Abstract
Proximal spinal muscular atrophy (SMA) is a leading genetic cause of infant death. Patients with SMA lose α-motor neurons in the ventral horn of the spinal cord which leads to skeletal muscle weakness and atrophy. SMA is the result of reduction in Survival Motor Neuron (SMN) expression. Transgenic mouse models of SMA have been generated and are extremely useful in understanding the mechanisms of motor neuron degeneration in SMA and in developing new therapeutic candidates for SMA patients. Several research groups have reported varying average lifespans of SMNΔ7 SMA mice (SMN2+/+;SMNΔ7+/+;mSmn−/−), the most commonly used mouse model for preclinical therapeutic candidate testing. One environmental factor that varied between research groups was maternal diet. In this study, we compared the effects of two different commercially available rodent chows (PicoLab20 Mouse diet and Harlan-Teklad 22/5 diet) on the survival and motor phenotype of the SMNΔ7 mouse model of SMA. Specifically, the PicoLab20 diet significantly extends the average lifespan of the SMNΔ7 SMA mice by approximately 25% and improved the motor phenotype as compared to the Harlan diet. These findings indicate that maternal diet alone can have considerable impact on the SMA phenotype.
Keywords: spinal muscular atrophy, motor neuron disease, diet, survival motor neuron, plastin-3, motor behavior
INTRODUCTION
Proximal spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease characterized by selective loss of α motor neurons in the anterior horn of the spinal cord [1]. This leads to atrophy of limb and trunk muscles. In humans, the SMN (survival motor neuron) gene is duplicated and the two SMN genes (SMN1 and SMN2) differ by several non-polymorphic nucleotides, however, a single nucleotide (C→T) within an exon splice enhancer of exon 7 is central to SMA development [2;3]. SMN1 transcripts produce full-length SMN (FL-SMN) protein. In contrast, the majority of SMN2 transcripts lack exon 7 (SMNΔ7) and therefore produce SMNΔ7 protein; however, 10-20% of SMN2 transcripts are correctly spliced and produce FL-SMN protein. SMA results from deletions or mutations of the SMN1 (survival motor neuron) gene; the SMN2 gene remains intact [4]. SMA disease severity typically depends on the copy number of SMN2 and the levels of SMN protein [5-7].
Development of therapeutics for the treatment of SMA is an active area of research. Several animal models of SMA are used to test potential therapeutic strategies and to study the mechanism(s) of disease [8]. The SMNΔ7 SMA mouse model accurately mimics the human disease [9;10] and has been used extensively to test potential SMA therapies [11]. In the absence of treatment, the reported average lifespan of the SMNΔ7 SMA mice varies from 13 days to 18 days [9;10;12;13] indicating there may be environmental factors which influence the lifespan of this SMA mouse model. Environmental factors are important modifiers of neurological phenotypes and can partially explain differences in phenotype observed between research laboratories [14]. It is important to identify the environmental variables which influence the lifespan of this mouse model in order to accurately compare lifespan data between research institutions and to properly control for these variables in preclinical therapeutics trials.
Careful examination of SMNΔ7 mouse breeding colonies from two different laboratories that showed different average lifespans of SMNΔ7 SMA mice (i.e. Ohio State University and the University of Missouri) reveals that the breeder mice and as a result their SMNΔ7 SMA pups were maintained on different diets (Harlan-Teklad 22/5 and PicoLab20 Mouse diets, respectively). In this study, we examined the effect of maternal diet on the phenotype of the SMNΔ7 SMA mouse model. We find the maternal diet is a powerful environmental modifier of the SMNΔ7 SMA mouse phenotype accounting for most, if not all, of the variability in lifespan observed between two independent research institutions.
