reconstitution 24 h after aDC depletion would be sufficient to promote increased survival and accumulation of CD8 T cells in the lungs. Therefore, groups of mice were influenza virus infected with or without aDC depletion. 24 h after DC depletion, mice were reconstituted i.n. with purified pulmonary pDCs or CD8α+ DCs as in Fig. 2 E. On day 5 p.i., the lungs were assessed for frequencies (Fig. 4 A) and total numbers of tetramer+ CD8 T cells (Fig. 4 C), as well as the frequency of tetramer+ CD8 T cell apoptosis as measured by active caspase 3/7 expression (Fig. 4, B and D). Similar to our previous results, we observed a significant rescue in the frequency and number of pulmonary tetramer+ CD8 T cells after reconstitution with purified pDCs or CD8α+ DCs relative to nonreconstituted aDC-depleted control mice (Fig. 4, A and C). Consistent with the rescue of pulmonary IAV-specific CD8 T cell numbers, we also observed a significant reduction in the frequency of apoptotic CD8 T cells in the lungs on day 5 p.i. after reconstitution with either pDCs or CD8α+ DCs (Fig. 4, B and D), with levels of CD8 T cell apoptosis being similar to those of nondepleted control CD8 T cells. A similar trend was also seen when examining the frequency of active caspase 8+ T cells (unpublished data). Collectively, these results suggest that virus-specific CD8 T cell interactions with pulmonary pDCs and CD8α+ DCs provide key survival signals, allowing T cells to accumulate to high numbers in the lungs after IAV infection.
Figure 4.
Pulmonary DC reconstitution of aDC-depleted mice results in decreased apoptosis of IAV-specific CD8 T cells in the lungs. Groups of BALB/c mice were infected with a sublethal dose of IAV with or without aDC depletion as in Fig. 1. On day 3 p.i., groups of aDC-depleted mice were reconstituted i.n. with 2.5 × 104 pulmonary pDCs (light gray bars) or CD8α+ DCs (dark gray bars) purified from the lungs of IAV-infected donors. On day 5 p.i., the frequency (A) and number (C) of tetramer+ CD8 T cells, and the frequency of apoptotic, tetramer+ CD8 T cells (B and D) per lung were measured. Data in A are representative FACS plots gated on CD3+CD8+ T cells. Numbers of tetramer+ CD8 T cells in the lungs were determined by subtracting background staining using the media control (A, top). Data in B are representative FACS plots gated on CD3+CD8+tetramer+ T cells. Data in C and D are pooled from two separate experiments (n = 6 mice/group). Means ± SEM are shown. *, P ≤ 0.05 relative to undepleted control group.
aDC depletion at 48 h p.i. results in decreased pulmonary IL-15 expression
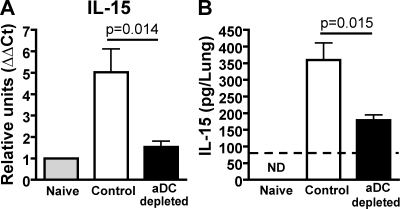
Given our results suggesting that donor pulmonary DCs were positively regulating virus-specific CD8 T cell responses in the lungs of aDC-depleted mice through the promotion of increased CD8 T cell survival, and our previous studies demonstrating that the rescue of IAV-specific, aDC-depleted CD8 T cell responses requires direct DC–T cell interactions, MHC I, viral antigens, and specific DC subsets, we next sought to identify what additional survival signals the pDCs and CD8α+ DCs were providing. Therefore, we performed focused superarray analysis on whole lung homogenates from day 6 infected control and aDC-depleted lungs as well as on purified DC subsets. Interestingly, we observed decreased expression of IL-15 mRNA in aDC-depleted lungs. Further, IL-15 mRNA expression was more robustly expressed by pDCs and CD8α+ DCs (unpublished data), the two DC subsets significantly reduced in the lungs of aDC-depleted mice and known to restore pulmonary CD8 T cell responses upon reconstitution (McGill et al., 2008) relative to other pulmonary APC populations. Similar to our initial array results, quantitative real-time RT-PCR (qRT-PCR) analysis for IL-15 expression by naive, day 6 p.i. control IAV-infected and day 6 p.i. aDC-depleted lung homogenates showed that IAV infection induces an approximately fivefold increase in pulmonary IL-15 mRNA expression relative to naive control lungs (Fig. 5 A). Strikingly, however, aDC depletion at 48 h p.i. resulted in a significant reduction in IL-15 mRNA expression, with expression levels reduced to approximately twofold gene induction over naive (Fig. 5 A). This change in mRNA expression was reflected at the protein level, as we observed a significant reduction in IL-15 protein expression by aDC-depleted lungs relative to nondepleted controls (Fig. 5 B). Collectively, these results demonstrated that aDC depletion at 48 h p.i. results in a significant reduction in the expression of IL-15 message and protein in the lungs during IAV infection.
Figure 5.
aDC depletion at 48 h p.i. results in decreased pulmonary IL-15 mRNA and protein expression. Groups of BALB/c mice were infected with a sublethal dose of IAV with or without aDC depletion as in Fig. 1. (A) On day 6 p.i., lungs were removed and analyzed using qRT-PCR analysis for IL-15 expression. Results were normalized to GAPDH expression, and then to expression of IL-15 mRNA by naive lungs to calculate the ΔΔCt. Data are pooled from two separate experiments and represent means ± SEM (n = 3–6 mice/group). (B) On day 6 p.i., lung homogenates from groups of naive, control, and aDC-depleted mice were analyzed for IL-15 protein expression by ELISA as described in Materials and methods. The dashed line represents the limit of detection of the assay. Data are representative of four separate experiments and represent means ± SEM (n = 5 mice/group). ND, not detected.
