Abstract
Objective:
To investigate whether longitudinal declines in cognition are associated with higher fibrillar amyloid-beta (Aβ) deposition in vivo in individuals without dementia.
Method:
[11C]PiB images were obtained to measure fibrillar Aβ burden in 57 participants without dementia from the Baltimore Longitudinal Study of Aging. Participants (33 men, 24 women) had a mean (SD) age of 78.7 (6.2) years. Six participants (4 men, 2 women) had mild cognitive impairment defined as Clinical Dementia Rating = 0.5. To measure [11C]PiB retention, distribution volume ratios (DVR) for 15 regions of interest were estimated by fitting a simplified reference tissue model to the measured time activity curves. Mixed effects regression was used to predict cognitive trajectories over time using data before and including time of PiB (mean follow-up 10.8 years), with mean cortical DVR, age at baseline, sex, and education as independent predictors. Voxel-based analysis identified local associations.
Results:
[11C]PiB retention was higher in older individuals. Greater declines over time in mental status and verbal learning and memory, but not visual memory, were associated significantly with higher PiB retention. Voxel-based analysis showed significant associations in frontal and lateral temporal regions.
Conclusions:
Higher Aβ deposition is associated with greater longitudinal decline in mental status and verbal memory in the preceding years. The differential association for verbal but not visual memory may reflect the greater reliance of verbal word list learning on prefrontal regions, which show early Aβ deposition. Prospective imaging may help distinguish between individuals with evolving neuropathology who develop accelerated cognitive decline vs those with normal aging.
GLOSSARY
- AD
= Alzheimer disease;
- BLSA
= Baltimore Longitudinal Study of Aging;
- BVRT
= Benton Visual Retention Test;
- CDR
= Clinical Dementia Rating;
- CVLT
= California Verbal Learning Test;
- DVR
= distribution volume ratio;
- MCI
= mild cognitive impairment;
- MMSE
= Mini-Mental State Examination;
- ROI
= regions of interest.
Postmortem studies of associations between amyloid-beta (Aβ) burden and antemortem cognition yield conflicting findings.1,2 We found that longitudinal cognitive trajectories were similar between cognitively normal individuals with and without Alzheimer disease (AD) neuropathology and that both groups differed from individuals with mild cognitive impairment (MCI) or AD, who showed marked cognitive decline.3
Radiotracers for in vivo imaging of fibrillar Aβ burden allow prospective investigation of relationships between cognitive performance and Aβ. Consistent with postmortem studies, 20% to 30% of clinically normal individuals show Aβ deposition on imaging.4,5 Across the spectrum of cognitive function, lower memory correlates cross-sectionally with higher [11C]PiB.6,7 The only study investigating longitudinal changes in cognition in relation to Aβ deposition found that slopes of word list recall prior to [11C]PiB imaging correlated with Aβ deposition in elderly subjects without dementia who subsequently progressed to MCI/AD but not in individuals who remained cognitively normal.8
To further evaluate relationships between longitudinal cognitive change and in vivo Aβ deposition, we studied 57 participants without dementia of the neuroimaging substudy of the Baltimore Longitudinal Study of Aging (BLSA) who had up to 13 serial cognitive assessments prior to and concurrent with [11C]PiB evaluation of Aβ. We hypothesized that trajectories of cognitive change over time, particularly on tests of episodic memory, would be associated with in vivo Aβ deposition in a group of individuals who remained cognitively healthy as well as those who developed very mild cognitive impairment during the follow-up.
METHODS
Study participants.
Fifty-seven participants without dementia (33 men, 24 women; mean [SD] age 78.7 [6.2]) of the Baltimore Longitudinal Study of Aging neuroimaging study (BLSA-NI)9 were included. They were ascertained from the initial 61 BLSA-NI participants consecutively assessed with [11C]PiB from June 2005 to March 2007, after excluding 2 participants with clinical stroke, 1 with a brain injury, and 1 unable to tolerate MRI. The 57 individuals were representative of the entire BLSA-NI with respect to baseline age, sex, race, and education. Exclusionary criteria at entry into the neuroimaging study included CNS disease, severe cardiovascular disease, severe pulmonary disease, or metastatic cancer. All participants underwent neuropsychological evaluation in conjunction with each neuroimaging visit, and serial assessments performed in conjunction with neuroimaging visits prior to and concurrent with (1994–2007) the [11C]PiB study were analyzed.