MATERIALS AND METHODS
Mice
SMNΔ7 SMA mice (SMN2+/+;SMNΔ7+/+;mSmn−/−) were generated from males and females of the genotype SMN2+/+;SMNΔ7+/+;mSmn+/− (line 4299; FVB.Cg-Tg(SMN2*delta7)4299Ahmb Tg(SMN2)89Ahmb Smn1tm1Msd). These mice can be obtained from Jackson (#005025). Breeder mice were provided with ad libitum water and rodent chow; these mice received either the Harlan-Teklad 22/5 rodent diet (#8640; Teklad) or the PicoLab20 Mouse diet (#5058; Purina). Neonatal offspring were genotyped using a PCR-based assay on genomic DNA from tail biopsies as described previously [9]. Birth is defined as postnatal day (PND) 01 for these experiments. All experiments were conducted in accordance with the protocols described in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee and the Animal Care and Use Committee of the University of Missouri.
Behavior Analysis
A cohort of SMNΔ7 SMA mice within each diet group were assessed for changes in righting reflex success and latency, spontaneous locomotor activity and pivoting activity as described previously [10].
Histology
Processing, sectioning and Cresyl violet staining of PND11 spinal cords was accomplished as described previously [15].
Clinical Chemistry
At PND12 mice were anesthetized by isoflurane and then sacrificed by decapitation. Whole blood was collected and glucose was measured using the OneTouch (New Brunswick, NJ) UltraMini glucose monitor. Serum was separated from red blood cells by centrifugation and then frozen at −80°C for later analysis. Serum fatty acids were quantified using a kit from Zenbio (Research Triangle Park, NC). β-Hydroxybutyrate levels were quantified using a β-Hydroxybutyrate kit from BioVision (Mountain View, CA).
Immunoblot
Immunoblot analysis of spinal cord extracts was completed as described previously [15]. The following primary antibodies were used: mouse anti-SMN mAb (clone 8, BD Biosciences; 1:5000), goat anti-plastin-3 pAb (Santa Cruz Biotechnology; 1:200) and mouse anti-β-actin mAb (clone AC-15, Sigma-Aldrich; 1:10 000).
Statistical Analysis
Data are expressed as means ± standard errors. Data were analyzed either by two-tailed Student’s t-test or by 1 way ANOVA followed by Newman-Keuls multiple comparison post-hoc test. Kaplan-Meier curves were generated from the survival and onset of loss of body mass data and tested using the Mantel-Cox log rank test. For all tests p<0.05 was considered significant. All statistical analyses were performed with either SPSS v.17 or Graphpad Prism v.5.
RESULTS
Effect of Transition from Harlan-Teklad 22/5 to PicoLab20 Mouse Diets on Survival and Motor Phenotype
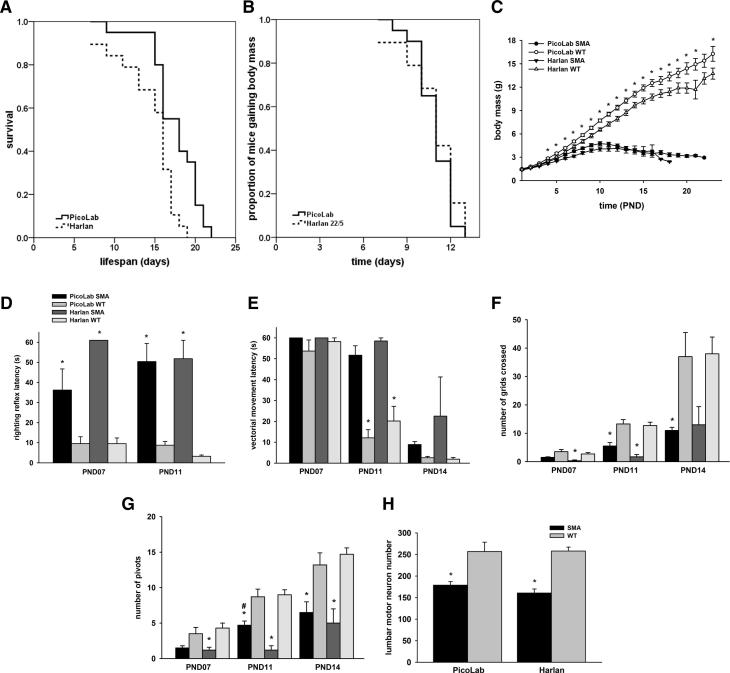
We began our study by making the observation that SMNΔ7 SMA mice live on average 13-14 days in the Ohio State University vivarium [9;10;15] while the same model lives on average 16-17 days in the vivarium at the University of Missouri [12;13]. A potential contributing factor to the observed lifespan difference was the diet fed to the dams: Harlan-Teklad 22/5 (Ohio State) or PicoLab20 Mouse chow (Missouri). While the diets differ modestly in several ingredients, the most prominent difference