Pulmonary pDCs and CD8α+ DCs express IL-15 mRNA and surface IL-15Rα
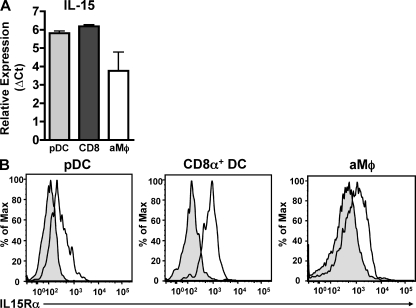
Given the previously described role for IL-15 in promoting effector CD8 T cell responses (Akbar et al., 1996; Bulfone-Paus et al., 1997; Vella et al., 1998; Schluns et al., 2002; Rausch et al., 2006; Yajima et al., 2006), its potential ability to promote CD8 T cell survival (Akbar et al., 1996; Bulfone-Paus et al., 1997; Vella et al., 1998; Rausch et al., 2006; Yajima et al., 2006), and the ability of the purified pulmonary DC subsets that express robust levels of IL-15 mRNA to rescue IAV-specific CD8 T cells from apoptosis in the lungs after aDC depletion, we next determined if these subsets were indeed capable of making and presenting IL-15 to IAV-specific CD8 T cells in the lungs. We first confirmed the results of our arrays by performing qRT-PCR for IL-15 mRNA expression on DC subsets purified from the lungs of day 6 p.i. donors. As seen in Fig. 6 A, both pDCs and CD8α+ DCs express high levels of IL-15 mRNA. In contrast, aMϕs, another prevalent APC population in the lungs during IAV infection that is incapable of rescuing IAV-specific CD8 T cell responses, expressed lower levels of IL-15 mRNA (∼60% of pDCs/CD8α+ DCs).
Figure 6.
Pulmonary pDCs and CD8α+ DCs express IL-15 and IL-15Rα. (A) Groups of BALB/c mice were infected with a sublethal dose of IAV. On day 6 p.i., lungs were pooled and pDCs, CD8α+ DCs, and aMϕs were FACS purified and analyzed by qRT-PCR analysis for IL-15 expression. Results were normalized to GAPDH expression. Data are pooled from three separate experiments and represent means ± SEM. (B) Groups of BALB/c mice were infected with a sublethal dose of IAV. On day 6 p.i., pDCs, CD8α+ DCs, and aMϕs in the lungs were analyzed for surface expression of IL-15Rα (continuous line) versus isotype control (shaded) by flow cytometry. Data are representative of three separate experiments.
Because the ability to trans-present IL-15 to other cells requires surface expression of IL-15Rα, we next verified that both pDCs and CD8α+ DCs expressed detectable levels of the receptor. As seen in Fig. 6 B, pDCs, CD8α+ DCs, and aMϕs all expressed IL-15Rα on their surface, suggesting they could potentially trans-present IL-15 to other cell types.
IL-15 presented on the surface of pulmonary DC subsets promotes increased accumulation of IAV-specific CD8 T cells in the lungs of aDC-depleted mice
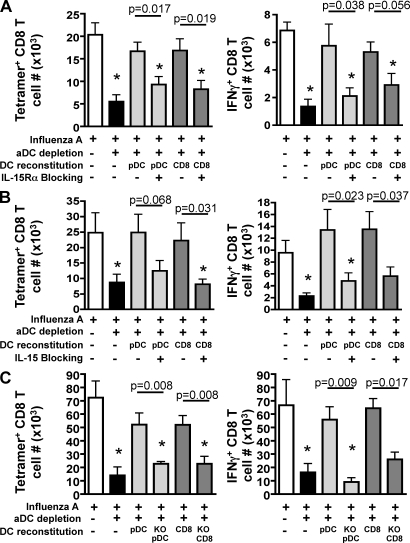
Having demonstrated that pDCs and CD8α+ DCs have the potential to trans-present IL-15 to other cell types, that aDC-depleted lungs express reduced levels of IL-15 protein, and that reconstitution with pDCs or CD8α+ DCs is able to rescue CD8 T cell responses in the lungs of aDC-depleted mice, we next directly determined the role of pulmonary DC–expressed IL-15 and IL-15Rα in our aDC depletion/reconstitution system. To this end, BALB/c mice were infected with a sublethal dose of IAV with or without aDC depletion at 48 h p.i. On day 3 p.i., pulmonary pDCs or CD8α+ DCs were purified and then coated in vitro with anti–mouse IL-15Rα (Fig. 7 A) or IL-15 (Fig. 7 B) blocking antibodies before adoptive transfer into aDC-depleted host mice. As seen in Fig. 7 A, although adoptive transfer of control pDCs or CD8α+ DCs into aDC-depleted lungs promotes increased IAV-specific CD8 T cell responses as measured by tetramer or intracellular staining for IFN-γ, adoptive transfer of pDCs or CD8α+ DCs coated with anti–IL-15Rα blocking antibodies ablates the rescue of pulmonary IAV-specific CD8 T cell responses in the lungs of DC-reconstituted mice. Similarly, using our CL-4 adoptive transfer system, we observed that reconstitution with pDCs or CD8α+ DCs rescued the numbers of virus-specific CL-4 T cells in the lungs, but blockade of IL-15Rα on the DCs before their adoptive transfer prevented the DC-mediated rescue of CL-4 CD8 T cell numbers in the lungs of aDC-depleted mice (Fig. S3). Like the results observed in Fig. 7 A, if pulmonary DC subsets are instead coated with anti–IL-15 blocking antibodies before their transfer into aDC-depleted lungs, there is again an ablation of the rescue effect mediated by control pulmonary pDCs or CD8α+ DCs (Fig. 7 B). Although coating of DCs with IL-15 or IL-15Rα blocking antibodies before their adoptive transfer in vivo could potentially result in their clearance and hence a shortened half-life, we did not observe significant differences in DC recovery from the lungs when comparing uncoated, or anti–IL-15 or anti–IL-15Rα antibody–coated pDCs or CD8α+ DCs (unpublished data). This suggests that differences observed in the ability of the DCs to promote IAV-specific CD8 T cell rescue in this experiment were not a result of enhanced clearance of the DCs in vivo because of Fc-mediated mechanisms.
Figure 7.