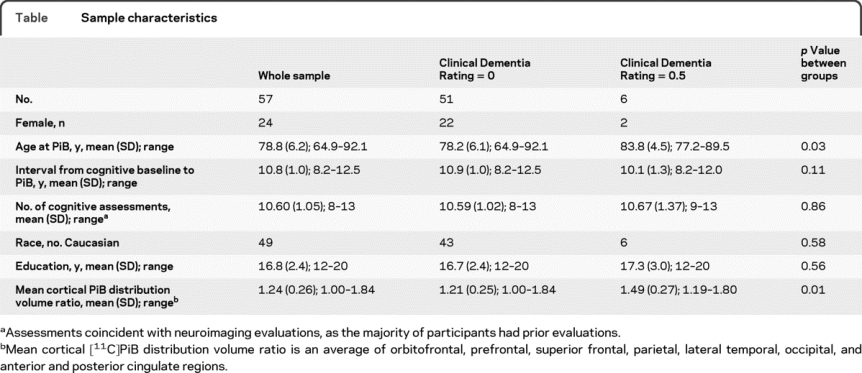
The Clinical Dementia Rating (CDR) scale,10 typically informant-based, was administered in conjunction with the [11C]PiB imaging study. The CDR also was administered during prior imaging visits when participants scored 3 or greater on the Blessed Information Memory Concentration11 test and at each visit for autopsy study participants (about 50% of sample). Cognitive status was determined by consensus diagnosis according to established procedures.3,12 At the time of the PiB study, 6 individuals had a CDR total score of 0.5 but only one met consensus criteria for MCI. Demographic and clinical characteristics of participants are presented in the table.
Table Sample characteristics
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from each participant at each imaging visit. This study was approved by the Institutional Review Boards of the NIA Intramural Research Program and Johns Hopkins Medical Institutions.
[11C]PiB PET studies.
Dynamic [11C]PiB PET studies (37 time frames over 90 minutes) were performed in 3-dimensional mode on a GE Advance scanner. Participants were fitted with a thermoplastic mask for PET imaging to minimize motion during scanning. The PET scanning started immediately after IV bolus injection of a mean (SD) 14.5 (0.9) mCi of [11C]PiB with a mean (SD) specific activity of 5.7 (range 0.98 to 14.62) Ci/μmol. Dynamic images were reconstructed using filtered back-projection with a ramp filter (image size = 128 × 128, pixel size = 2 × 2 mm, slice thickness = 4.25 mm), yielding a spatial resolution of about 4.5 mm FWHM at the center of field of view. Transmission scans in 2D mode were used for attenuation correction of the emission scans.
MRI-based region of interest definition.
Volumetric T1-weighted MRI scans (124 slices, matrix = 256 × 256, pixel size = 0.94 × 0.94 mm, slice thickness = 1.5 mm) acquired at 1.5 T were coregistered to the mean of the first 20-minute dynamic PET images for each participant using the mutual information method in the Statistical Parametric Mapping software (SPM2; Wellcome Department of Cognitive Neurology, London, UK). In addition to cerebellum, which was used as a reference region, 15 regions of interest (ROIs; 1 = caudate; 2 = putamen; 3 = thalamus; 4 = lateral temporal; 5 = mesial temporal; 6 = orbital frontal; 7 = prefrontal; 8 = occipital; 9 = superior frontal; 10 = parietal; 11 = anterior cingulate; 12 = posterior cingulate; 13 = pons; 14 = midbrain; 15 = white matter) were manually drawn on the coregistered MRIs and used for ROI definition on the PET scans.13
Quantification of distribution volume ratios.