is within the total fat content (9% versus 5.2%; PicoLab20 versus Harlan-Teklad, respectively; Supplementary Table). In the first set of experiments, breeding animals that had been exclusively raised on Harlan-Teklad 22/5 were transitioned to PicoLab20 Mouse diets (hereafter referred to as Harlan-to-PicoLab). In the offspring from the Harlan-to-PicoLab group, SMNΔ7 SMA mice survived on average 21% longer than those SMNΔ7 SMA mice from dams that were exclusively fed the Harlan-Teklad 22/5 diet (Fig. 1A; 17.6 ± 0.7 days on the PicoLab20 diet (n=20) as compared to 14.5 ± 0.8 days on the Harlan-Teklad 22/5 diet (n=19); χ2=9.759, p=0.002).
Fig 1.
Effect of diet on survival and motor phenotype of SMNΔ7 SMA mice. (A). Kaplan-Meier survival plots of SMNΔ7 SMA mice from dams transitioned from the Harlan-Teklad 22/5 diet to the PicoLab20 Mouse diet. (B). Kaplan-Meier analysis of the onset of body mass loss an indicator of the late stages of disease in SMNΔ7 SMA mice from dams fed the PicoLab20 Mouse diet or the Harlan-Teklad 22/5 diet. (C). Body mass curves for SMNΔ7 SMA mice as well as WT littermates from dams fed either the PicoLab 20 Mouse or the Harlan-Teklad 22/5 diets. Key: *, p<0.05 when comparing SMNΔ7 SMA to WT mice. (D) Righting reflex latencies for SMNΔ7 SMA mice as well as WT littermates at PND07 and PND11 from dams fed either the PicoLab20 Mouse diet of the Harlan-Teklad 22/5 diet. (E) Vectorial movement latencies for SMNΔ7 SMA mice as well as WT littermates at PND07, PND11 and PND14 from dams fed either the PicoLab20 Mouse diet of the Harlan-Teklad 22/5 diet. (F) Spontaneous locomotor activity—as measured by the number of grids crossed in 1 minute—for SMNΔ7 SMA mice as well as WT littermates at PND07, PND11 and PND14 from dams fed either the PicoLab20 Mouse diet of the Harlan-Teklad 22/5 diet. (G) The number of 90° pivots in 1 minute for SMNΔ7 SMA mice as well as WT littermates at PND07, PND11 and PND14 from dams fed either the PicoLab20 Mouse diet of the Harlan-Teklad 22/5 diet. Key: *, p<0.05 when comparing SMNΔ7 SMA to WT mice; #, p<0.05 when comparing PicoLab20-fed to Harlan-Teklad 22/5-fed mice. (H). Effect of diet on motor neuron numbers in the lumbar spinal cords of SMNΔ7 SMA mice at PND11. Key: *, p<0.05 when comparing SMNΔ7 SMA to WT mice.
To begin to understand why these cohorts of SMA animals exhibit differing life spans, we examined several phenotypic parameters of the SMA mice within these two cohorts. In addition to lifespan, the onset of body mass loss, an indicator for the end-stage of disease [10], was determined for the PicoLab20 Mouse and Harlan-Teklad SMA animals. The onset of body mass loss, while modestly different, is not significantly different between SMA mice on the two diets (Fig. 1B; 10.9 ± 0.3 days vs. 10.8 ± 0.4 days; χ2=0.371, p=0.543).There is a trend for PicoLab20-diet fed SMA mice to weigh more than Harlan 22/5 diet-fed SMA mice especially at PND07-PND11 but this trend is not statistically significant (Fig. 1C). PicoLab20-fed WT mice (i.e. non-SMA pups), however, have significantly larger body masses than those WT mice fed with the Harlan 22/5 diet.
