Abstract
Pro-inflammatory cytokines are produced in the gastric mucosa by inflammatory cells activated by chronic Helicobacter pylori (H. pylori) infection. Polymorphisms of these cytokine genes are associated with individual differences in gastric mucosal cytokine mRNA level, which result in differences in gastric mucosal inflammation, acid inhibition and gastroduodenal disease risk in response to H. pylori infection. Although polymorphisms of interleukin (IL)-1B, IL-1RN and TNF-A have been reported to relate well with gastric cancer and peptic ulcer risk, those of IL-2, IL-4, IL-6 and IL-8 genes are unclear. In combined analyses using data from previous studies, we found that the risk of gastric non-cardia cancer development was significantly associated with IL-4-168 C allele (OR: 0.81, 95% CI: 0.69-1.00) and IL-4-590 T allele carrier status (0.61, 0.53-0.73), and IL-6-174 G/G genotype (2.02, 1.31-3.10). In peptic ulcer development, IL-2-330 G and IL-4-590 T allele carriers had a significantly decreased risk (0.37, 0.27-0.50 and 0.58, 0.34-0.99, respectively). Moreover, IL-2, IL-4, IL-6 and IL-8 gene genotypes prevalence differs among populations. The inflammatory cytokine gene polymorphisms (e.g. IL-4-590 and IL-6-572 for gastric cancer, and IL-4-590, IL-6-572 and IL-8-251 for peptic ulcer) have a more potent influence on development of gastroduodenal diseases in Western than East Asian populations. These cytokine gene polymorphisms, as well as those of IL-1B, IL-1RN and TNF-A, may be used to identify groups at higher risk of gastric cancer and peptic ulcer, and those suitable for their prevention by H. pylori eradication therapy in Western populations.
Keywords: Helicobacter pylori, Cytokines, Genetic polymorphism, Stomach neoplasms, Peptic ulcer
INTRODUCTION
Helicobacter pylori (H. pylori) infects > 50% of the world’s population, and is particularly prevalent in developing countries (> 90%)[1-3]. Chronic H. pylori infection relates not only to the development of upper gastrointestinal diseases, such as peptic ulcer diseases, gastric adenoma, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma, but also with some extra-gastrointestinal disorders, such as idiopathic thrombocytopenic purpura, chronic idiopathic urticaria and iron-deficiency anemia[4-12]. Prevention and treatment of H. pylori-related disease has therefore relied on eradication therapy as first-line treatment[4-12].
The key pathophysiological event in H. pylori infection of gastric mucosa is the induction of a gastric mucosal inflammatory response. Following infection, neutrophils and mononuclear cells activated by H. pylori and their products infiltrate H. pylori-infected gastric mucosa and stimulate the transcription and synthesis of several pro-inflammatory cytokines [e.g. interleukin (IL)-1β, IL-2, IL-6, IL-8 and tumor necrosis factor (TNF)-α] and anti-inflammatory cytokines (e.g. IL-4 and IL-10)[13]. The increased production of inflammatory cytokines in response to H. pylori infection results in enhanced gastric mucosal inflammation, through binding to specific receptors on target cells.
Most of these inflammatory cytokine genes have genetic variations that influence cytokine levels in the gastric mucosa. Levels of mucosal IL-1β, for example, the most studied inflammatory cytokine, differ significantly among the different genotypes in three polymorphisms, IL-1B-511, -31 and IL-RN[13,14]. Carriers of the IL-1B-511 T, -31 C and IL-RN *2 alleles have significantly higher IL-1β levels than those of the other allele[13]. Consistent with this difference, carriers of the IL-1B-511 T, IL-1B-31 C alleles and IL-1RN *2/*2 (2 repeats of 86 bp) genotype show enhanced suppression of gastric acid secretion, which results in more rapid development of gastric atrophy, and a consequently greater risk of developing gastric cancer than in those with the IL-1B-511C, IL-1B-31 T and IL-1RN*1 alleles[13-18]. However, although IL-2, IL-4, IL-6 and IL-8 levels in gastric mucosa are reported to increase in patients with H. pylori infection[19,20], it remains unknown whether these inflammatory cytokine polymorphisms are associated with gastroduodenal disease development in a similar way as those with IL-1B and TNF-A. Previously reported associations with disease risk and cytokine gene polymorphisms of IL-2, IL-4, IL-6 and IL-8 are controversial, however, owing to either or both type 2 error and geographical differences (Tables 1, 2, 3, 4).
Table 1.
Association of IL-2 polymorphism and gastroduodenal diseases
| Position | Disease | Authors | Year | n | |||||
| -330 T/G | T/T | T/G | G/G | ||||||
| GC | Wu et al[37] | 2009 | GC | 1026 | 491 | 441 | 94 | GCC: T/T: 0.7 (0.4-1.0) | |
| NUD | 1083 | 516 | 480 | 87 | |||||
| GC | Shin et al[35] | 2008 | GC | 122 | 79 | 35 | 8 | NS | |
| NUD | 100 | 72 | 16 | 12 | |||||
| PU | Shin et al[35] | 2008 | PU | 220 | 159 | 45 | 16 | NS | |
| NUD | 100 | 72 | 16 | 12 | |||||
| Atrophy | Togawa et al[34] | 2005 | Atrophy | 152 | 80 | 63 | 9 | T/T: 2.8 (1.3-6.2) | |
| NUD | 443 | 202 | 196 | 45 | |||||
| -384 G/T | G/G | G/T | T/T | ||||||
| GCC | Savage et al[36] | 2004 | GC | 87 | 16 | 47 | 20 | NS | |
| NUD | 379 | 96 | 174 | 109 | |||||
| +114 G/T | G/G | G/T | T/T | ||||||
| GCC | Savage et al[36] | 2004 | GC | 82 | 33 | 35 | 14 | NS | |
| NUD | 377 | 149 | 148 | 80 |
GC: Gastric cancer; GCC: Gastric cardia cancer; PU: Peptic ulcer; IL: Interleukin; NUD: Non-ulcer dyspepsia; NS: Not significant.
Table 2.
Association of IL-4 polymorphism and gastroduodenal diseases
| Position | Disease | Authors | Year | n | |||||
| -168 T/C | T/T | T/C | C/C | ||||||
| GC | Wu et al[37] | 2009 | GC | 1042 | 744 | 271 | 27 | C carrier: 0.8 (0.7-1.0) | |
| NUD | 1099 | 743 | 332 | 24 | |||||
| -590 C/T | C/C | C/T | T/T | ||||||
| GC | Zambon et al[57] | 2008 | GC | 40 | 32 | 7 | 1 | NS | |
| NUD | 171 | 124 | 43 | 4 | |||||
| GC | García-González et al[56] | 2007 | GC | 404 | 283 | 107 | 14 | NS | |
| NUD | 404 | 267 | 123 | 14 | |||||
| GC | Lai et al[54] | 2005 | GC | 123 | 83 | 38 | 2 | NS | |
| NUD | 162 | 105 | 50 | 7 | |||||
| GC | El-Omar et al[53] | 2003 | GC | 122 | 78 | 37 | 7 | NS | |
| NUD | 209 | 153 | 46 | 10 | |||||
| GC | Wu et al[52] | 2003 | GC | 220 | 146 | 69 | 5 | C carrier (diffuse-type): 1.6 (1.0-2.7) | |
| NUD | 230 | 163 | 55 | 12 | |||||
| DU | Zambon et al[57] | 2008 | DU | 171 | 124 | 43 | 4 | NS | |
| NUD | 107 | 79 | 26 | 2 | |||||
| Atrophy | Kato et al[51] | 2006 | Atrophy | 788 | 398 | 308 | 82 | NS | |
| Dys | 115 | 51 | 48 | 16 | |||||
| NUD | 1020 | 506 | 414 | 100 | |||||
| -33 C/T | C/C | C/T | T/T | ||||||
| Atrophy | Togawa et al[34] | 2005 | Atrophy | 157 | 10 | 70 | 77 | T/C: 2.2 (1.0-4.9) | |
| NUD | 452 | 42 | 183 | 227 | |||||
| 984, 2983 | AA/AA | AA/GC | AA/GA | ||||||
| GC | Seno et al[55] | 2007 | Atrophy | 100 | 52 | 29 | 7 | AA/GA: 0.3 (0.1-0.9) | |
| NUD | 93 | 40 | 27 | 17 |
Although Seno et al[55] investigated nine SNPs (IL-4-590, -33, 3437, 3557, 4047, 4144, 4271, 4367 and 8427), data concerning the exclusion of IL-4+984 and 2983 were unclear. Dys: Dysplasia; DU: Duodenal ulcer.
Table 3.
