Abstract
Adoptive transfer of large numbers of tumor-reactive CD8+ cytotoxic T lymphocytes (CTLs) expanded and differentiated in vitro has shown promising clinical activity against cancer. However, such protocols are complicated by extensive ex vivo manipulations of tumor-reactive cells and have largely focused on CD8+ CTLs, with much less emphasis on the role and contribution of CD4+ T cells. Using a mouse model of advanced melanoma, we found that transfer of small numbers of naive tumor-reactive CD4+ T cells into lymphopenic recipients induces substantial T cell expansion, differentiation, and regression of large established tumors without the need for in vitro manipulation. Surprisingly, CD4+ T cells developed cytotoxic activity, and tumor rejection was dependent on class II–restricted recognition of tumors by tumor-reactive CD4+ T cells. Furthermore, blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on the transferred CD4+ T cells resulted in greater expansion of effector T cells, diminished accumulation of tumor-reactive regulatory T cells, and superior antitumor activity capable of inducing regression of spontaneous mouse melanoma. These findings suggest a novel potential therapeutic role for cytotoxic CD4+ T cells and CTLA-4 blockade in cancer immunotherapy, and demonstrate the potential advantages of differentiating tumor-reactive CD4+ cells in vivo over current protocols favoring in vitro expansion and differentiation.
Although CD4+ T cells are critical for orchestrating immunological processes, cancer immunotherapy has focused primarily on tumor-reactive CD8+ CTLs, largely because of their capacity to directly engage and kill transformed cells. Adoptive cellular therapy (ACT) using large numbers of tumor-reactive CD8+ CTLs expanded and differentiated in vitro has shown significant clinical promise after lymphoablative conditioning, where responses occur in up to 70% of patients from whom tumor-reactive lymphocytes can be isolated (Dudley et al., 2008; Muranski and Restifo, 2009). Furthermore, the use of gene therapy to introduce tumor-reactive TCRs in autologous lymphocytes has been used as an option for those patients from whom tumor-reactive lymphocytes cannot be isolated (Morgan et al., 2006; Burns et al., 2009). However, these strategies are all complicated by ex vivo manipulations and complex mixtures of cytokines and growth factors required to prevent their terminal differentiation, and to allow them to maintain a pool of effector and memory tumor-reactive T cells after transfer in vivo (Gattinoni et al., 2005b, 2009).
Although the role of CD4+ Th cells in enhancing and sustaining CD8+ T cell responses is well established (Pardoll and Topalian, 1998; Antony et al., 2005), recent evidence suggests more direct roles, lending support to the use of tumor-reactive CD4+ T cells for cancer immunotherapy (Mumberg et al., 1999; Corthay et al., 2005; Perez-Diez et al., 2007). After expansion, in vitro, Th17-polarized tumor-reactive CD4+ cells are capable of rejecting established melanoma (Muranski et al., 2008). Furthermore, adoptive transfer of a large number of CD4+ T cells expanded from a single tumor-reactive T cell clone resulted in a complete response lasting 2 yr in a melanoma patient (Hunder et al., 2008), although the precise cellular mechanisms regulating and driving tumor rejection remain unclear. Despite these encouraging data, incorporation of CD4+ T cells into ACT protocols remains challenging because of technical difficulties in acquiring and expanding helper cells to the numbers thought necessary for ACT (Muranski and Restifo, 2009). Interestingly, a growing body of evidence supports the idea that activation and differentiation of small numbers of adoptively transferred antigen-specific T cells in vivo can result in efficacious long-lived immunity (Hataye et al., 2006; Blair and Lefrançois, 2007), and that these responses can be further enhanced by host conditioning (Gonzalez et al., 2002; Rizzuto et al., 2009). Similar strategies could greatly affect ACT, perhaps circumventing the problems associated with priming, expansion, and differentiation of tumor-reactive CD4+ T cells in vitro. A greater understanding of the role of CD4+ T cells and of the mechanisms regulating their function in adoptive cancer immunotherapy may therefore inform and enhance the development of these approaches.
We used a CD4+ TCR transgenic (Tg) mouse specific for a melanoma differentiation antigen (tyrosinase-related protein 1 [Trp1]) as a model for the study of self-/tumor-reactive CD4+ T cells during cancer immunotherapy (Muranski et al., 2008). We demonstrate that transfer of very small numbers of naive tumor-/self-reactive CD4+Trp1+ cells into irradiated recipients is followed by marked expansion and, most importantly, differentiation into cytotoxic CD4+ T cells, which efficiently eliminated established melanoma tumors in a mouse model of advanced disease. Furthermore, combination of this CD4-ACT protocol with blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on T cells resulted in increased expansion of effector T (Teff) cells, reduced numbers of T reg cells, higher IFN-γ levels, heightened in vivo cytotoxicity, and improved antitumor activity. Additional experiments testing the efficacy of this novel combinatorial approach demonstrated regression of large melanoma lesions in a mouse model of autochthonous spontaneous melanoma, underlining the potential significance of our findings.
These results highlight the relevance of the CD4+ T cell compartment, and particularly of cytotoxic CD4+ T cells, in cancer immunotherapy, while providing evidence that T cell differentiation in vivo may afford advantages over current approaches using differentiation and expansion in vitro. Finally, the data inform the rational development of combinatorial strategies incorporating CD4-ACT and CTLA-4 blockade to generate more potent and durable antitumor responses.
RESULTS
Transfer of small numbers of CD4+Trp1+ T cells in combination with radiation and CTLA-4 blockade produces potent rejection of established B16/BL6 melanoma tumors
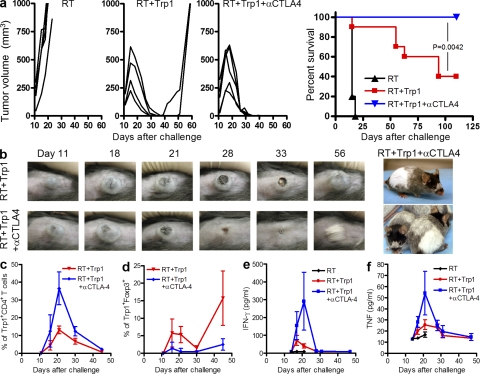
To determine whether activation and differentiation of small numbers of naive tumor-reactive CD4+ T cells in vivo could drive rejection of established tumors, we used naive CD4+Trp1+ cells isolated from Trp1 Tg mice. C57BL/6 mice were challenged with a lethal dose (2.5 × 105 cells) of the poorly immunogenic B16/BL6 mouse melanoma line. 10–12 d after challenge, when tumors were clearly visible (Fig. 1 b), mice received 5 Gy of irradiation (radiation therapy [RT]) followed by adoptive transfer of naive CD4+Trp1+ cells (CD4-ACT). To assess the potential combinatorial efficacy of CTLA-4 blockade and CD4-ACT, a cohort of mice also received anti–CTLA-4 mAb. RT by itself failed to induce regression of established tumors (Fig. 1 a). Additional controls including anti–CTLA-4 + RT or anti–CTLA-4 + CD4+Trp1+ also failed to induce regression (unpublished data). Remarkably, transfer of as few as 50,000 CD4+Trp1+ T cells into irradiated hosts was sufficient to induce initial regression of large established melanoma tumors and depigmentation in all mice (Fig. 1 a). However, in the absence of anti–CTLA-4, the combination of RT and CD4-ACT failed to eradicate tumors completely, and tumors recrudesced in up to 60% of mice (Fig. 1 a). The addition of anti–CTLA-4 produced long-term protection in all mice (Fig. 1 a), as well as development of disseminated depigmentation (Fig. 1 b, far right). Analysis of T cell dynamics in the blood of treated mice showed rapid expansion of CD4+Trp1+ cells after transfer into irradiated mice (Fig. 1 c). CTLA-4 blockade increased expansion by two- to threefold, reaching up to 40% of blood lymphocytes (Fig. 1 c), while simultaneously preventing the expansion and accumulation of CD4+Trp1+Foxp3+ cells (Fig. 1 d).
Figure 1.