To assess whether motor function was also influenced by maternal diet, we compared the neonatal motor behaviors of SMNΔ7 SMA mice from dams fed either the Picolab or Harlan diet. Consistent with previous reports [10], surface righting reflex responses are impaired in SMNΔ7 SMA mice fed on either diet relative to WT littermates (Fig. 1D). When the righting reflexes were compared between the PicoLab20-fed SMNΔ7 SMA mice and the Harlan-Teklad-fed mice, the PicoLab20 cohort exhibited a slightly better righting reflex than SMNΔ7 Harlan-Teklad SMA mice at PND07 but this reduction is not statistically significant. Vectorial movement latency—the time it takes a neonatal mouse to move in one direction a distance that is greater than its body length [10]—is significantly longer in SMNΔ7 SMA mice relative to age-matched WT mice fed either diet at PND11 and PND14 (Fig. 1E). Spontaneous locomotor activity is significantly impaired in SMNΔ7 SMA mice for both diets at all ages tested (Fig. 1F), however the number of grids crossed by SMNΔ7 SMA mice on the PicoLab20 diet tended to be greater than those crossed by SMNΔ7 SMA mice on the Harlan-Teklad diet but the difference is not statistically significant. Pivoting responses are reduced SMNΔ7 SMA mice on either diet relative to WT littermates (Fig. 1G). Interestingly, SMNΔ7 SMA mice on the PicoLab20 diet pivoted more frequently than SMNΔ7 SMA mice on the Harlan-Teklad diet especially at PND11.
Loss of motor neurons in the lumbar spinal cord of SMNΔ7 SMA mice begins at PND09 with the magnitude of motor neuron loss increasing over time as the disease progresses phenotypically [9]. To determine whether diet impacts motor neuron survival, we determined the number of lumbar spinal motor neurons in SMNΔ7 SMA mice as well as WT littermates from dams fed on either the PicoLab20 or the Harlan-Teklad 22/5 diets. In both diets, SMNΔ7 SMA mice have fewer motor neurons in the lumbar spinal cord at PND11 than age-matched WT mice (Fig. 1H). There is no difference, however, in motor neuron counts between SMNΔ7 SMA mice fed on the PicoLab20 diet and on the Harlan 22/5 diet.
Effect of Diet on Blood Metabolite Levels in SMNΔ7 SMA Mice
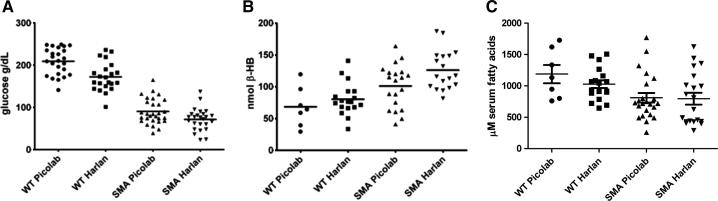
To further characterize the differences between the SMNΔ7 SMA mice fed the two diets, we measured energy metabolite levels in the blood of these mice at PND12—a time point after the onset of significant motor neuron loss [9]. Strikingly, WT mice had nearly twice the blood glucose concentration of SMNΔ7 SMA mice regardless of diet (Fig. 2A). Diet, however, did influence the glucose levels of the pups because both WT and SMNΔ7 SMA pups from PicoLab20-fed dams had significantly higher blood glucose levels than their WT or SMNΔ7 SMA counterparts from Harlan-Teklad-fed dams (p=0.0004 for WT and p=0.02 for SMNΔ7 SMA). Infact, there is a positive correlation (Pearson R = 0.7061; p<0.0001) between body mass and blood glucose levels in SMNΔ7 SMA mice from either Harlan-Teklad or PicoLab20-fed dams. We found β-hydroxybutyrate levels significantly elevated in SMNΔ7 SMA mice as compared to their WT littermates (Fig. 4B) and the SMNΔ7 SMA mice on the Harlan-Teklad diet had significantly higher β-hydroxybutyrate levels than the SMA mice on the PicoLab20 diet. Consistent with these findings, low physiological concentrations of blood glucose are known to stimulate the liver to produce ketone bodies such as β-hydroxybutyrate from acetyl CoA. With both diets, serum free fatty acid levels tended to be lower in SMNΔ7 SMA mice than in age-matched WT littermates (Fig. 4C). Also, serum free fatty acid levels tended to be higher in mice fed on the PicoLab20 diet as compared to genotype-matched Harlan-Teklad diet-fed mice but these differences were not statistically significant.