The distribution volume ratios (DVRs) of ROIs were estimated by simultaneous fitting of a simplified reference tissue model using linear regression with spatial constraints (SRTM-LRSC) to the 15 measured ROI time activity curves.14 The mean cortical DVR was calculated by averaging DVR values from orbitofrontal, prefrontal, superior frontal, parietal, lateral temporal, occipital, and anterior and posterior cingulate regions. In addition, DVR images were generated for voxel-wise analysis.14,15 Parametric images were spatially normalized using an R1 template (R1 = K1/K1 [reference tissue ], the target to reference tissue ratio of tracer transport rate constant from vascular space to tissue),14 and the mean parametric images for the 51 cognitively normal individuals and the 6 individuals with CDR = 0.5 were calculated.
Neuropsychological testing.
A battery of 12 neuropsychological tests was administered at each neuroimaging visit to evaluate mental status, word knowledge and verbal ability, memory, language, verbal fluency, attention, executive function, and spatial ability. The present analyses focused on longitudinal changes in mental status, verbal and visual memory, and executive function. Mental status was assessed with the Mini-Mental State Examination (MMSE); verbal memory was assessed using the California Verbal Learning Test (CVLT) and visual memory with the Benton Visual Retention Test (BVRT); the Trail Making Test Part A assessed attention and visual scanning; and Trail Making Test Part B and category (animals, fruits, vegetables) and letter fluency (FAS) assessed executive function.
Statistical analysis.
Two sample t tests and χ2 tests were used to compare sample characteristics presented in the table.
Associations between [11C]PiB mean cortical DVR and cognitive change over time were examined using linear mixed models (Proc Mixed; SAS v. 9.1; SAS Institute Inc.; Cary, NC), which allows analysis of all available data despite missing values (approximately 5%–8% by test). These associations were investigated without (model 1) and with (model 2) adjustment for age at baseline cognitive assessment for each dependent measure, separately. (Analysis of a third set of models, adding adjustment for sex, yielded similar results and is not presented.) [11C]PiB mean cortical DVR (PiB mDVR), interval (years) from baseline assessment, and their interactions were modeled as fixed covariates. Years of education were also modeled as a fixed covariate, and random effects included intercept and interval. The main effect of PiB mDVR indicates whether Aβ burden is associated with cognitive performance at baseline assessment, whereas the PiB mDVR × interval effect tests the primary hypothesis of the relationship between Aβ burden and longitudinal cognitive change over time. Analyses were repeated excluding the 6 participants with CDR = 0.5. Individual trajectories and rates of cognitive change, adjusted for baseline age, were estimated from the linear mixed effects models for illustration of associations between PiB mDVR and cognitive change and for use in voxel-based analysis of regional associations.
Parametric images and SPM 5 (Statistical Parametric Mapping 5; Wellcome Department of Imaging Neuroscience, London, UK) were used to investigate voxel-wise associations between [11C]PiB DVR and longitudinal change in cognitive function. To decrease the number of analyses and potential false positive errors, voxel-based correlation analyses of regional patterns were conducted only for those dependent measures showing significant associations between PiB mDVR and cognitive change. Parametric PiB DVR images were smoothed with a Gaussian filter of 8, 8, 8 mm in the x, y, and z planes. Correlation analyses investigated the relationships between the estimated rates of cognitive change, adjusted for baseline age, and local PiB retention. SPM analyses employed a significance threshold of p ≤ 0.005, with a spatial extent of 100 voxels.
RESULTS
Fibrillar Aβ burden as a function of age and cognitive status.