Association of IL-6 polymorphism and gastroduodenal diseases
| Position | Disease | Authors | Year | n | |||||
| -174 C/G | C/C | C/G | G/G | ||||||
| GC | Gatti et al[81] | 2007 | GC | 56 | 1 | 13 | 42 | GG: 11.0 (1.2-96.7) | |
| NUD | 112 | 11 | 53 | 48 | |||||
| GCC | Deans et al[82] | 2007 | GC | 197 | 43 | 83 | 71 | NS | |
| NUD | 224 | 44 | 101 | 79 | |||||
| GC | Kamangar et al[83] | 2006 | GC | 102 | 27 | 54 | 21 | GC: 2.2 (1.2-4.0) vs G/G | |
| NUD | 152 | 43 | 58 | 51 | |||||
| GC | El-Omar et al[53] | 2003 | GC | 123 | 16 | 52 | 55 | NS | |
| NUD | 209 | 28 | 98 | 83 | |||||
| GC | Hwang et al[77] | 2003 | GC | 60 | 2 | 9 | 49 | - | |
| PU | Chakravorty et al[84] | 2008 | GU | 91 | 1 | 18 | 72 | NS | |
| NUD | 62 | 1 | 7 | 54 | |||||
| DU | Hwang et al[77] | 2003 | DU | 60 | 0 | 0 | 30 | - | |
| -572 G/C | C/C | C/G | G/G | ||||||
| GC | Kang et al[85] | 2009 | GC | 284 | 154 | 113 | 17 | NS | |
| NUD | 278 | 140 | 123 | 15 | |||||
| GC | Hwang et al[77] | 2003 | GC | 60 | 19 | 29 | 12 | - | |
| PU | Kang et al[85] | 2009 | PU | 434 | 249 | 167 | 20 | DU: GG 0.3 (0.1-0.9) | |
| NUD | 278 | 140 | 123 | 15 | |||||
| PU | Chakravorty et al[84] | 2008 | PU | 91 | 57 | 27 | 7 | NS | |
| NUD | 62 | 37 | 20 | 5 | |||||
| DU | Hwang et al[77] | 2003 | DU | 60 | 21 | 20 | 19 | - | |
| -597 G/A | G/G | G/A | A/A | ||||||
| GC | Kamangar et al[83] | 2006 | GC | 110 | 25 | 59 | 26 | NS | |
| NUD | 203 | 61 | 86 | 56 | |||||
| GC | Hwang et al[77] | 2003 | GC | 60 | 50 | 8 | 2 | - | |
| PU | Chakravorty et al[84] | 2008 | PU | 91 | 53 | 29 | 10 | NS | |
| NUD | 62 | 41 | 16 | 5 | |||||
| DU | Hwang et al[77] | 2003 | GC | 60 | 52 | 8 | 0 | - | |
| -634 C/T | C/C | C/T | T/T | ||||||
| GC | Liao et al[72] | 2008 | GC | 155 | 96 | 55 | 4 | NS | |
| NUD | 211 | 118 | 84 | 9 |
Although Kang et al and Savage et al investigated the association with IL-6-174 C/G polymorphism and gastric cancer, data were not described in detail (> 99% of patients were of the IL-6-174 G/G genotype).
Table 4.
Association of IL-8 polymorphism and gastroduodenal diseases
| Disease | Authors | Year | n | ||||||
| -251 A/T | T/T | T/A | A/A | ||||||
| GC | Kang et al[85] | 2009 | GC | 284 | 106 | 136 | 43 | AA: 2.0 (1.2-3.6) | |
| NUD | 275 | 125 | 125 | 25 | |||||
| GC | Canedo et al[94] | 2008 | GC | 333 | 111 | 169 | 53 | NS | |
| NUD | 880 | 265 | 445 | 170 | |||||
| GC | Garza-Gonzalez et al[58] | 2007 | GC | 78 | 15 | 47 | 16 | A carrier: 2.1 (1.1-4.2) | |
| NUD | 230 | 76 | 107 | 47 | |||||
| GC | Kamali-Sarvestani et al[95] | 2006 | GC | 19 | 4 | 6 | 9 | AT: 4.5 (1.5-12.9) | |
| NUD | 153 | 57 | 74 | 22 | |||||
| GC | Shirai et al[96] | 2006 | GC | 181 | 83 | 78 | 20 | MSI (+): TT 5.2 (1.5-18.0) | |
| NUD | 268 | 211 | 208 | 49 | |||||
| GC | Savage et al[97] | 2006 | GC | 287 | 71 | 140 | 76 | NS | |
| NUD | 426 | 106 | 205 | 117 | |||||
| GC | Kamangar et al[83] | 2006 | GC | 112 | 42 | 56 | 14 | NS | |
| NUD | 207 | 72 | 111 | 24 | |||||
| GC | Taguchi et al[98] | 2005 | GC | 396 | 161 | 191 | 44 | AA: 2.2 (1.1-4.6) | |
| NUD | 252 | 125 | 105 | 22 | |||||
| GC | Lee et al[99] | 2005 | GC | 470 | 198 | 213 | 59 | TT: 1.9 (1.3-3.0) | |
| NUD | 308 | 108 | 138 | 62 | |||||
| GC | Ohyauchi et al[100] | 2005 | GC | 212 | 93 | 106 | 13 | A carrier: 1.8 (1.1-2.8) | |
| NUD | 195 | 106 | 74 | 15 | |||||
| GCC | Savage et al[101] | 2004 | GC | 88 | 26 | 39 | 23 | AA: 2.0 (1.0-3.8) | |
| NUD | 429 | 147 | 207 | 75 | |||||
| GC | Lu et al[102] | 2005 | GC | 250 | 94 | 102 | 54 | AA: 1.9 (1.2-3.2) | |
| NUD | 300 | 119 | 144 | 37 | |||||
| PU | Kang et al[85] | 2009 | PU | 447 | 160 | 223 | 64 | GU: AA: 2.7 (1.5-4.8) | |
| NUD | 275 | 125 | 125 | 25 | |||||
| PU | Garza-Gonzalez et al[58] | 2007 | PU | 29 | 11 | 14 | 4 | NS | |
| NUD | 230 | 76 | 107 | 47 | |||||
| GU | Kamali-Sarvestani et al[95] | 2006 | GU | 61 | 19 | 28 | 14 | NS | |
| NUD | 153 | 57 | 74 | 22 | |||||
| GU | Ohyauchi et al[100] | 2005 | PU | 283 | 134 | 127 | 22 | GU:A carrier: 1.8 (1.1-3.0) | |
| NUD | 195 | 106 | 74 | 15 | |||||
| PU | Chakravorty et al[84] | 2008 | PU | 91 | 20 | 46 | 25 | NS | |
| NUD | 62 | 18 | 28 | 16 | |||||
| DU | Hofner et al[103] | 2007 | DU | 85 | 15 | 49 | 21 | AA: 2.3 (1.5-6.4) | |
| NUD | 211 | 61 | 106 | 44 | |||||
| DU | Gyulai et al[104] | 2004 | DU | 69 | 11 | 45 | 13 | A carrier: 4.4 (1.9-10.5) | |
| NUD | 47 | 21 | 17 | 9 | |||||
| IM | Leung et al[105] | 2006 | IM | 123 | 23 | 56 | 44 | NS | |
| NUD | 179 | 36 | 92 | 51 | |||||
| Atrophy | Taguchi et al[98] | 2005 | Atrophy | 215 | 90 | 99 | 26 | AA: 2.4 (1.1-4.9) | |
| NUD | 252 | 125 | 105 | 22 | |||||
| NUD | Hamajima et al[106] | 2003 | NUD | 448 | 234 | 177 | 37 | - | |
| +396 T/G | T/T | T/G | G/G | ||||||
| GC | Kamangar et al[83] | 2006 | GC | 111 | 42 | 55 | 14 | NS | |
| NUD | 208 | 72 | 112 | 24 | |||||
| GCC | Savage et al[101] | 2004 | GC | 86 | 29 | 33 | 24 | GG: 2.1 (1.1-3.9) | |
| NUD | 402 | 152 | 181 | 69 | |||||
| +781 C/T | C/C | C/T | T/T | ||||||
| GC | Kamangar et al[83] | 2006 | GC | 111 | 47 | 52 | 12 | NS | |
| NUD | 208 | 81 | 105 | 22 | |||||
| GCC | Savage et al[101] | 2004 | GC | 85 | 28 | 41 | 16 | NS | |
| NUD | 406 | 167 | 177 | 62 |
Although Seno et al[55] investigated six SNPs (IL-4-352, 289, 294, 680, 2217 and 2670), data were not described in detail. IM: Intestinal metaplasia.
Here, we review differences in the risk of development of peptic ulcer and gastric cancer by different inflammatory cytokine gene polymorphisms of IL-2, IL-4, IL-6 and IL-8.
IL-2 POLYMORPHISM AND GASTRODUODENAL DISEASES
IL-2, a 15-kDa α-helical cytokine of the Th1 type produced exclusively by activated T cells, promotes the proliferation of lymphocytes, macrophages and NK cells[21]. IL-2 potently regulates the immune response, and plays important roles in the differentiation of CD41-positive T cells into Th1 and Th2 effector subsets, while inhibiting T-helper 17 differentiation[22,23]. In T cells, IL-2 binding to the IL-2 receptor activates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, as well as mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling, which results in the transcription of pro-inflammatory cytokine genes. Through these pathways, IL-2 upregulates the expression of CD25 and IL-2Rβ, modulates genes involved in cell cycle regulation, and promotes T-cell survival and differentiation into effector and memory cells[24,25]. IL-2 contributes to the induction and transmission of inflammatory immune responses, including H. pylori-induced gastric inflammation.
Two kinds of single nucleotide polymorphism (SNP) occur in IL-2-330 and -384 (4q26-q27) of the promoter region, which affect IL-2 production[26,27]. IL-2 expression level with deletion of the IL-2-289 to -361 region was significantly decreased compared with that with the normal gene. IL-2-330 polymorphism located in this region is therefore considered to have particular influence on IL-2 levels[26,27]. In fact, IL-2 production in the IL-2-330 G/G genotype is about threefold greater than that of the IL-2-330 T/G or T/T genotypes in healthy subjects[28]. Consistent with this difference, an association between the IL-2-330 polymorphism and susceptibility to some inflammatory and immune diseases, such as rheumatoid arthritis, psoriasis and multiple sclerosis, has been reported[29-31]. IL-2 is therefore also thought to induce H. pylori-associated gastroduodenal diseases by regulating Th1 immune responses[32] and inhibiting gastric acid secretion[33].
