Abstract
Background:
In spinal muscular atrophy (SMA), weakness, decreased endurance, and fatigue limit mobility. Scales have been developed to measure function across the wide spectrum of disease severity. However, these scales typically are observer dependent, and scores are based on sums across Likert-scaled items. The Six-Minute Walk Test (6MWT) is an objective, easily administered, and standardized evaluation of functional exercise capacity that has been proven reliable in other neurologic disorders and in children.
Methods:
To study the performance of the 6MWT in SMA, 18 ambulatory participants were evaluated in a cross-sectional study. Clinical measures were 6MWT, 10-m walk/run, Hammersmith Functional Motor Scale–Expanded (HFMSE), forced vital capacity, and handheld dynamometry. Associations between the 6MWT total distance and other outcomes were analyzed using Spearman correlation coefficients. A paired t test was used to compare the mean distance walked in the first and sixth minutes.
Results:
The 6MWT was associated with the HFMSE score (r = 0.83, p < 0.0001), 10-m walk/run (r = −0.87, p < 0.0001), and knee flexor strength (r = 0.62, p = 0.01). Gait velocity decreased during successive minutes in nearly all participants. The average first minute distance (57.5 m) was significantly more than the sixth minute distance (48 m) (p = 0.0003).
Conclusion:
The Six-Minute Walk Test (6MWT) can be safely performed in ambulatory patients with spinal muscular atrophy (SMA), correlates with established outcome measures, and is sensitive to fatigue-related changes. The 6MWT is a promising candidate outcome measure for clinical trials in ambulatory subjects with SMA.
GLOSSARY
- FVC
= forced vital capacity;
- HFMSE
= Hammersmith Functional Motor Scale–Expanded;
- HHD
= handheld dynamometry;
- 6MWT
= Six-Minute Walk Test;
- SMA
= spinal muscular atrophy.
Spinal muscular atrophy (SMA) is the second most common neuromuscular disease of childhood,1 and in its mildest form, type 3, individuals have proximal weakness and impaired ambulation. In addition, fatigue is a common symptom2,3 and also may impair function and endurance. However, existing motor function measures do not capture fatigue.4
Identification of a common genetic mutation simplifies diagnosis and has led to the discovery of potentially effective treatments.5 The rapid assessment of the effectiveness of these compounds requires the development of validated outcome measures. Global assessments have the advantage that they can be used over a wide range of function.6 Assessments of walking ability and endurance are direct measures of functional mobility and are thus considered inherently clinically meaningful, and may be more sensitive to change.
The Six-Minute Walk Test (6MWT) is an objective evaluation of exercise capacity and is representative of a person's ability because the test intensity is self-selected.7 The 6MWT has been used to assess function8,9 and has been accepted by regulatory agencies as a clinically meaningful endpoint10 in other neurologic disorders. The 6MWT also has been shown in other pediatric disorders and in healthy children to be a valid and reliable measure,11 demonstrates good test-retest reliability,12,13 and is sensitive to change.14,15 Normative standards for children,16 adolescents,16,17 and adults18 are available. The goals of this study were to evaluate the validity and feasibility of the 6MWT in patients with SMA and to determine whether it captures the clinical phenomenon of fatigue.
METHODS
Subjects.
Eighteen ambulatory participants (4–48 years old, 8 female and 10 male) from an ongoing multicenter natural history study of SMA were included in this cross-sectional study. The 6MWT evaluations were added to the protocol partway through the natural history study and were performed between July 2008 and July 2009 at the 3 participating sites (Columbia University, Children's Hospital Boston, Children's Hospital of Philadelphia). Each participant had genetic confirmation of SMA with a homozygous deletion of exon 7 in the SMN1 gene. Eleven of the 18 participants had clinical symptoms before age 3 years, also termed type 3a19; the others had clinical symptoms after age 3 years, termed type 3b. Informed consent for the institutional review board–approved study was obtained from all participants. Participants had to have been diagnosed with SMA before age 19 years, could not have unstable medical conditions that would preclude participation, had to live within reasonable driving distance from the clinical site, and had to be able to walk safely without assistance. To reduce selection bias, all patients seen in the neuromuscular clinics who fulfilled eligibility criteria were offered enrollment. Additional recruitment efforts included a study Web site and interactions with family groups. The use of assistive devices such as ankle foot orthoses, crutches, walkers, or canes was not permitted during any functional or gait assessments.