Fig. 2.
Effect of diet on blood metabolite levels in SMNΔ7 SMA mice. (A). Blood glucose levels for SMNΔ7 SMA mice as well as WT littermates at PND12 from dams fed either the PicoLab20 Mouse diet of the Harlan-Teklad 22/5 diet. (B). Blood β-hydroxybutyrate levels for SMNΔ7 SMA mice as well as WT littermates at PND12 from dams fed either the PicoLab20 Mouse diet of the Harlan-Teklad 22/5 diet. (C). Blood free fatty acids levels for SMNΔ7 SMA mice as well as WT littermates at PND12 from dams fed either the PicoLab20 Mouse diet of the Harlan-Teklad 22/5 diet.
Fig. 4.
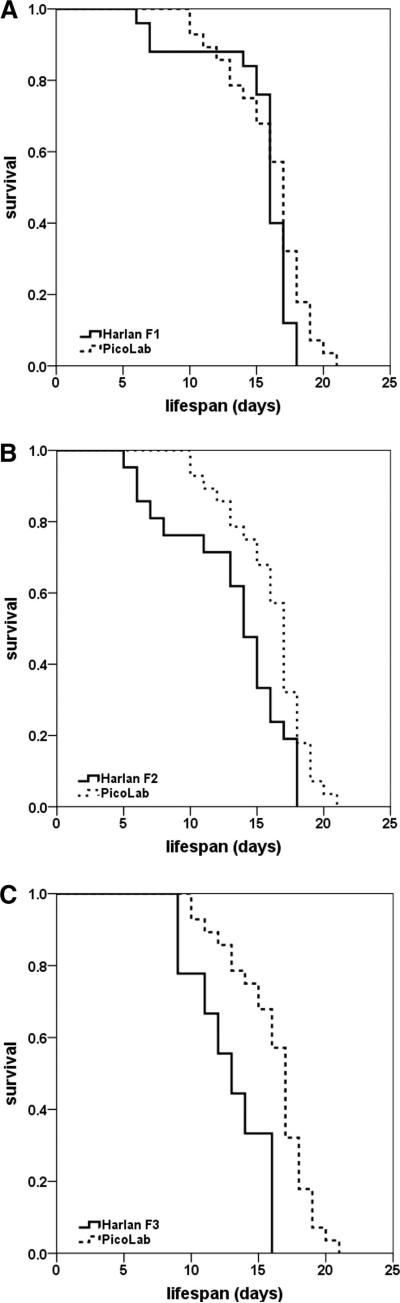
Kaplan-Meier survival plots of SMNΔ7 SMA mice from dams transitioned from the PicoLab20 Mouse diet to the Harlan-Teklad 22/5 diet for 1 (A), 2 (B) and 3 (C) generations.
