Abstract
We studied Alzheimer’s disease (AD) pathology in the precuneus and surrounding brain areas. Anatomically, the precuneus corresponds to the medial portion of human cerebral cortical Brodmann Area 7. This study utilized patients from the University of Kentucky Alzheimer’s Disease Center autopsy cohort. Data from 47 brains were used comprising patients of differing antemortem cognitive impairment severities, each with longitudinal clinical data and extensive neuropathological data. We assessed whether the precuneus and surrounding areas are differentially vulnerable to AD-type pathological lesions (diffuse amyloid plaques, neuritic amyloid plaques, and neurofibrillary tangles). Eleven areas of brain were evaluated for each case: amygdala, hippocampal CA1, subiculum, entorhinal cortex, frontal cortex, superior and middle temporal gyri, inferior parietal lobule, occipital cortex, posterior cingulate gyrus, Brodmann Area 31, and the precuneus proper. Like other areas of neocortex, the precuneus demonstrated increased diffuse and neuritic amyloid plaques early in the evolution in AD, and increased neurofibrillary tangles late in AD. Correcting for the antemortem cognitive status of the patients, there was no evidence of an increase in the density of AD-type pathology in the precuneus or neighboring areas relative to other areas of cerebral neocortex. Our results are not consistent with the idea that the precuneus is involved in a special way with plaques or tangles relative to other areas of neocortex.
Keywords: Precuneus, Posterior cingulate, Parietal, MCI, Cognition, Neuropathology, Pathology, Tissue
Previous studies have indicated that the precuneus area of the cerebral cortex may be specifically vulnerable to early changes of Alzheimer’s disease (AD). These prior studies include a variety of neuro-imaging modalities: structural analyses (magnetic resonance imaging, MRI), functional studies of metabolism (both positron emission tomography [PET] and functional MRI [fMRI]), as well as PET scans using ligands that bind amyloid plaques ([1,7–9,12–16,21,22,26] and see reviews [3,4]). However, there has never been a study that focuses on neuropathology of the precuneus in AD.
The precuneus itself has been defined as the mesial portion of Brodmann Area (BA) 7 [6]. Immediately ventral to the precuneus is a transitional area, BA 31, and between BA 31 and the corpus callosum is the posterior cingulate gyrus (PCG, also known as BA 23). The anterior boundary of the precuneus is the marginal branch of the cingulate sulcus, whereas the parieto-occipital sulcus provides the posterior boundary [6]. The anatomy is illustrated in Fig. 1. The function of the precuneus is not well known but may pertain to higher-order representation of self [4,6,25].
Fig. 1.
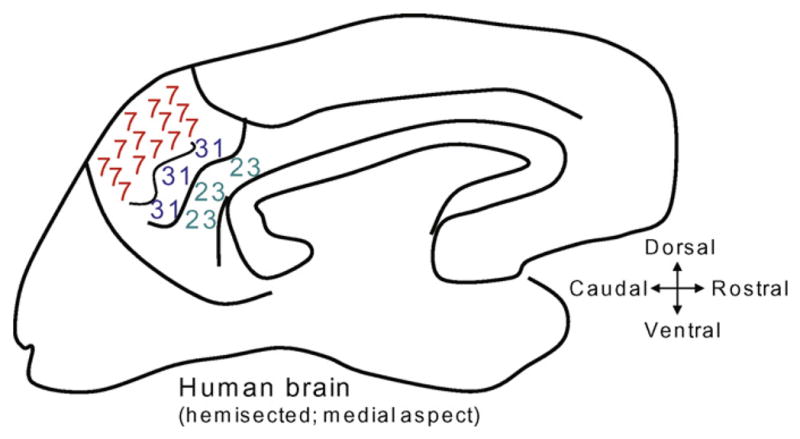
A cartoon shows the medial surface of a human brain that has been hemi-sected sagitally. The numbers refer to Brodmann Area (BA) fields. The precuneus is the mesial area of BA 7. Inferior to the precuneus is BA 31. The posterior cingulate gyrus (BA 23) is immediately dorsal to the posterior half of the corpus callosum.