Standard protocol approvals, registrations, and patient consents.
All participants or guardians of participants signed informed consent approved by the individual institutional review boards at the 3 sites. The study was registered with ClinicalTrials.gov (NCT00443066).
Outcome measures.
Six-Minute Walk Test.
Participants were instructed to walk as fast as possible along a 25-m linear marked course on a linoleum floor, turn around a marker cone, and then return in the opposite direction and to repeat this loop as often as possible for 6 minutes. The course had a start line, placed horizontally at the beginning, with smaller horizontal lines placed every 1 m. The participants were comfortably dressed with rubber-soled shoes appropriate for walking. Assistive devices were not permitted, such as ankle braces, walkers, crutches, or canes. Participants were permitted to rest, without sitting, if necessary. Running or jogging was not permitted. Distance walked each minute and time to complete each 25-m segment were recorded. Standard encouragement using even, neutral tones was used for each patient, adopted from American Thoracic Society guidelines.7 Falls, if any, were recorded as adverse events.
10-m walk/run.
This test measures the time it takes a participant to walk or run 10 m as fast as possible without compromising the participant's safety. In SMA, the 10-m walk/run time correlated with knee extensor and flexor strength and discriminated between younger and older ambulant patients.20 The 10-m walk/run test is a good measure of walking ability with minimal, if any, endurance demands.
Hammersmith Functional Motor Scale–Expanded.
The Hammersmith Functional Motor Scale–Expanded (HFMSE), a 33-item scale designed to assess motor function in patients with SMA type 2 and type 3, is associated with minimal participant burden, requires only standard equipment, and is completed in less than 15 minutes on average.21 The HFMSE showed excellent test-retest reliability and is correlated with other clinical and physiologic measures in SMA.22
Forced vital capacity.
Pulmonary function was assessed by measuring forced vital capacity (FVC) as percent predicted for age and height.23 Pulmonary measures have been validated for use as outcomes in SMA, and among these, FVC was the most reliable measure.24 The best of 3 trials was recorded.
Handheld dynamometry.
Handheld dynamometry (HHD) was used to quantify strength. In SMA, good interrater and intrarater reliability has been shown for measures of strength in elbow flexors, knee extensors, and knee flexors,25 and composite leg scores have been shown to be sensitive to change.26 HHD scores have also been shown to be correlated with timed function tests and to discriminate between walkers and nonwalkers.20 Strength of the knee flexors and knee extensors was assessed on the participant's preferred side. While seated, the participant was evaluated using the “break” method, where the participant was instructed to push as hard as possible on a stable myometer for 3 to 5 seconds before this effort was overcome by the evaluator. The best of 3 trials was recorded. The clinical evaluators were trained in HHD, and interrater reliability was established before collection of these data.
Data analysis.
Walking distances and average velocities were summarized for each minute (1–6) separately and cumulatively, overall and by SMA type. Total distances for the 6MWT were compared with normative values (children <19 years old,16 adults ≥19 years old18) and predicted distances based on maintenance of the average velocity observed for the first 25 m. Associations between the 6MWT total distance and other outcomes were analyzed using Spearman correlation coefficients because of the presence of unusual values for some outcomes. A paired t test was used to compare the mean distances walked in the first and sixth minutes.
The sample size of 18 participants was determined by the number of ambulatory subjects with SMA type 3 who were enrolled in the ongoing natural history study and had a 6MWT assessment; it was not prospectively determined based on statistical considerations.