Pulmonary pDC– and CD8α+ DC–mediated rescue of IAV-specific CD8 T cell responses from aDC-depleted mice requires IL-15 trans-presentation. Groups of BALB/c mice were infected with a sublethal dose of IAV with or without aDC depletion as in Fig. 1. On day 3 p.i., groups of aDC-depleted mice were reconstituted i.n. with 2.5 × 104 purified pulmonary pDCs (light gray bars) or CD8α+ DCs (dark gray bars) that were left untreated or blocked in vitro with (A) anti–IL-15Rα antibody or (B) anti–IL-15 antibody before adoptive transfer. On day 6 p.i., the number of pulmonary antigen-specific CD8 T cells was enumerated by tetramer staining (left) or ICS for IFN-γ (right). Data are pooled from three separate experiments and represent means ± SEM (n = 9–12 mice/group). (C) Groups of C57BL/6 mice were infected with a sublethal dose of IAV and aDCs depleted at 48 h p.i. (black bars), whereas controls remained undepleted (white bars). On day 3 p.i., groups of aDC-depleted mice were then reconstituted i.n. with 2.5 × 104 purified pulmonary pDCs (light gray bars) or CD8α+ DCs (dark gray bars) that were purified from IAV-infected C57BL/6 IL-15−/− (KO) or wild-type IL-15+/+ donors. On day 7 p.i., the number of pulmonary IAV-specific CD8 T cells was enumerated by tetramer staining (left) or ICS for IFN-γ (right). Data are pooled from two separate experiments and represent means ± SEM (n = 6–7 mice/group). *, P ≤ 0.05 relative to undepleted control group.
However, given this potential limitation of our in vivo antibody blocking approach, we confirmed our results using the adoptive transfer of IL-15−/− pulmonary DC subsets. To this end, we infected wild-type C57BL/6 host mice with a sublethal dose of IAV strain A/PR/8/34 (H1N1). Mice were then aDC depleted at 48 h p.i., followed by i.n. reconstitution on day 3 p.i. with pulmonary pDC or CD8α+ DC subsets purified from the lungs of day 6 A/PR/8/34-infected wild-type or IL-15−/− C57BL/6 donor mice. On day 7 p.i., the numbers of IAV PA224- and NP366-specific CD8 T cells (Fig. 7 C) in the lungs were measured by tetramer staining and intracellular cytokine staining (ICS) for IFN-γ. In agreement with our results using the IL-15 or IL-15Rα blocking antibodies (Fig. 7, A and B; and Fig. S3), reconstitution with IL-15−/− pulmonary DCs ablated donor DC rescue of the IAV-specific CD8 T cell response (Fig. 7 C). Importantly, given that these experiments were performed in IL-15+/+ mice, this trans-presentation must be mediated specifically by pulmonary pDCs or CD8α+ DCs, as the presence of other IL-15–expressing cells in the lungs could not compensate for the absence of the IL-15+/+ pulmonary DC subsets.
IL-15 presented on the surface of pulmonary DC subsets promotes increased survival of IAV-specific CD8 T cells in the lungs of aDC-depleted mice
Given our observation that blocking IL-15 or IL-15Rα on the surface of pDCs and CD8α+ DCs before their adoptive transfer ablated the rescue of influenza-specific CD8 T cell responses in the lungs of aDC-depleted mice, and the documented role of IL-15 in promoting effector CD8 T cell survival in vitro, we next hypothesized that DC IL-15–induced signaling may be providing survival signals to activated CD8 T cells in vivo, therein allowing increased accumulation of IAV-specific CD8 T cell responses in the lungs of aDC-depleted mice. Therefore, we infected BALB/c mice with IAV, depleted aDCs at 48 h p.i., and reconstituted them with purified control pulmonary DC subsets, anti–IL-15 blocking antibody–coated pulmonary DC subsets, or anti–IL-15Rα blocking antibody–coated DC subsets as in Fig. 7 (A and B). On day 5 p.i., the frequency of expression of active caspase 3/7 by tetramer+ CD8 T cells in the lungs was measured as in Figs. 3 and 4. Similar to our previous results, we observed significantly increased levels of apoptosis as measured by an increased frequency of active caspase 3/7+ cells among the lung tetramer+ CD8 T cell population of aDC-depleted mice compared with nondepleted controls (Fig. S4). Reconstitution with IL-15 or IL-15Rα antibody–coated pulmonary pDCs or CD8α+ DCs did not result in a significant reduction in the frequency of apoptotic, IAV-specific CD8 T cells in the lungs of aDC-depleted mice.