We sought to test the hypothesis that the precuneus is an area of the brain where the neuropathology of AD is more severe than in other brain areas. The University of Kentucky Alzheimer’s Disease Center (UK ADC) follows a large clinical cohort longitudinally with yearly in-depth neuropsychological mental status assessments. All subjects enrolled have agreed to autopsy and brain donation following death. This manuscript includes analysis of the clinicopathological indices including premortem cognitive status and quantitative measures of neurofibrillary tangles (NFTs), diffuse amyloid plaques (DPs), and neuritic amyloid plaques (NPs). NFT, DP, and NP lesion densities are routinely counted at the UK ADC and can be compared across different neuroanatomical regions [19]. We found no evidence for a disproportionate increase in precuneus AD-type pathology relative to other neocortical brain areas, at any point in the evolution of AD.
Patients who had come to autopsy from UK ADC cohorts were the basis for the study. Research protocols were approved by the UK IRB. Details of UK ADC recruitment were described previously [19,24]. All patients had post-mortem consensus conference diagnoses of AD, MCI, or non-demented—no dementia with Lewy bodies or vascular disease patients were included. Only patients who had Mini-Mental Status Examination (MMSE) scores within 2 years of death were used in this project. We gathered recent patients that represented a gamut of cognitive impairment, focusing on the relatively modestly impaired patients (see below). Mental status testing of our subjects has been described previously [23]. The present study focuses on the MMSE conducted closest to the date of death as the ‘severity metric’ for cognitive impairment. The MMSE score was chosen as it was the most consistently available measure obtained from both normal and demented subjects over the course of their evaluations. Other than the range of interest for cognitive impairment and the lack of concomitant pathologies, this represents a convenience sample and we chose the cases randomly from recent autopsies at the UK ADC. Since we had no prior knowledge about the severity of pathology in the regions studied, we had no bias in terms of case selection that would have affected our main outcome measures (i.e. pathology severity in the precuneus, BA 31, and PCG relative to other areas of neocortex).
The main focus of the analyses was a comparison of lesion counts between neocortical fields. Pathological assessments were as described in detail previously [19,20]. Briefly, a total of at least 25 sections were taken from each brain that included precuneus (mesial BA 7), BA 31, PCG (BA 23), middle frontal gyrus (BA 9), superior and middle temporal gyri (BA 21 and 22), inferior parietal lobule (BA 39 and 40), occipital lobe including primary visual area (Occ; BA 17 and 18). A gross anatomical photograph to show the areas selected near the precuneus is provided as Supplemental file 1. Thorough neuropathological investigation was performed including assessment of infarctions and Lewy bodies (via alpha-synuclein immunohistochemistry) as previously described [19]. Sectioning was performed either fresh or after fixation. Most of the posterior cingulate gyri sections were sectioned twice, both fresh and after fixation, and the lesion counts were not affected (data not shown). Lesions were counted as previously described [19]; silver impregnation and counts are routinely performed at the UK ADC. Senile plaques were counted using a 10× objective (field size, 2.35 mm2 in the five most involved fields in each section of the regions described earlier. The most involved fields were determined by studying the whole section and marking it. Senile plaques were separated into DPs (plaques without surrounding argyrophilic degenerating neurites) and NPs (plaques with multiple argyrophilic neurites) in each region. Neurofibrillary tangles were counted with a 20× objective (field size, 0.586 mm2) in the five most involved fields of each section of the regions described earlier. An arithmetic mean was calculated from the count of the five most involved fields for DPs (number of DPs per 2.35 mm2), NPs (number of NPs per 2.35 mm2), and NFTs (number of NFTs per 0.586 mm2) for each region. The same neuropathological analyst and the same microscope were used for all counts generated.
Means were compared using a linear mixed model with between subject factor MMSE subgroup, within subject factor brain region, and an unstructured covariance structure for the repeated measurements across regions. Since the primary hypothesis centers about counts in the precuneus region, Dunnett’s many to one t-test procedure was used to compare the mean counts for each region with the mean count for the precuneus region.