RESULTS
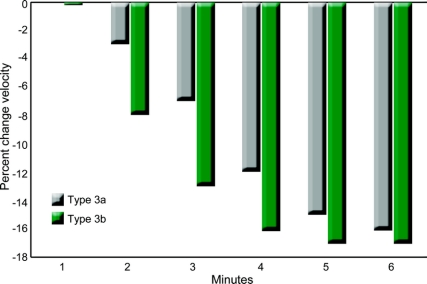
Eighteen participants performed the 6MWT; their clinical characteristics, including 6MWT distances, are summarized in the table, overall and by SMA type. The 6MWT distance was significantly associated with HFMSE score (r = 0.83, p < 0.0001), 10-m walk/run time (r = −0.87, p < 0.0001), and knee flexion HHD (r = 0.62, p = 0.01). The associations with FVC (r = 0.35, p = 0.21) and knee extension HHD (r = 0.36, p = 0.17) were considerably weaker. The mean distances walked during the first (57.5 m) and sixth (48.0 m) minutes were significantly different (p = 0.0003); this mean difference (9.5 m) was nearly the same for participants with type 3a (9.8 m) and type 3b (9.0 m). The mean velocity for each successive minute walked during the 6MWT decreased when compared with the mean velocity walked in the first minute for participants with type 3a and 3b (figure). Participants with type 3a had slower average velocities at each minute. In 17 (94%) of the 18 participants, the 6MWT distance was less than the predicted distance determined by the first 25-m velocity, and in all subjects, the 6MWT distance was less than that predicted for their age and gender. One participant fell while performing the 6MWT but was able to get up unassisted and complete the test, and no one sustained any injury.
Table Clinical characteristics of participants by spinal muscular atrophy type
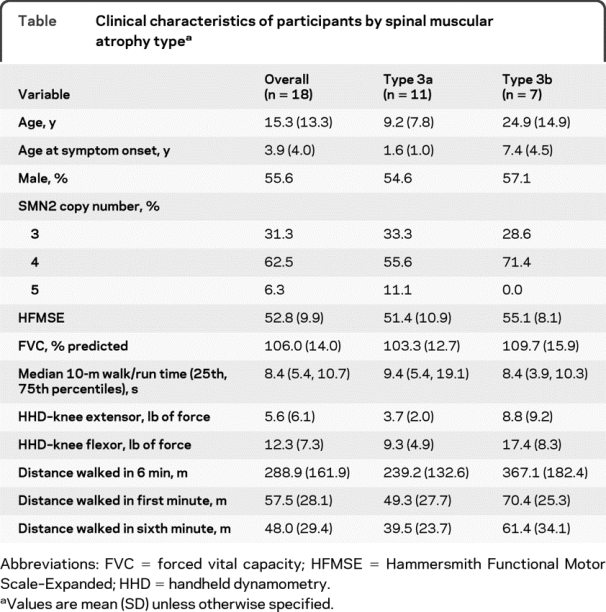
Figure Percent change in average velocity for each minute during the Six-Minute Walk Test
Percent change in average velocity for each minute compared with the average velocity walked in the first minute during the Six-Minute Walk Test (6MWT) for spinal muscular atrophy (SMA) types 3a and 3b. The average velocity for each minute walked during the 6MWT decreased when compared with the average velocity walked in the first minute for participants with type 3a and 3b. Participants with SMA type 3a had slower average velocities at each minute.
DISCUSSION
The 6MWT is a validated measure of exercise capacity and motor function that has been widely applied in the study of several other neuromuscular diseases and numerous other disease states. The ability to quantify functional endurance9,27 and the sensitivity to change28 makes the 6MWT an attractive clinical outcome measure. It has been accepted by European (European Medicines Agency)10,29 and US (Food and Drug Administration) regulators10,30 as a primary endpoint in late phase clinical trials. It is easily administered and requires no special equipment or training. Future research is needed to determine the functional importance of changes in the 6MWT distance and in other measures of motor function (e.g., HFMSE).
In our sample, the 6MWT was highly correlated with the HFMSE but not with FVC (figure e-1 on the Neurology® Web site at www.neurology.org). The 10-m walk/run test, an assessment of gait but not endurance, was also associated with the 6MWT (figure e-1). Subnormal performance on the 6MWT could be due to pulmonary or motor limitations. In SMA type 3, respiratory complications are uncommon, with the majority of patients having normal pulmonary function31; this was evident in our sample (mean FVC% 106, range 84–127). Therefore, the 6MWT in SMA is thought to reflect motor limitations, although poor cardiopulmonary fitness cannot be excluded as a component. We did not study concurrent heart rate change during the 6MWT to address this possibility. The 10-m walk/run test has been considered as an outcome measure because it is quick and less burdensome. However, although it correlates with the 6MWT, it does not measure endurance demands.