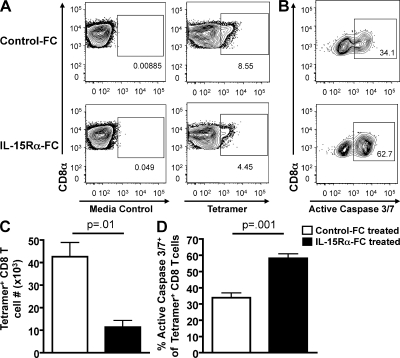
Our results thus far suggest a model whereby pulmonary DCs trans-present IL-15 to effector CD8 T cells to promote T cell survival and accumulation therein. We have examined the role of IL-15 by using an aDC depletion and pulmonary DC reconstitution approach, whereby we eliminate a variety of pulmonary DC–mediated signals and examine the role of IL-15 in this context. We next sought to determine the role of IL-15 alone, in an otherwise pulmonary DC–sufficient environment, in promoting effector CD8 T cell survival after IAV infection. To this end, we chose to block IL-15 presentation in the lungs through the use of recombinant mouse IL-15Rα/Fc fusion protein. Therefore, we infected BALB/c mice with a sublethal dose of IAV, treated groups of mice i.n. on days 3, 4, and 5 p.i. with mouse IL-15Rα/Fc fusion protein or control human Fc fragments, and analyzed the lungs on day 6 p.i. for the frequency (Fig. 8 A) and total number (Fig. 8 C) of tetramer+ IAV-specific CD8 T cells as well as the frequency of apoptotic IAV-specific CD8 T cells (Fig. 8, B and D). In agreement with our results using the aDC depletion/reconstitution system (Fig. 7), the administration of the blocking mouse IL-15Rα/Fc fusion protein resulted in significantly reduced numbers of pulmonary influenza-specific CD8 T cells compared with mice receiving control Fc fragments. Additionally, we observed a significantly increased frequency of IAV-specific CD8 T cell apoptosis in IL-15Rα/Fc chimera–treated lungs relative to control-treated lungs. In agreement with our results blocking IL-15 trans-presentation using the IL-15Rα/Fc chimera, IAV infection of IL-15−/− mice also resulted in significantly reduced frequencies and numbers of IAV-specific CD8 T cells in the lungs (Fig. S5), further confirming an important role for IL-15 trans-presentation in regulating the magnitude of the pulmonary effector CD8 T cell response. Importantly, these results demonstrate a novel role for pulmonary IL-15 signaling in regulating the magnitude of antigen-specific CD8 T cell responses in the lungs after IAV infection, and lend support to our results suggesting that pulmonary DCs are providing these IL-15–mediated signals to CD8 T cells during infection via direct cell-to-cell contact and trans-presentation by IL-15Rα. Collectively, these results suggest that the DC-mediated rescue of IAV-specific CD8 T cells from death in the lungs of aDC-depleted mice requires IL-15 trans-presentation by pulmonary DC subsets. The results further suggest a model whereby IAV-specific CD8 T cells require a two-hit model of priming to accumulate sufficient numbers to control IAV infection, with the first interaction being that of LN priming to initiate programmed activation, differentiation, and proliferation, and with the second interaction being in the lungs with pDCs or CD8α+ DCs bearing MHC I and IAV-derived antigen as well as IL-15Rα trans-presenting IL-15.
Figure 8.
IL-15Rα–Fc administration results in reduced IAV-specific CD8 T cell responses and increased CD8 T cell apoptosis in the lungs after IAV infection. Groups of BALB/c mice were infected with a sublethal dose of IAV. On days 3, 4, and 5 p.i., mice were i.n. administered IL-15Rα–Fc (black bars) or control human Fc fragments (white bars). On day 6 p.i., the lungs were analyzed for (A) the frequency and (C) number of tetramer+ CD8 T cells or (B and D) for apoptosis of tetramer+ CD8 T cells. Representative FACS plots are gated on (A) CD3+CD8+ T cells or (B) CD3+CD8+tetramer+ T cells. Numbers of tetramer+ CD8 T cells in the lungs were determined by subtracting background staining using the media control (A, left). Data are representative of two separate experiments and represent means ± SEM (n = 3–5 mice/group).
DISCUSSION
Our previous studies have demonstrated that the ability to rescue IAV-specific CD8 T cell responses in the lungs of aDC-depleted mice was specific to pulmonary pDCs, CD8α+ DCs, and interstitial DCs, as adoptive transfer of aDCs or aMϕs or the presence of MHC I–viral antigen–bearing epithelial cells in the lungs of aDC-depleted mice is not sufficient to rescue the T cell response therein (McGill et al., 2008). Although it remains unclear why aDCs and aMϕs are not sufficient to rescue IAV-specific CD8 T cell responses in the lungs, our preliminary results showed that aDCs and aMϕs express reduced levels of IL-15 (Fig. 6 and not depicted) when compared with pDCs and CD8α+ DCs, which express high levels of IL-15 mRNA. Further, our preliminary experiments suggest that after addition of exogenous IL-15, adoptively transferred aDCs may be able to rescue IAV-specific CD8 T cell responses in aDC-depleted mice. These results suggest that the reduced levels of IL-15 expression by aMϕs and aDCs may be a key factor in preventing aMϕs and aDCs from rescuing the T cell response in DC-depleted mice. Further, given the abundance of late co-stimulatory molecules and cytokine signals previously demonstrated to regulate the magnitude of IAV-specific CD8 T cell responses, including 4-1BB (Bertram et al., 2002; Lin et al., 2009), OX40L (Humphreys et al., 2003; Bansal-Pakala et al., 2004), CD70 (Halstead et al., 2002; Dolfi and Katsikis, 2007), and IFN-α (Marrack et al., 1999; Price et al., 2000; Kolumam et al., 2005), it is possible that in addition to IL-15, MHC I and viral antigen, pulmonary DCs may provide additional signals that are necessary for the rescue of virus-specific CD8 T cell response in the lungs of aDC-depleted mice.
In addition to the important role that pDCs and CD8α+ DCs play in regulating IAV-specific T cell immunity, Aldridge et al. (2009) have recently demonstrated an important role for TNF/iNOS-producing DCs (tipDCs) in interacting with antigen-specific CD8 T cells after IAV infection (Aldridge et al., 2009). Elimination of tipDC recruitment to the lungs using a CCR2−/− mouse resulted in a significant reduction in the number of virus-specific CD8 T cells in the lungs after infection. Reconstitution of the lungs with purified tipDCs restored the virus-specific CD8 T cell response in the lungs via a mechanism that required viral antigen. These results expand on our previously published findings and suggest that in addition to interstitial DCs, pDCs, and CD8α+ DCs, tipDCs are also capable of interacting with pulmonary CD8 T cells to promote increased effector responses in the lungs. It remains unclear, however, what mechanism is responsible for increased CD8 T cell responses after tipDC reconstitution, and it will be important to determine if tipDCs are likewise promoting increased CD8 T cell survival via IL-15 trans-presentation, or if tipDCs use a unique mechanism to promote CD8 T cell accumulation in the lungs (Aldridge et al., 2009).