Transfer of small numbers of CD4+Trp1+ T cells in combination with radiation and CTLA-4 blockade produces potent rejection of established B16/BL6 melanoma tumors. Tumor-bearing mice were treated at day 10 with 5 Gy of RT and were injected or not with 50,000 naive tumor-reactive CD4+Trp1+ cells and anti–CTLA-4. (a) Data are presented as tumor growth in each mouse (three left panels; representative of at least three independent experiments; n = 5 mice per group) and cumulative survival from two independent experiments (far right panel; n = 10 mice per group). (b) Representative images of tumors in mice treated with 5 Gy of RT and CD4+Trp1+ cells in the absence (top) or presence (bottom) of anti–CTLA-4. The far right panel shows disseminated depigmentation in mice surviving therapy. (c–f) Blood samples from mice treated with 5 Gy of RT (black line), 5 Gy + CD4+Trp1+ (red line), and 5 Gy + CD4+Trp1+ + anti–CTLA-4 (blue line) were monitored at different time points after tumor challenge. Data are representative of three independent experiments (n = 5 mice per group). c shows the percentage of CD4+Trp1+ T cells from blood lymphocytes, whereas d depicts the percentage of Foxp3+ cells within the CD4+Trp1+ population. Serum samples were analyzed over time and tested for levels of IFN-γ (e) and TNF (f). Error bars represent means ± SD.
Although mice receiving RT monotherapy showed no changes in serum levels of IFN-γ (Fig. 1 e) and TNF (Fig. 1 f), both inflammatory cytokines were increased significantly after CD4-ACT in irradiated hosts (Fig. 1, e and f). Cytokine production was markedly increased after CTLA-4 blockade (Fig. 1, e and f), correlating with the increase in CD4+Trp1+ cells (Fig. 1 c) and heightened tumor rejection (Fig. 1 a). These data demonstrate that adoptive transfer of small numbers of naive tumor-reactive CD4+ T cells into irradiated recipients can mediate potent rejection of established melanoma. Incorporation of CTLA-4 blockade significantly enhanced T cell expansion, cytokine secretion, and antitumor activity, conferring complete rejection and durable protection in all treated mice.
Combination of CD4+Trp1+ ACT and CTLA-4 blockade enhances activation and differentiation of effector CD4+Trp1+ T cells, and reduces the number of T reg cells
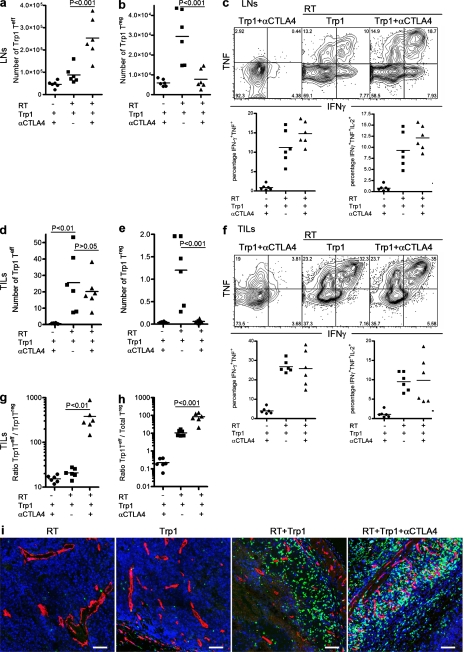
To further characterize the antitumor response induced by CD4+Trp1+ T cells, we analyzed their expansion, differentiation, and function in LNs and tumors 8 d after transfer. CD4+Trp1+ Teff cells (Foxp3−) were detectable in low numbers in LNs of nonirradiated recipients, increasing in number when transferred after RT (Fig. 2 a). CTLA-4 blockade significantly increased expansion of CD4+Trp1+ Teff cells transferred into irradiated hosts (Fig. 2 a), while preventing expansion of CD4+Trp1+Foxp3+ T reg cells (Fig. 2 b). The combination of RT and CD4-ACT resulted in a marked Th1-like phenotype, with CD4+Trp1+ T cells secreting copious amounts of IFN-γ, TNF, and IL-2. (Fig. 2 c and Fig. S1). Contrary to its effect on the absolute number of CD4+Trp1+ Teff and T reg cells, CTLA-4 blockade had no impact on the frequency of IL-2, IFN-γ, or TNF single, double, or triple producers in LNs (Fig. 2 c and Fig. S1) or tumors (Fig. 2 f and Fig. S2). Remarkably, in addition to IFN-γ, TNF, and IL-2, in vivo activation of tumor-reactive CD4+Trp1+ T cell also resulted in their differentiation into granzyme B–producing CD4+ T cells in both the periphery and in tumors (Figs. S1 and S2).
Figure 2.
Combination of CD4+Trp1+ ACT and CTLA-4 blockade enhances activation and differentiation of CD4+Trp1+ Teff cells and reduces the number of T reg cells. (a–e) Tumor-bearing mice were treated at day 10 with or without 5 Gy of RT followed by transfer of 50,000 CD4+Trp1+ cells in the presence or absence of anti–CTLA-4 mAb. 8 d after therapy (day 18 after tumor challenge), mice were euthanized and numbers of CD4+Trp1+Foxp3− cells (Teff cells; a and d) and CD4+Trp1+Foxp3+ cells (T reg cells; b and e) were analyzed in LNs (a and b) and tumors (d and e). Numbers of CD4+Trp1+ Teff and T reg cells in tumors (d and e) were calculated as described in Materials and methods. (g and h) Proportions of CD4+Trp1+ Teff cells to CD4+Trp1+ T reg cells (g) and CD4+Trp1+ Teff cells to total T reg cells (h) were also calculated from tumor samples. CD4+Trp1+ cells from LNs (c) and tumor samples (f) were restimulated ex vivo, and IFN-γ, TNF, and IL-2 secretion was determined on a per cell basis. (i) In a parallel experiment, tumors were dissected and fresh frozen in optimum cutting temperature solution, cut, and stained for DAPI (blue), CD31 (red), and CD4 (green). Samples were analyzed by confocal microscopy with a 20X water immersion objective. Bars, 50 µm. Images showing whole-tumor immunofluorescence are shown in Fig. S3. Data are representative of three independent experiments (n = 3 mice per group). Horizontal bars represent means.
Analysis of tumor-infiltrating CD4+Trp1+ T cells also highlighted the importance of RT for T cell accumulation (Fig. 2 d) and differentiation (Fig. 2 f and Fig. S2). Although CTLA-4 blockade failed to further increase the absolute number of intratumoral CD4+Trp1+ Teff cells in irradiated recipients, it markedly reduced the number of tumor-infiltrating CD4+Trp1+Foxp3+ T reg cells, mirroring changes in the LNs and blood. The intratumor ratio of Trp1+ Teff cells to Trp1+ T reg cells (Fig. 2 g) and of CD4+Trp1+ Teff cells to total T reg cells (Fig. 2 h) increased several fold upon CTLA-4 blockade. Finally, histological analysis of T cell infiltrates confirmed lack of infiltration after monotherapy with RT or CD4-ACT, whereas their combination significantly enhanced CD4+Trp1+ accumulation within tumors (Fig. 2 I; and Figs. S3 and S4). These data are consistent with those obtained from blood and support a role for RT in the activation and differentiation of tumor-reactive CD4+Trp1+ Teff cells and for CTLA-4 blockade in driving further expansion of such cells while preventing T reg cell accumulation.
Rejection of established tumors depends on IFN-γ secretion by CD4+Trp1+ T cells and is independent of TNF and endogenous T, B, and NK cells
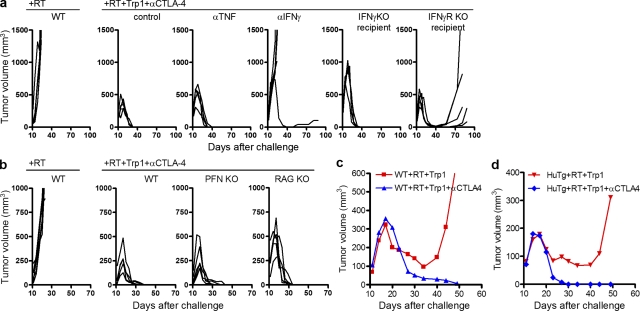
To determine whether IFN-γ and TNF were directly involved in tumor rejection, we used both neutralizing antibodies and a variety of knockout mice as tumor recipients. TNF blockade during treatment with RT, CD4-ACT, and CTLA-4 blockade (triple therapy) had no impact on tumor rejection (Fig. 3 a). In contrast, neutralization of IFN-γ prevented rejection in four out of five mice, suggesting an important mechanistic role for IFN-γ in tumor rejection (Fig. 3 a). Complete tumor eradication was observed after triple therapy using IFN-γ−/− recipient mice, indicating that IFN-γ secreted by the transferred CD4+Trp1+ cells, and not by the host cells, is critical for antitumor activity (Fig. 3 a). IFN-γR−/− mice also rejected established tumors after triple therapy, suggesting that the target cell for IFN-γ could (at least in an initial phase) be the tumor itself (Fig. 3 a). However, all IFN-γR−/− recipients regrew pigmented tumors 40–70 d after the primary challenge, suggesting that IFN-γ–sensitive cells other than the tumor are potentially important for complete tumor eradication.