Interestingly, in this study, knee flexor but not knee extensor strength was moderately associated with distance walked during the 6MWT (figure e-1). Previous reports in SMA demonstrated correlations between HHD of both knee flexors and extensors with the 10-m walk/run test, timed rising from the floor, and time to climb stairs. However, none of these tests assessed exercise capacity or endurance.20 Similar findings associating quadriceps strength and functional measures have been demonstrated in other neuromuscular disorders.32 Normal walking relies on only a few major muscle groups: hip and knee extensors, ankle plantar and dorsiflexors, and hip abductors.33 The differences found in our study may reflect the unique requirements or compensatory strategies of functional ambulation in SMA and warrant further detailed, kinematic evaluation.
Fatigue is a typical symptom in some neurologic diseases34 and a common physical symptom of patients with SMA. This symptom is more evident with patients with SMA type 3 than with patients with more severe forms of the disease.3 Severity of subjective fatigue can be evaluated using validated questionnaires,35,36 but this may not further our understanding of the pathophysiologic mechanisms underlying fatigue in SMA. Electrophysiologic fatigue is evaluated at the peripheral level using electromyography, and centrally with motor cortex stimulation or assessment of movement-related cortical EEG potentials.37 In a large sample of patients with neuromuscular disease, including fascioscapulohumeral dystrophy, myotonic dystrophy, and hereditary motor and sensory neuropathy, despite a significantly higher frequency of reports of subjective fatigue compared with age-matched controls, neither central nor peripheral electrophysiologic fatigue was detectable by these laboratory means.38 Similarly, quantitative strength testing using single maximal voluntary isometric contraction did not capture fatigue in patients with SMA.4 Other possible mechanisms of disease-related functional deficits and fatigue may occur at the neuromuscular junction as suggested by preclinical studies in transgenic models of SMA.39 Indeed, in a mouse model of mild SMA, repetitive stimulation at high frequency resulted in intermittent neurotransmission failures.39 Such failures would likely result in excessive fatigue, akin to the reduced endurance we observed in patients subjected to the 6MWT. Neuromuscular junction dysfunction, however, has not been evaluated directly in patients with SMA. Therapeutic strategies that successfully treat motor fatigue would be expected to improve daily function and quality of life in ambulatory patients with SMA. The 6MWT may be a useful outcome measure in clinical trials for such therapeutic interventions.
Unlike boys with Duchenne muscular dystrophy and healthy individuals,10 in our study, patients with SMA did not maintain consistent walking speed throughout the 6MWT, with a 17% decrement in performance from the first to the sixth minute. Furthermore, the walking speed assessed during the 10-m walk/run test does not capture deficits in endurance, as might be expected because it allows for running and may not be a good predictor of ability to function in the community.40 The 6MWT may serve as valuable tool to quantify fatigue in this population. Furthermore, in addition to absolute distance and deviations from normative values, the change in walking speed during the 6MWT should be investigated as a useful measure of fatigue in clinical trials.
Our study indicates that the 6MWT can be safely performed in ambulatory patients with SMA. In this sample, only 1 fall occurred during the test, which did not result in injury, and the participant was able to rise unassisted and complete the test. The test correlates with standard measures of motor function, including another timed walking test. It is easily administered and could serve as a clinical outcome measure in clinical trials of ambulatory patients. Patients with SMA do not maintain consistent walking speeds throughout the 6MWT, indicating a clinically relevant aspect of motor fatigue; pathophysiologic correlates to this finding need to be explored. Although we attempted to minimize selection bias through recruitment efforts and the use of broad eligibility criteria, caution is needed regarding the generalizability of our results. It is essential that 6MWT methods be standardized for future international prospective studies across SMA centers. Larger studies are needed to confirm its reliability and sensitivity in this population.
AUTHOR CONTRIBUTIONS
Dr. McDermott performed the statistical analyses.