Four studies have investigated the associations with IL-2-330 (three studies), +114 (one study) and +384 (one study) polymorphisms and development of atrophic gastritis (one study), peptic ulcer (one study) or gastric cancer (three studies) (Table 1)[34-37]. With regard to IL-2-330 polymorphism, Wu et al[37] have reported that subjects carrying the T allele, a low producer allele, have a significantly reduced risk of gastric cardia cancer (OR: 0.68, 95% CI: 0.46-0.99) compared with those with the G/G genotype. IL-2-330 polymorphisms may contribute to the etiology of gastric cardia cancer in Chinese populations[37]. However, Shin et al[35] failed to demonstrate a significant association with IL-2-330 polymorphism and gastric cancer development in the Chinese, while Togawa et al[34] conversely have reported that the IL-2-330 T/T genotype increased the risk of gastric cancer-related gastric atrophy (OR: 2.78, 95% CI: 1.26-6.17) in the Japanese. The results for IL-2-330 polymorphism are thus controversial. Moreover, no significant association was seen for IL-2-384 and +114 polymorphisms and gastric cancer development[36].
When combined, the results of previous studies of IL-2-330 polymorphism[34,35,37] surprisingly have shown that the risk of peptic ulcer development is 0.57 (95% CI: 0.33-0.98) for the G/G genotype and 0.37 (0.27-0.50) for G allele carriers compared with the T/T genotype (Table 5). However, no association with IL-2-330 polymorphism was seen for the risk of gastric non-cardia cancer. This finding is inconsistent with the first hypothesis, which states that patients with the IL-2 high producer genotype have an increased risk of gastric cancer and gastric ulcer development. Togawa et al[34] have speculated that one possible reason is that a higher IL-2 level is thought to enhance the immune response to eradicate H. pylori, and thereby decrease gastric mucosal inflammation. Moreover, an IL-2 promoter construct in a cell line shows higher levels of gene expression with the IL-2-330 G allele, whereas the transcriptional effect of this polymorphism in lymphocytes shows that the IL-2-330 G allele is associated with a lower expression of IL-2[30]. In fact, many studies have shown that the IL-2-330 T/T genotype increases the risk of a number of diseases, such as Takayasu’s disease[38], subacute sclerosing panencephalitis[39] and schizophrenia[40].
Table 5.
ORs for gastric non-cardia cancer and peptic ulcer development in IL-2-330 polymorphism
| Genotype | 1NUD (n) | Cancer (n) | OR | 95% CI | P value | Ulcer (n) | OR | 95% CI | P value | |
| IL-2-330 | T/T | 870 | 258 | - | 159 | - | ||||
| G/T | 755 | 209 | 0.93 | 0.76-1.15 | 0.51 | 45 | 0.33 | 0.23-0.46 | < 0.01 | |
| G/G | 153 | 35 | 0.77 | 0.52-1.14 | 0.20 | 16 | 0.57 | 0.33-0.98 | 0.04 | |
| G carrier | 908 | 244 | 0.91 | 0.74-1.11 | 0.33 | 61 | 0.37 | 0.27-0.50 | < 0.01 |
1NUD includes gastritis without gastric cancer and peptic ulcer, and atrophic gastritis patients. Because we deleted a number of gastric cardia cancer patients, a number of cancer patients shown in this Table do not match that in Table 1.
All studies that have investigated the relationship of IL-2-330 polymorphism and disease development to date were in Asian populations[34,35,37]. Further studies, including those in Western populations, will be necessary to solve this discrepancy and establish this relationship.
IL-4 POLYMORPHISM AND GASTRODUODENAL DISEASES
IL-4 is an anti-inflammatory cytokine, which inhibits gastric mucosal H. pylori-induced inflammation and atrophy by decreasing interferon γ (IFN-γ), which plays an important role in Th1 immune responses. IL-4 also plays a central role in the maturation of T-helper cells to the Th2 phenotype. With a shift from a Th1 to a Th2 cell pattern, IL-4 can enhance the production of anti-inflammatory cytokines (e.g. IL-10 and IL-13), including that of IL-4[41,42], and suppress the production of monocyte-derived pro-inflammatory cytokines (e.g. IL-1β, IL-6 and IL-8)[42].
IL-4 is overproduced in H. pylori-infected gastric mucosa. However, gastric mucosal inflammation has been shown to significantly reduce IL-4 administration[19,20], and IL-4-deficient mice infected with H. pylori show severe gastric inflammation compared with wild-type mice[43,44]. A balance between Th1 and Th2 cytokines by IL-4 therefore crucially influences the outcome of H. pylori infection. Moreover, IL-4 is reportedly associated with cancer development via its suppression of inflammation, and directly inhibits the growth of human melanoma, renal cell carcinoma and gastric cancer cells[45].
The family of the IL-4 gene, which encodes IL-4, is located on chromosome 5q31-33, which contains the IL-3, IL-4, IL-5, IL-9, IL-13, IL-15 genes as well as the interferon-regulatory factor and granulocyte-macrophage colony-stimulating factor (GM-CSF)[46]. There are two common polymorphisms in the IL-4 gene, -590 C/T and a 70-bp sequence variable number tandem repeat at intron 3; and many minor polymorphisms, such as -168, -33, 3437, 3557, 4047, 4144, 4271, 4367, 8427[47,48]. The IL-4-590 polymorphism is located upstream of all known control elements of IL-4, such as the negative regulatory element, the NF-r recognition sequence, and the TATA box[49]. Individuals with the IL-4-590 T/T genotype can produce IL-4 at higher levels than those with the C/C genotype[48]. IL-4 polymorphism is reportedly associated with the risk of cancer development (e.g. colorectal cancer[50]), and the Th2 T-cell response represented by IL-4 is expected to play a protective role in the development of cancer.
Seven studies have investigated the association of IL-4-590 polymorphism and atrophic gastritis (one study[51]), gastric cancer (five studies[52-56]), and duodenal ulcer development (one study[57]) (Table 2). In 2003, Wu et al[52] first reported that a higher prevalence of diffuse-type gastric cancer (OR: 1.64, 95% CI: 1.01-2.67), particularly in gastric cardia cancer (2.44, 1.13-5.27), is observed in IL-4-590 C allele carriers, a low producer allele, compared with the IL-4-590 T/T genotype, which suggests that low production of IL-4 is responsible for the development of gastric cancer. However, other studies have failed to demonstrate any significant association of IL-4 polymorphisms with disease risk[37,53,54,57,58]. In a combined-analysis of IL-4-590 C/T polymorphism[52-56], however, the risk of gastric non-cardia cancer development was 0.68 (95% CI: 0.57-0.80) for the C/T genotype, 0.36 (0.24-0.53) for the T/T genotype and 0.61 (0.53-0.73) for T allele carriers (Table 6). Moreover, the risk of peptic ulcer development in T allele carriers (0.58, 0.34-0.99) was significantly lower (Table 6). This protective effect of IL-4-590 polymorphism is therefore significant for gastric non-cardia cancer and peptic ulcer patients with a higher producer genotype.
Table 6.
ORs for gastric non-cardia cancer and peptic ulcer development with IL-4-168 and -590 polymorphisms
| Genotype | 1NUD (n) | Cancer (n) | OR | 95% CI | P value | Ulcer (n) | OR | 95% CI | P value | |
| IL-4-168 | T/T | 743 | 744 | - | ||||||
| T/C | 332 | 271 | 0.81 | 0.67-0.98 | 0.03 | |||||
| C/C | 24 | 27 | 1.12 | 0.64-2.00 | 0.70 | |||||
| C carrier | 356 | 298 | 0.81 | 0.69-1.00 | 0.05 | |||||
| IL-4-590 | C/C | 1716 | 591 | - | 46 | - | ||||
| C/T | 1039 | 242 | 0.68 | 0.57-0.80 | < 0.01 | 18 | 0.65 | 0.37-1.10 | 0.12 | |
| T/T | 229 | 28 | 0.36 | 0.24-0.53 | < 0.01 | 2 | 0.33 | 0.08-1.35 | 0.12 | |
| T carrier | 1268 | 270 | 0.61 | 0.53-0.73 | < 0.01 | 20 | 0.58 | 0.34-0.99 | 0.05 |
1NUD includes patients with gastritis without gastric cancer and peptic ulcer, and atrophic gastritis. Because we deleted a number of gastric cardia cancer patients, the number of cancer patients in this Table does not match that in Table 2.
The prevalence of IL-4-590 C/C, C/T and T/T genotypes differs between Western and Asian populations (Table 7). The prevalence of C/C, C/T and T/T genotypes in a Western population with gastric cancer was 69.8% (362/518), 26.1% (135/) and 4.1% (21/), respectively, whereas that in those with non-ulcer dyspepsia (NUD) was 55.9% (1448/2592), 36.0% (934/) and 8.1% (210/). In a Western population, the risks for gastric non-cardia cancer and peptic ulcer development were 0.55 (95% CI: 0.46-0.67) and 0.55 (0.32-0.94) for T allele carriers, respectively (Table 8). In an Asian population, in contrast, no significant difference was seen between subjects with gastric cancer and NUD. This difference in the influence of IL-4-590 polymorphism on disease development may have a geographic basis, and the effect appears to be stronger in Western populations (Table 8).
Table 7.