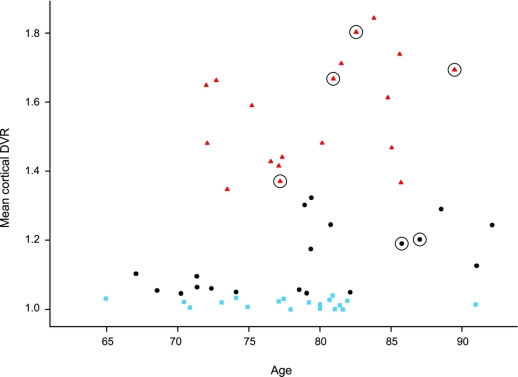
Mean PiB DVR images for cognitively normal individuals and the 6 individuals with CDR = 0.5 are shown in figure e-1 on the Neurology® Web site at www.neurology.org. Increased [11C]PiB retention is apparent in frontal, lateral temporal, and posterior cingulate/precuneus regions in older adults with CDR = 0.5. The distributions of PiB mDVR values as a function of age by tertile of PiB mDVR are presented in figure 1. Due to the non-normal distribution of PiB retention, we used median regression to investigate the relationship between age and mDVR. There was an increase in mDVR with age (p = 0.05; β = 0.008; χ2 [1 df] = 3.77). In addition, mDVR values for 4 of the 6 individuals with memory loss by CDR fell within the top tertile of mDVR values.
Figure 1 Distribution of mean cortical [11C]PiB retention in older adults without dementia by tertile of mean distribution volume ratio
Individuals in the highest tertile are shown as red triangles, in the middle by black circles, and the lowest by blue squares. The 6 older adults with CDR = 0.5 are shown by circled symbols.
Mean cortical Aβ burden and longitudinal cognitive decline.
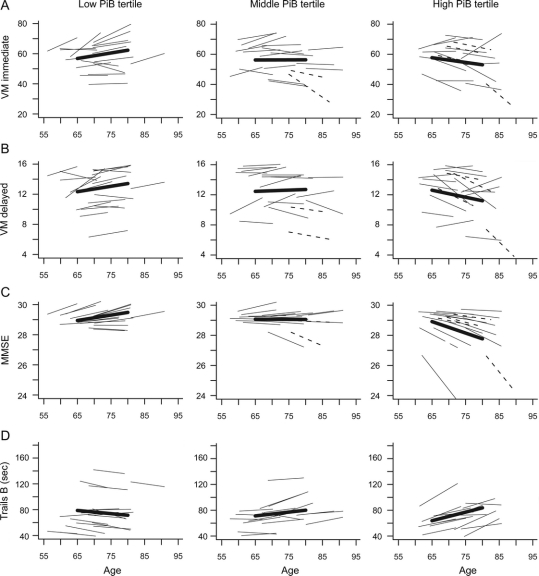
PiB mDVR was not associated significantly with baseline level of cognitive performance for mental status or measures of domain-specific cognitive performance under any model. (Similar results were obtained restricting the regression analysis to the baseline cognitive assessment only.) However, in analyses including all participants, higher PiB mDVR was associated with longitudinal decline in mental status (MMSE) and verbal immediate (sum of 5 CVLT List A trials) and delayed free recall under both models (measuring cognitive change prior to and concurrent with the [11C]PiB scan). PiB mDVR was not associated significantly with longitudinal change in measures of executive function. Estimates (SE) of these associations reflect change in rates of annual declines in scores per unit of greater PiB retention with adjustment for baseline age and education: MMSE −0.13 (0.05), CVLT Immediate Recall −1.35 (0.44), CVLT Delayed Recall −0.32 (0.11), all p ≤ 0.01. To illustrate these associations, mean and estimated individual trajectories of cognitive change are shown by tertiles of mDVR (<1.045, 1.045–1.345, >1.345) for MMSE, CVLT immediate, and delayed free recall in figure 2, A through C.
Figure 2 Predicted longitudinal trajectories by tertile of PiB mean distribution volume ratio for cognitive tests showing significant longitudinal decline in association with higher Aβ burden
Mean trajectories for each tertile are shown by bolded lines, and trajectories for participants with CDR = 0.5 are shown as dashed lines. (A) Verbal episodic memory, immediate recall (p < 0.01); (B) verbal episodic memory, delayed recall (p < 0.01); (C) Mental Status (p = 0.01); (D) executive function measured by Trails B in CDR = 0 individuals (p = 0.02). Note that higher scores reflect poorer performance for Trails B. CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination; VM = verbal memory.