DISCLOSURE
Ms. Montes reports no disclosures. Dr. McDermott has received honoraria from the Michael J. Fox Foundation; has served as a consultant to the New York State Department of Health, the American Epilepsy Society, the Michael J. Fox Foundation, and Teva Pharmaceutical Industries Ltd.; has received research support from Medivation, Inc., Boehringer Ingelheim, NeuroSearch Sweden AB, and Forest Laboratories, Inc.; and receives research support from the NIH (NS42372 [Co-I], HD44430 [Co-I], DE16280 [Co-I], NS52619 [PI], NS45686 [Co-I], NS46487 [Co-I], NS50095 [Co-I], NS49639 [Co-I], AR52274 [Co-I], NS50573 [Co-I], HL80107 [Co-I], RR24160 [Co-I], NS58259 [Co-I], and NS48843 [Co-I]), the US Food and Drug Administration, the Michael J. Fox Foundation, the SMA Foundation, and the Muscular Dystrophy Association. Mr. Martens reports no disclosures. Dr. Dunaway reports no disclosures. Dr. Glanzman has received travel expenses for educational activities not funded by industry; and has received research support from PTC Therapeutics, Inc., the NIH (RO1 NS043264-04 A1 [clinical evaluator] and NIDRR 60662 [Site PI]) and from the SMA Foundation. Dr. Riley, Ms. Quigley, and Ms. Montgomery report no disclosures. Dr. Sproule has received funding for travel from UCB and for lectures or educational activities not funded by industry; his spouse is an employee of Pfizer Inc; receives research support from PTC Therapeutics, Inc. and the NIH/NINDS (K12NS01698) Neurological Science Academic Development Award; and holds stock and stock options in Pfizer Inc. Dr. Tawil receives research support from the NIH (N01-AR-50-227450 [role: co-PI]), the SMA Foundation, and from the Fields Family Foundation. Dr. Chung receives research support from the SMA Foundation, NHLBI U01 HL098163, NICHD HD057036, NIDDK DK52431, RC2 HD064525, NCI U01CA069398, DK-04-021, DK63608, US Department of Defense, and the Doris Duke Charitable Foundation. Dr. Darras receives royalties from publishing in UpToDate; serves as a consultant to and receives speaker honoraria from Genzyme Corporation; receives research support from PTC Therapeutics, Inc., the NIH (NIAMS 2P01 NS040828-6A11 [Co-PI] and NINDS HHSN265200423611C, [PI]), the SMA Foundation, the Muscular Dystrophy Foundation, the New England Research Institutes/SMA Foundation, and from the Slaney Family Fund for SMA. Dr. De Vivo serves on scientific advisory boards for the SMA Foundation, the Colleen Giblin Foundation, the Pediatric Neurotransmitter Disease Association, the International Reye Syndrome Foundation, and the Will Foundation; receives royalties from publishing The Molecular Basis and Genetic Basis of Neurologic and Psychiatric Disease, 4th ed. (Lippincott Williams & Wilkins, 2008); and receives research support from the NIH (1 UL1 RR024156-01 [investigator], NINDS 5K12NS01698 [program director] and 5R01NS37949 [PI]), and NICHD (5P01HD32062 [PI]), the SMA Foundation, the Colleen Giblin Foundation,the Will Foundation, and the Pediatric Neurotransmitter Disease Association. Dr. Kaufmann conducted the work reported here while she was a full-time employee of Columbia University. This report is not related to her current work at NINDS. Dr. Kaufmann has received consulting honoraria from the SMA Foundation. She has received travel reimbursement to investigator meetings from the NIH, SMA Foundation, the MDA, Santhera Pharmaceuticals and PTC Therapeutics, Inc.; she serves on the editorial board of Neuromuscular Disorders; has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; has received research support from Santhera Pharmaceuticals, Penwest Pharmaceuticals Co., PTC Therapeutics, Inc., the NIH (RO1 NS48125 NINDS, PI); Department of Defense, and the SMA Foundation. Her spouse serves on the editorial board of Pediatric Pulmonology. Dr. Finkel serves on advisory boards for PTC Therapeutics, Inc., DuchenneConnect, Families of SMA, the National Fabry Disease Foundation, and TREAT-NMD; has received travel expenses for lectures not funded by industry; receives research support from PTC Therapeutics, Inc., Genzyme Corporation, Santhera Pharmaceuticals, the NIH (U54 AR0526446-03 [Co-I] and 1U54 NS0657-12-01 [Co-I]), the SMA Foundation, the Muscular Dystrophy Association, and the Foundation for the Eradication of Duchenne; and his spouse serves on the editorial board of Arthritis Research and Therapy, holds and has received license fees for numerous patents related to T cell activation and HIV, and receives research support from Merck Serono and the NIH in the field of T cell activation, HIV and genomics of juvenile arthritis.