Prevalence of inflammatory cytokine gene genotypes in East Asian and Western populations
| Gene | Population | NUD | GC | PU | ||||||
| IL-2-330 | T/T | T/G | G/G | T/T | T/G | G/G | T/T | T/G | G/G | |
| Asian | 870 | 755 | 153 | 258 | 209 | 35 | 159 | 45 | 16 | |
| Western | ||||||||||
| IL-4-590 | C/C | C/T | T/T | C/C | C/T | T/T | C/C | C/T | T/T | |
| Asian | 268 | 105 | 19 | 229 | 107 | 7 | ||||
| Western | 1448 | 934 | 210 | 362 | 135 | 21 | ||||
| IL-6-174 | C/C | C/G | G/G | C/C | C/G | G/G | C/C | C/G | G/G | |
| Asian | 0 | 0 | 30 | 0 | 0 | 30 | ||||
| Western | 126 | 310 | 261 | 34 | 82 | 112 | 1 | 26 | 94 | |
| IL-6-572 | C/C | C/G | G/G | C/C | C/G | G/G | C/C | C/G | G/G | |
| Asian | 140 | 123 | 15 | 170 | 126 | 18 | 165 | 170 | 19 | |
| Western | 37 | 20 | 5 | 3 | 16 | 11 | 62 | 34 | 25 | |
| IL-6-597 | G/G | G/A | A/A | G/G | G/A | A/A | G/G | G/A | A/A | |
| Asian | 30 | 0 | 0 | 30 | 0 | 0 | ||||
| Western | 102 | 102 | 61 | 45 | 67 | 28 | 75 | 37 | 10 | |
| IL-8-251 | T/T | T/A | A/A | T/T | T/A | A/A | T/T | T/A | A/A | |
| Asian | 1324 | 1425 | 443 | 735 | 826 | 233 | 294 | 350 | 86 | |
| Western | 676 | 1093 | 449 | 243 | 418 | 168 | 76 | 182 | 77 | |
| IL-8+396 | T/T | T/G | G/G | T/T | T/G | G/G | T/T | T/G | G/G | |
| Asian | 152 | 181 | 69 | |||||||
| Western | 72 | 112 | 24 | 27 | 43 | 12 | ||||
| IL-8+781 | C/C | C/T | T/T | C/C | C/T | T/T | C/C | C/T | T/T | |
| Asian | 167 | 177 | 62 | |||||||
| Western | 81 | 105 | 22 | 29 | 41 | 11 |
Table 8.
Comparison of the incidence of gastric non-cardia cancer and peptic ulcer in Asian and Western populations
| Disease | Gene | Reference | Genotype |
Western |
Asian |
||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||||
| GC | IL-4-590 | C/C | C/T | 0.57 | 0.41-0.72 | < 0.01 | 1.12 | 0.87-1.65 | 0.28 |
| T/T | 0.40 | 0.25-0.64 | < 0.01 | 0.43 | 0.18-1.04 | 0.06 | |||
| T carrier | 0.55 | 0.46-0.67 | < 0.01 | 1.08 | 0.79-1.45 | 0.17 | |||
| IL-6-572 | C/C | C/G | 9.87 | 2.56-37.98 | < 0.01 | 0.85 | 0.61-1.19 | 0.34 | |
| G/G | 21.13 | 5.56-131.98 | < 0.01 | 0.99 | 0.48-2.03 | 0.97 | |||
| G carrier | 13.32 | 3.64-48.69 | < 0.01 | 0.87 | 0.63-1.20 | 0.38 | |||
| IL-8-251 | T/T | T/A | 1.04 | 0.86-1.25 | 0.69 | 1.06 | 0.93-1.20 | 0.38 | |
| A/A | 1.04 | 0.83-1.31 | 0.73 | 0.95 | 0.79-1.14 | 0.56 | |||
| A carrier | 1.04 | 0.87-1.24 | 0.67 | 1.03 | 0.92-1.16 | 0.61 | |||
| GU | IL-4-590 | C/C | C/T | 0.61 | 0.35-1.05 | 0.08 | |||
| T/T | 0.30 | 0.07-1.24 | 0.10 | ||||||
| T carrier | 0.55 | 0.32-0.94 | 0.03 | ||||||
| IL-6-572 | C/C | C/G | 1.31 | 0.07-2.56 | 0.42 | 0.77 | 0.55-1.05 | 0.07 | |
| G/G | 2.98 | 1.05-8.47 | 0.04 | 0.67 | 0.33-1.34 | 2.66 | |||
| G carrier | 1.65 | 0.89-3.04 | 1.65 | 0.74 | 0.55-1.01 | 0.06 | |||
| IL-8-251 | T/T | T/A | 1.48 | 1.12-1.97 | < 0.01 | 1.11 | 0.93-1.31 | 0.25 | |
| A/A | 1.53 | 1.09-2.14 | 0.01 | 0.87 | 0.67-1.14 | 0.31 | |||
| A carrier | 1.49 | 1.14-1.96 | < 0.01 | 1.05 | 0.89-1.24 | 0.55 | |||
With regard to minor polymorphisms of IL-4, IL-4-168, -33, 984/2983 SNPs have been reported by one study each[34,37,55]. Compared with the IL-4-168 C/C high producer genotype[37], the IL-4-168 T allele carrier was associated with a significantly decreased gastric cancer risk (OR: 0.83, 95% CI: 0.69-1.00). Further, this significant protective effect was also seen for gastric cardia cancer patients (0.73, 0.56-0.95)[37].
Thus, a significant protective effect against gastric non-cardia cancer was seen with the higher producer genotype IL-4-590 and -168 polymorphisms, particularly in Western populations.
IL-6 POLYMORPHISM AND GASTRODUODENAL DISEASES
Il-6, a multifunctional cytokine produced by immune and many non-immune cells including monocytes, lymphocytes, macrophages, and endothelial and intestinal epithelial cells, functions as both an inflammatory mediator and endocrine regulator[59]. IL-6 plays an important role in host defense mechanisms as a messenger between innate and adaptive systems, by stimulating IFN-γ production in T cells and promoting immunoglobulin secretion in activated B cells[60].
High serum levels of IL-6 family cytokines have been reported in various gastrointestinal cancer cells[61]. IL-6 and IL-11 belong to the IL-6 cytokines family, which includes ciliary neurotrophic factor, cardiotrophin-1, cardiotrophin-like cytokine, leukemia inhibitory factor, oncostatin M, and IL-27. These act as ligands for the signaling receptor subunit gp130[62,63]. IL-6 requires specific α receptor subunits and gp130 homodimers of signal transducing receptor[63]. Recently, mice with a mutation in gp130 (gp130 757F/F mouse) have been established to enhance chronic gastric inflammation and develop gastric neoplasms without H. pylori infection, via an imbalance between STAT3 and Y-759/SHP-2 signaling[64]. The presence of the Y757F mutation in the gp130 receptor promotes the failure of SHP-2 phosphorylation and subsequent activation of the pro-apoptotic Ras/Erk and PI3K/AKT pathways, which results in massive STAT3 activation. STAT3 hyperactivity suppresses the cytostatic effect of the stroma on cell proliferation[65]. Moreover, STAT3 also induces epithelial cell expression of IL-11[66]. These signaling events promote an oncogenic program in which the expression of anti-apoptotic, pro-angiogenic, and pro-proliferative genes results in inflammation-associated gastric tumorigenesis[66]. The IL-6 family signaling system is therefore an attractive research target in gastric cancer pathogenesis.
Mucosal IL-6 levels increase in H. pylori-associated gastritis[66,67] and dramatically decrease after eradication of infection[68]. IL-6 mRNA levels in gastric mucosa correlate with the level of gastric mucosal inflammation[67,69]. Serum levels of IL-6 are higher in patients with gastric cancer than gastritis[70]. IL-6 plays an important role as a prognostic factor in advanced gastric cancer and lymph node metastasis[71], and a serum IL-6 level > 13 pg/mL correlates with tumor progression and poor survival after resection[72].
The IL-6 gene is located on chromosome 7p21 and the SNPs at the 5’ flanking region of the IL-6 promoter have been identified as IL-6-174, -572 and -597[73]. IL-6-174 G allele carriers produce higher levels of IL-6 than those with the C/C genotype[74], and have a higher prevalence of systemic juvenile-onset chronic arthritis, lipid abnormalities[75] and insulin resistance[76]. IL-6-174 G and -597 G allele carriers are closely linked regardless of ethnic group or disease status[77]. The IL-6-572 G allele is also associated with a higher serum IL-6 level than IL-6-572 C/C allele[78], and is a risk factor for diabetic nephropathy and lung cancer with asthma/atopy[79,80].
Six studies of the IL-6-174 polymorphism[53,77,81-84], three of IL-6-572[77,84,85], three of IL-6-597[77,83,84] and one of IL-6+634[72] in relation to the development of gastric cancer and peptic ulcer have appeared (Table 3). Gatti et al[81] have reported that the IL-6-174 G allele carriers account for a significantly higher incidence of gastric cancer than NUD patients (98.2%, 55/56 and 90.2%, 101/112, respectively). However, Kamangar et al[83] have demonstrated that, compared with G/G genotype IL-6, the low producer genotype IL-6-174G/C has an increased risk of gastric cancer, while other studies have shown no significant relationship of IL-6-174 polymorphism with gastric diseases. The association of this polymorphism with these conditions thus remains unclear. In contrast, frequencies of the IL-6-572 G/G genotype (OR: 0.3, 95% CI: 0.1-0.9) and of G allele carriers (0.5, 0.4-0.8) are lower in H. pylori-positive patients with duodenal ulcer than in those with NUD[85].
In a combined analysis of IL-6-174 C/G polymorphism[13,53,81-84], the risk of gastric non-cardia cancer was 2.02 (1.31-3.10) for the G/G compared with C/C genotype (Table 9). Moreover, the risk of gastric ulcer was significantly higher with the G/G genotype (58.86, 8.27-433.4) and G allele carriers (33.10, 4.59-233.8) (Table 9). However, the prevalence of IL-6-174 C/C, C/T and T/T genotypes differs among populations, with the C/C genotype being less common in East Asian, South Asian and Latin American populations (0%-9.8%) than in North American and European populations (13.4%-28.3%)[13,53,81-84]. In East Asians in particular, the IL-6-174 polymorphism has a C allele frequency of < 1%, not only in patients with gastric cancer, but also in those with NUD[36,77,85]. This polymorphism may therefore not be useful in identifying the association with disease development in Asian populations. Although the prevalence of gastric cancer is higher in East Asians and Latin Americans than Caucasians, the difference in IL-6-174 polymorphism may nevertheless explain the difference in prevalence between Asian and Western countries.