Figure 3.
Rejection of established tumors depends on IFN-γ secretion by CD4+Trp1+ T cells and is independent of TNF and endogenous T, B, and NK cells. Tumor-bearing mice were irradiated at day 10 with 5 Gy followed or not by treatment with 50,000 CD4+Trp1+ cells and anti–CTLA-4 mAb. Tumor growth was measured and data are presented for each independent mouse. (a) Mice received only 5 Gy (left) or 5 Gy + CD4+Trp1+ + anti–CTLA-4 with control antibody or with neutralizing anti-TNF or anti–IFN-γ antibody. Two further groups included IFN-γ−/− and IFN-γR−/− recipients treated at day 10 with 5 Gy, 50,000 CD4+Trp1+ cells, and anti–CTLA-4. (b) In a separate experiment, recipient PFN−/− and Rag−/− mice were compared with wild-type mice for their capacity to reject established melanoma after treatment at day 10 with 5 Gy, 50,000 CD4+Trp1+ cells, and anti–CTLA-4. (c and d) Mean tumor growth in mice treated with 5 Gy of RT + CD4+Trp1+ cells in the presence (blue line) or absence (red line) of anti–CTLA-4. Mice used for this set of experiments were either wild-type C57BL/6 (c) or CTLA-4 human Tg (d) mice in which anti–CTLA-4 antibodies will only block CTLA-4 on the transferred CD4+Trp1+ cells. Data are representative of at least two independent experiments (n = 5 mice per group).
Remarkably, tumor rejection appeared to be independent of endogenous CD4, CD8, and B cells because RAG−/− recipients were capable of rejecting established melanoma after triple therapy (Fig. 3 b). Tumors were also rejected in perforin−/− (PFN−/−) recipient mice treated with triple therapy, suggesting that rejection is independent of endogenous PFN-dependent killing activity (i.e., mediated by CD8 and NK cells). This was further confirmed by depletion experiments with anti-CD8 and anti-NK1.1 mAbs, which failed to prevent tumor rejection (unpublished data). Finally, to determine the cellular targets of anti–CTLA-4, we used recipient mice expressing human CTLA-4 instead of mouse CTLA-4 (Peggs et al., 2009). In these mice, anti–mouse CTLA-4 mAbs block CTLA-4 only on the transferred CD4+Trp1+ cells. As shown previously (Fig. 1 a), RT and CD4-ACT resulted in partial rejection in wild-type mice, whereas incorporation of anti–mouse CTLA-4 resulted in complete tumor rejection (Fig. 3 c). Similar results were obtained when using human CTLA-4 mice as recipients, suggesting that CTLA-4 blockade on only the transferred cells is sufficient to mediate complete protection (Fig. 3 d). Collectively, the data support a direct role for the transferred CD4+Trp1+ T cells in tumor rejection, mediated most likely through the action of IFN-γ. This activity is independent of endogenous CD4, CD8, and B cells, or NK cells, suggesting a direct effect of the transferred CD4+Trp1+ cells on the tumor.
Antitumor activity after CD4+Trp1+ T cell transfer is specific to B16/BL6 melanoma and does not prevent growth of an unrelated tumor
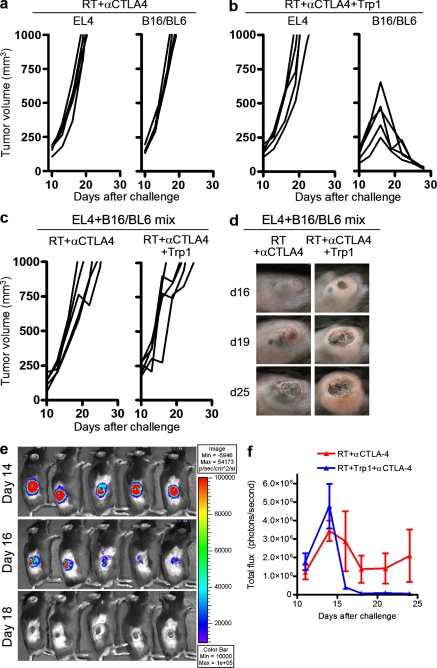
To determine whether the antitumor activity was tumor specific and not mediated through nonspecific mechanisms (i.e., activation of other cellular subsets such as inflammatory myeloid-derived cells; Mumberg et al., 1999; Corthay et al., 2005), we challenged mice with B16/BL6 melanoma in one flank and EL-4 lymphoma in the contralateral flank. Without CD4+Trp1+ transfer, both EL-4 and B16/BL6 tumors continued to grow (Fig. 4 a), whereas triple therapy resulted in complete rejection of B16/BL6 melanoma without influencing the growth of the unrelated EL-4 tumor (Fig. 4 b). To exclude the possibility that the inflammatory milieu created after triple therapy induces local rejection of tumors regardless of specific recognition by CD4+Trp1+ cells, we injected a mixture of B16/BL6 and EL-4 in the same site and followed tumor progression after therapy. Triple therapy did not result in rejection of the tumor mixture (Fig. 4 c), arguing against bystander tumor rejection. In the absence of CD4+Trp1+ transfer, the tumor contained a mixture of B16/BL6 melanoma and EL-4 (black tumors), whereas after CD4+Trp1+ T cell transfer there was no detectable melanoma within the growing tumor (off-white tumors; Fig. 4 d). To further verify that B16/BL6 melanoma was being rejected in the coinjection setting, mice were challenged with a mixture of EL-4 and B16/BL6-luciferase and treated at day 10 with triple therapy. The luciferase signal was completely eliminated in the triple therapy group but not in control mice lacking CD4-ACT (Fig. 4, e and f). The data argue against a nonspecific or bystander mechanism of tumor rejection and, together with the tumor rejection data in IFN-γR−/− mice, suggest a direct impact of transferred CD4+Trp1+ T cells and IFN-γ on melanoma cells.
Figure 4.
Antitumor activity after CD4+Trp1+ T cell transfer is specific to B16/BL6 melanoma and does not prevent growth of an unrelated tumor. (a–e) Mice were challenged with B16/BL6 melanoma and EL-4 in opposing flanks (a and b) or coinjected in the same flank (c–e). 10 d after tumor challenge, all mice received 5 Gy of RT and anti–CTLA-4 mAb. Half of the mice were also treated with 50,000 CD4+Trp1+ T cells and all mice were monitored for tumor growth (a–c). (d) Representative images of mice challenged with B16/BL6 and EL-4 in the same site shows the growth of a pigmented tumor in the absence of CD4+Trp1+ transfer and growth of a nonpigmented tumor after administration of 50,000 tumor-reactive CD4+Trp1+ T cells Data are representative of three independent experiments (n = 5 mice per group). To verify that B16/BL6 melanoma is rejected in the coinjection setting, mice were challenged with EL-4 and B16/BL6-luciferase in the same site, and light emission was determined over time after tumor challenge and triple therapy. (e) Representative images of luciferase signal disappearing from the tumor after therapy. (f) Quantification of luciferase signal and tumor growth. Data are representative of two independent experiments (n = 5 mice per group). Error bars represent means ± SD.