ACKNOWLEDGMENT
The authors thank the Outcome Measures Subcommittee of the International Coordinating Committee for SMA Clinical Trials for their meaningful discussions and efforts to standardize SMA outcome measures. The authors also thank Dr. Umrao Monani for his helpful comments regarding possible pathophysiological mechanisms of fatigue in SMA. They also thank the members of the PNCR Network for SMA Clinical Trials Research, the PNCR Network External Advisory Board, and the Muscle Study Group for their guidance, and the research participants and their families for their generous gift of time and effort.
Supplementary Material
Address correspondence and reprint requests to Ms. Jacqueline Montes, SMA Clinical Research Center, Department of Neurology, Columbia University, 180 Ft. Washington Ave., 5th Floor, New York, NY 10032 jm598@columbia.edu
Supplemental data at www.neurology.org
Study funding: Supported by the Spinal Muscular Atrophy (SMA) Foundation, which had no role in the conduct of this study or the parent SMA natural history study.
Disclosure: Author disclosures are provided at the end of the article.
Received September 16, 2009. Accepted in final form December 14, 2009.
REFERENCES
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases: a world survey. Neuromuscul Disord 1991;1:19–29. [DOI] [PubMed] [Google Scholar]
- 2.Piepers S, van den Berg LH, Brugman F, et al. A natural history study of late onset spinal muscular atrophy types 3b and 4. J Neurol 2008;255:1400–1404. [DOI] [PubMed] [Google Scholar]
- 3.de Groot IJ, de Witte LP. Physical complaints in ageing persons with spinal muscular atrophy. J Rehabil Med 2005;37:258–262. [DOI] [PubMed] [Google Scholar]
- 4.Iannaccone ST, White M, Browne R, Russman B, Buncher R, Samaha FJ. Muscle fatigue in spinal muscular atrophy. J Child Neurol 1997;12:321–326. [DOI] [PubMed] [Google Scholar]
- 5.Sumner CJ. Therapeutics development for spinal muscular atrophy. NeuroRx 2006;3:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montes J, Gordon AM, Pandya S, De Vivo DC, Kaufmann P. Clinical outcome measures in spinal muscular atrophy. J Child Neurol 2009;24:968–978. [DOI] [PubMed] [Google Scholar]
- 7.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest 2001;119:256–270. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi Y, Katsuno M, Banno H, et al. Walking capacity evaluated by the 6-minute walk test in spinal and bulbar muscular atrophy. Muscle Nerve 2008;38:964–971. [DOI] [PubMed] [Google Scholar]
- 9.Andersson C, Asztalos L, Mattsson E. Six-minute walk test in adults with cerebral palsy: a study of reliability. Clin Rehabil 2006;20:488–495. [DOI] [PubMed] [Google Scholar]
- 10.McDonald CM, Henricson EK, Han JJ. The 6-minute walk test (6MWT) as a clinical trial outcome measure in Duchenne/Becker muscular dystrophy (DMD/BMD). Presented at the 13th International WMS Congress; September 29–October 2, 2008; New Castle Gateshead.