Table 9.
ORs for the development of gastric non-cardia cancer with the IL-6-174, +572, +597 and +634 polymorphisms
| Genotype | 1NUD (n) | Cancer (n) | OR | 95% CI | P value | Ulcer (n) | OR | 95% CI | P value | |
| IL-6-174 | C/C | 126 | 34 | - | 1 | |||||
| C/G | 310 | 82 | 0.98 | 0.63-1.54 | 0.93 | 26 | 10.57 | 1.42-78.73 | 0.02 | |
| G/G | 261 | 142 | 2.02 | 1.31-3.10 | < 0.01 | 124 | 59.86 | 8.27-433.4 | < 0.01 | |
| G carrier | 571 | 224 | 1.45 | 0.97-2.19 | 0.06 | 150 | 33.10 | 4.59-238.8 | < 0.01 | |
| IL-6+572 | C/C | 177 | 173 | - | 327 | - | ||||
| C/G | 143 | 143 | 1.02 | 0.75-1.40 | 0.89 | 214 | 0.81 | 0.61-1.07 | 0.15 | |
| G/G | 20 | 29 | 1.48 | 0.81-2.72 | 0.20 | 44 | 1.19 | 0.68-2.08 | 0.54 | |
| G carrier | 163 | 172 | 1.08 | 0.80-1.46 | 0.61 | 258 | 0.86 | 0.66-1.12 | 0.26 | |
| IL-6+597 | G/G | 102 | 75 | - | - | |||||
| G/A | 102 | 67 | 0.89 | 0.58-1.37 | 0.61 | |||||
| A/A | 61 | 28 | 0.62 | 0.37-1.07 | 0.09 | |||||
| A carrier | 163 | 95 | 0.79 | 0.54-1.17 | 0.24 | |||||
| IL-6+634 | C/C | 118 | 96 | - | ||||||
| C/T | 84 | 55 | 0.81 | 0.52-1.24 | 0.33 | |||||
| T/T | 9 | 4 | 0.45 | 0.16-1.83 | 0.32 | |||||
| T carrier | 93 | 59 | 0.78 | 0.51-1.12 | 0.25 |
1NUD includes patients with gastritis without gastric cancer and peptic ulcer, and atrophic gastritis. Because we deleted a number of gastric cardia cancer patients, the number of cancer patients shown in this Table does not match that in Table 3.
On combined analysis, IL-6-572, IL-6-597 and IL-6+643 polymorphisms have shown no significant relationship with gastric disease. When patients are divided into Asian and Western populations, however, a clear difference in the prevalence of IL-6-572 genotypes is seen (Table 7). The risk of gastric non-cardia cancer and peptic ulcer development in Western populations was 21.13 (95% CI: 5.56-131.98) and 2.98 (1.05-8.47) for the IL-6-572 G/G genotype, respectively (Table 8). In contrast, no significant different has been seen between Asians with gastric cancer or NUD.
This influence of the IL-6-174 and IL-6-572 polymorphisms on disease development may have been due to geographic differences. Furthermore, the influence of IL-4-590 polymorphism on gastroduodenal diseases is particularly strong in Western populations.
IL-8 POLYMORPHISM AND GASTRODUODENAL DISEASES
IL-8, a member of the CXC chemokine family, which was originally identified as a potent chemoattractant for neutrophils and lymphocytes, induces not only cell proliferation and migration, but also angiogenesis. IL-8 is produced by gastric epithelial cells during H. pylori infection, particularly in the cag-pathogenicity-island-positive strain of H. pylori, one of the major virulence factors[86,87]. In addition, IL-8 protein levels are 10-fold higher in gastric cancer than in normal gastric tissue[68], and directly correlate with the vascularity of the tumors[88]. The transfection of gastric cancer cells with the IL-8 gene enhances their tumorigenesis and angiogenesis in the gastric wall of nude mice[88]. Increased IL-8 levels may amplify the inflammatory response to H. pylori by recruiting neutrophils and monocytes, thereby resulting in an advanced degree of gastritis, which ultimately predisposes to the development of gastric cancer.
There are three common polymorphisms in the IL-8 gene, -251 A/T, 396 T/G and 781 C/T[47,48]. Of these, IL-8-251 A allele carrier status is associated with increased IL-8 production[89]. Consistent with these differences, IL-8-251 polymorphism influences cancer risk, including that of lung[90], colorectal[91], bladder[92], and prostate cancer[93].
Seventeen studies of IL-8-251 polymorphism[58,83-85,94-106], two of IL-6+396[83,101] and two of IL-6+781[83,101] in relation to the development of gastric cancer and peptic ulcer have appeared. Of these, six studies have shown a significantly increased risk of gastric cancer for the IL-8-251 A/A high producer genotype or A allele carriers[58,85,95,98,100-102], while four have shown an increase for peptic ulcer[85,100,103,104] and one for gastric mucosal atrophy[98]. The IL-8-251 A/A genotype is more common in H. pylori-positive patients with gastric cancer (OR: 2.0, 95% CI: 1.2-3.6) or gastric ulcer (2.7, 1.5-4.8) than in those with NUD[85]. In addition, the IL-8-251 A/A genotype is associated with a higher risk for the intestinal than the diffuse type of gastric cancer[85]. Moreover, Taguchi et al[98] have reported that the IL-8-251 A/A genotype correlates with a higher risk of lymph node and liver metastasis, and is histopathologically associated with more severe neutrophil infiltration in non-cancerous gastric mucosa adjacent to cancer. These results may be due to the tumorigenic and angiogenic functions of IL-8 modulating the growth and invasive behavior of malignant tumors by autocrine and paracrine mechanisms, and suggest that genetic variants of IL-8 potentially affect the prognosis of gastric cancer. Nevertheless, several contrary studies have also appeared. Lee et al[99], for example, have reported that, compared with the IL-8-251 A/A high producer genotype, the A/T genotype had a > 60% risk of gastric cancer (1.62, 1.07-2.46), while the T/T genotype had a > 90% risk (1.93, 1.26-2.95), particularly in the diffuse type; whereas Shirai et al[96] have reported that the IL-8-251 T/T genotype is significantly associated with an increased risk of microsatellite instability (MSI)-high gastric cancer, which is more frequently associated with active H. pylori infection than in microsatellite-stable (MSS) cases, compared to MSI-low/MSS gastric cancer (5.2, 1.5-18.0) and NUD (3.7, 1.1-12.4).
Most studies that have reported positive associations for gastric cancer risk have been conducted in Asian populations[98,100-102], whereas those that have reported negative findings were conducted in Western populations[83,97]. In a combined analysis of the IL-8-251 polymorphism, no association was seen for the risk of gastric non-cardia cancer development (Table 10). However, the prevalence of IL-8-251 genotypes differs between Western and Asian populations (Table 7), and the risk for peptic ulcer in Western populations is higher [1.53 (1.09-2.14) for the IL-8-251 A/A genotype and 1.49 (1.14-1.96) for A allele carriers] (Table 8). The IL-8-251 polymorphism more potently influences the development of peptic ulcer in Western than East Asian populations.
Table 10.
ORs for the development of gastric non-cardia cancer with IL-8-251, +396 and +781 polymorphisms
| Genotype | NUD (n) | Cancer (n) | OR | 95% CI | P value | Ulcer (n) | OR | 95% CI | P value | |
| IL-8-251 | T/T | 2000 | 978 | - | 370 | |||||
| T/A | 2518 | 1244 | 1.01 | 0.91-1.12 | 0.38 | 532 | 1.14 | 0.99-1.32 | 0.07 | |
| A/A | 892 | 401 | 0.92 | 0.80-1.06 | 0.24 | 163 | 0.99 | 0.81-1.21 | 0.90 | |
| A carrier | 3410 | 1645 | 0.99 | 0.90-1.09 | 0.18 | 695 | 1.10 | 0.96-1.26 | 0.17 | |
| IL-8+396 | T/T | 224 | 27 | - | ||||||
| T/G | 293 | 43 | 1.22 | 0.73-2.03 | 0.44 | |||||
| G/G | 93 | 12 | 1.07 | 0.52-2.20 | 0.85 | |||||
| G carrier | 385 | 55 | 1.18 | 0.73-1.93 | 0.50 | |||||
| IL-8+781 | C/C | 248 | 29 | - | ||||||
| C/T | 282 | 41 | 1.24 | 0.75-2.06 | 0.39 | |||||
| T/T | 84 | 11 | 1.12 | 0.54-2.34 | 0.76 | |||||
| T carrier | 366 | 52 | 1.22 | 0.75-1.97 | 0.43 |
NUD includes patients with gastritis without gastric cancer and peptic ulcer, and atrophic gastritis. Because we deleted a number of gastric cardia cancer patients, the number of cancer patients shown in this Table does not match that in Table 4.
In combined analysis, the IL-8+396 and IL-8+781 polymorphisms have no significant relationship with the incidence of gastric disease (Table 10). However, Savage et al[101] have reported that the IL-8-251/+396/+781 AGT/AGC haplotype is associated with a fourfold increased risk of gastric cardia cancer. This haplotypic analysis will help identify groups with a higher risk of disease and should be investigated in a larger study.