Tumor-reactive CD4+Trp1+ T cells develop class II–dependent killing activity
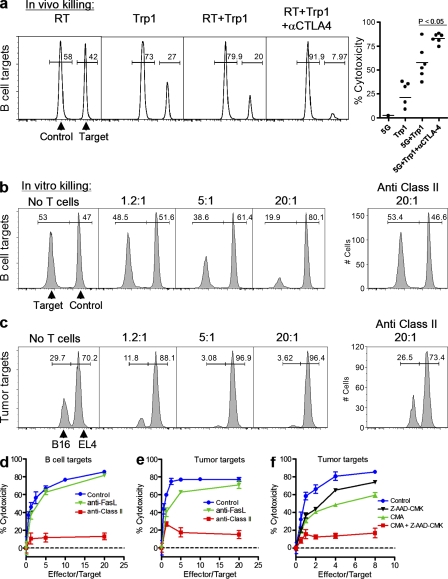
To determine if the antitumor responses achieved after triple therapy were caused by acquired cytotoxic activity and direct tumor killing by CD4+Trp1+ T cells, we performed a series of in vivo and in vitro killing experiments. Mice were challenged with tumors and treated at day 10 with or without RT, CD4+Trp1+ T cells, and anti–CTLA-4. CFSE-labeled B cell targets were injected i.v. 17 d after tumor challenge, and in vivo killing activity was determined 14–16 h later by quantification of the CFSEhigh peak (corresponding to target cells loaded with the class II–restricted peptide recognized by CD4+Trp1+ cells) relative to the unloaded CFSElow peak. CD4+Trp1+ cells acquired potent in vivo killing activity after transfer into irradiated recipients (Fig. 5 a). This activity was significantly enhanced after CTLA-4 blockade, whereas little to no activity was detected with CD4-ACT or RT alone (Fig. 5 a, right). Killing activity was confirmed in vitro, where CD4+Trp1+ cells isolated from triple therapy–treated mice and expanded in vitro were extremely efficient at eliminating peptide-loaded B cell targets (Fig. 5, b and d) or B16/BL6 targets (Fig. 5, c and e). Importantly, although killing activity was largely inhibited by antibodies blocking class II, blockade of FAS–FASL interactions did not significantly impair lysis of B cell targets or tumor cell targets (Fig. 5, d and e). Additional experiments blocking either FASL (MFL4 clone) or TRAIL (N2B2 clone) in vivo also failed to prevent tumor rejection in mice treated with triple therapy (unpublished data). In contrast, and in keeping with the high levels of granzyme B expressed by tumor-reactive CD4+Trp1+ cells (Figs. S1 and S2), simultaneous blockade of granzyme B and PFN activity in vitro synergized to prevent lysis of tumor targets (Fig. 5 f). Collectively, these data suggest that the cytolytic activity acquired by tumor-reactive CD4+Trp1+ T cells depends on direct recognition of target cells through class II and degranulation of granzyme-containing lytic granules.
Figure 5.
Tumor-reactive CD4+Trp1+ T cells develop class II–dependent killing activity. (a) 10 d after tumor challenge, tumor-bearing mice were treated or not with 5 Gy of RT, 50,000 CD4+Trp1+ cells, 5 Gy + CD4+Trp1+, or 5 Gy + CD4+Trp1+ + anti–CTLA-4 mAb. 7 d after therapy (day 17 after tumor challenge), all mice were injected with CFSE-labeled B cell targets. CFSEhigh (5 µM) cells were also loaded with the class II–restricted peptide recognized by CD4+Trp1+ cells, whereas CFSElow (0.5 µM) cells were used as a control population. 14–16 h after i.v. injection of CFSE targets, mice were sacrificed and in vivo killing activity was quantified in single-cell suspensions from the spleens of each mouse. Data are representative of three independent experiments (n = 3 mice per group). Horizontal bars represent means. (b–f) CD4+Trp1+ T cells were primed in vivo and expanded in vitro to allow analysis of their in vitro killing activity. Tumor-reactive in vivo–primed CD4+Trp1+ T cells were incubated at different ratios with CFSE-loaded spleen targets (b and d) or B16/BL6 and EL-4 tumor targets (c, e, and f). (b and d) CFSElow B cell targets were also loaded with the class II–restricted peptide recognized by Trp1 cells, whereas CFSEhigh cells were used as the control population. (c, e, and f) B16/BL6 cells were loaded with 0.5 µM CFSE, whereas EL-4 control tumor cells were loaded with 5 µM CFSE. Blocking anti–class II or anti-FASL antibodies were used at a final concentration of 50 µg/ml for all in vitro experiments (b–e). (f) In vitro killing of tumor targets was also tested in presence of 0.5 µM concanamycin A (PFN inhibitor) and/or 25 µM Z-AAD-CMK. In vitro killing activity was determined 12–14 h after initiation of the assay by quantifying the decrease of the target population in comparison to the control population, as described in Materials and methods. Data are representative of four independent experiments. Numbers in a–c indicate percentages. Error bars in d–f represent means ± SD.
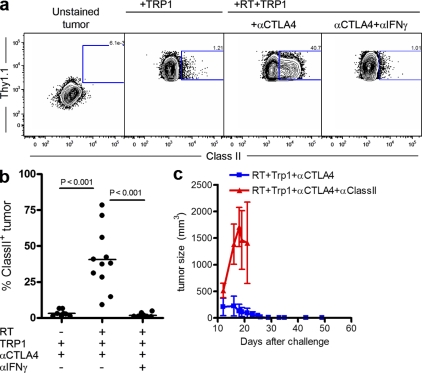
Direct recognition and specific destruction of tumor targets in vitro suggests a requirement for class II expression by the target cells in vivo. To test whether B16/BL6 could express class II in vivo, we challenged mice with a traceable Thy1.1-expressing B16/BL6 melanoma cell line and quantified class II expression on Thy1.1-positive cells after therapy (Fig. 6, a and b). Class II was not detected on the Thy1.1-expressing tumor cells in the untreated group or on tumors from mice treated only with CD4+Trp1+ and anti–CTLA-4 (Fig. 6, a and b). In contrast, high levels of class II could be found on tumor cells after triple therapy. Furthermore, although RT alone failed to induce class II up-regulation, the combination of RT and CD4+Trp1+ was as efficient as triple therapy at inducing class II up-regulation on tumor cells (not depicted).
Figure 6.
B16 melanoma up-regulates class II expression in vivo in an IFN-γ–dependent manner and are direct targets of cytotoxic CD4+Trp1+ T cells. (a) In vivo class II expression by tumor cells from mice receiving either CD4+Trp1+ cells alone or in combination with 5 Gy of RT and in the presence or absence of blocking anti–CTLA-4 and neutralizing IFN-γ. Mice were treated at day 10 after challenge with B16/BL6 melanoma expressing Thy1.1 and sacrificed 7 d after initiation of therapy (day 17 after tumor). Single-cell suspensions of tumors were analyzed for MHC class II levels by gating in Thy1.1-positive cells. Numbers indicate percentages. (b) Quantification of class II expression by tumors in vivo shown as cumulative data from three independent experiments (n = 3 mice per group). Horizontal bars represent means. (c) CD4+Trp1+ T cells were primed in vivo and expanded in vitro to allow analysis of their antitumor activity upon retransfer into tumor-bearing CII−/− recipient mice. In brief, 10 d after tumor challenge, MHC CII−/− recipient mice were treated with 5 Gy of RT, primed CD4+Tpr1+ cells, and anti–CTLA-4. Half of the mice were also treated with 200 µg of blocking anti–class II antibody every 3 d, and tumor growth was monitored over time. Data are representative of two independent experiments (n = 5 mice per group). Error bars represent means ± SEM.
A possible explanation for the high levels of class II expression could be the copious amounts of IFN-γ produced by tumor-reactive CD4+Trp1+, as IFN-γ has been shown to up-regulate expression of MHC molecules in several different cell types (Boehm et al., 1997; Boss, 1997). In support of this mechanism, IFN-γ was absolutely necessary for class II up-regulation, which was completely ablated by administration of neutralizing antibodies (Fig. 6, a and b).
Finally, to test the direct class II dependence of in vivo recognition and rejection of melanoma tumors, we adoptively transferred CD4+Trp1+ T cells that had been primed in vivo into tumor-bearing class II knockout (CII−/−) recipients. Activated cells were used because of a preliminary observation that class II expression on recipient cells is required for priming of tumor-reactive CD4+ T cells, as transfer of naive CD4+Trp1+ T cells into irradiated CII−/− mice failed to eradicate established tumors (unpublished data). Transfer of activated CD4+Trp1+ T cells into irradiated CII−/− mice followed by CTLA-4 blockade resulted in complete tumor eradication. Notably, tumor rejection was completely ablated by antibodies blocking class II MHC (Fig. 6 c). Collectively, the data support a model in which IFN-γ secreted by activated CD4+Trp1+ T cells induces class II up-regulation on tumor cells, converting them into targets for cytotoxic CD4+Trp1+ T cells.