Effect of Diet on SMN and Plastin-3 Protein Levels in SMNΔ7 SMA Mice
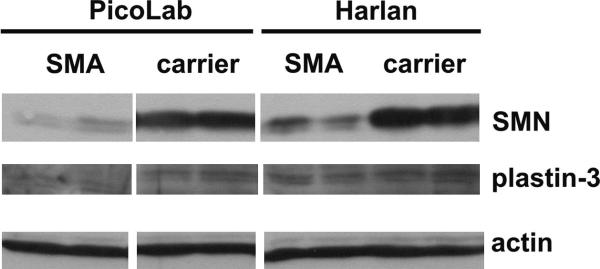
Studies using transgenic mice have shown that increasing SMN2 copy number [16] or increasing SMN protein levels in neurons [17] ameliorates the phenotype of SMA mice. We wanted to determine if the protective effect of the PicoLab20 diet on SMNΔ7 SMA mice results from increasing SMN protein levels in the spinal cord. SMN protein expression in the spinal cord of SMA mice fed on the PicoLab20 diet were similarly low at PND08 (before the onset of motor neuron loss) compared to SMA mice fed on the Harlan 22/5 diet (Figure 3). The β-actin band shows that equivalent amounts of protein were loaded in each lane. Recent studies have identified plastin-3 (T-plastin) as a putative modifier of the SMA phenotype in patients [18]. Therefore, plastin-3 protein levels in the spinal cord were examined and shown not to change appreciably between diets or between genotypes (i.e., SMA vs. carrier; Figure 3).
Fig. 3.
Effect of diet on the expression of SMN and plastin-3 proteins in the spinal cords of SMNΔ7 SMA mice at PND08. The β-actin band shows that equivalent amounts of protein were loaded in each lane.
Effect of Transition from PicoLab20 to Harlan-Teklad 22/5 Mouse Diets on Survival
The previous experiments involved animals that were transitioned from a Harlan diet to a PicoLab20 diet. To further extend these results and the importance of diet in the SMA phenotype, animals from a PicoLab20-fed colony were transitioned to Harlan chow. Interestingly, the average lifespan of the first generation (F1) of SMNΔ7 SMA pups on the Harlan diet trended approximately 5% lower than the PicoLab20 pups, however, the results were not statistically significant (Fig. 4A, 16.1±0.5 days (n=28) compared to 15.2±0.7 days (n=25); χ2=2.800, p=0.094). The second generation of animals fed on the Harlan diet (F2 generation from the original PicoLab20-transitioned parents) resulted in an average life span significantly shorter than the PicoLab20 cohort, but was still slightly longer than the pups reared exclusively on the PicoLab20 chow (Fig. 4B; 16.1±0.5 days compared to 13.1±0.9 days (n=21); χ2=6.092, p=0.014). In the analysis of the F3 PicoLab20-to-Harlan transition animals, an even shorter life span was recorded demonstrating that disease severity was increasing in successive generations on the Harlan low fat chow (Fig. 4C, 16.1±0.5 days compared to 12.9±0.9 days (n=9); χ2=10.880, p=0.001). The life span of the F3 animals was consistent with the original Harlan life span data and previously published work [9;10]. Furthermore, the analysis of subsequent generations did not detect a further deviation in life span (data not shown) suggesting that the SMA phenotype for the animals receiving the transition diet had stabilized. Taken together, these results suggest that a component of the diet significantly impacts the severity of the SMA phenotype and that the component is passed from dam to pup since none of the pups reach weaning age.
DISCUSSION
In this study, we demonstrate that maternal diet can significantly modify survival and the motor neuron disease phenotype in the SMNΔ7 mouse model for SMA. SMNΔ7 SMA mice from dams that were fed a diet with 9% fat (PicoLab20) survived on average 21% longer than those SMA mice from dams that were fed a lower fat (5%) diet (Harlan-Teklad 22/5). The effect of dietary fat on the lifespan of mice has been observed in many different transgenic mouse lines. For example, transgenic mice with an amyotrophic lateral sclerosis (ALS)-linked superoxide dismutase 1 (SOD1(G86R)) mutation survive longer when fed on a high fat (26%) diet than on a low fat (5%) diet [19]. Taguchi and colleagues [20] reported that mice which express reduced levels of insulin receptor substrate-2 (Irs2+/−) live up to 18% longer than strain-matched controls (Irs2+/+). Selman et al. [21] did not observe any differences in lifespan between Irs2+/+ and Irs2+/− mice in their colony. One difference between the two colonies is that the Taguchi colony was maintained on a 9% fat diet while the Selman colony was on a 5% fat diet [21;22]; it is possible that the differences in lifespan extension observed between these colonies could be the result of difference in fat content in their diets. Acetylcholinesterase knockout (AChE−/−) mice die within 14 days after birth due to apparent starvation caused by muscle weakness [23]. Supplementation of the maternal diet with a higher fat pellets and oral feeding of AChE−/− pups with lipid-enriched formula marked extended their lifespan past weaning to ~100 days [23].