Although this study suggests a novel role for pulmonary DC IL-15 trans-presentation to CD8 T cells in the lungs after IAV infection, previous reports have shown that IL-15 can regulate CD8 T cell responses both in vivo and in vitro. For example, IL-15−/− mice undergoing a primary infection by vesicular stomatitis virus have an ∼50% reduction in the CD8 T cell response compared with wild-type controls (Schluns et al., 2002). Similarly, IL-15−/− mice infected with M. tuberculosis exhibit significantly reduced numbers of CD8 T cells in the lungs and mediastinal LN (Rausch et al., 2006). Importantly, although these cells were shown to undergo similar rates of proliferation compared with wild-type CD8 T cells as measured by BrdU incorporation, annexin V staining revealed a significant increase in the level of apoptosis in IL-15−/− CD8 T cells relative to wild-type controls (Rausch et al., 2006). These results are in accordance with several other reports suggesting that IL-15 is crucial for promoting the survival of activated mouse CD8 T cells both in vitro and in vivo. These include experiments demonstrating the importance of IL-15 after cytokine starvation induced by IL-2 withdrawal (Akbar et al., 1996), after in vitro activation with the superantigen staphylococcal enterotoxin A (Vella et al., 1998), and after systemic administration of the anti-Fas monoclonal antibody Jo2 (Bulfone-Paus et al., 1997). Interestingly, however, IL-15−/− mice infected with lymphocytic choriomeningitis virus mount a robust primary CTL response (Becker et al., 2002). Collectively, these results suggest that the requirement for IL-15 by effector CD8 T cells may differ depending on the infectious agent and type of ensuing immune response.
At this time, the molecular mechanisms regulating the IL-15–mediated rescue of virus-specific CD8 T cells by pulmonary DC subsets in the lungs remain unclear. Previous reports examining the molecular basis for IL-15–mediated protection from apoptosis after IL-2 withdrawal in vitro suggested that although IL-15 signaling did not inhibit the expression of the proapoptotic molecules Bax or Bcl-xS (Akbar et al., 1996), it promoted strong induction of the important antiapoptotic molecules Bcl-2 and Bcl-xL (Akbar et al., 1996; Yajima et al., 2006). Bcl-2 has been demonstrated to play a critical role in activated CD8 T cell survival, and the balance between proapoptotic Bim and Bcl-2 is key in regulating activated CD8 T cell survival in a variety of model systems (Marrack and Kappler, 2004). In accordance with these findings, our preliminary results suggest that pulmonary aDC-depleted virus-specific CD8 T cells express lower levels of Bcl-2 but increased levels of Bim compared with CD8 T cells from non-aDC–depleted mice (unpublished data). Collectively, these results indicate that the increased apoptosis of aDC-depleted T cells may result from an imbalance in the Bim/Bcl-2 ratio. Further, IL-15–mediated signaling may be one of the important factors promoting Bcl-2 up-regulation after T cell activation, thereby determining subsequent survival or apoptosis of CD8 T cells. In the future, it will be important to perform a more detailed molecular analysis to determine the role of IL-15 in promoting virus-specific CD8 T cell survival in the lungs after IAV infection.
In summary, we have demonstrated that aDC depletion at 48 h p.i. results in increased apoptosis of virus-specific CD8 T cells in the lungs and significantly reduced levels of pulmonary IL-15 expression after IAV infection. The defect in CD8 T cell survival can be rescued by reconstitution of pulmonary pDCs and CD8α+ DCs via a mechanism that requires direct DC trans-presentation of IL-15 to IAV-specific CD8 T cells in the lungs. These novel findings suggest a key checkpoint in regulating effector T cell survival during pulmonary immune responses, and demonstrate an important and previously unrecognized role for DC-presented IL-15 in regulating the magnitude of virus-specific CD8 T cell responses in the lungs, and therein lend further support to the recently proposed two-hit model of T cell activation (McGill et al., 2008).
MATERIALS AND METHODS
Mice.
6–12-wk-old female BALB/c mice and 6–12-wk-old male and female C57BL/6 mice were purchased from the National Cancer Institute. C57BL/6 IL-15−/− (C57BL/6NTac-IL15tm1Imx) mice were purchased from Taconic. CL-4 TCR transgenic mice (H-2d; CD90.1+) were purchased from the Jackson Laboratory, and CL-4 TCR transgenic mice (H-2d; CD90.2+) were a gift from L. Sherman (The Scripps Research Institute, La Jolla, CA). Both CL-4 transgenic strains are specific to the HA533-541 epitope of H1 and H2 IAVs. BALB/c CD90.1+ congenic mice were a gift of R. Enelow (Dartmouth College, Hanover, NH) and J. Harty (University of Iowa, Iowa City, IA). All mice were housed, bred, and maintained in the animal care facility at the University of Iowa. Experiments were conducted according to federal and institutional guidelines and were approved by the University of Iowa Animal Care and Use Committee.
Virus infection.
Mouse-adapted IAVs A/PR/8/34 and A/JAPAN/305/57 and influenza B virus B/Lee/40 were grown in the allantoic fluid of 10-d-old embryonated chicken eggs for 2 d at 37°C, as previously described (Legge and Braciale, 2005). Allantoic fluid was harvested and stored at −80°C. The A2 strain of RSV was a gift from B.S. Graham (National Institutes of Health, Bethesda, MD) and was propagated in HEp-2 cells as previously described (Olson and Varga, 2007). BALB/c mice were anesthetized with isofluorane and infected i.n. with either 5,875 or 587 tissue culture infectious units (TCIU) of mouse-adapted A/JAPAN/305/57, 7.50 × 104 egg infectious units of mouse-adapted influenza B/Lee/40, or 3 × 106 PFU of the A2 strain of RSV, as previously described (Legge and Braciale, 2005; Olson and Varga, 2007). C57BL/6 mice were anesthetized with isofluorane and infected i.n. with 1,066 TCIU of A/PR/8/34 in 50 µl of Iscove’s media, as previously described (Legge and Braciale, 2005).
Clodronate-liposome treatment.
Pulmonary DC and macrophage depletion was performed by treatment with liposomes containing dichloromethylene bisphosphonate (clodronate; McGill et al., 2008). Clodronate was a gift of Roche (Mannheim, Germany). It was encapsulated in liposomes as previously described (Van Rooijen and Sanders, 1994). Phosphatidylcholine (LIPOID E PC) was obtained from Lipoid GmbH. Cholesterol was purchased from Sigma-Aldrich. At 48 h p.i., mice were anesthetized by isofluorane inhalation and administered 75 µl of clodronate-liposomes or PBS-liposomes i.n.