Triple therapy mediates tumor regression in a mouse model of spontaneous melanoma
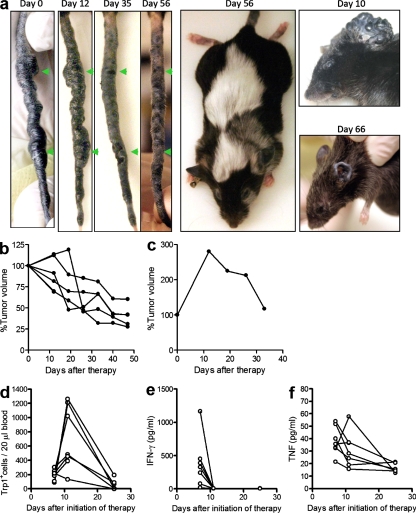
To test the efficacy of this new combinatorial therapy in a stringent and more realistic model, we applied our treatment regimen to a mouse model of spontaneous melanoma. Glutamate receptor 1 (Grm-1) Tg mice overexpress the metabotropic Grm-1 driven by the melanocyte-specific promoter of dopachrome tautomerase and develop tumors in the tail and pinnae of the ear between 4 and 6 mo of age (Pollock et al., 2003). Mice received triple therapy after development of large melanoma lesions between 10 and 13 mo of age. Although untreated tumors never regress spontaneously in this model (Pollock et al., 2003), a remarkable regression of tumors in the ears, eyes, and tails was observed after triple therapy (Fig. 7, a and b). CD4+Trp1+ cells expanded greatly after transfer into irradiated mice (Fig. 7 d), as observed in the transplantable B16/BL6 melanoma model (Fig. 1 c). High levels of IFN-γ and TNF were also detected in the blood of treated mice, occurring with a faster kinetic than with the transplantable melanoma line (Fig. 7, e and f). The data suggest that the efficacy of this therapeutic regimen is not limited to transplantable tumor models but is also evident in a more stringent model of spontaneous and disseminated disease, which is thought to better represent human malignancies. Finally, it illustrates the potency of this therapy, as demonstrated by its ability to mediate regression of many tumor lesions developing for up to 4 mo at different sites in the mouse.
Figure 7.
Triple therapy mediates tumor regression in a mouse model of spontaneous melanoma. Grm-1 Tg mice spontaneously develop melanoma tumors between 4–6 mo of age. After development of large tumor lesions (10–13 mo of age), mice were treated with 5 Gy of RT in combination with tumor-reactive CD4+Trp1+ T cells and anti–CTLA-4, as described in Materials and methods. Tumor progression was monitored weekly by gross morphological examination. The individual tumor lesions were measured with calipers weekly for each animal. The total tumor volume for each mouse was considered as 100% on the first day of treatment. (a) Representative images of treated mice and tumor regression. (b) Tumor volume after triple therapy. (c) One mouse with aggressive disease continued to grow tumor up to day 10 after therapy when tumor volume started to decrease, similar to what is observed in the transplantable B16/BL6 model. (d and e) Mice were also monitored for expansion of CD4+Trp1+ T cells in the blood (d) and cytokine levels in the serum (e) of treated mice. Data are representative of two independent experiments (n = 7–10 mice).
DISCUSSION
Adoptive transfer of high numbers of tumor-reactive lymphocytes into lymphoablated patients is a promising therapy for late metastatic disease, particularly metastatic melanoma (Dudley et al., 2002, 2008). Although initially focused on CD8+ CTL responses (Yee et al., 2002), recent preclinical and clinical studies have redirected attention to CD4+ T cells, which are thought to provide the necessary help to the effector CD8+ CTL compartment (Hunder et al., 2008). In this paper, we demonstrate that CD4+ T cells transferred into lymphopenic mice can mediate rejection of large vascularized melanoma tumors, particularly when combined with CTLA-4 blockade. These data are significant for several reasons: (a) CD4-mediated rejection does not require expansion and manipulation in vitro, but occurs after activation and differentiation of small numbers of naive T cells in vivo in a lymphodepleted host; (b) tumor-reactive CD4+ T cells acquire cytotoxic activity and directly reject the tumor in an MHC class II–dependent manner; and (c) CTLA-4 blockade greatly enhances therapeutic efficacy of CD4-ACT, informing a potential strategy for future clinical studies. Finally, this therapy is effective in a mouse model of spontaneous melanoma, reinforcing the potential pathophysiological relevance of our findings. The data offer novel mechanistic insights into the role and function of tumor-reactive CD4+ T cells, and have significant clinical relevance for ACT, including approaches incorporating gene therapy. Collectively, they suggest that a small number of naive T cells transduced to express tumor-reactive TCRs might be sufficient to drive tumor rejection after differentiation in vivo, offering an alternative to the extensive ex vivo manipulation that is required to achieve high numbers of in vitro–differentiated tumor-reactive T cells.
The data demonstrate that RT in combination with CD4+Trp1+ transfer results in expansion, intratumor accumulation, and differentiation of the transferred cells into IFN-γ–, TNF-, IL-2–, and granzyme B–producing effector cells, whereas RT or CD4+Trp1+ transfer alone fail to mediate these changes (Figs. 1 and 2). Contrary to most studies of ACT using T cells activated in vitro, tumors continued to grow after CD4-ACT, and started to necrose and regress several days thereafter (Fig. 1 b). This may reflect a requirement for priming, expansion in the lymphopenic environment, and differentiation of the transferred cells before acquisition of full antitumor activity. Although slower to manifest than the antitumor effect observed after ACT with cells expanded in vitro (Hanson et al., 2000; Overwijk et al., 2003; Spiotto et al., 2004; Antony et al., 2005), priming in vivo in the lymphopenic hosts is extremely efficient, resulting in rejection of large tumors of up to 600 mm3.
Transfer of CD4+Trp1+ cells into nonirradiated tumor-bearing RAG−/− mice, which lack B and T cells, also resulted in extensive proliferation, cytokine production, and tumor rejection (unpublished data), suggesting that the main role of RT in this model is the induction of lymphopenia and/or the elimination of cytokine sinks limiting tumor-reactive T cell responses (Gattinoni et al., 2005a; Muranski et al., 2006). Importantly, in RAG-sufficient hosts, RT may also contribute by sensitizing the tumor stroma (Zhang et al., 2007) and by increasing the expression of adhesion molecules on the tumor vasculature, which would render the tumor susceptible to T cell infiltration (Ganss et al., 2002; Lugade et al., 2005; Quezada et al., 2008).
Although total body irradiation has been successfully used in several major cancer centers across the United States (Muranski et al., 2006; Dudley et al., 2008), clinical application of our findings may benefit from future studies addressing alternatives to lymphodepletion. If the main contribution of RT is to induce lymphopenia, then conditioning of recipients by lymphodepleting chemotherapy regimens should also create an environment favorable to tumor rejection by adoptive transfer of small numbers of tumor-reactive CD4+ T cells. Finally, lymphodepletion may not be the only way to fully activate tumor-reactive CD4+ T cells; thus, alternatives including activation of the APC compartment (i.e., CD40 and TLR agonists) should be addressed in future studies.
The addition of CTLA-4 blockade to RT and CD4+Trp1+ transfer further enhanced antitumor responses, inducing higher total numbers of CD4+Trp1+ cells and higher levels of TNF and IFN-γ in serum samples (Fig. 1). CTLA-4 blockade did not modify cytokine production on a per cell basis but increased the number of tumor-reactive cells, accounting for the overall increase in levels of inflammatory cytokines in the serum. Equally important, CTLA-4 blockade resulted in a significant reduction in the absolute number of CD4+Trp1+Foxp3+ and endogenous T reg cells in the periphery and in the tumors. Collectively, these changes may create a state of combined hyperactivation and reduced regulation, correlating with increased cytotoxicity (Fig. 5 a) and resulting in complete tumor rejection. Notably, restricting CTLA-4 blockade to the transferred cells was sufficient to induce maximal antitumor activity, suggesting a strategy for future combinatorial approaches incorporating TCR transfer and CTLA-4 ablation on the same cell, which may avoid adverse immune events associated with systemic administration of anti–CTLA-4 antibodies (Peggs et al., 2006).