Results are shown in Figs. 2 and 3. The neuropathology counts are presented stratified by MMSE scores: 30 (N = 7); 26–29 (N = 20); 20–25 (N = 10); 11–19 (N = 5); and 0–10 (N = 5), and see Table 1. Note that the largest group in the present study included people in MMSE score range 26–29, because we hypothesize that these are in the early stage of cognitive decline. Four of the patients in this group had the diagnosis of MCI, and four were early AD.
Fig. 2.
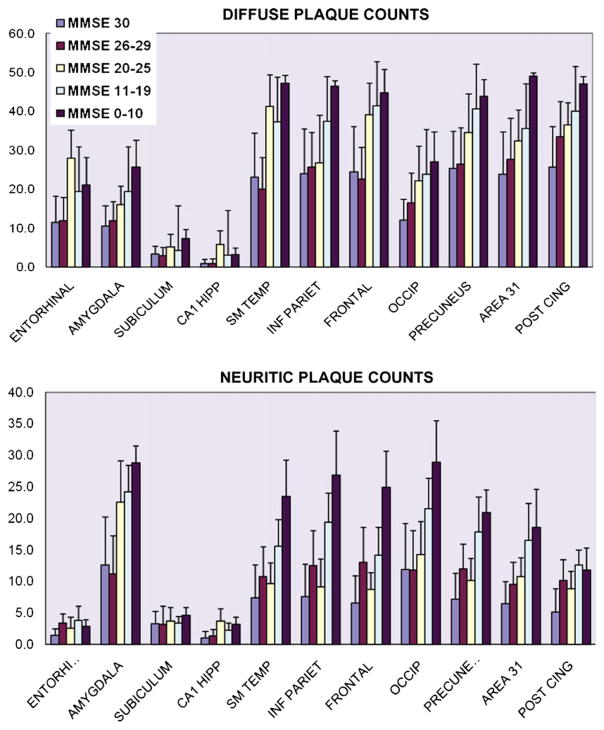
A chart shows the results of amyloid plaque counts from 11 different brain areas. Diffuse plaques (amyloid plaques without surrounding argyrophilic swollen neurites) are analyzed separately from neuritic plaques. The results are stratified by MMSE scores. Note that the results are similar for both subtypes of amyloid plaques. All neocortical regions, as well as the amygdala, have increased plaque counts as MMSE scores decrease. However, there is no relative increase in plaque counts referent to the precuneus or surrounding areas relative to other neocortical areas. Note that ordinate values are different because diffuse plaque densities are higher than neuritic plaque densities. Error bars indicate S.D.
Fig. 3.
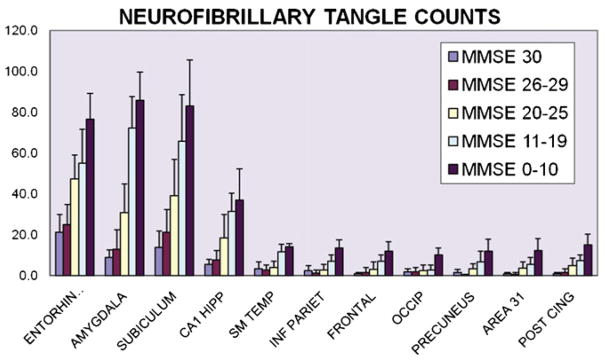
A chart shows the results of neurofibrillary tangle (NFT) counts from 11 different brain areas. NFT counts tend to be higher in allocortical areas of the mesial temporal lobe, which are associated with the hippocampal formation and amygdala. As with the amyloid plaque counts, there is no evidence of a selective increase in NFT counts in the precuneus, Brodmann area 31, or posterior cingulate gyrus. Error bars indicate S.D.
Table 1.