Peptides.
Influenza virus peptides HA204-212 (LYQNVGTYV), HA529-537 (IYATVAGSL), and NP147-155 (TYQRTRALV) for BALB/c, and PA224 (SSLENFRAYV) and NP366 (ASNENMETM) for C57BL/6 were synthesized by BioSynthesis Inc.
MHC I tetramers.
IAV tetramers HA204 (H-2K(d)/LYQNVGTYV), HA529 (H-2K(d)/IYATVAGSL), and NP147 (H2K(d)/TYQRTRALV) for BALB/c, and PA224 (H2D(b)/SSLENFRAYV) and NP366 (H2D(b)/ASNENMETM) for C57BL/6, or RSV tetramer M282 (H-2K(d)/SYGSINNI) were obtained from the National Institutes of Health Tetramer Core Facility.
Preparation of cells.
Lungs were pressed through wire mesh to obtain a single-cell suspension, which was then enumerated by trypan blue exclusion. For DC preparations, lungs were digested for 25 min at 25°C in media containing 1 mg/ml collagenase (Sigma-Aldrich) and 0.02 mg/ml DNase (Sigma-Aldrich) before single-cell preparation. DCs for reconstitution experiments were purified from the lungs of day 6 A/JAPAN/305/57- or A/PR/8/34-infected BALB/c or IL-15+/+ and IL-15−/− C57BL/6 donors, respectively, using MACS technology (Miltenyi Biotec) according to the manufacturer’s instructions, or by FACS as previously described (McGill et al., 2008). Purified cells were resuspended in Iscove’s DMEM, and 2.5 × 104 cells in 50 µl were adoptively transferred i.n. to host mice on day 3 p.i. (McGill et al., 2008).
Purification and adoptive transfer of CL-4 T cells.
Single-cell suspensions of splenic cells from CL-4 mice were labeled with CD8α microbeads and purified according to the manufacturer’s instructions (Miltenyi Biotec). 5 × 105 purified CD90.2+ CL-4 cells were then adoptively transferred i.v. into BALB/c CD90.1+ congenic mice, or 5 × 105 purified CD90.1+ CL-4 cells were adoptively transferred i.v. into BALB/c CD90.2+ mice. 24 h later, the host mice were infected with influenza as described.
Antibodies and reagents.
The following reagents were used for these studies: rat anti–mouse CD3ε (145-2C11), rat anti–mouse CD8α (53-6.7), rat anti–mouse IFN-γ (XMG1.2), rat anti–mouse IA/IE (M5/114.15.2), rat anti–mouse CD11b (M1/70), mouse anti–rat CD90.1 (OX-7), hamster anti–mouse CD11c (HL3), mouse anti–human Ki67 (B56), and 7-AAD purchased from BD; mouse anti–mouse CD90.2 (5a-8), rat anti–mouse CD45R (RA3-6B2), and recombinant human annexin V purchased from Invitrogen; and polyclonal goat anti–mouse IL-15Rα and polyclonal goat anti–mouse IL-15 purchased from R&D Systems. i.n. CFSE labeling was performed as previously described (Legge and Braciale, 2003; McGill and Legge, 2008, 2009). BrdU incorporation was assessed using a BrdU Flow kit (BD) according to the manufacturer’s instructions, as previously described (McGill and Legge, 2008, 2009). FAM caspase 3/7 and caspase 8 kits (Vybrant) were purchased from Invitrogen and used according to the manufacturer’s instructions. For surface staining, 106 isolated cells were stained with antibody or tetramer for 30 min at 4°C and then fixed using FACS Lysing Solution (BD). For ICS, fixed cells were permeabilized and labeled with antibodies in FACS buffer containing 0.5% saponin (Acros Organics) for 1 h at 4°C. All flow cytometry data were acquired on a FACSCalibur or FACSCanto II (both from BD) and analyzed using FlowJo software (Tree Star, Inc.).
IL-15 ELISA.
The IL-15 ELISA Duo kit was purchased from R&D Systems. Control and aDC-depleted lungs were harvested on day 6 p.i., homogenized in 0.5 ml of ELISA diluent (1% BSA in PBS) plus Complete Mini EASYpack Protease Inhibitor Cocktail Tablets (Roche), and plated in 100-µl aliquots. ELISA plates were incubated overnight at 4°C. ELISAs were read according to the manufacturer’s instructions.
qRT-PCR analysis for IL-15 mRNA expression.
Total RNA was isolated from whole-lung homogenates or from FACS-purified pulmonary DC subsets using TRIzol according to the manufacturer’s instructions (Invitrogen). cDNA was transcribed from 1 µg RNA using 1 µM 18-mer Oligo dT primer and 1 U/µl AMV RT (Promega) in the presence of 1 U/µl RNase OUT (Invitrogen), 10 mM dNTP, 25 mM MgCl, and 10× ThermoStart PCR reaction buffer (Thermo Fisher Scientific) at 42°C for 60 min in a 20-µl reaction volume. The qRT-PCR was performed using a sequence detector apparatus (ABI Prism 7000; Applied Biosystems) for 40 cycles in a two-temperature profile: a denaturation period of 15 s at 94°C followed by a 1-min annealing/extension period at 60°C. All PCR reactions were performed in 25-µl final volumes comprising the following components: 100 ng cDNA, TaqMan Universal PCR Master Mix, 450 nM of primers, and 125 nM of probes. The following primers and probes were used (Overbergh et al., 1999; Abebe et al., 2006): IL-15 forward, 5′-CATCCATCTCGTGCTACTTGTGTT-3′; IL-15 reverse, 5′-CATCTATCCAGTTGGCCTCTGTTT-3′; IL-15 probe, FAM-AGGGAGACCTACACTGACACAGCCCAAAA-BHQ; GAPDH forward, 5′-TTCACCACCATGGAGAAGGC-3′; GAPDH reverse, 5′-GGCATGGACTGTGGTCATGA-3′; GAPDH probe, FAM-TGCATCCTGCACCACCAACTGCTTAG-BHQ.