Although previous studies have indicated that Th17 cells polarized in vitro are capable of inducing tumor rejection (Muranski et al., 2008), we did not observe differentiation of CD4+Trp1+ cells into IL-17 producers in vivo (unpublished data). In the previous study, tumor rejection was dependent mostly on IFN-γ despite the requirement for differentiation into Th17 cells, in accordance with studies suggesting a possible reversion of Th17 into Th1 cells (Shi et al., 2008; Lee et al., 2009; Martin-Orozco et al., 2009). In contrast, we believe that priming of tumor-reactive CD4+Trp1+ T cells in vivo in a lymphopenic environment leads directly to a Th1-like phenotype. Surprisingly, these cells also expressed high levels of granzyme B in the periphery and tumors (Figs. S1 and S2), which correlated with acquisition of granzyme-dependent cytotoxic activity (Fig. 5) and potent rejection of large established tumors.
The acquisition of cytotoxic activity by transferred tumor-reactive CD4+ T cells is particularly striking. This distinguishes our findings from previous work showing that CD4+ T cells can help rejection of less well-established tumors through indirect effects of IFN-γ (Mumberg et al., 1999) on NK cells (Perez-Diez et al., 2007) and tumor-infiltrating macrophages (Greenberg et al., 1985; Hung et al., 1998; Corthay et al., 2005; Corthay, 2007). Interestingly, CD4+Trp1+ cells developed all the hallmarks of CD4+ Th cells with the additional benefit of exhibiting cytolytic activity. CD4+ CTLs targeting viral antigens (Paludan et al., 2002; Hegde et al., 2005; Heller et al., 2006) and alloantigens (Holloway et al., 2005; Spaapen et al., 2008) have been described previously, but the demonstration of similar activity in a more physiological model for self-/tumor antigen emphasizes the promise of these cells in cancer immunotherapy. Further identification and induction of tumor-reactive CD4+ T cells with cytotoxic activities in cancer patients may offer significant advantages for the treatment of human malignancies. These cells could be isolated and minimally expanded before reinfusion into conditioned recipients. The feasibility of such strategies is supported by the potent tumor rejection observed in CII−/− mice after transfer of CD4+Trp1+ T cells that had been originally primed in vivo and then minimally expanded before transfer into lymphopenic tumor-bearing mice (Fig. 6 c). Furthermore, similar approaches for the isolation of tumor-reactive lymphocytes after immunotherapy followed by autologous transfer into conditioned recipients have been previously demonstrated in preclinical (Quezada et al., 2008) and clinical settings (Rapoport et al., 2005; June, 2007).
Other aspects of CD4+Trp1+ T cell function were equally critical for tumor eradication. The Th1-like phenotype that developed after transfer into a lymphopenic environment was characterized by the production of high levels of TNF, IL-2, and IFN-γ. IFN-γ was clearly required for tumor rejection and appeared to directly affect tumor cells in our model, because tumor regression was observed in IFN-γR−/− recipients. In addition, MHC II expression by tumor cells depended on IFN-γ because its neutralization prevented MHC II up-regulation (Fig. 6, a and b). This is in keeping with previous work on autoimmunity demonstrating that IFN-γ secreted by CD4+ T cells can mediate up-regulation of class II on target cells (Wu et al., 1999, 2000). Remarkably, tumors recrudesced in all IFN-γR−/− recipients after initial regression, suggesting that an IFN-γ–sensitive cell type other than the tumor may have a role in mediating long-term protection, in accordance with previous observations using CD8-ACT in which rejection of the tumor stroma was critical for complete tumor eradication (Spiotto et al., 2004; Zhang et al., 2007).
Based on these data, we propose a model in which tumor-reactive CD4+Trp1+ T cells transferred into lymphopenic mice expand, differentiate into IFN-γ–secreting, cytotoxic CD4+ cells, and accumulate within the tumor. IFN-γ induces up-regulation of MHC II on tumor cells, rendering them targets for the killer activity of CD4+Trp1+ cells. Finally, cytotoxic activity depends absolutely on class II expression by the tumor and correlates with high levels of granzyme B within CD4+Trp1+ cells in LNs and particularly in tumors, where it is expressed by 40–50% of infiltrating CD4+Trp1+ T cells (Figs. S1 and S2). As part of a more global view and in addition to their direct impact on the tumor, IFN-γ–secreting CD4+Trp1+ cells may also induce a cascade of events involving priming of cytolytic CD8+ T cells through activation of DCs and additional tumor destruction through activation of NK cells. Furthermore, high levels of IFN-γ in the tumor may also lead to activation of type I macrophages, which will also favorably affect antitumor activity.
In conclusion, we believe that these data greatly inform our basic understanding of the importance of tumor-reactive CD4+ T cells in the context of ACT. Equally importantly, they support development of clinical strategies focusing on exploiting the function of cytotoxic tumor-reactive CD4+ T cells generated after transfer and activation in vivo, which may obviate requirements for extensive and possibly detrimental manipulation in vitro before adoptive transfer. Finally, the augmented potency of the transferred tumor-specific T cells observed upon blockade of coinhibitory molecules such as CTLA-4 provides a basis for the improvement of ongoing ACT trials as well as for the development of future combinatorial trials.
MATERIALS AND METHODS
Mice.
6–8-wk-old C57BL/6, B6.SJL, RAG2−/−, Prf−/−, IFN-γ−/−, IFN-γR−/−, and MHC CII−/− Δ78 mice were purchased from the Jackson Laboratory. Trp1 Tg mice carry TCR α and β transgenes on a background homozygous for the targeted mutation Rag1tm1Mom and homozygous for the white-based brown radiation–induced mutation of Trp1, Tyrp1B-w (Muranski et al., 2008). These mutant mice express an MHC class II–restricted (I-Ab) TCR recognizing the endogenous melanocyte differentiation antigen–minimal TRP-1 epitope corresponding to amino acids 113–127. Trp1 Tg mice were crossed to B6.SJL mice, and their progeny were intercrossed to generate CD45.1+/+Trp1 Tg mice (also on the Tyrp1B-WRAG−/− background). Grm-1 Tg mice (Pollock et al., 2003) were provided by S. Chen (Rutgers, The State University of New Jersey, Piscataway, NJ). Grm-1 Tg mice overexpress the metabotropic Grm-1 driven by the melanocyte-specific promoter of dopachrome tautomerase. Mice develop spontaneous melanoma lesions primarily in the tail and pinnae of the ear between 4 and 6 mo of age. Mice were housed in specific pathogen-free conditions in accordance with institutional guidelines. All animal experiments were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee.
Cell lines.
The poorly immunogenic B16/BL6 cell line was originally obtained from I.J. Fidler (M.D. Anderson Cancer Center, Houston, TX). The lymphoma EL-4 was obtained from the American Type Culture Collection. B16/BL6 cells were transduced and selected to constitutively express either Thy1.1 or Renilla luciferase.
Antibodies.
Anti–CTLA-4 (9H10), anti-NK1.1 (PK136), anti-CD8 (2.43), anti–IFN-γ (XMG1.2), anti-TNF (XT3.11), and anti–I-Ab (M5/114) were purchased from BioXCell and administered i.p. Antibodies for flow cytometry and immunofluorescence were purchased from eBioscience and BD.
Tumor challenge and treatments.
Mice were challenged intradermally (i.d.) with 2.5 × 105 B16/BL6 tumor cells on day 0. In some experiments, mice received an additional i.d. challenge of 2.5–5 × 105 EL-4 lymphoma cells on the opposite flank, or a mix of both tumors in one unique site. On day 10, mice were treated or not with 5 Gy of total body irradiation, 50,000 CD4+Trp1+ cells i.v., and 200 µg anti–CTLA-4 i.p. Mice received additional anti–CTLA-4 injections of 100 µg on days 13, 16, 20, and 24. For neutralization and blocking experiments, 200 µg anti–IFN-γ, anti-TNF, or anti–I-Ab was administered 1 d before initiation of therapy and every 2 d after that for a total of six injections. CD4+Trp1+ for adoptive transfers was isolated from Trp1 Tg mice using CD4 magnetic beads (Miltenyi Biotec).
Phenotypic and functional analyses.