The relationships between mental status (p < 0.01) and CVLT immediate recall (p = 0.02) remained significant in both models after excluding the 6 participants with CDR = 0.5, but PiB mDVR was no longer associated with decline in CVLT delayed free recall (p = 0.06). Estimates (SE) of these associations with adjustment for baseline age and education were as follows: MMSE −0.15 (0.05), CVLT Immediate Recall −1.03 (0.45), CVLT Delayed Recall −0.22 (0.11). Additionally, longitudinal decline in executive function (Trails B seconds) was associated with higher PiB mDVR when only CDR = 0 individuals were included in the analysis (estimate = 2.86, SE = 1.20, p = 0.02; figure 2D).
Regional Aβ burden and cognitive decline.
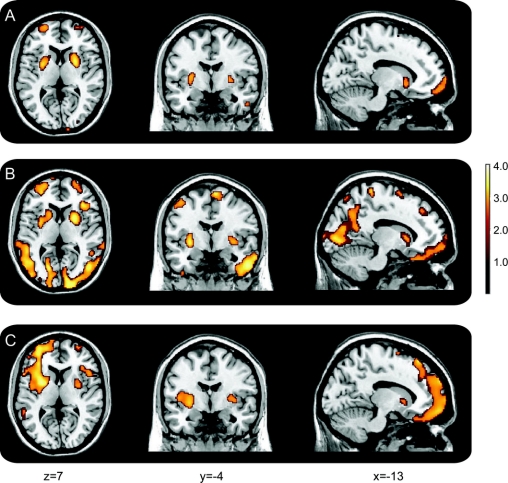
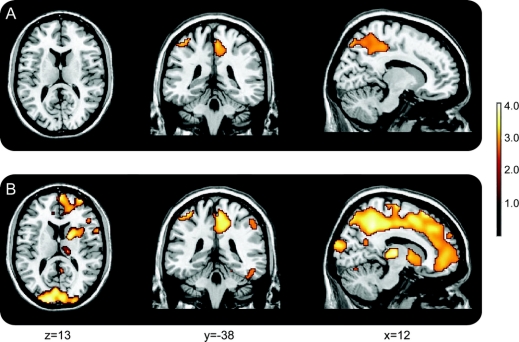
Correlations between regional Aβ burden and declines in CVLT immediate and delayed verbal memory and mental status performance are presented in table e-1 and figure 3. Voxel-based analysis, adjusted for baseline age, showed that higher Aβ deposition in frontal and temporal regions and putamen was associated with greater decline in verbal memory and mental status. Declines in MMSE were associated with greater PiB retention in inferior, middle, and superior frontal regions, as well as left middle temporal gyrus and right insula. Declines in CVLT immediate and delayed free recall were associated with Aβ deposition in putamen, inferior, middle, and superior frontal regions, temporal pole, lingual gyrus, and precuneus, with more widespread correlations in lateral temporal regions for delayed free recall. There were no significant associations observed for the posterior cingulate region or medial temporal regions. The relationship between rates of change in Trails B, a measure of executive function, and Aβ burden was more robust in analyses restricted to cognitively normal individuals with CDR = 0 (table e-2, figure 4), confirming the analysis based on mDVR. Higher levels of Aβ deposition in frontal and parietal regions, including precuneus, were associated with declines in executive function.
Figure 3 Voxel-based associations between regional Aβ load and slopes of longitudinal changes in episodic memory and mental status
(A) CVLT immediate recall; (B) CVLT delayed recall; (C) MMSE. CVLT = California Verbal Learning Test; MMSE = Mini-Mental State Examination.
Figure 4 Voxel-based associations between regional Aβ load and slopes of longitudinal change in executive function
Declining performance on Trails B over time is more robustly related to [11C]PiB retention in normal older adults. (A) Whole sample including 6 individuals with Clinical Dementia Rating = 0.5; (B) subset of 51 individuals with CDR = 0.