Summary of association between H. pylori-related diseases and cytokine polymorphisms
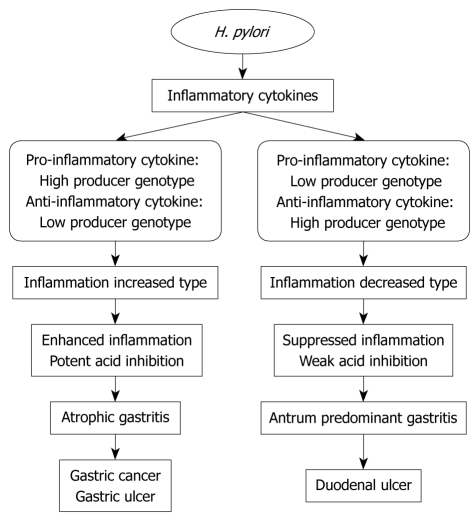
In general, gastric mucosal inflammation in H. pylori infection of gastric mucosa is exacerbated in patients with high producer alleles of pro-inflammatory cytokines and low producer alleles of anti-inflammatory cytokines, which results in a higher risk for the development of gastric cancer and gastric ulcer (Figure 1). In contrast, low producer allele carriers of pro-inflammatory cytokines and high producer allele carriers of anti-inflammatory cytokines have mild gastric mucosal inflammation (Figure 1). A summary of the association between H. pylori-related diseases and cytokine polymorphisms is shown in Table 11. As important points, the prevalence of cytokine gene genotypes differs between Western and Asian populations. Although Asian populations have been reported to be associated with IL-1B-511, IL-10, TNF-A polymorphisms and development of peptic ulcer and gastric cancer[13,18], the influence of IL-4, -6 and -8 polymorphisms on the diseases in the current review may be lower. In Western studies, combination analysis of several cytokine gene genotypes is related to development of diseases[53]. As shown in Table 11, because IL-4-590, IL-6-174, IL-6-572 and IL-8-251 polymorphisms in Western populations relate to development of gastroduodenal diseases, combination analysis including these gene polymorphisms with previously reported IL-1 and TNF-A is expected to increase detection of elevated risk of diseases. These findings should be further evaluated in a larger population.
Figure 1.
Scheme of the association of inflammatory cytokine polymorphisms and gastroduodenal disease development.
Table 11.
Summary of association with inflammatory cytokine genotypes and gastroduodenal diseases in East Asian and Western populations
| Gene |
Western |
Asian |
|||
| PU | GC | PU | GC | ||
| IL-2-330 | G carrier (vs T) | - | - | ↓ | NS |
| IL-4-590 | T carrier (vs C) | ↓ | ↓ | NS | - |
| IL-6-174 | G carrier (vs C) | ↑ | ↑ | - | - |
| IL-6-572 | G carrier (vs C) | NS | ↑ | NS | NS |
| IL-6-597 | A carrier (vs G) | NS | NS | - | - |
| IL-8-251 | A carrier (vs T) | ↑ | NS | NS | NS |
| IL-8+396 | G carrier (vs T) | - | NS | - | - |
| IL-8+781 | T carrier (vs C) | - | NS | - | - |
↓ decrease in risk of disease; ↑ increase in risk of disease.
CONCLUSION
Many genetic factors are associated with the development of H. pylori-related diseases. Of these, we have reviewed here the important role of inflammatory cytokines (IL-2, IL-4, IL-6 and IL-8) and their polymorphisms in H. pylori-related diseases. We recommend intensive endoscopic screening and/or eradication therapy for patients at higher risk of gastric cancer based on genetic inflammatory cytokine polymorphisms, albeit that we are unsure whether all factors should be determined. Further data to refine this recommendation are therefore required.
Footnotes
Peer reviewer: Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Perez-Perez GI, Taylor DN, Bodhidatta L, Wongsrichanalai J, Baze WB, Dunn BE, Echeverria PD, Blaser MJ. Seroprevalence of Helicobacter pylori infections in Thailand. J Infect Dis. 1990;161:1237–1241. doi: 10.1093/infdis/161.6.1237. [DOI] [PubMed] [Google Scholar]
- 2.Rocha GA, Queiroz DM, Mendes EN, Oliveira AM, Moura SB, Barbosa MT, Mendes CC, Lima Júnior GF, Oliveira CA. Indirect immunofluorescence determination of the frequency of anti-H. pylori antibodies in Brazilian blood donors. Braz J Med Biol Res. 1992;25:683–689. [PubMed] [Google Scholar]
- 3.Souto FJ, Fontes CJ, Rocha GA, de Oliveira AM, Mendes EN, Queiroz DM. Prevalence of Helicobacter pylori infection in a rural area of the state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 1998;93:171–174. doi: 10.1590/s0074-02761998000200006. [DOI] [PubMed] [Google Scholar]
- 4.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 5.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, Oguma K, Okada H, Shiratori Y. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–1042. doi: 10.1111/j.1572-0241.2005.41384.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Wotherspoon AC, Doglioni C, de Boni M, Spencer J, Isaacson PG. Antibiotic treatment for low-grade gastric MALT lymphoma. Lancet. 1994;343:1503. [PubMed] [Google Scholar]
- 8.Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244–1252. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 9.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 10.Tebbe B, Geilen CC, Schulzke JD, Bojarski C, Radenhausen M, Orfanos CE. Helicobacter pylori infection and chronic urticaria. J Am Acad Dermatol. 1996;34:685–686. doi: 10.1016/s0190-9622(96)80086-7. [DOI] [PubMed] [Google Scholar]
- 11.Annibale B, Marignani M, Monarca B, Antonelli G, Marcheggiano A, Martino G, Mandelli F, Caprilli R, Delle Fave G. Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med. 1999;131:668–672. doi: 10.7326/0003-4819-131-9-199911020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto M, Kajimura M, Shirai N, Furuta T, Kanaoka S, Ikuma M, Sato Y, Hishida A. Outcome of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Intern Med. 2006;45:405–409. doi: 10.2169/internalmedicine.45.1473. [DOI] [PubMed] [Google Scholar]
- 13.Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 14.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 15.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Inaba T, Yamamoto K, Okada H, Yokota K, Oguma K, et al. Interleukin-1beta genetic polymorphism influences the effect of cytochrome P 2C19 genotype on the cure rate of 1-week triple therapy for Helicobacter pylori infection. Am J Gastroenterol. 2003;98:2403–2408. doi: 10.1111/j.1572-0241.2003.07707.x. [DOI] [PubMed] [Google Scholar]
- 16.Furuta T, Shirai N, Takashima M, Xiao F, Sugimura H. Effect of genotypic differences in interleukin-1 beta on gastric acid secretion in Japanese patients infected with Helicobacter pylori. Am J Med. 2002;112:141–143. doi: 10.1016/s0002-9343(01)01036-1. [DOI] [PubMed] [Google Scholar]
- 17.Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F, et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823–829. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto M, Furuta T, Shirai N, Nakamura A, Xiao F, Kajimura M, Sugimura H, Hishida A. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. J Gastroenterol Hepatol. 2007;22:51–59. doi: 10.1111/j.1440-1746.2006.04442.x. [DOI] [PubMed] [Google Scholar]
- 19.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 20.Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, Low MJ, Merchant JL. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc Natl Acad Sci USA. 2003;100:12944–12949. doi: 10.1073/pnas.2135193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker E, Leemhuis T, Roeder W. Murine B lymphoma cell lines release functionally active interleukin 2 after stimulation with Staphylococcus aureus. J Immunol. 1988;140:859–865. [PubMed] [Google Scholar]
- 22.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki T, Liu ZJ, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian EL, Permutter RM, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 25.Dooms H, Kahn E, Knoechel B, Abbas AK. IL-2 induces a competitive survival advantage in T lymphocytes. J Immunol. 2004;172:5973–5979. doi: 10.4049/jimmunol.172.10.5973. [DOI] [PubMed] [Google Scholar]
- 26.Williams TM, Eisenberg L, Burlein JE, Norris CA, Pancer S, Yao D, Burger S, Kamoun M, Kant JA. Two regions within the human IL-2 gene promoter are important for inducible IL-2 expression. J Immunol. 1988;141:662–666. [PubMed] [Google Scholar]
- 27.John S, Turner D, Donn R, Sinnott P, Worthington J, Ollier WE, Hutchinson IV, Hajeer AH. Two novel biallelic polymorphisms in the IL-2 gene. Eur J Immunogenet. 1998;25:419–420. doi: 10.1046/j.1365-2370.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann SC, Stanley EM, Darrin Cox E, Craighead N, DiMercurio BS, Koziol DE, Harlan DM, Kirk AD, Blair PJ. Association of cytokine polymorphic inheritance and in vitro cytokine production in anti-CD3/CD28-stimulated peripheral blood lymphocytes. Transplantation. 2001;72:1444–1450. doi: 10.1097/00007890-200110270-00019. [DOI] [PubMed] [Google Scholar]
- 29.Pawlik A, Kurzawski M, Florczak M, Gawronska Szklarz B, Herczyńska M. IL1beta+3953 exon 5 and IL-2 -330 promoter polymorphisms in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:159–164. [PubMed] [Google Scholar]