Unlike what is observed in AChE−/− mice, supplemental feeding of SMNΔ7 SMA mice using a lipid-enriched formula does not significantly alter their survival [12;24]. Several factors could account for the discrepancy between these studies [12;24] and the results presented here including the composition of diet/supplementation (PicoLab20 Mouse diet vs. lipid-enriched infant formula with/without B vitamins mixture), the starting point for intervention (before birth vs. PND06/PND09), the frequency of administration (ad libitum vs. 2-3 times a day) and route of administration (through maternal milk or through a feeding needle). Interestingly though, SMNΔ7 SMA are more responsive to the protective effects of trichostatin A (TSA) [25] if they also receive nutritional supplementation [24]. It is possible that their nutritional supplementation may have enhanced the absorption of TSA into the bloodstream and/or increased its biodistribution into the CNS. It has been established that diet can markedly affect the absorption, biodistribution and metabolism of drugs (reviewed in [26]). It would be interesting to see if diet can affect the levels of TSA in the CNS and, hence, further enhance the ameliorative effects of TSA on SMNΔ7 SMA mice.
Besides fat content, the diets also differ in the concentration of metabolizable energy with the higher fat diet also having a greater density of calories/gram. This likely leads to a higher concentration of calories in the dam’s milk as evidenced by the increase of weight in WT pups who nursed from dams fed the higher fat PicoLab diet (data not shown). As SMA mice have clear gross motor function impairment, it is possible they have difficulty nursing although there is no evidence of maternal abandonment of SMNΔ7 SMA mice which would, in turn, lead to reduced nursing frequency even when these mice reach the end-stage of disease (M.E.R.B., unpublished observations). A higher density of calories in the dam’s milk would allow them to consume more calories for the same effort and could explain why the higher fat diet extends the lifespan of the SMNΔ7 SMA mouse model.
We show here that, irrespective of diet, SMNΔ7 SMA mice exhibit lower blood glucose levels (hypoglycemia) at PND12 which is during the end-stage of disease. Furthermore, blood glucose levels correlate with body mass at PND12. It has been reported [27;28] that SMA patients develop hypoglycemia in response to fasting. This hypoglycemic response to fasting is not unique to SMA, however, as patients with SMA-independent severe muscle wasting (i.e. those with congenital myopathy and Duchenne muscular dystrophy) also become hypoglycemic after fasting [28]. It is important to note that blood glucose levels are higher in PicoLab20-fed mice than in Harlan-Teklad 22/5-fed mice. This elevation in blood glucose levels most likely does not result from direct consumption of these diets as they have similar carbohydrate contents (53% vs. 51.2%; Supplementary Table).
A considerable amount of SMA research focuses upon translational studies and analyzing compounds in SMA animal models. The results presented here demonstrate how seemingly simple components such as diet can significantly impact lifespan of the SMA mice. It is, therefore, imperative to consider standardizing the methodology in future drug development studies allowing results to be compared uniformly without underlying contributions from factors such as diet fat content. We would strongly recommend that a standardized set of criteria be assembled for and applied to preclinical testing of SMA therapeutic candidates.