DISCUSSION
In this series of 57 BLSA-NI participants with longitudinal cognitive assessments, we found that higher [11C]PiB retention was associated with steeper trajectories of cognitive decline in the years preceding and concurrent with the PiB evaluation. Specifically, higher [11C]PiB was associated with longitudinal decline in mental status and verbal episodic memory although mean cortical [11C]PiB retention was not related to baseline level of performance on any of the cognitive measures. Six individuals in our sample had mild memory loss by CDR, but only one met consensus criteria for MCI at the time of the study. Associations between higher Aβ burden and decline in mental status and immediate free recall remained significant after exclusion of these individuals, and additionally, decline in executive function was associated with higher [11C]PiB mDVR after exclusion of individuals with mild memory loss. These results provide evidence that in vivo measures of Aβ deposition with PET and [11C]PiB are associated with longitudinal cognitive change even in clinically normal individuals.
Our findings of associations between [11C]PiB retention and longitudinal decline in verbal episodic memory in clinically normal individuals extend observations of cross-sectional associations reported in some studies,7,16 although we did not find significant relationships between baseline cognitive function and Aβ burden. Higher [11C]PiB retention in association with greater longitudinal decline in verbal recall is also consistent with the sole longitudinal report,8 which indicated that older adults without dementia who show declines in word list recall are more likely to have higher [11C]PiB retention compared to individuals with stable clinical status and verbal recall. Furthermore, our findings hold even after excluding individuals who had developed very mild cognitive impairment, suggesting that associations between PiB retention and cognitive change emerge early in the disease process before clinical symptoms are apparent.
Although higher [11C]PiB retention was associated with decline in verbal recall performance, we observed no significant associations between [11C]PiB retention and longitudinal change in BVRT performance, a measure of short-term visual memory which declines early in the course of AD.3,12 The lack of association between BVRT performance and [11C]PiB retention has also been reported in cross-sectional studies.7 It is possible that the lack of an association for BVRT reflects greater sensitivity of this measure to mesial temporal dysfunction and the low level of Aβ deposition visualized by [11C]PiB in mesial temporal structures. In contrast, the CVLT is sensitive to frontal as well as temporal function, due to its greater dependence on the capacity to organize serial and semantic information. Voxel-based analyses offer support for this explanation, as CVLT associations with [11C]PiB retention were observed for prefrontal and lateral temporal but not mesial temporal regions. This explanation also receives some support from our observed association between decline in frontal executive function, as measured by Trails B, and [11C]PiB retention in individuals with CDR = 0 (which reached only trend level in the entire sample, p < 0.10). Perhaps, future tools for in vivo measurement of the neurofibrillary tangles that are present early in mesial temporal structures would show different patterns of regional correlations across cognitive domains.
Another issue raised by our observations of associations between elevated [11C]PiB retention and longitudinal declines in verbal episodic memory and executive function is the apparent inconsistency with postmortem findings indicating similar trajectories of longitudinal cognitive performance in clinically normal individuals with and without AD pathology.3 In postmortem studies, we compared antemortem cognitive function in a selected group of individuals who remained clinically stable until death despite AD pathology (asymptomatic AD) with groups of clinically and neuropathologically normal controls and clinically and neuropathologically abnormal AD and MCI. In contrast, our in vivo [11C]PiB studies include a mixed group of individuals who will remain cognitively normal as well as those who will go on to develop cognitive impairment and AD. An important difference between our autopsy sample and the in vivo study is the age at which Aβ burden was measured. Mean ages (years) in the autopsy sample were 78.6 for controls, 85.8 for cognitively normal subjects with pathology, and 89.4 for subjects with MCI/AD, whereas mean age in the imaging sample was 78.8 years. Thus, participants with elevated Aβ burden in the imaging sample were less likely to have passed fully through the risk period for cognitive impairment, and individuals with elevated [11C]PiB values and cognitive decline may represent those most likely to develop AD.8 An additional advantage of in vivo imaging of Aβ deposition is that it enables prospective evaluation of individuals with elevated Aβ burden who remain cognitively stable vs those who eventually show more marked cognitive decline and impairment. Such studies may aid in identifying possible protective and compensatory factors that allow some individuals with neuropathology to retain cognitive health.