Cases used for the study.
| MMSE group | N | Average age | Average MMSE |
|---|---|---|---|
| 0–10 | 5 | 85.6 | 5.2 |
| 11–19 | 5 | 85.0 | 16.0 |
| 20–25 | 10 | 87.0 | 22.4 |
| 26–29 | 20 | 84.5 | 28.2 |
| 30 | 7 | 87.1 | 30.0 |
Throughout the neocortex and in the amygdala, the counts for DPs and NPs increased in persons in correlation to the severity of cognitive impairment. The NFTs increase in the neocortex is less dramatic than in the medial temporal lobe structures associated with allocortex. As a rule, the density of plaques and tangles in the precuneus and surrounding structures (area 31 or posterior cingulate gyri) was in proportion to that of other neocortical regions. There is no group for any subtype of AD-type pathology where the density of pathology is greater in the precuneus than all the other areas.
Dunnett’s t-tests were performed to compare the density of DPs, NPs, and NFTs counted in the precuneus versus the other ten brain areas evaluated. These statistical analyses showed that the only regions that had mean NFT counts significantly different from the precuneus region were the entorhinal, amygdala, subiculum, and CA1 hippocampus. The regions that differed from the precuneus on mean DP counts were the entorhinal, amygdala, subiculum, CA1 hippocampus, and the occipital cortex (P < 0.0002 by Dunnett’s adjustment, 42df). For NP counts, those differing significantly from the precuneus were the entorhinal, subiculum, CA1 hippocampus, amygdala, and PCG (P < .0003, 42df). In sum, the AD-type pathology in the precuneus was only statistically different from other neocortical region for DPs versus the occipital cortex, and for NPs versus PCG. AD-type lesions counts from all other areas of neocortex were not statistically different from those in the precuneus.
This present study is, to the best of our knowledge, the first to compare the AD-type lesion densities across different areas of the brain that include specifically the precuneus and adjacent BA 31. Brains were evaluated across a range of antemortem cognitive impairments. We hypothesized that the density of AD-type pathology would be disproportionately higher in the precuneus relative to other neocortical regions. To test this hypothesis, we compared the counts of the densities of DPs, NPs, and NFTs in 12 different areas of the brain in 47 different patients from the UK ADC autopsy cohort. The results did not support our hypothesis as the data from these patients did not show a disproportionate increase in AD-type pathology in the precuneus or surrounding areas of the neocortex.
The precuneus is functionally related to the superior parietal cortex [6]. A prior study showed that amyloid plaque density in the superior parietal cortex is high in a subset of patients with poor language scores [5]. Another study found correlation between neurofibrillary tangle densities in the superior parietal, posterior cingulate, and occipital cortex and constructional apraxia [10]. No prior study has noted a selective increase in pathology in the superior parietal cortex in early AD overall. The present study included analysis of the PCG, and showed no significant increase in DPs, NPs, or NFTs in comparison to other areas of the neocortex; this result has also been found in a prior study of PCG comparing non-demented, MCI, and AD patients [17]. It is notable that the precuneus (or BA 31 or PCG) has not been highlighted as an area of early involvement by researchers who have studied many brains extremely thoroughly (see [2,11]).
The present research was motivated partly by neuro-imaging studies using MRI, fMRI, and PET scans that indicate precuneus involvement early in the pathogenesis of AD [1,3,4,7–9,12,14,16,22] The reason(s) for the apparent discrepancy between our findings and that of various neuro-imaging-based studies is not known. We did not correct for brain atrophy; the analyses are quantitative but not stereological, as we evaluated densities of DPs, NPs, and NFTs. However, the lack of correction for atrophy would not explain the apparent discrepancy with regard to the studies on amyloid binding PET ligands [12,18,22]. It is noteworthy, moreover, that the neuro-imaging findings have never been corroborated by any post-mortem study in humans. There may be some reason for systematic bias in the neuro-imaging studies due to the anatomical location of the precuneus, although speculation on this topic is beyond the scope of the current study.