Mice used for functional experiments were sacrificed on day 18 after tumor implantation, and LN cells and tumor-infiltrating lymphocytes were isolated as previously described (Quezada et al., 2006, 2008). Tumor-infiltrating CD4+ T cells were purified using CD4 positive selection (FlowComp; Invitrogen) according to the manufacturer’s instructions. Purified CD4+ T cells from tumors or bulk cells from LNs were restimulated for 2 h at 37°C with 5 × 104 DCs and 2 µM of Trp1 peptide followed by addition of brefeldin A (BD) for 2 more hours.
Flow cytometry and quantification.
Samples were stained with anti-CD45.1–Pacific Blue (A20) and anti-CD4–PerCP-Cy5.5 (GK1.5), fixed, and permeabilized (eBioscience) according to the manufacturer’s instructions, and stained with anti-Foxp3–PE (FJK-16s). Flow cytometry reference beads (PeakFlow blue; Invitrogen) were mixed with the samples before analysis to normalize for the volume of the sample acquired. The absolute number of CD4+Trp1+ cells per tumor was calculated using the following formula: number of CD4+Trp1+ cells = (count of CD4+Trp1+ cells/count of beads)/tumor weight. For functional analysis, restimulated samples were stained as described, fixed, and permeabilized using Cytofix/Cytoperm (BD), followed by anti–IFN-γ–Alexa Fluor 488 (XMG1.2), anti-TNF–PE (MP6-XT22) or anti–IL-2–PE (JES6-5H4), and anti–granzyme B–allophycocyanin (GB11).
Immunofluorescence.
Tumors were flash frozen in optimal cutting temperature solution (Sakura). 10-µm sections were cut with a microcryotome (Leica), fixed for 10 min in cold acetone, and stained with anti-CD4–Alexa Fluor 488, anti-CD31–PE, and DAPI, and analyzed on an inverted confocal microscope (LSM; Leica) under a 20× water immersion objective.
Blood cytokine measurements.
Serum from blood samples was analyzed using a Mouse Inflammation Cytometry Bead Array (BD) according to the manufacturer’s instructions.
In vivo cytotoxicity.
Splenocytes from B6.SJL mice were labeled with 5 or 0.5 µM CFSE. CFSEhigh splenocytes were loaded with 20 µM of Trp1 peptide (Bio-Synthesis) for 2 h at 37°C. On day 17 after tumor challenge (7 d after CD4+Trp1+ transfer), 5 × 105 cells of a 50:50 mixture of Trp1 peptide–loaded and –unloaded splenocytes were transferred by tail vein injection. 14–16 h later, mice were sacrificed and spleens and LNs were removed, and the percentage of loaded and unloaded B220+ splenocytes was analyzed. Cytotoxicity was calculated using the following formula: cytotoxicity = 100% × (1−((unloaded/loaded)control/(unloaded/loaded)experimental)).
Trp1 in vitro expansion and in vitro cytotoxicity.
7 d after adoptive transfer into irradiated tumor-bearing mice, CD4+Trp1+ cells were purified from LNs using CD4 beads (Miltenyi Biotec) and cultured with equal numbers of DCs and 2 µM of Trp1 peptide. After 3 d, cell cultures were supplemented with DCs, 30 U/ml IL-2, and 2 µM of Trp1 peptide. After 3 d, cells were harvested and used for in vitro cytotoxicity assays or for transfer into tumor-bearing CII−/− recipients. To determine in vitro killing of tumor targets, B16/BL6 tumor cells were loaded with 0.5 mM CFSE, whereas EL-4 control cells were loaded with 5 mM CFSE. Fluorescent reference beads were mixed with the samples before analysis to normalize for the volume of the sample acquired. Blocking anti–I-Ab and anti-FASL (MFL4 clone) mAbs were used at a final concentration of 50 µg/ml, whereas concanamycin A (Sigma-Aldrich) and the granzyme B inhibitor Z-AAD-CMK (Enzo Biochem, Inc.) were used at 0.5 and 25 µM, respectively.
Statistical analyses.
Data were analyzed using Prism 5.0 (GraphPad Software, Inc.). Experiments were repeated two to three times. Statistical significance was determined by a Student’s t test (between two groups or conditions) or analysis of variance with a post-hoc test (three or more groups or conditions). Tumor survival data were analyzed with the Kaplan-Meier method. The log-rank test was used to compare survival curves for different subgroups on univariate analyses. P < 0.05 was considered statistically significant.
Online supplemental material.
Figs. S1 and S2 show the levels of intracellular cytokines (IFN-γ, TNF, and IL-2) and granzyme B produced by CD4+Trp1+ T cells isolated from LNs (Fig. S1) and tumors (Fig. S2) of mice receiving different treatments. Fig. S3 depicts histological analysis of whole tumors from mice treated with RT with or without CD4+Trp1+ T cells and anti–CTLA-4. Fig. S4 illustrates tumor infiltration by CD4+Vβ14+ T cells after treatment with RT and CD4+Trp1+ T cells with and without anti–CTLA-4. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091918/DC1.
Supplementary Material
Acknowledgments
We would like to thank Dr. S. Chen for providing the Grm-1 Tg mice, and Dr. A. Jungbluth for critical help with microscopy. We also would like to thank W. Montalvo, J. Geddes, and J. Lu for technical assistance, and E. Corse and P. Savage for critical evaluation of the work.
S.A. Quezada is a Research Fellow funded by the Irvington Institute Fellowship Program of the Cancer Research Institute and a junior member of the Millennium Nucleus on Immunology and Immunotherapy, Pontifícia Universidad Católica de Chile. T.R. Simpson is supported by a Canadian Institutes of Health Doctoral Research Award. K.S. Peggs is currently an investigator at the Department of Haematology, University College London Cancer Institute, University College London, and receives funding from the Leukaemia Research Fund. J.P. Allison is an investigator of the Howard Hughes Medical Institute and holds the David H. Koch Chair in Immunological Studies at the Memorial Sloan-Kettering Cancer Center. This work was also supported by the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by W.H. Goodwin and A. Goodwin, and the Swim Across America Foundation.
J.P. Allison is an inventor of intellectual property licensed by the University of California to Medarex, and is a consultant for Medarex and Bristol Meyers Squibb. The authors have no further conflicting financial interests.