Comparisons were made by the comparative threshold cycle method, with GAPDH serving as the comparator. For whole-lung homogenates, data are presented as fold change relative to naive lung samples, which are set to a value of 1.
IL-15Rα/Fc treatment.
The recombinant mouse IL-15Rα/Fc chimera was purchased from R&D Systems, and control human Fc fragments were purchased from the Jackson Laboratory. Mice were anesthetized using isofluorane and administered 5 µg IL-15Rα–Fc or control-Fc i.n. on days 3, 4, and 5 p.i.
Statistical analysis.
Statistical significance of the difference between two sets of data was assessed using an unpaired, one-tailed t test or a paired t test for control and experimental data groups that could be paired. Differences were considered to be statistically significant at P < 0.05.
Online supplemental material.
Fig. S1 depicts expression of the transcription factor Ki67 by endogenous polyclonal and transgenic virus-specific CD8 T cells in the lungs of control, aDC-depleted, and pulmonary DC–reconstituted mice at various time points p.i. Fig. S2 depicts the frequency of CL-4 IAV-specific CD8 T cells undergoing apoptosis in the lungs after aDC depletion. Fig. S3 shows the effect of blocking pulmonary DC–expressed IL-15Rα on the numbers of CL-4 CD8 T cells in the lungs of aDC-depleted mice. Fig. S4 depicts the effects of blocking pulmonary DC–expressed IL-15 or IL-15Rα on virus-specific CD8 T cell apoptosis in the lungs of aDC-depleted mice. Fig. S5 shows the frequency and numbers of antigen-specific CD8 T cells present in the lungs of wild-type and IL-15−/− mice after IAV infection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091711/DC1.
Supplementary Material
Acknowledgments
We thank Drs. J. Harty, S. Varga, and T. Waldschmidt for critical reading of this manuscript.
This work was supported by grants from the National Institutes of Health (AI071085 and AI076989 to K.L. Legge).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- aDC
- alveolar and airway DC
- aMϕ
- alveolar macrophage
- CL-4
- clone-4
- IAV
- influenza A virus
- ICS
- intracellular cytokine staining
- i.n.
- intranasal
- pDC
- plasmacytoid DC
- p.i.
- post infection
- qRT-PCR
- quantitative real-time RT-PCR
- RSV
- respiratory syncytial virus
- tipDC
- TNF/iNOS-producing DC
References
- Abebe F., Mustafa T., Nerland A.H., Bjune G.A. 2006. Cytokine profile during latent and slowly progressive primary tuberculosis: a possible role for interleukin-15 in mediating clinical disease. Clin. Exp. Immunol. 143:180–192 10.1111/j.1365-2249.2005.02976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A.N., Borthwick N.J., Wickremasinghe R.G., Panayoitidis P., Pilling D., Bofill M., Krajewski S., Reed J.C., Salmon M. 1996. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 26:294–299 10.1002/eji.1830260204 [DOI] [PubMed] [Google Scholar]
- Alam A., Cohen L.Y., Aouad S., Sékaly R.P. 1999. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 190:1879–1890 10.1084/jem.190.12.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J.R., Jr., Moseley C.E., Boltz D.A., Negovetich N.J., Reynolds C., Franks J., Brown S.A., Doherty P.C., Webster R.G., Thomas P.G. 2009. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. USA. 106:5306–5311 10.1073/pnas.0900655106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal-Pakala P., Halteman B.S., Cheng M.H., Croft M. 2004. Costimulation of CD8 T cell responses by OX40. J. Immunol. 172:4821–4825 [DOI] [PubMed] [Google Scholar]
- Becker T.C., Wherry E.J., Boone D., Murali-Krishna K., Antia R., Ma A., Ahmed R. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram E.M., Lau P., Watts T.H. 2002. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 168:3777–3785 [DOI] [PubMed] [Google Scholar]
- Budagian V., Bulanova E., Paus R., Bulfone-Paus S. 2006. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 17:259–280 10.1016/j.cytogfr.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Bulfone-Paus S., Ungureanu D., Pohl T., Lindner G., Paus R., Rückert R., Krause H., Kunzendorf U. 1997. Interleukin-15 protects from lethal apoptosis in vivo. Nat. Med. 3:1124–1128 10.1038/nm1097-1124 [DOI] [PubMed] [Google Scholar]
- Burkett P.R., Koka R., Chien M., Chai S., Chan F., Ma A., Boone D.L. 2003. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. USA. 100:4724–4729 10.1073/pnas.0737048100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett P.R., Koka R., Chien M., Chai S., Boone D.L., Ma A. 2004. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 200:825–834 10.1084/jem.20041389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J.M., Johnson C.M., Mescher M.F. 2003a. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165–5171 [DOI] [PubMed] [Google Scholar]
- Curtsinger J.M., Lins D.C., Mescher M.F. 2003b. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197:1141–1151 10.1084/jem.20021910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T.M., Seder R.A., Sher A. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156:735–741 [PubMed] [Google Scholar]
- Dolfi D.V., Katsikis P.D. 2007. CD28 and CD27 costimulation of CD8+ T cells: a story of survival. Adv. Exp. Med. Biol. 590:149–170 10.1007/978-0-387-34814-8_11 [DOI] [PubMed] [Google Scholar]
- Dubois S.P., Waldmann T.A., Müller J.R. 2005. Survival adjustment of mature dendritic cells by IL-15. Proc. Natl. Acad. Sci. USA. 102:8662–8667 10.1073/pnas.0503360102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Sivakumar P.V., Glaccum M., Kennedy M.K., Bevan M.J., Benoist C., Mathis D., Butz E.A. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522 10.1084/jem.20020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead E.S., Mueller Y.M., Altman J.D., Katsikis P.D. 2002. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat. Immunol. 3:536–541 10.1038/ni798 [DOI] [PubMed] [Google Scholar]
- Humphreys I.R., Walzl G., Edwards L., Rae A., Hill S., Hussell T. 2003. A critical role for OX40 in T cell–mediated immunopathology during lung viral infection. J. Exp. Med. 198:1237–1242 10.1084/jem.20030351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M., Takehara T., Tatsumi T., Kanto T., Groh V., Spies T., Suzuki T., Miyagi T., Hayashi N. 2003. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J. Immunol. 171:5423–5429 [DOI] [PubMed] [Google Scholar]