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr. Arthur Burghes for providing laboratory space for some of these experiments. This study was supported in part by Families of SMA (MERB), FightSMA (CLL) and National Institutes of Health (CLL, R01NS41584 and R01HD054413). FFR was supported by a National Institutes of Health Training Grant (T32GM008396).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol.Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- [2].Lorson CL, Hahnen E, Androphy EJ, et al. A single nucleotide in the SMN gene regulates splicing an is responsible for spinal muscular atrophy. Proc.Natl.Acad.Sci.U.S.A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Monani UR, Lorson CL, Parsons DW, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum.Mol.Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- [4].Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- [5].Coovert DD, Le TT, McAndrew PE, et al. The survival motor neuron protein in spinal muscular atrophy. Hum.Mol.Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- [6].Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat.Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- [7].McAndrew PE, Parsons DW, Simard LR, et al. Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am.J.Hum.Genet. 1997;60:1411–1422. doi: 10.1086/515465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Butchbach MER, Burghes AHM. Perspectives on models of spinal muscular atrophy for drug discovery. DrugDisc.TodayDis.Models. 2004;1:151–156. [Google Scholar]
- [9].Le TT, Pham LT, Butchbach MER, et al. SMNΔ7, the major product of the centromeric survival motor neuron gene (SMN2), extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum.Mol.Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- [10].Butchbach MER, Edwards JD, Burghes AHM. Abnormal motor phenotype in the SMNΔ7 mouse model of spinal muscular atrophy. Neurobiol.Dis. 2007;27:207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burghes AHM, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nature Rev.Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rose FF, Jr, Mattis VB, Rindt H, et al. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum.Mol.Genet. 2009;18:997–1005. doi: 10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mattis VB, Ebert AD, Fosso MY, et al. Delivery of a read-through inducing compound, TC007, lessens the severity of a SMA animal model. Hum.Mol.Genet. 2009;18:3906–3913. doi: 10.1093/hmg/ddp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wahlsten D, Metten P, Phillips TJ, et al. Different data from different labs: lessons from studies of gene-environment interactions. J.Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- [15].Butchbach MER, Singh J, Þorsteindóttir M, et al. Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Hum.Mol.Genet. 2009 doi: 10.1093/hmg/ddp510. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Monani UR, Sendtner M, Coovert DD, et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum.Mol.Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- [17].Gavrilina TO, McGovern VL, Workman E, et al. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle specific SMN expression has no phenotypic effect. Hum.Mol.Genet. 2008;17:1063–1075. doi: 10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oprea GE, Kröber S, McWhorter ML, et al. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320:524–527. doi: 10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dupuis L, Oudart H, René F, et al. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc.Natl.Acad.Sci.U.S.A. 2004;101:11159–11164. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- [21].Selman C, Lingard S, Gems D, et al. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis”. Science. 2008;320:1012b. doi: 10.1126/science.1152366. [DOI] [PubMed] [Google Scholar]
- [22].Taguchi A, White MF. Response to comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis”. Science. 2008;320:1012c. doi: 10.1126/science.1152366. [DOI] [PubMed] [Google Scholar]
- [23].Duysen EG, Stribley JA, Fry DL, et al. Rescue of the acetylcholinesterase knockout mouse by feeding a liquid diet; phenotype of the adult acetylcholinesterase deficient mouse. Dev.Brain Res. 2002;137:43–54. doi: 10.1016/s0165-3806(02)00367-x. [DOI] [PubMed] [Google Scholar]
- [24].Narver HL, Kong L, Burnett BG, et al. Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Ann.Neurol. 2008;64:465–470. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Avila AM, Burnett BG, Taye AA, et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J.Clin.Invest. 2007;117:659–671. doi: 10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Welling PG. Influence of food and diet on gastrointestinal drug absorption: a review. J.Pharmacokinet.Biopharm. 1977;5:291–334. doi: 10.1007/BF01061694. [DOI] [PubMed] [Google Scholar]
- [27].Bruce AK, Jacobsen E, Dossing H, et al. Hypoglycaemia in spinal muscular atrophy. Lancet. 1995;346:609–610. doi: 10.1016/s0140-6736(95)91439-0. [DOI] [PubMed] [Google Scholar]
- [28].Ørngreen MC, Zacho M, Hebert A, et al. Patients with severe muscle wasting are prone to develop hypoglycemia during fasting. Neurology. 2003;61:997–1000. doi: 10.1212/01.wnl.0000086813.59722.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.