Footnotes
Abbreviations used:
- ACT
- adoptive cellular therapy
- CII−/−
- class II knockout
- CTLA-4
- CTL-associated antigen 4
- Grm-1
- glutamate receptor 1
- PFN
- perforin
- RT
- radiation therapy
- Teff
- effector T
- Tg
- transgenic
- Trp1
- tyrosinase-related protein 1
References
- Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D.C., Chan C.C., Klebanoff C.A., Overwijk W.W., et al. 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D.A., Lefrançois L. 2007. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc. Natl. Acad. Sci. USA. 104:15045–15050 10.1073/pnas.0703767104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J.C. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749–795 10.1146/annurev.immunol.15.1.749 [DOI] [PubMed] [Google Scholar]
- Boss J.M. 1997. Regulation of transcription of MHC class II genes. Curr. Opin. Immunol. 9:107–113 10.1016/S0952-7915(97)80166-5 [DOI] [PubMed] [Google Scholar]
- Burns W.R., Zheng Z., Rosenberg S.A., Morgan R.A. 2009. Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood. 114:2888–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthay A. 2007. CD4+ T cells cooperate with macrophages for specific elimination of MHC class II-negative cancer cells. Adv. Exp. Med. Biol. 590:195–208 10.1007/978-0-387-34814-8_14 [DOI] [PubMed] [Google Scholar]
- Corthay A., Skovseth D.K., Lundin K.U., Røsjø E., Omholt H., Hofgaard P.O., Haraldsen G., Bogen B. 2005. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 22:371–383 10.1016/j.immuni.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Dudley M.E., Wunderlich J.R., Robbins P.F., Yang J.C., Hwu P., Schwartzentruber D.J., Topalian S.L., Sherry R., Restifo N.P., Hubicki A.M., et al. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 298:850–854 10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E., Yang J.C., Sherry R., Hughes M.S., Royal R., Kammula U., Robbins P.F., Huang J., Citrin D.E., Leitman S.F., et al. 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26:5233–5239 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganss R., Ryschich E., Klar E., Arnold B., Hämmerling G.J. 2002. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 62:1462–1470 [PubMed] [Google Scholar]
- Gattinoni L., Finkelstein S.E., Klebanoff C.A., Antony P.A., Palmer D.C., Spiess P.J., Hwang L.N., Yu Z., Wrzesinski C., Heimann D.M., et al. 2005a. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202:907–912 10.1084/jem.20050732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Klebanoff C.A., Palmer D.C., Wrzesinski C., Kerstann K., Yu Z., Finkelstein S.E., Theoret M.R., Rosenberg S.A., Restifo N.P. 2005b. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115:1616–1626 10.1172/JCI24480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Zhong X.S., Palmer D.C., Ji Y., Hinrichs C.S., Yu Z., Wrzesinski C., Boni A., Cassard L., Garvin L.M., et al. 2009. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M., Quezada S.A., Blazar B.R., Panoskaltsis-Mortari A., Rudensky A.Y., Noelle R.J. 2002. The balance between donor T cell anergy and suppression versus lethal graft-versus-host disease is determined by host conditioning. J. Immunol. 169:5581–5589 [DOI] [PubMed] [Google Scholar]
- Greenberg P.D., Kern D.E., Cheever M.A. 1985. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2− T cells. Tumor eradication does not require participation of cytotoxic T cells. J. Exp. Med. 161:1122–1134 10.1084/jem.161.5.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson H.L., Donermeyer D.L., Ikeda H., White J.M., Shankaran V., Old L.J., Shiku H., Schreiber R.D., Allen P.M. 2000. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 13:265–276 10.1016/S1074-7613(00)00026-1 [DOI] [PubMed] [Google Scholar]
- Hataye J., Moon J.J., Khoruts A., Reilly C., Jenkins M.K. 2006. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 312:114–116 10.1126/science.1124228 [DOI] [PubMed] [Google Scholar]
- Hegde N.R., Dunn C., Lewinsohn D.M., Jarvis M.A., Nelson J.A., Johnson D.C. 2005. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. J. Exp. Med. 202:1109–1119 10.1084/jem.20050162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K.N., Gurer C., Münz C. 2006. Virus-specific CD4+ T cells: ready for direct attack. J. Exp. Med. 203:805–808 10.1084/jem.20060215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P.A., Kaldenhoven N., Kok-Schoemaker H.M., Dijk M., Otten H.G., Tilanus M., Postma S., Mutis T., Lokhorst H.M., Bloem A.C. 2005. A class II-restricted cytotoxic T-cell clone recognizes a human minor histocompatibility antigen with a restricted tissue distribution. Br. J. Haematol. 128:73–81 10.1111/j.1365-2141.2004.05283.x [DOI] [PubMed] [Google Scholar]
- Hunder N.N., Wallen H., Cao J., Hendricks D.W., Reilly J.Z., Rodmyre R., Jungbluth A., Gnjatic S., Thompson J.A., Yee C. 2008. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 358:2698–2703 10.1056/NEJMoa0800251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. 1998. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188:2357–2368 10.1084/jem.188.12.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C.H. 2007. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 117:1466–1476 10.1172/JCI32446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity. 30:92–107 10.1016/j.immuni.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugade A.A., Moran J.P., Gerber S.A., Rose R.C., Frelinger J.G., Lord E.M. 2005. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174:7516–7523 [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N., Chung Y., Chang S.H., Wang Y.H., Dong C. 2009. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur. J. Immunol. 39:216–224 10.1002/eji.200838475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P., et al. 2006. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 314:126–129 10.1126/science.1129003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Monach P.A., Wanderling S., Philip M., Toledano A.Y., Schreiber R.D., Schreiber H. 1999. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc. Natl. Acad. Sci. USA. 96:8633–8638 10.1073/pnas.96.15.8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Restifo N.P. 2009. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr. Opin. Immunol. 21:200–208 10.1016/j.coi.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Boni A., Wrzesinski C., Citrin D.E., Rosenberg S.A., Childs R., Restifo N.P. 2006. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat. Clin. Pract. Oncol. 3:668–681 10.1038/ncponc0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Boni A., Antony P.A., Cassard L., Irvine K.R., Kaiser A., Paulos C.M., Palmer D.C., Touloukian C.E., Ptak K., et al. 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 112:362–373 10.1182/blood-2007-11-120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk W.W., Theoret M.R., Finkelstein S.E., Surman D.R., de Jong L.A., Vyth-Dreese F.A., Dellemijn T.A., Antony P.A., Spiess P.J., Palmer D.C., et al. 2003. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198:569–580 10.1084/jem.20030590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan C., Bickham K., Nikiforow S., Tsang M.L., Goodman K., Hanekom W.A., Fonteneau J.F., Stevanović S., Münz C. 2002. Epstein-Barr nuclear antigen 1-specific CD4(+) Th1 cells kill Burkitt’s lymphoma cells. J. Immunol. 169:1593–1603 [DOI] [PubMed] [Google Scholar]
- Pardoll D.M., Topalian S.L. 1998. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 10:588–594 10.1016/S0952-7915(98)80228-8 [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Quezada S.A., Korman A.J., Allison J.P. 2006. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr. Opin. Immunol. 18:206–213 [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Quezada S.A., Chambers C.A., Korman A.J., Allison J.P. 2009. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies. J. Exp. Med. 206:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Diez A., Joncker N.T., Choi K., Chan W.F., Anderson C.C., Lantz O., Matzinger P. 2007. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 109:5346–5354 10.1182/blood-2006-10-051318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock P.M., Cohen-Solal K., Sood R., Namkoong J., Martino J.J., Koganti A., Zhu H., Robbins C., Makalowska I., Shin S.S., et al. 2003. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat. Genet. 34:108–112 10.1038/ng1148 [DOI] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Curran M.A., Allison J.P. 2006. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116:1935–1945 10.1172/JCI27745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Simpson T.R., Shen Y., Littman D.R., Allison J.P. 2008. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J. Exp. Med. 205:2125–2138 10.1084/jem.20080099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport A.P., Stadtmauer E.A., Aqui N., Badros A., Cotte J., Chrisley L., Veloso E., Zheng Z., Westphal S., Mair R., et al. 2005. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat. Med. 11:1230–1237 10.1038/nm1310 [DOI] [PubMed] [Google Scholar]
- Rizzuto G.A., Merghoub T., Hirschhorn-Cymerman D., Liu C., Lesokhin A.M., Sahawneh D., Zhong H., Panageas K.S., Perales M.A., Altan-Bonnet G., et al. 2009. Self-antigen–specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J. Exp. Med. 206:849–866 10.1084/jem.20081382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Cox C.A., Vistica B.P., Tan C., Wawrousek E.F., Gery I. 2008. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J. Immunol. 181:7205–7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaapen R.M., Lokhorst H.M., van den Oudenalder K., Otterud B.E., Dolstra H., Leppert M.F., Minnema M.C., Bloem A.C., Mutis T. 2008. Toward targeting B cell cancers with CD4+ CTLs: identification of a CD19-encoded minor histocompatibility antigen using a novel genome-wide analysis. J. Exp. Med. 205:2863–2872 10.1084/jem.20080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotto M.T., Rowley D.A., Schreiber H. 2004. Bystander elimination of antigen loss variants in established tumors. Nat. Med. 10:294–298 10.1038/nm999 [DOI] [PubMed] [Google Scholar]
- Wu Z., Biro P.A., Mirakian R., Hammond L., Curcio F., Ambesi-Impiombato F.S., Bottazzo G.F. 1999. HLA-DMB expression by thyrocytes: indication of the antigen-processing and possible presenting capability of thyroid cells. Clin. Exp. Immunol. 116:62–69 10.1046/j.1365-2249.1999.00831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Biro P.A., Mirakian R., Curcio F., Ambesi-Impiombato F.S., Bottazzo G.F. 2000. Transcriptional regulation of the MHC II gene DRA in untransformed human thyrocytes. Int. Immunol. 12:405–413 10.1093/intimm/12.4.405 [DOI] [PubMed] [Google Scholar]
- Yee C., Thompson J.A., Byrd D., Riddell S.R., Roche P., Celis E., Greenberg P.D. 2002. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. USA. 99:16168–16173 10.1073/pnas.242600099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Bowerman N.A., Salama J.K., Schmidt H., Spiotto M.T., Schietinger A., Yu P., Fu Y.X., Weichselbaum R.R., Rowley D.A., et al. 2007. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J. Exp. Med. 204:49–55 10.1084/jem.20062056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.