- Kaech S.M., Ahmed R. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat. Immunol. 2:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.R., Hwang K.A., Park S.H., Kang I. 2008. IL-7 and IL-15: biology and roles in T-cell immunity in health and disease. Crit. Rev. Immunol. 28:325–339 [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Dubois S., Sato N., Sabzevari H., Sakai Y., Waldmann T.A., Tagaya Y. 2005. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 105:721–727 10.1182/blood-2003-12-4187 [DOI] [PubMed] [Google Scholar]
- Kolumam G.A., Thomas S., Thompson L.J., Sprent J., Murali-Krishna K. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637–650 10.1084/jem.20050821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C.W., Braciale T.J. 2004. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J. Immunol. 173:1209–1218 [DOI] [PubMed] [Google Scholar]
- Lawrence C.W., Ream R.M., Braciale T.J. 2005. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J. Immunol. 174:5332–5340 [DOI] [PubMed] [Google Scholar]
- Legge K.L., Braciale T.J. 2003. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 18:265–277 10.1016/S1074-7613(03)00023-2 [DOI] [PubMed] [Google Scholar]
- Legge K.L., Braciale T.J. 2005. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 23:649–659 10.1016/j.immuni.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Lin G.H., Sedgmen B.J., Moraes T.J., Snell L.M., Topham D.J., Watts T.H. 2009. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J. Immunol. 182:934–947 [DOI] [PubMed] [Google Scholar]
- Liu T., Nishimura H., Matsuguchi T., Yoshikai Y. 2000. Differences in interleukin-12 and -15 production by dendritic cells at the early stage of Listeria monocytogenes infection between BALB/c and C57 BL/6 mice. Cell. Immunol. 202:31–40 10.1006/cimm.2000.1644 [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. 2004. Control of T cell viability. Annu. Rev. Immunol. 22:765–787 10.1146/annurev.immunol.22.012703.104554 [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J., Mitchell T. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521–530 10.1084/jem.189.3.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei F., Schiavoni G., Belardelli F., Tough D.F. 2001. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 167:1179–1187 [DOI] [PubMed] [Google Scholar]
- Mattei F., Bracci L., Tough D.F., Belardelli F., Schiavoni G. 2009. Type I IFN regulate DC turnover in vivo. Eur. J. Immunol. 39:1807–1818 10.1002/eji.200939233 [DOI] [PubMed] [Google Scholar]
- McGill J., Legge K.L. 2008. Continued proliferation of influenza-specific T cells in the lungs during the early stages of influenza virus infections. Katz J.M., editor Options for the Control of Influenza. International Medical Press, London: 162–164 [Google Scholar]
- McGill J., Legge K.L. 2009. Cutting edge: contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J. Immunol. 183:4177–4181 10.4049/jimmunol.0901109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill J., Van Rooijen N., Legge K.L. 2008. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J. Exp. Med. 205:1635–1646 10.1084/jem.20080314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso T., Calosso L., Zucca M., Millesimo M., Ravarino D., Giovarelli M., Malavasi F., Ponzi A.N., Paus R., Bulfone-Paus S. 1999. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 93:3531–3539 [PubMed] [Google Scholar]
- Olson M.R., Varga S.M. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 179:5415–5424 [DOI] [PubMed] [Google Scholar]
- Overbergh L., Valckx D., Waer M., Mathieu C. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 11:305–312 10.1006/cyto.1998.0426 [DOI] [PubMed] [Google Scholar]
- Price G.E., Gaszewska-Mastarlarz A., Moskophidis D. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996–4003 10.1128/JVI.74.9.3996-4003.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Dillon S., Joseph C., Curiel T., Banchereau J., Mohamadzadeh M. 2004. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur. J. Immunol. 34:66–73 10.1002/eji.200324567 [DOI] [PubMed] [Google Scholar]
- Rausch A., Hessmann M., Hölscher A., Schreiber T., Bulfone-Paus S., Ehlers S., Hölscher C. 2006. Interleukin-15 mediates protection against experimental tuberculosis: a role for NKG2D-dependent effector mechanisms of CD8+ T cells. Eur. J. Immunol. 36:1156–1167 10.1002/eji.200535290 [DOI] [PubMed] [Google Scholar]
- Sandau M.M., Schluns K.S., Lefrancois L., Jameson S.C. 2004. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J. Immunol. 173:6537–6541 [DOI] [PubMed] [Google Scholar]
- Schluns K.S., Williams K., Ma A., Zheng X.X., Lefrançois L. 2002. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827–4831 [DOI] [PubMed] [Google Scholar]
- Schluns K.S., Klonowski K.D., Lefrançois L. 2004. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 103:988–994 10.1182/blood-2003-08-2814 [DOI] [PubMed] [Google Scholar]
- Topham D.J., Tripp R.A., Doherty P.C. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197–5200 [PubMed] [Google Scholar]
- Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146 10.1038/nri1001 [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 174:83–93 10.1016/0022-1759(94)90012-4 [DOI] [PubMed] [Google Scholar]
- Vella A.T., Dow S., Potter T.A., Kappler J., Marrack P. 1998. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 95:3810–3815 10.1073/pnas.95.7.3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P., Pamer E.G. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 166:5864–5868 [DOI] [PubMed] [Google Scholar]
- Wong P., Pamer E.G. 2004. Disparate in vitro and in vivo requirements for IL-2 during antigen-independent CD8 T cell expansion. J. Immunol. 172:2171–2176 [DOI] [PubMed] [Google Scholar]
- Yajima T., Yoshihara K., Nakazato K., Kumabe S., Koyasu S., Sad S., Shen H., Kuwano H., Yoshikai Y. 2006. IL-15 regulates CD8+ T cell contraction during primary infection. J. Immunol. 176:507–515 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.