- 30.Matesanz F, Fedetz M, Leyva L, Delgado C, Fernández O, Alcina A. Effects of the multiple sclerosis associated -330 promoter polymorphism in IL2 allelic expression. J Neuroimmunol. 2004;148:212–217. doi: 10.1016/j.jneuroim.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Kim YK, Pyo CW, Choi HB, Kim SY, Kim TY, Kim TG. Associations of IL-2 and IL-4 gene polymorphisms with psoriasis in the Korean population. J Dermatol Sci. 2007;48:133–139. doi: 10.1016/j.jdermsci.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 33.Padol IT, Hunt RH. Effect of Th1 cytokines on acid secretion in pharmacologically characterised mouse gastric glands. Gut. 2004;53:1075–1081. doi: 10.1136/gut.2003.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togawa S, Joh T, Itoh M, Katsuda N, Ito H, Matsuo K, Tajima K, Hamajima N. Interleukin-2 gene polymorphisms associated with increased risk of gastric atrophy from Helicobacter pylori infection. Helicobacter. 2005;10:172–178. doi: 10.1111/j.1523-5378.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 35.Shin WG, Jang JS, Kim HS, Kim SJ, Kim KH, Jang MK, Lee JH, Kim HJ, Kim HY. Polymorphisms of interleukin-1 and interleukin-2 genes in patients with gastric cancer in Korea. J Gastroenterol Hepatol. 2008;23:1567–1573. doi: 10.1111/j.1440-1746.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 36.Savage SA, Abnet CC, Haque K, Mark SD, Qiao YL, Dong ZW, Dawsey SM, Taylor PR, Chanock SJ. Polymorphisms in interleukin -2, -6, and -10 are not associated with gastric cardia or esophageal cancer in a high-risk chinese population. Cancer Epidemiol Biomarkers Prev. 2004;13:1547–1549. [PubMed] [Google Scholar]
- 37.Wu J, Lu Y, Ding YB, Ke Q, Hu ZB, Yan ZG, Xue Y, Zhou Y, Hua ZL, Shu YQ, et al. Promoter polymorphisms of IL2, IL4, and risk of gastric cancer in a high-risk Chinese population. Mol Carcinog. 2009;48:626–632. doi: 10.1002/mc.20502. [DOI] [PubMed] [Google Scholar]
- 38.Saruhan-Direskeneli G, Biçakçigil M, Yilmaz V, Kamali S, Aksu K, Fresko I, Akkoç N, Kiraz S, Ozer HT, Tunç E, et al. Interleukin (IL)-12, IL-2, and IL-6 gene polymorphisms in Takayasu's arteritis from Turkey. Hum Immunol. 2006;67:735–740. doi: 10.1016/j.humimm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz V, Demirbilek V, Gürses C, Yentür SP, Uysal S, Yapici Z, Yilmaz G, Muncey A, Cokar O, Onal E, et al. Interleukin (IL)-12, IL-2, interferon-gamma gene polymorphisms in subacute sclerosing panencephalitis patients. J Neurovirol. 2007;13:410–415. doi: 10.1080/13550280701455383. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz MJ, Krönig H, Riedel M, Dehning S, Douhet A, Spellmann I, Ackenheil M, Möller HJ, Müller N. IL-2 and IL-4 polymorphisms as candidate genes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:72–76. doi: 10.1007/s00406-005-0603-9. [DOI] [PubMed] [Google Scholar]
- 41.Vercelli D, Jabara HH, Lauener RP, Geha RS. IL-4 inhibits the synthesis of IFN-gamma and induces the synthesis of IgE in human mixed lymphocyte cultures. J Immunol. 1990;144:570–573. [PubMed] [Google Scholar]
- 42.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 43.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 44.Ren Z, Pang G, Clancy R, Li LC, Lee CS, Batey R, Borody T, Dunkley M. Shift of the gastric T-cell response in gastric carcinoma. J Gastroenterol Hepatol. 2001;16:142–148. doi: 10.1046/j.1440-1746.2001.02385.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoon DS, Okun E, Banez M, Irie RF, Morton DL. Interleukin 4 alone and with gamma-interferon or alpha-tumor necrosis factor inhibits cell growth and modulates cell surface antigens on human renal cell carcinomas. Cancer Res. 1991;51:5687–5693. [PubMed] [Google Scholar]
- 46.Saltman DL, Dolganov GM, Warrington JA, Wasmuth JJ, Lovett M. A physical map of 15 loci on human chromosome 5q23-q33 by two-color fluorescence in situ hybridization. Genomics. 1993;16:726–732. doi: 10.1006/geno.1993.1254. [DOI] [PubMed] [Google Scholar]
- 47.Mout R, Willemze R, Landegent JE. Repeat polymorphisms in the interleukin-4 gene (IL4) Nucleic Acids Res. 1991;19:3763. doi: 10.1093/nar/19.13.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenwasser LJ, Klemm DJ, Dresback JK, Inamura H, Mascali JJ, Klinnert M, Borish L. Promoter polymorphisms in the chromosome 5 gene cluster in asthma and atopy. Clin Exp Allergy. 1995;25 Suppl 2:74–78; discussion 95-96. doi: 10.1111/j.1365-2222.1995.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 49.Walley AJ, Cookson WO. Investigation of an interleukin-4 promoter polymorphism for associations with asthma and atopy. J Med Genet. 1996;33:689–692. doi: 10.1136/jmg.33.8.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landi S, Bottari F, Gemignani F, Gioia-Patricola L, Guino E, Osorio A, de Oca J, Capella G, Canzian F, Moreno V. Interleukin-4 and interleukin-4 receptor polymorphisms and colorectal cancer risk. Eur J Cancer. 2007;43:762–768. doi: 10.1016/j.ejca.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Kato I, Canzian F, Franceschi S, Plummer M, van Doorn LJ, Lu Y, Gioia-Patricola L, Vivas J, Lopez G, Severson RK, et al. Genetic polymorphisms in anti-inflammatory cytokine signaling and the prevalence of gastric precancerous lesions in Venezuela. Cancer Causes Control. 2006;17:1183–1191. doi: 10.1007/s10552-006-0060-4. [DOI] [PubMed] [Google Scholar]
- 52.Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT, Lin JT. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer. 2003;104:617–623. doi: 10.1002/ijc.10987. [DOI] [PubMed] [Google Scholar]
- 53.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 54.Lai KC, Chen WC, Jeng LB, Li SY, Chou MC, Tsai FJ. Association of genetic polymorphisms of MK, IL-4, p16, p21, p53 genes and human gastric cancer in Taiwan. Eur J Surg Oncol. 2005;31:1135–1140. doi: 10.1016/j.ejso.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Seno H, Satoh K, Tsuji S, Shiratsuchi T, Harada Y, Hamajima N, Sugano K, Kawano S, Chiba T. Novel interleukin-4 and interleukin-1 receptor antagonist gene variations associated with non-cardia gastric cancer in Japan: comprehensive analysis of 207 polymorphisms of 11 cytokine genes. J Gastroenterol Hepatol. 2007;22:729–737. doi: 10.1111/j.1440-1746.2007.04934.x. [DOI] [PubMed] [Google Scholar]
- 56.García-González MA, Lanas A, Quintero E, Nicolás D, Parra-Blanco A, Strunk M, Benito R, Angel Simón M, Santolaria S, Sopeña F, et al. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am J Gastroenterol. 2007;102:1878–1892. doi: 10.1111/j.1572-0241.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 57.Zambon CF, Basso D, Marchet A, Fasolo M, Stranges A, Schiavon S, Navaglia F, Greco E, Fogar P, Falda A, et al. IL-4 -588C>T polymorphism and IL-4 receptor alpha [Ex5+14A>G; Ex11+828A>G] haplotype concur in selecting H. pylori cagA subtype infections. Clin Chim Acta. 2008;389:139–145. doi: 10.1016/j.cca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Garza-Gonzalez E, Bosques-Padilla FJ, Mendoza-Ibarra SI, Flores-Gutierrez JP, Maldonado-Garza HJ, Perez-Perez GI. Assessment of the toll-like receptor 4 Asp299Gly, Thr399Ile and interleukin-8 -251 polymorphisms in the risk for the development of distal gastric cancer. BMC Cancer. 2007;7:70. doi: 10.1186/1471-2407-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine. 2001;16:79–86. doi: 10.1006/cyto.2001.0982. [DOI] [PubMed] [Google Scholar]
- 60.Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuo K, Oka M, Murase K, Soda H, Isomoto H, Takeshima F, Mizuta Y, Murata I, Kohno S. Expression of interleukin 6 and its receptor in human gastric and colorectal cancers. J Int Med Res. 2003;31:69–75. doi: 10.1177/147323000303100202. [DOI] [PubMed] [Google Scholar]
- 62.Peters M, Müller AM, Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood. 1998;92:3495–3504. [PubMed] [Google Scholar]
- 63.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howlett M, Judd LM, Jenkins B, La Gruta NL, Grail D, Ernst M, Giraud AS. Differential regulation of gastric tumor growth by cytokines that signal exclusively through the coreceptor gp130. Gastroenterology. 2005;129:1005–1018. doi: 10.1053/j.gastro.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 65.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 66.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 67.Harris PR, Smythies LE, Smith PD, Dubois A. Inflammatory cytokine mRNA expression during early and persistent Helicobacter pylori infection in nonhuman primates. J Infect Dis. 2000;181:783–786. doi: 10.1086/315257. [DOI] [PubMed] [Google Scholar]
- 68.Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY. Relation between cytokines and Helicobacter pylori in gastric cancer. Helicobacter. 2001;6:116–124. doi: 10.1046/j.1523-5378.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 69.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 70.Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, Aoki T, Ohnuma S, Aoki T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124–131. doi: 10.1007/s10120-005-0315-x. [DOI] [PubMed] [Google Scholar]