There are some limitations to our study design. Amyloid plaques and NFTs are not the only pathological findings in AD brains, and this study is limited to description of plaque and tangle densities. Also, even in non-demented individuals, amyloid plaques are relatively abundant in multiple areas of the cerebral cortex, so it is difficult to pinpoint the particular location in which amyloid plaques are first seen. This problem derives from the fact that the correlation between cognitive impairment severity and amyloid plaque densities is relatively poor [19]. Another drawback to this study is the limited sample size. We anticipate future neuropathological studies will be performed to corroborate our finding that the precuneus shows no special vulnerability to plaques and tangles in AD brains.
Supplementary Material
Acknowledgments
We are deeply grateful to all of the participants in our longitudinal aging study and to the patients with Alzheimer’s disease in our Alzheimer’s Disease Center’s research clinic. We thank Ann Tudor, Paula Thomason, Dr. Huaichen Liu, and Sonya Anderson for technical support, and Gregory Cooper, MD, PhD, Nancy Stiles, MD, and Allison Caban-Holt, PhD, for clinical evaluations. We also thank Randy L. Buckner, PhD, for stimulating discussion.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.neulet.2008.11.006.
Footnotes
Note added to proof
Ann C McKee and colleagues (JNEN 65[6]: p. 621–630) observed recently that Brodmann Area 19 is involved by AD-type pathology in non-demented individuals. We sampled the precuneus in parietal lobe) and Brodmann areas 17 and 18, but not Brodmann area 19.
Funding/Support: This study was supported by grant 5-P30-AG028383 and K08 NS050110 from the National Institutes of Health, Bethesda, MD.
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caramelli P, Robitaille Y, Laroche-Cholette A, Nitrini R, Gauvreau D, Joanette Y, Lecours AR. Structural correlates of cognitive deficits in a selected group of patients with Alzheimer’s disease. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:184–190. [PubMed] [Google Scholar]
- 6.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 7.Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, Miller BL, Weiner MW. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain. 2007;130:1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 9.Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Detection of grey matter loss in mild Alzheimer’s disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannakopoulos P, Duc M, Gold G, Hof PR, Michel JP, Bouras C. Pathologic correlates of apraxia in Alzheimer disease. Arch Neurol. 1998;55:689–695. doi: 10.1001/archneur.55.5.689. [DOI] [PubMed] [Google Scholar]
- 11.Giannakopoulos P, Hof PR, Michel JP, Guimon J, Bouras C. Cerebral cortex pathology in aging and Alzheimer’s disease: a quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain Res Brain Res Rev. 1997;25:217–245. doi: 10.1016/s0165-0173(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 12.Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007;80(Spec No 2):S160–S167. doi: 10.1259/bjr/97295129. [DOI] [PubMed] [Google Scholar]
- 13.Hirono N, Hashimoto M, Ishii K, Kazui H, Mori E. One-year change in cerebral glucose metabolism in patients with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2004;16:488–492. doi: 10.1176/jnp.16.4.488. [DOI] [PubMed] [Google Scholar]
- 14.Ibanez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, Rapoport SI, Schapiro MB, Horwitz B. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50:1585–1593. doi: 10.1212/wnl.50.6.1585. [DOI] [PubMed] [Google Scholar]
- 15.Karas G, Scheltens P, Rombouts S, van Schijndel R, Klein M, Jones B, van der Flier W, Vrenken H, Barkhof F. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- 16.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 17.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 18.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 19.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson PT, Kryscio RJ, Abner EL, Schmitt FA, Jicha GA, Mendiondo MS, Cooper G, Smith CB, Markesbery WR. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with Alzheimer’s disease and AD + DLB. J Alzheimers Dis. 2008 doi: 10.3233/JAD-2009-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrella JR, Wang L, Krishnan S, Slavin MJ, Prince SE, Tran TT, Doraiswamy PM. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245:224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- 22.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal aging: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. Gerontologist. 2001;41:716–722. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- 25.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 26.Vannini P, Almkvist O, Dierks T, Lehmann C, Wahlund LO. Reduced neuronal efficacy in progressive mild cognitive impairment: a prospective fMRI study on visuospatial processing. Psychiatry Res. 2007;156:43–57. doi: 10.1016/j.pscychresns.2007.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.