- 11.Li AM, Yin J, Yu CC, et al. The six-minute walk test in healthy children: reliability and validity. Eur Respir J 2005;25:1057–1060. [DOI] [PubMed] [Google Scholar]
- 12.Maher CA, Williams MT, Olds TS. The six-minute walk test for children with cerebral palsy. Int J Rehabil Res 2008;31:185–188. [DOI] [PubMed] [Google Scholar]
- 13.Cunha MT, Rozov T, de Oliveira RC, Jardim JR. Six-minute walk test in children and adolescents with cystic fibrosis. Pediatr Pulmonol 2006;41:618–622. [DOI] [PubMed] [Google Scholar]
- 14.Harmatz P, Giugliani R, Schwartz IV, et al. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab 2008;94:469–475. [DOI] [PubMed] [Google Scholar]
- 15.Maiya S, Hislop AA, Flynn Y, Haworth SG. Response to bosentan in children with pulmonary hypertension. Heart 2006;92:664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr 2007;150:395–399, 399.e391-e392. [DOI] [PubMed] [Google Scholar]
- 17.Li AM, Yin J, Au JT, et al. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am J Respir Crit Care Med 2007;176:174–180. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil 2001;21:87–93. [DOI] [PubMed] [Google Scholar]
- 19.Zerres K, Rudnik-Schoneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa-Petrusewicz I. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci 1997;146:67–72. [DOI] [PubMed] [Google Scholar]
- 20.Merlini L, Bertini E, Minetti C, et al. Motor function-muscle strength relationship in spinal muscular atrophy. Muscle Nerve 2004;29:548–552. [DOI] [PubMed] [Google Scholar]
- 21.Glanzman AM, O'Hagen JM, McDermott MP, et al. Validation of the Expanded Hammersmith Functional Motor Scale in SMA type II and III. Presented at the 12th Annual International Spinal Muscular Atrophy Research Group Meeting; June 19–22, 2008; Boston.
- 22.O'Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord 2007;17:693–697. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993;15:75–88. [DOI] [PubMed] [Google Scholar]
- 24.Iannaccone ST. Outcome measures for pediatric spinal muscular atrophy. Arch Neurol 2002;59:1445–1450. [DOI] [PubMed] [Google Scholar]
- 25.Merlini L, Mazzone ES, Solari A, Morandi L. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve 2002;26:64–70. [DOI] [PubMed] [Google Scholar]
- 26.Merlini L, Solari A, Vita G, et al. Role of gabapentin in spinal muscular atrophy: results of a multicenter, randomized Italian study. J Child Neurol 2003;18:537–541. [DOI] [PubMed] [Google Scholar]
- 27.Brown CD, Benditt JO, Sciurba FC, et al. Exercise testing in severe emphysema: association with quality of life and lung function. COPD 2008;5:117–124. [DOI] [PubMed] [Google Scholar]
- 28.Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther 2009;89:569–579. [DOI] [PubMed] [Google Scholar]
- 29.Wraith JE, Clarke LA, Beck M, et al. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase). J Pediatr 2004;144:581–588. [DOI] [PubMed] [Google Scholar]
- 30.Muenzer J, Wraith JE, Beck M, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med 2006;8:465–473. [DOI] [PubMed] [Google Scholar]
- 31.Samaha FJ, Buncher CR, Russman BS, et al. Pulmonary function in spinal muscular atrophy. J Child Neurol 1994;9:326–329. [DOI] [PubMed] [Google Scholar]
- 32.Lindeman E, Leffers P, Reulen J, Spaans F, Drukker J. Quadriceps strength and timed motor performances in myotonic dystrophy, Charcot-Marie-Tooth disease, and healthy subjects. Clin Rehabil 1998;12:127–135. [DOI] [PubMed] [Google Scholar]
- 33.Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture 2003;17:159–169. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 2004;363:978–988. [DOI] [PubMed] [Google Scholar]
- 35.Beurskens AJ, Bultmann U, Kant I, Vercoulen JH, Bleijenberg G, Swaen GM. Fatigue among working people: validity of a questionnaire measure. Occup Environ Med 2000;57:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res 2004;56:157–170. [DOI] [PubMed] [Google Scholar]
- 37.Zwarts MJ, Bleijenberg G, van Engelen BG. Clinical neurophysiology of fatigue. Clin Neurophysiol 2008;119:2–10. [DOI] [PubMed] [Google Scholar]
- 38.Schillings ML, Kalkman JS, Janssen HM, van Engelen BG, Bleijenberg G, Zwarts MJ. Experienced and physiological fatigue in neuromuscular disorders. Clin Neurophysiol 2007;118:292–300. [DOI] [PubMed] [Google Scholar]
- 39.Kariya S, Park GH, Maeno-Hikichi Y, et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet 2008;17:2552–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dean CM, Richards CL, Malouin F. Walking speed over 10 metres overestimates locomotor capacity after stroke. Clin Rehabil 2001;15:415–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.