Limitations of our study include the small sample size and the fact that [11C]PiB imaging was not possible at baseline cognitive assessment. In addition, the BLSA is a highly educated community dwelling sample, which limits the generality of our findings. However, the incidence of AD in the BLSA12 and the rates of brain changes17 are comparable to other samples. Our study also has several strengths. BLSA-NI participants were followed prospectively for at least 8 years prior to initial [11C]PiB evaluation, and cognitive status was determined within the context of these longitudinal assessments, with 51 participants rated as CDR = 0 at the time of [11C]PiB evaluation. While we do not yet know which individuals will eventually develop cognitive impairment, our analyses represent a snapshot in time and the fact that most individuals have had stable cognition over ≥8 years increases the likelihood that the majority of participants will remain clinically normal through the next few years of follow-up. Another strength of our study is that our outcome measures of episodic memory, including the CVLT and BVRT, are not used in determination of diagnostic status. Thus, associations between [11C]PiB retention and change on these measures are determined independently of clinical determinations of cognitive status.
Continued longitudinal assessments of Aβ deposition in conjunction with cognitive performance and diagnostic status will provide insights into behavioral manifestations and the pathobiology of disease progression at the earliest stages of the neurodegenerative disease process. In vivo imaging of neuropathology has the potential to identify not only risk factors for clinical manifestation of disease, but also to elucidate possible protective and compensatory factors that maintain cognitive health in successful aging.
ACKNOWLEDGMENT
The authors thank the staff of the PET facility at Johns Hopkins University and the neuroimaging staff of the NIA for their assistance.
DISCLOSURE
GE Healthcare holds a license agreement with the University of Pittsburgh based on the PiB technology described in this manuscript. Drs. Klunk and Mathis are co-inventors of PiB and, as such, have a financial interest in this license agreement. Dr. Resnick serves as Action Editor for Brain and Cognition; and receives research support from the NIH/NIA Intramural Research Program. Dr. Sojkova, Dr. Zhou, Mr. An, Dr. Ye, Dr. Holt report no disclosures. Dr. Dannals serves as editor of the Journal of Labelled Compounds and Radiopharmaceuticals. Dr. Mathis serves on a scientific advisory board for Neuroptix Corporation; has received funding for travel and speaker honoraria from Elan Corporation, GE Healthcare, Bayer-Schering Pharma, IBA, and Takeda Pharmaceutical Company Limited; serves on the editorial board of Nuclear Medicine and Biology; may accrue revenue on over 20 active US and international patents, 1996-present re: amyloid imaging agents; serves as a consultant for GE Healthcare, Elan Corporation, Wyeth, and Novartis; estimates that 30% of his academic effort is spent on PiB imaging, which might benefit the commercial license holder of the technology, GE Healthcare, and the University of Pittsburgh as the licensor of the technology; has received/receives research support from GE Healthcare, Neuroptix Corporation, the NIH (AG018402 [PI]), the US Department of Energy, the Dana Foundation, and the Anonymous Foundation; holds stock options in Neuroptix Corporation; and has received license fees and will receive future royalties from GE Healthcare (amyloid imaging agents for brain applications) and Neuroptix Corporation (amyloid imaging agents for eye applications). Dr. Klunk serves on scientific advisory boards for GE Healthcare, Neuroptix Corporation, Elan Corporation/Janssen, Roche, AstraZeneca; has received funding for travel from Elan Corporation/Janssen, Roche, AstraZeneca, and for lectures or educational activities not funded by industry; holds patents US 7,270,800 (plus related), issued 2007: PiB PET Imaging and US 6,168,776 (plus related), issued 2001: Chrysamine-G Derivatives for imaging and therapy; has received honoraria from Cerebrio LLC; receives research support from Neuroptix Corporation, the NIH (NIA 5 R37 AG025516 [PI], NIA 1 P01 AG025204 [PI], NIA 1 U01 AG028526 [PI], NIA 5 P50 AG005133 [Co-I], NIA 2 R01 AG018402 [Co-I], NIA 5 U01 AG024904-03S3 [Co-I], NIBIB 1 R01 AG026240 [Co-I], NIA 1 R01 AG031110 [Co-I]), and the Anonymous Foundation; has received license fees and will receive future royalties from GE Healthcare (PiB PET Imaging) and Neuroptix Corporation (Chrysamine G Derivatives); and holds stock in Neuroptix Corporation. Dr. Ferrucci serves as Editor-in-Chief of the Journals of Gerontology Medical Sciences. Dr. Kraut serves on the editorial board of the Journal of Magnetic Resonance Imaging; receives royalty from publication of Neural Basis of Semantic Memory (Cambridge University Press, 2007); receives research support from the NIH (NIMH 2 R01 MH085328-05A1 [Co-I], NIMH 1 R01 MH077852-1 [Co-I], NIA 300-N01-AG40012 [Co-I]), and from the Veterans Administration. Dr. Wong serves on editorial advisory boards for Neuropsychopharmacology, the Journal of Cerebral Blood Flow and Metabolism, and Molecular Imaging and Biology; and has received/receives research support from Avid Radiopharmaceuticals, Inc., Amgen, Bristol-Myers Squibb, Intra-Cellular Therapies, Inc., Eli Lilly and Company, Merck Serono, Orexigen Therapeutics, Inc., Otsuka Pharmaceutical Co., Ltd., Roche, sanofi-aventis, the NIH (NIAAA R01AA12839 [PI], NIDA K24, DA00412 [PI], NIMH R01 MH078175 [PI], NIAAA R01 AA010158 [Co-I], NIDA R21DA020777 [Co-I], NIMH R33 MH079017 [Co-I], NIMH R01 MH64823 [Co-I], NIMH U01 MH075378 [Co-I], NIDA R21 DA016182 [Co-I], NIDA R01DA022433 [PI on subcontract]), the DANA Foundation, and the Rett Syndrome Research Foundation.
Supplementary Material
Address correspondence and reprint requests to Dr. Susan M. Resnick, Laboratory of Personality and Cognition, National Institute on Aging, 251 Bayview Blvd./BRC Room 4B335, Baltimore, MD 21224-6825 susan.resnick@nih.gov
Supplemental data at www.neurology.org
e-Pub ahead of print on February 10, 2010, at www.neurology.org.
Study funding: Supported in part by the Intramural Research Program of the NIH/NIA, N01-AG-3-2124, and K24 DA00412 (D.F.W.). A portion of that support was through a R&D contract with MedStar Research Institute. Dr. Klunk and Mathis's contributions were supported by NIH/NIA P01-AG025204, R37 AG025516 and P50-AG005133.
Disclosure: Author disclosures are provided at the end of the article.
Received August 5, 2009. Accepted in final form December 21, 2009.
REFERENCES
- 1.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 1992;42:631–639. [DOI] [PubMed] [Google Scholar]
- 2.Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging 1995;16:285–298. [DOI] [PubMed] [Google Scholar]
- 3.Driscoll I, Resnick SM, Troncoso JC, An Y, O'Brien R, Zonderman AB. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann Neurol 2006;60:688–695. [DOI] [PubMed] [Google Scholar]
- 4.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452. [DOI] [PubMed] [Google Scholar]
- 5.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008;65:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR, Jr., Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 2007;130:2837–2844. [DOI] [PubMed] [Google Scholar]
- 8.Villemagne VL, Pike KE, Darby D, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia 2008;46:1688–1697. [DOI] [PubMed] [Google Scholar]
- 9.Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex 2000;10:464–472. [DOI] [PubMed] [Google Scholar]
- 10.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 11.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114:797–811. [DOI] [PubMed] [Google Scholar]
- 12.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology 2000;54:2072–2077. [DOI] [PubMed] [Google Scholar]
- 13.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Resnick SM, Ye W, et al. Using a reference tissue model with spatial constraint to quantify [11C]Pittsburgh compound B PET for early diagnosis of Alzheimer's disease. Neuroimage 2007;36:298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Endres CJ, Brasic JR, Huang SC, Wong DF. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage 2003;18:975–989. [DOI] [PubMed] [Google Scholar]
- 16.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 2008;29:1456–1465. [DOI] [PubMed] [Google Scholar]
- 17.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 2003;23:3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.