- 72.Liao WC, Lin JT, Wu CY, Huang SP, Lin MT, Wu AS, Huang YJ, Wu MS. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin Cancer Res. 2008;14:428–434. doi: 10.1158/1078-0432.CCR-07-1032. [DOI] [PubMed] [Google Scholar]
- 73.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 74.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernández-Real JM, Broch M, Vendrell J, Richart C, Ricart W. Interleukin-6 gene polymorphism and lipid abnormalities in healthy subjects. J Clin Endocrinol Metab. 2000;85:1334–1339. doi: 10.1210/jcem.85.3.6555. [DOI] [PubMed] [Google Scholar]
- 76.Fernández-Real JM, Broch M, Vendrell J, Gutiérrez C, Casamitjana R, Pugeat M, Richart C, Ricart W. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49:517–520. doi: 10.2337/diabetes.49.3.517. [DOI] [PubMed] [Google Scholar]
- 77.Hwang IR, Hsu PI, Peterson LE, Gutierrez O, Kim JG, Graham DY, Yamaoka Y. Interleukin-6 genetic polymorphisms are not related to Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2003;8:142–148. doi: 10.1046/j.1523-5378.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 78.Jerrard-Dunne P, Sitzer M, Risley P, Buehler A, von Kegler S, Markus HS. Inflammatory gene load is associated with enhanced inflammation and early carotid atherosclerosis in smokers. Stroke. 2004;35:2438–2443. doi: 10.1161/01.STR.0000144681.46696.b3. [DOI] [PubMed] [Google Scholar]
- 79.Kitamura A, Hasegawa G, Obayashi H, Kamiuchi K, Ishii M, Yano M, Tanaka T, Yamaguchi M, Shigeta H, Ogata M, et al. Interleukin-6 polymorphism (-634C/G) in the promotor region and the progression of diabetic nephropathy in type 2 diabetes. Diabet Med. 2002;19:1000–1005. doi: 10.1046/j.1464-5491.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- 80.Seow A, Ng DP, Choo S, Eng P, Poh WT, Ming T, Wang YT. Joint effect of asthma/atopy and an IL-6 gene polymorphism on lung cancer risk among lifetime non-smoking Chinese women. Carcinogenesis. 2006;27:1240–1244. doi: 10.1093/carcin/bgi309. [DOI] [PubMed] [Google Scholar]
- 81.Gatti LL, Burbano RR, Zambaldi-Tunes M, de-Lábio RW, de Assumpção PP, de Arruda Cardoso-Smith M, Marques-Payão SL. Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res. 2007;38:551–555. doi: 10.1016/j.arcmed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Deans C, Rose-Zerilli M, Wigmore S, Ross J, Howell M, Jackson A, Grimble R, Fearon K. Host cytokine genotype is related to adverse prognosis and systemic inflammation in gastro-oesophageal cancer. Ann Surg Oncol. 2007;14:329–339. doi: 10.1245/s10434-006-9122-9. [DOI] [PubMed] [Google Scholar]
- 83.Kamangar F, Abnet CC, Hutchinson AA, Newschaffer CJ, Helzlsouer K, Shugart YY, Pietinen P, Dawsey SM, Albanes D, Virtamo J, et al. Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland) Cancer Causes Control. 2006;17:117–125. doi: 10.1007/s10552-005-0439-7. [DOI] [PubMed] [Google Scholar]
- 84.Chakravorty M, Datta De D, Choudhury A, Santra A, Roychoudhury S. Association of specific haplotype of TNFalpha with Helicobacter pylori-mediated duodenal ulcer in eastern Indian population. J Genet. 2008;87:299–304. doi: 10.1007/s12041-008-0048-9. [DOI] [PubMed] [Google Scholar]
- 85.Kang JM, Kim N, Lee DH, Park JH, Lee MK, Kim JS, Jung HC, Song IS. The effects of genetic polymorphisms of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. J Clin Gastroenterol. 2009;43:420–428. doi: 10.1097/MCG.0b013e318178d1d3. [DOI] [PubMed] [Google Scholar]
- 86.Yamaoka Y, Kita M, Kodama T, Sawai N, Tanahashi T, Kashima K, Imanishi J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY. Relation between clinical presentation, Helicobacter pylori density, interleukin 1beta and 8 production, and cagA status. Gut. 1999;45:804–811. doi: 10.1136/gut.45.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, et al. Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer. 1999;81:647–653. doi: 10.1038/sj.bjc.6690742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55:1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campa D, Zienolddiny S, Maggini V, Skaug V, Haugen A, Canzian F. Association of a common polymorphism in the cyclooxygenase 2 gene with risk of non-small cell lung cancer. Carcinogenesis. 2004;25:229–235. doi: 10.1093/carcin/bgh008. [DOI] [PubMed] [Google Scholar]
- 91.Landi S, Moreno V, Gioia-Patricola L, Guino E, Navarro M, de Oca J, Capella G, Canzian F. Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res. 2003;63:3560–3566. [PubMed] [Google Scholar]
- 92.Leibovici D, Grossman HB, Dinney CP, Millikan RE, Lerner S, Wang Y, Gu J, Dong Q, Wu X. Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. J Clin Oncol. 2005;23:5746–5756. doi: 10.1200/JCO.2005.01.598. [DOI] [PubMed] [Google Scholar]
- 93.McCarron SL, Edwards S, Evans PR, Gibbs R, Dearnaley DP, Dowe A, Southgate C, Easton DF, Eeles RA, Howell WM. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002;62:3369–3372. [PubMed] [Google Scholar]
- 94.Canedo P, Castanheira-Vale AJ, Lunet N, Pereira F, Figueiredo C, Gioia-Patricola L, Canzian F, Moreira H, Suriano G, Barros H, et al. The interleukin-8-251*T/*A polymorphism is not associated with risk for gastric carcinoma development in a Portuguese population. Eur J Cancer Prev. 2008;17:28–32. doi: 10.1097/CEJ.0b013e32809b4d0f. [DOI] [PubMed] [Google Scholar]
- 95.Kamali-Sarvestani E, Bazargani A, Masoudian M, Lankarani K, Taghavi AR, Saberifiroozi M. Association of H pylori cagA and vacA genotypes and IL-8 gene polymorphisms with clinical outcome of infection in Iranian patients with gastrointestinal diseases. World J Gastroenterol. 2006;12:5205–5210. doi: 10.3748/wjg.v12.i32.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shirai K, Ohmiya N, Taguchi A, Mabuchi N, Yatsuya H, Itoh A, Hirooka Y, Niwa Y, Mori N, Goto H. Interleukin-8 gene polymorphism associated with susceptibility to non-cardia gastric carcinoma with microsatellite instability. J Gastroenterol Hepatol. 2006;21:1129–1135. doi: 10.1111/j.1440-1746.2006.04443.x. [DOI] [PubMed] [Google Scholar]
- 97.Savage SA, Hou L, Lissowska J, Chow WH, Zatonski W, Chanock SJ, Yeager M. Interleukin-8 polymorphisms are not associated with gastric cancer risk in a Polish population. Cancer Epidemiol Biomarkers Prev. 2006;15:589–591. doi: 10.1158/1055-9965.EPI-05-0887. [DOI] [PubMed] [Google Scholar]
- 98.Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487–2493. doi: 10.1158/1055-9965.EPI-05-0326. [DOI] [PubMed] [Google Scholar]
- 99.Lee WP, Tai DI, Lan KH, Li AF, Hsu HC, Lin EJ, Lin YP, Sheu ML, Li CP, Chang FY, et al. The -251T allele of the interleukin-8 promoter is associated with increased risk of gastric carcinoma featuring diffuse-type histopathology in Chinese population. Clin Cancer Res. 2005;11:6431–6441. doi: 10.1158/1078-0432.CCR-05-0942. [DOI] [PubMed] [Google Scholar]
- 100.Ohyauchi M, Imatani A, Yonechi M, Asano N, Miura A, Iijima K, Koike T, Sekine H, Ohara S, Shimosegawa T. The polymorphism interleukin 8 -251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54:330–335. doi: 10.1136/gut.2003.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Savage SA, Abnet CC, Mark SD, Qiao YL, Dong ZW, Dawsey SM, Taylor PR, Chanock SJ. Variants of the IL8 and IL8RB genes and risk for gastric cardia adenocarcinoma and esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:2251–2257. [PubMed] [Google Scholar]
- 102.Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631–636. doi: 10.1093/carcin/bgh349. [DOI] [PubMed] [Google Scholar]
- 103.Hofner P, Gyulai Z, Kiss ZF, Tiszai A, Tiszlavicz L, Tóth G, Szõke D, Molnár B, Lonovics J, Tulassay Z, et al. Genetic polymorphisms of NOD1 and IL-8, but not polymorphisms of TLR4 genes, are associated with Helicobacter pylori-induced duodenal ulcer and gastritis. Helicobacter. 2007;12:124–131. doi: 10.1111/j.1523-5378.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- 104.Gyulai Z, Klausz G, Tiszai A, Lénárt Z, Kása IT, Lonovics J, Mándi Y. Genetic polymorphism of interleukin-8 (IL-8) is associated with Helicobacter pylori-induced duodenal ulcer. Eur Cytokine Netw. 2004;15:353–358. [PubMed] [Google Scholar]
- 105.Leung WK, Chan MC, To KF, Man EP, Ng EK, Chu ES, Lau JY, Lin SR, Sung JJ. H. pylori genotypes and cytokine gene polymorphisms influence the development of gastric intestinal metaplasia in a Chinese population. Am J Gastroenterol. 2006;101:714–720. doi: 10.1111/j.1572-0241.2006.00560.x. [DOI] [PubMed] [Google Scholar]
- 106.Hamajima N, Shibata A, Katsuda N, Matsuo K, Ito H, Saito T, Tajima K, Tominaga S. Subjects with TNF-A-857TT and -1031TT genotypes showed the highest Helicobacter pylori seropositive rate compared with those with other genotypes. Gastric Cancer. 2003;6:230–236. doi: 10.1007/s10120-003-0258-z. [DOI] [PubMed] [Google Scholar]